Abstract
Background
Atrial fibrillation (AF) is a common, growing, and costly medical condition. We aimed to evaluate the impact of a management algorithm for symptomatic AF that used an emergency department observation unit on hospital admission rates and patient outcomes.
Methods and Results
This retrospective cohort study compared 563 patients who presented consecutively in the year after implementation of the algorithm, from July 2013 through June 2014 (intervention group), with 627 patients in a historical cohort (preintervention group) who presented consecutively from July 2011 through June 2012. All patients who consented to have their records used for chart review were included if they had a primary final emergency department diagnosis of AF. We observed no significant differences in age, sex, vital signs, body mass index, or CHADS2 (congestive heart failure, hypertension, age, diabetes mellitus, and prior stroke or transient ischemic attack) score between the preintervention and intervention groups. The rate of inpatient admission was significantly lower in the intervention group (from 45% to 36%; P<0.001). The groups were not significantly different with regard to rates of return emergency department visits (19% versus 17%; P=0.48), hospitalization (18% versus 16%; P=0.22), or adverse events (2% versus 2%; P=0.95) within 30 days. Emergency department observation unit admissions were 40% (P<0.001) less costly than inpatient hospital admissions of ≤1 day's duration.
Conclusions
Implementation of an emergency department observation unit AF algorithm was associated with significantly decreased hospital admissions without increasing the rates of return emergency department visits, hospitalization, or adverse events within 30 days.
Keywords: anticoagulants, arrhythmia, fibrillation
Subject Categories: Atrial Fibrillation, Arrhythmias, Cost-Effectiveness, Quality and Outcomes
Introduction
Atrial fibrillation (AF) is the most common cardiac dysrhythmia, with an estimated prevalence of 0.5% to 1% in the general population,1 and it accounts for ≈0.5% of all emergency department (ED) visits.2 Since the 1980s, the annual hospitalizations associated with AF have nearly tripled, a trend that is projected to continue.3, 4 The growing cost of AF, currently estimated to exceed $6 billion annually, is primarily attributable to the rising cost of hospitalization.5, 6 However, the initial diagnosis, evaluation, and management of AF often occur in the ED.3
Management of AF in the ED markedly varies worldwide, particularly regarding the selection of rate versus rhythm control.7, 8, 9 Elective electrical cardioversion (ECV) is increasingly accepted as safe and effective management of acute‐onset AF.10, 11, 12 In the United States, 60% to 70% of patients who present to the ED for AF are admitted.3, 13, 14
Given the explosive growth of this already prevalent condition and the challenge of hospital bed shortages, various solutions have been proposed, including protocols using aggressive electrical rhythm restoration and an ED observation unit (EDOU).15 Initial feasibility studies have indicated potential reductions in length of stay and cost without increasing adverse events or readmission rates.16, 17, 18
To reduce unnecessary hospital admissions and improve the cost and quality of care, a multidisciplinary team that included physicians and allied health staff representing cardiology, primary care, emergency medicine, and thrombophilia collaborated to create a management algorithm for patients presenting to the ED with symptomatic AF. This algorithm, which includes the use of ECV and the EDOU, was implemented on July 18, 2013. We sought to evaluate the impact of this algorithm on admission rates and patient outcomes.
Methods
Study Design
A retrospective cohort study was conducted to compare the characteristics, dispositions, and outcomes of patients who presented before and after implementation of a practice algorithm for AF management. We compared consecutive patients who received a final ED diagnosis of AF from July 2011 and June 2012 (preintervention cohort) with those who presented after inception of the algorithm, from July 2013 through June 2014 (intervention cohort). We separated the cohorts by a year to potentially avoid capturing any effects of preliminary discussions about the algorithm during its creation. All study subjects provided written consent to have their medical records reviewed for research purposes. Patients were excluded if they did not consent to retrospective review or were younger than 18 years. The Mayo Clinic Institutional Review Board approved the research protocol.
Setting
The Mayo Clinic ED sees ≈80 000 patients annually and serves a 1265‐bed hospital that includes 5 primary cardiology services (with a daily census of ≈60 patients), 6 catheterization procedure rooms, and a 16‐bed cardiac intensive care unit. Approximately 6500 interventional cardiology procedures are performed annually, including 500 for the treatment of AF. About 3% of patients seen in the ED are admitted to the 9‐bed EDOU annually. Exclusions to EDOU admission include necessity for restraints, 1:1 nursing care, inability to complete activities of daily living independently, behavioral problems, or isolation precautions. EDOU admission is further limited by room availability.
