Abstract
Background
Inadvertent damage to leads for transvenous pacemakers, implantable cardioverter‐defibrillators, and cardiac resynchronization therapy defibrillators is an important complication associated with generator‐replacement procedures. We sought to estimate the incidence and costs associated with transvenous lead damage following cardiac implantable electronic device replacement.
Methods and Results
Using the Truven Health Analytics MarketScan Commercial Research Database, we identified health care claims between 2009 and 2013 for lead damage following generator replacement. Patients were identified by claims with a procedure code for cardiac implantable electronic device replacement and then evaluated for 1 year. All follow‐up visits for lead damage were identified, and incidence, risk factors, and hospitalization costs were determined. A total of 22 557 patients with pacemakers, 20 632 with implantable cardioverter‐defibrillators, and 2063 with cardiac resynchronization therapy defibrillators met selection criteria. Incidence of lead damage was 0.46% for pacemaker replacement, 1.27% for implantable cardioverter‐defibrillator replacement, and 1.94% for cardiac resynchronization therapy defibrillator replacement procedures (P<0.001). After adjusting for patient characteristics, patients with implantable cardioverter‐defibrillators and cardiac resynchronization therapy defibrillators demonstrated risk of lead damage that was, respectively, double (hazard ratio 2.00, 95% CI 1.57–2.55) and >2.5 times (hazard ratio 2.58, 95% CI 1.73–3.83) that of patients with pacemakers. Lead revision or repair procedures were associated with increased inpatient hospitalization costs (mean