AF Management Algorithm
In the summer of 2013, a multidisciplinary team with representatives from emergency medicine, cardiology, primary care, and thrombophilia collaborated to create a practice algorithm for the management of patients who presented to the ED with a primary diagnosis of symptomatic AF. The goals were to improve quality of care and to reduce costly, unnecessary admissions. Identified challenges included the need to develop consistent recommendations for initiating anticoagulation therapy appropriately, arrange for reliable, prompt outpatient follow‐up, and standardize rate‐control strategies in terms of drug selection, dosing, and transition from intravenous to oral medication. The practice algorithm is summarized in Figure 1.
Figure 1.
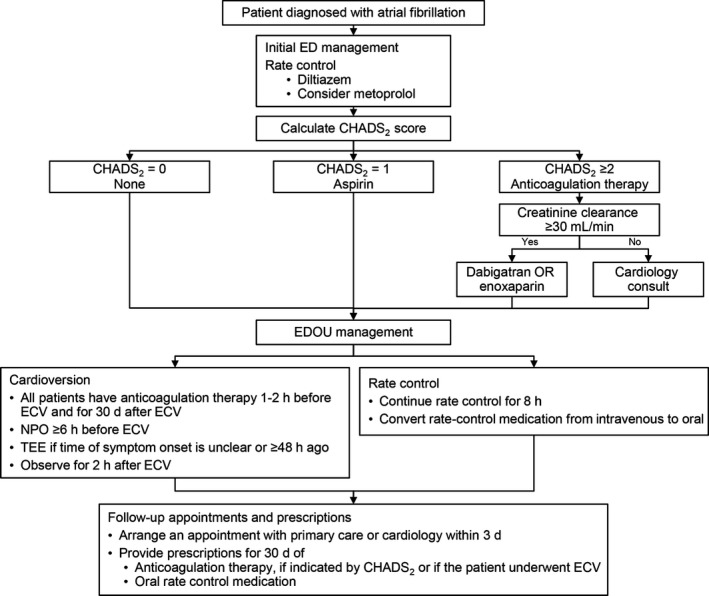
Management algorithm for atrial fibrillation. CHADS 2 indicates congestive heart failure, hypertension, age, diabetes mellitus, and prior stroke or transient ischemic attack; ECV, electrical cardioversion; ED, emergency department; EDOU, emergency department observation unit; NPO, nil per os (nothing by mouth); TEE, transesophageal echocardiography.
Initially, patients were assessed for stability, which was primarily determined by the discretion of the provider, with instability being suggested by chest pain, ST‐segment changes concerning for ischemia, respiratory distress, hypoxia, or hypotension. Patients deemed unstable were excluded from the treatment algorithm and were instead resuscitated as indicated by the clinical scenario.
After the initial evaluation and assessment of stability, intravenous (IV) diltiazem was recommended as the initial medication for rate control at an initial dose of 0.1 to 0.25 mg/kg or 10 to 20 mg IV over 2 minutes. This dose could be repeated 15 minutes later if the heart rate remained >110 beats per minute (bpm) and blood pressure remained adequate. Simultaneously, 30 mg of oral diltiazem was recommended to be administered after the initial bolus was given, which would be scheduled to be given every 6 hours while the patient remained in the ED. If the patient's heart rate continued to be >110 bpm after 2 doses of intravenous diltiazem and 30 mg of oral diltiazem, providers were instructed to consider initiation of a continuous diltiazem infusion at 5 mg/h titrated to a heart rate of 80 to 110 bpm, increased in increments of 3 to 5 mg/h every 5 minutes, as blood pressure allowed.
The need for anticoagulation therapy was determined by the CHADS2 (congestive heart failure, hypertension, age, diabetes mellitus, prior stroke or transient ischemic attack) score, a clinical prediction rule that estimates the yearly risk of stroke in patients with AF, with higher scores indicating increased risk.19 Patients with CHADS2 scores ≥1 had anticoagulation therapy initiated, and patients with scores ≥2 received a 30‐day prescription for dabigatran. Patients received standard treatment with low‐molecular‐weight heparin and warfarin if they had contraindications to dabigatran or if clinical judgment indicated that dabigatran was suboptimal therapy. Cardiology consultations were recommended for complex clinical scenarios, including patients with inadequate creatinine clearance.
Notably, the algorithm was updated in June of 2015 to recommend the use of the CHA2DS2–vascular disease and sex category (‐Vasc) and HAS‐BLED (defined as hemorrhage involving a critical anatomic site, for example, intracranial, or a bleed requiring hospitalization, transfusion of ≥2 units of packed cells, or associated with a decrease in hemoglobin level of ≥2 g/L) scores to estimate the patient's respective risks of embolization and bleeding, to inform shared decision making between the provider and the patient regarding the initiation of anticoagulation.20, 21 The algorithm also was updated to recommend the use of apixaban or rivaroxaban as first‐line therapy in favor of dabigatran, which was the preferential choice throughout the study period.
Early cardioversion was considered for (1) patients with ≥6 hours of nil per os status, a clear time of symptom onset, and symptoms of <48 hours' duration or (2) patients receiving long‐term anticoagulation treatment, with a therapeutic international normalized ratio. The patient's preferences played a vital role in these decisions.
All patients who underwent ECV received a 30‐day prescription for anticoagulation medication, regardless of CHADS2 score, and were anticoagulated for a minimum of 1 hour before the procedure. This guideline was based on the consensus of local experts in thrombophilia and cardiology involved in the creation of the algorithm that the risk of embolization from atrial stunning after ECV, even in patients with <48 hours of arrhythmia, outweighed the risk of bleeding from anticoagulation in these patients.
Patients were transferred to the EDOU after initial stabilization and selection of a strategy. Once in the EDOU, management was largely nurse driven, with the observation unit nurse directed to titrate IV medications to achieve a steady heart rate, coordinate timing of cardioversion if planned, notify the cardiologist on call if a consultation was requested, and arrange for prompt follow‐up. After rate control was achieved, the patient was transitioned to oral rate control medication. Alternatively, if ECV was chosen, transesophageal echocardiography was performed if there was uncertainty regarding the onset of AF or if the duration was >48 hours. Before discharge, patients had a follow‐up appointment scheduled with their primary care provider or a cardiologist within 3 to 5 days. Patients also received a 30‐day prescription for the selected method of anticoagulation if indicated.
Data Collection and Processing
Electronic health records (EHRs) with a final ED diagnosis of AF were identified by a data quality analyst. For each patient, the following data were then extracted: date and time of visit, patient age, sex, diagnosis, disposition, medications administered, and length of hospitalization. Then, a focused chart review was performed by a resident emergency medicine physician and a registered nurse quality improvement coordinator. The following data were extracted and stored in a spreadsheet (Excel; Microsoft Corp): history of hypertension, heart failure, diabetes mellitus, cerebrovascular accident (CVA) or transient ischemic attack, body mass index, and cardioversion performed as part of the index visit. Charts were further reviewed for any of the following events within 30 days of the index visit: outpatient follow‐up, hospital admission, and major adverse events (defined as bleeding, CVA, myocardial infarction, cardiac arrest, or death). Reviewers agreed on and documented predetermined definitions of historical and outcome features: heart failure was considered present, regardless of preserved systolic function, if a diagnosis was made by a cardiologist; hypertension was considered present if the patient had a previous diagnosis of hypertension; cerebrovascular disease included a diagnosis of stroke, CVA, or transient ischemic attack; and adverse events were included only if they did not occur as part of the index visit or admission. These historical features were used to calculate the CHADS2 score. If no adverse events were noted in the EHR, we assumed that they did not occur.
To estimate potential cost differences between EDOU admissions and inpatient admissions, we performed a subgroup analysis of the intervention group, comparing patients admitted to the EDOU with patients with an inpatient hospitalization of ≤1 day's duration. Patients were compared only with others from the same 1‐year period to limit variability caused by changes in reimbursement. We determined costs for each patient by extracting the internal cost of every billed service within the index visit from an existing internal financial decision support system. Costs that occurred in 2013 were multiplied by 1.01442 to account for inflation before comparing them with 2014 costs. Institutional policy prohibits publication of costs in dollar amounts; instead, we present the relative difference in cost between groups. The limitation on inpatient stay (≤1 day) was selected to minimize the effect of the inherent selection bias regarding directing patients toward inpatient hospitalization versus EDOU admission. Presumably, patients with an inpatient length of stay of ≤1 day likely could have been cared for similarly in an EDOU setting.
Data Analysis
The primary outcome measure of our investigation was the inpatient admission rate. Secondary outcomes were short‐term (30‐day) events, including return ED visits, readmission, adverse events, and outpatient follow‐up. Inpatient admission was defined as any admission to the hospital, regardless of hospital status or duration of hospitalization, and included patients admitted under observation status. Patients admitted to the EDOU were considered separately. Additionally, within the intervention group, we investigated features associated with cardioversion, as well as compared features associated with inpatient versus EDOU admission.
Data analysis was performed with the SAS software package (SAS Institute Inc). Continuous variables are reported as medians and IQRs, and categorical variables are reported as frequency counts and percentages. Comparisons of these features between patient groups of interest were evaluated using Wilcoxon rank sum, Fisher exact, and χ2 tests. All tests were 2‐tailed, and P values <0.05 were considered statistically significant.
Results
In the year after the EDOU algorithm was implemented, 563 patients who presented to the ED received a final primary diagnosis of AF (intervention group). These patients were compared with a historical cohort of 627 patients (preintervention group). Therefore, 1190 patients were included in the study. Clinical features of these 2 patient groups are summarized in Table 1. We observed no significant differences in age, sex, presenting vital signs, body mass index, or CHADS2 score. Diabetes mellitus was more common in patients who presented after initiation of the algorithm (22% versus 17%; P=0.02).
Table 1.
Baseline Characteristics of Patients Seen Before (Preintervention) or After (Intervention) Initiation of an EDOU Protocol for Managing Atrial Fibrillation
| Characteristic | Preintervention (n=627) | Intervention (n=563) | P Value |
|---|---|---|---|
| Age, median (IQR), y | 70 (60–79) | 69 (61–79) | 0.81 |
| Initial vital signs, median (IQR) | |||
| Temperature, °C | 36.7 (36.5–36.8) | 36.7 (36.5–36.8) | 0.81 |
| Pulse rate, beats/min | 120 (96–138) | 120 (94–137) | 0.41 |
| Systolic blood pressure, mm Hg | 131 (116–150) | 134 (118–152) | 0.29 |
| Body mass index, median (IQR), kg/m2 | 29.0 (25.0–34.0) | 28.9 (25.0–34.8) | 0.48 |
| CHADS2 score, median (IQR) | 2 (1–2) | 2 (1–3) | 0.30 |
| Male sex, n (%) | 349 (56) | 304 (54) | 0.56 |
| Comorbid conditions, n (%) | |||
| Congestive heart failure | 155 (25) | 150 (27) | 0.45 |
| Diabetes mellitus | 104 (17) | 124 (22) | 0.02 |
| History of cerebrovascular accident or transient ischemic attack | 65 (10) | 62 (11) | 0.72 |
| Hypertension | 407 (65) | 383 (68) | 0.26 |
| CHADS2 score, n (%) | 0.30 | ||
| 0 | 127 (20) | 105 (19) | |
| 1 | 186 (30) | 166 (29) | |
| 2 | 166 (26) | 147 (26) | |
| 3 | 99 (16) | 83 (15) | |
| 4 | 30 (5) | 46 (8) | |
| 5 | 16 (3) | 14 (2) | |
| 6 | 3 (<1) | 2 (<1) | |
| Cardioversion, n (%) | 451 (72) | 402 (71) | 0.84 |
| Disposition, n (%) | <0.001 | ||
| Discharge | 151 (24) | 131 (23) | |
| EDOU | 191 (30) | 232 (41) | |
| Inpatient | 285 (45) | 200 (36) | |
CHADS2 indicates congestive heart failure, hypertension, age, diabetes mellitus, and prior stroke or transient ischemic attack; EDOU, emergency department observation unit.
The absolute rate of inpatient admission was reduced after implementation of the algorithm (from 45% to 36%) and the rate of EDOU admission increased significantly (from 30% to 41%; P<0.001). The reduction in the inpatient admission rate represented a 20% relative reduction in overall admissions between groups. When compared with patients admitted to the EDOU, patients admitted to the hospital were older, had lower initial systolic blood pressure, and had more comorbid conditions (Table 2). Before implementation of the algorithm, 14.7% of patients admitted to the EDOU were converted to inpatient admission, whereas 18.1% were admitted after (P=0.34). Of the 232 patients admitted to the EDOU in the intervention group, 42 patients were converted to inpatient admissions. Converted patients generally were older (median 74 [IQR 61–80] versus 66 [IQR 59–76] years; P=0.04), and more had congestive heart failure (33% versus 17%; P=0.02).
Table 2.
Characteristics of Patients Admitted to the EDOU or Inpatient Service
| Characteristic | EDOU (n=232) | Inpatient (n=200) | P Value |
|---|---|---|---|
| Baseline | |||
| Age, median (IQR), y | 67 (60–77) | 74 (65–82) | <0.001 |
| Initial vital signs, median (IQR) | |||
| Temperature, °C | 36.7 (36.5–36.8) | 36.7 (36.5–36.9) | 0.25 |
| Pulse rate, beats/min | 122 (99–137) | 120 (96–140) | 0.84 |
| Systolic blood pressure, mm Hg | 137 (121–154) | 131 (114–150) | 0.02 |
| Body mass index, median (IQR), kg/m2 | 29.7 (25.3–35.5) | 28.4 (24.9–34.7) | 0.17 |
| CHADS2 score, median (IQR) | 1 (1–2) | 2 (1–3) | <0.001 |
| Male sex, n (%) | 125 (54) | 108 (54) | 0.98 |
| Comorbid conditions, n (%) | |||
| Congestive heart failure | 47 (20) | 82 (41) | <0.001 |
| Diabetes mellitus | 38 (16) | 65 (33) | <0.001 |
| History of cerebrovascular accident or transient ischemic attack | 16 (7) | 28 (14) | 0.02 |
| Hypertension | 147 (63) | 159 (80) | <0.001 |
| CHADS2 score, n (%) | |||
| 0 | 52 (22) | 12 (6) | <0.001 |
| 1 | 86 (37) | 50 (25) | |
| 2 | 53 (23) | 59 (30) | |
| 3 | 25 (11) | 44 (22) | |
| 4 | 13 (6) | 25 (13) | |
| 5 | 3 (1) | 8 (4) | |
| 6 | 0 (0) | 2 (1) | |
| Thirty‐day patient outcomes | |||
| Outpatient follow‐up, n (%) | 181 (78) | 164 (82) | 0.71 |
| Return ED visit | |||
| No. of patients (%) | 42 (18) | 27 (14) | 0.23 |
| No. of ED visits, No. of patients (%) (n=69) | 0.48 | ||
| 1 | 35 (83) | 24 (89) | |
| 2 | 4 (10) | 3 (11) | |
| 3 | 2 (5) | 0 (0) | |
| 4 | 1 (2) | 0 (0) | |
| Hospital admissions | |||
| No. of patients (%) | 34 (15) | 35 (18) | 0.36 |
| No. of hospital admissions, No. of patients (%) (n=69) | 0.71 | ||
| 1 | 30 (88) | 32 (91) | |
| 2 | 4 (12) | 3 (9) | |
| Adverse events, n (%) | 3 (1) | 7 (4) | 0.20 |
CHADS2 indicates congestive heart failure, hypertension, age, diabetes mellitus, and prior stroke or transient ischemic attack; ED, emergency department; EDOU, emergency department observation unit.
The percentage of patients who underwent ECV remained essentially unchanged (29% versus 28%; P=0.84) after implementation of the algorithm. A total of 337 patients in the study underwent ECV, with 37% of these procedures being performed by an emergency medicine physician in an ED setting and 63% occurring in the catheterization laboratory under the care of a cardiologist and anesthesiologist. This distribution did not change markedly between the preintervention and intervention cohorts. Patients undergoing ECV were younger, had lower CHADS2 scores, and were more often admitted to the EDOU compared with those who did not undergo ECV.
Outcome data are summarized in Table 3. For patients admitted to the inpatient service, the median length of stay was 2 days in both groups (P=0.30). Overall, 8 patients died before hospital discharge (3 in the preintervention group and 5 in the intervention group). We observed no significant differences in the rate of return ED visits (19% versus 17%; P=0.48), hospital admissions (18% versus 16%; P=0.22), or major adverse events (2% versus 2%; P=0.95) within 30 days of the index visit. Of the 12 adverse events that occurred in the preintervention group, 8 patients died within 30 days of their visit, 1 patient received a diagnosis of a non–ST‐elevation myocardial infarction, 1 patient received a diagnosis of CVA, and 2 patients had clinically significant bleeding. Eleven adverse events occurred in the intervention group: 5 deaths, 3 episodes of clinically significant bleeding, 1 CVA, 1 ST‐elevation myocardial infarction, and 1 cardiac arrest with return of spontaneous circulation.
Table 3.
Thirty‐Day Patient Outcomes Before (Preintervention) and After (Intervention) Initiation of an EDOU Algorithm for Managing Atrial Fibrillation
| Outcome | Preintervention (n=627) | Intervention (n=563) | P Value |
|---|---|---|---|
| Duration of hospitalization, median (IQR), d (n=548) | 2 (1–4) | 2 (1–4) | 0.66 |
| Outpatient follow‐up, n (%) | 491 (78) | 448 (80) | 0.59 |
| Return ED visit | |||
| No. of patients (%) | 116 (19) | 95 (17) | 0.48 |
| No. of ED visits, No. of patients (%) (n=211)a | 0.85 | ||
| 1 | 94 (81) | 78 (82) | |
| 2 | 17 (15) | 13 (14) | |
| 3 | 4 (3) | 3 (3) | |
| 4 | 1 (1) | 1 (1) | |
| Hospital admission | |||
| No. of patients (%) | 114 (18) | 87 (15) | 0.22 |
| No. of hospital admissions, n (%) (n=201)b | 0.53 | ||
| 1 | 99 (87) | 78 (90) | |
| 2 | 14 (12) | 9 (10) | |
| 3 | 1 (1) | 0 (0) | |
| Adverse events, n (%) (n=23)c | 0.95 | ||
| No. of patients (%) | 12 (2) | 11 (2) | |
| Death | 8 (37) | 5 (45) | |
| Cardiac arrest with return of spontaneous circulation | 0 (0) | 1 (9) | |
| Acute coronary syndrome | 1 (8) | 1 (9) | |
| Cerebrovascular accident | 1 (8) | 1 (9) | |
| Bleeding | 2 (17) | 3 (27) | |
ED indicates emergency department; EDOU, emergency department observation unit.
Preintervention group, n=115; intervention group, n=93.
Preintervention group, n=113; intervention group, n=79.
Preintervention group, n=12; intervention group, n=11.
For the subgroup analysis, we compared the median cost of the 232 EDOU admissions versus the 69 inpatient admissions with a hospital length of stay of ≤1 day. EDOU care was 40% less costly than hospitalization (P<0.001). The breakdown of these costs by category is shown in Figure 2. EDOU care was less costly with regard to room, professional, medication, laboratory, and ED costs, with >80% of the difference in cost between the 2 groups being accounted for by room and professional costs.
Figure 2.
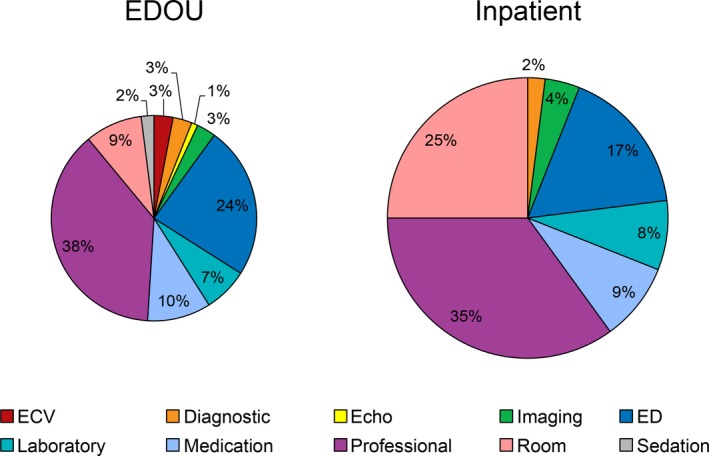
Relative total costs, stratified by category, for patients admitted to the EDOU vs inpatient service. Circle sizes are proportionate to the respective costs of care (40% lower for patients managed in the EDOU). “Inpatient” represents patients hospitalized for ≤1 day. Echo indicates echocardiography; ECV, electrical cardioversion; ED, emergency department; EDOU, emergency department observation unit; professional, cost charged by physicians or advanced practice providers.
Discussion
Our study demonstrates the impact of implementing a management algorithm using EDOU care for the treatment of AF. We report a 20% reduction in inpatient admissions without increasing return ED visits, hospitalization, or adverse events within 30 days. Further, we estimated a 40% cost reduction associated with EDOU care compared with inpatient hospitalizations of ≤1 day.
The advantage of our observational, retrospective design was the ability to evaluate an algorithm that is actively in practice, thereby enhancing the clinical applicability of our findings. Disadvantages of our single‐site retrospective review include reliance on chart review, which may have resulted in missed cases, inaccuracies in the medical history, missed procedures (eg, cardioversion), and incomplete capture of adverse events. Notably, our hospital system accounts for two‐thirds of local ED visits and serves as the regional referral center, and our institution possesses a highly integrated EHR that captures much of the surrounding primary care. Further, as the implementation of the algorithm was part of a quality improvement initiative, patients were identified and prospectively added to a quality improvement outcome collection database during the postintervention period; this outcome information was incorporated into the final analysis.
In addition to potential inaccuracies introduced by chart review, the consecutive preimplementation and postimplementation design of our study introduces the possibility that outcome results were confounded by ongoing changes in standard practice as well as by other health system improvements that were ongoing throughout this period. To our knowledge, there were no major national guideline changes or local system changes that would account for these results. In contrast, during the intervention period, our ED facility underwent a massive renovation. In fact, construction began 4 days after the introduction of the AF treatment algorithm. This renovation resulted in widely fluctuating availability of EDOU beds. These logistical challenges likely limited the full impact of the algorithm.
The retrospective nature of our study limited our ability to truly compare costs, given that patients who were admitted as inpatients likely did not meet criteria for the EDOU or were judged to be more ill than their EDOU counterparts. Understandably, patients admitted as inpatients were older and generally had more comorbidities (Table 2). To minimize the impact of this inherent referral bias, we limited our inpatient admission cost analysis to patients hospitalized for ≤1 day. We hypothesized that patients with very brief hospitalizations were unlikely to be vastly different from the EDOU population and were more likely admitted for logistical reasons, particularly lack of space in the EDOU.
Notably, a recently published, large, multicenter epidemiologic study reported a median length of hospital stay of 3 days (IQR 2–5 days) for patients admitted for AF.6 In our study, the median length of stay for patients admitted to the hospital in both the preintervention and the postintervention group was 2 days (IQR 1–4 days). Thus, we may have significantly underestimated the cost difference. However, we were unable to truly compare costs between these 2 approaches, nor were we able to compare preintervention versus postintervention costs because of marked changes in reimbursement during the study period.
The 40% difference in cost observed between the EDOU group and patients admitted with a hospital length of stay of ≤1 day was largely accounted for by reduced room and professional costs. EDOU room costs may be less compared with inpatient admissions primarily because of lengths of stay of <24 hours as EDOU room costs are calculated on an hourly basis. Further, EDOU care is less resource intensive in terms of professional costs as care is largely protocol driven and does not require transfer of care to an additional team or as frequently result in the consultation of a specialist. The lower cost of care we observed in the EDOU group is consistent with the findings of other studies reporting cost savings resulting from the use of EDOU care for the management of chest pain, asthma, transient ischemic attacks, and infections.22
Two previous investigations of the use of EDOU protocols for AF have been reported. In 2002, Koenig et al16 published a case series reporting the feasibility of an EDOU treatment protocol for AF, including 67 patients with symptoms of <48 hours' duration. The rate of inpatient admission in that study was 80%, with a 7% rate of EDOU admission and a 19% conversion rate from EDOU admission to inpatient admission. Six percent of patients returned to the ED within 7 days of the index visit. In 2008, our department published a prospective randomized trial evaluating an EDOU protocol for acute onset (<48 hours) AF that included 153 patients.17 Seventy‐five patients were randomized to EDOU management, and 9 patients (12%) were converted to inpatient admission. There was no significant difference between patients in the EDOU group versus those receiving routine inpatient care in repeat hospitalization or adverse events during the follow‐up period.
In contrast to the 2 aforementioned, prospective feasibility studies, the current study retrospectively examined the impact of an EDOU management algorithm for AF after its implementation into clinical practice. This allowed us to examine the effect of the algorithm on admission rates, which is not possible in the setting of a randomized trial. Further, we did not limit our study to acute‐onset AF, as was done in the earlier studies. Multidisciplinary collaboration was critical to create a broadly applicable algorithm, which provided guidance for rate and rhythm control strategies, prompt outpatient follow‐up, cardiology consultation within the EDOU, and initiation of long‐term anticoagulation therapy when appropriate.
Based on our data, patients with a final primary diagnosis of AF compose ≈0.5% to 1% of all visits to our ED, which is consistent with previous reports. Because of the retrospective, inclusive design of our study, we likely included patients who were older and had more comorbidities compared with patients reported in previous studies analyzing the impact of AF treatment protocols, particularly with regard to history of congestive heart failure and CVA.6, 13, 18 Our rates of adverse events were consistent with those previously reported.2
Even before the implementation of the AF algorithm, our admission rate was markedly lower than that previously reported nationally (45% versus >60%).13, 14 We postulate that our earlier randomized controlled trial, previous quality improvement initiatives, and availability of primary care and subspecialty follow‐up at our institution contributed to this difference. Before algorithm implementation, 30% of patients who received a final diagnosis were already being admitted to the EDOU. A practice algorithm such as ours would likely have the largest impact at a center that currently does not use an observation unit for acute AF management.
Conclusion
The implementation of an EDOU management algorithm for AF was associated with a 20% decrease in hospital admissions without increasing return visits, hospitalization, or adverse events within 30 days. EDOU care was 40% less expensive than hospitalizations of ≤1 day. Therefore, adoption of similar management algorithms would likely result in considerable cost savings for the management of this increasingly prevalent and costly medical condition.
Sources of Funding
Cost analysis support was provided by the Center for Clinical and Translational Science, which is supported by grant UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
Acknowledgments
We would like to thank Sue Visscher, PhD, for her guidance regarding cost analysis.
(J Am Heart Assoc. 2016;5:e002984 doi: 10.1161/JAHA.115.002984)
Portions of this manuscript have been published in abstract form: Acad Emerg Med. 2015;22 (suppl S1):S16.
References
- 1. Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey J‐Y, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–e198. [DOI] [PubMed] [Google Scholar]
- 2. Atzema CL, Austin PC, Miller E, Chong AS, Yun L, Dorian P. A population‐based description of atrial fibrillation in the emergency department, 2002 to 2010. Ann Emerg Med. 2013;62:570–577.e577. [DOI] [PubMed] [Google Scholar]
- 3. McDonald AJ, Pelletier AJ, Ellinor PT, Camargo CA Jr. Increasing US emergency department visit rates and subsequent hospital admissions for atrial fibrillation from 1993 to 2004. Ann Emerg Med. 2008;51:58–65. [DOI] [PubMed] [Google Scholar]
- 4. Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, McManus DD, Dupuis J, Ellinor PT, Benjamin EJ. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011;124:1982–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. [DOI] [PubMed] [Google Scholar]
- 6. Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, Shah N, Chothani A, Savani GT, Mehta K, Parikh V, Rathod A, Badheka AO, Lafferty J, Kowalski M, Mehta JL, Mitrani RD, Viles‐Gonzalez JF, Paydak H. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129:2371–2379. [DOI] [PubMed] [Google Scholar]
- 7. Stiell IG, Clement CM, Brison RJ, Rowe BH, Borgundvaag B, Langhan T, Lang E, Magee K, Stenstrom R, Perry JJ, Birnie D, Wells GA. Variation in management of recent‐onset atrial fibrillation and flutter among academic hospital emergency departments. Ann Emerg Med. 2011;57:13–21. [DOI] [PubMed] [Google Scholar]
- 8. Rogenstein C, Kelly AM, Mason S, Schneider S, Lang E, Clement CM, Stiell IG. An international view of how recent‐onset atrial fibrillation is treated in the emergency department. Acad Emerg Med. 2012;19:1255–1260. [DOI] [PubMed] [Google Scholar]
- 9. Coll‐Vinent B, Fuenzalida C, Garcia A, Martin A, Miro O. Management of acute atrial fibrillation in the emergency department: a systematic review of recent studies. Eur J Emerg Med. 2013;20:151–159. [DOI] [PubMed] [Google Scholar]
- 10. Mead GE, Elder AT, Flapan AD, Kelman A. Electrical cardioversion for atrial fibrillation and flutter. Cochrane Database Syst Rev. 2005;3:CD002903. [DOI] [PubMed] [Google Scholar]
- 11. Bellone A, Etteri M, Vettorello M, Bonetti C, Clerici D, Gini G, Maino C, Mariani M, Natalizi A, Nessi I, Rampoldi A, Colombo L. Cardioversion of acute atrial fibrillation in the emergency department: a prospective randomised trial. Emerg Med J. 2012;29:188–191. [DOI] [PubMed] [Google Scholar]
- 12. Cohn BG, Keim SM, Yealy DM. Is emergency department cardioversion of recent‐onset atrial fibrillation safe and effective? J Emerg Med. 2013;45:117–127. [DOI] [PubMed] [Google Scholar]
- 13. Zimetbaum P, Reynolds MR, Ho KK, Gaziano T, McDonald MJ, McClennen S, Berezin R, Josephson ME, Cohen DJ. Impact of a practice guideline for patients with atrial fibrillation on medical resource utilization and costs. Am J Cardiol. 2003;92:677–681. [DOI] [PubMed] [Google Scholar]
- 14. Sacchetti A, Williams J, Levi S, Akula D. Impact of emergency department management of atrial fibrillation on hospital charges. West J Emerg Med. 2013;14:55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stiell IG, Clement CM, Perry JJ, Vaillancourt C, Symington C, Dickinson G, Birnie D, Green MS. Association of the Ottawa Aggressive Protocol with rapid discharge of emergency department patients with recent‐onset atrial fibrillation or flutter. CJEM. 2010;12:181–191. [DOI] [PubMed] [Google Scholar]
- 16. Koenig BO, Ross MA, Jackson RE. An emergency department observation unit protocol for acute‐onset atrial fibrillation is feasible. Ann Emerg Med. 2002;39:374–381. [DOI] [PubMed] [Google Scholar]
- 17. Decker WW, Smars PA, Vaidyanathan L, Goyal DG, Boie ET, Stead LG, Packer DL, Meloy TD, Boggust AJ, Haro LH, Laudon DA, Lobl JK, Sadosty AT, Schears RM, Schiebel NE, Hodge DO, Shen WK. A prospective, randomized trial of an emergency department observation unit for acute onset atrial fibrillation. Ann Emerg Med. 2008;52:322–328. [DOI] [PubMed] [Google Scholar]
- 18. Elmouchi DA, VanOosterhout S, Muthusamy P, Khan M, Puetz C, Davis AT, Brown MD. Impact of an emergency department‐initiated clinical protocol for the evaluation and treatment of atrial fibrillation. Crit Pathw Cardiol. 2014;13:43–48. [DOI] [PubMed] [Google Scholar]
- 19. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 20. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 21. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 22. Baugh CW, Venkatesh AK, Bohan JS. Emergency department observation units: a clinical and financial benefit for hospitals. Health Care Manage Rev. 2011;36:28–37. [DOI] [PubMed] [Google Scholar]


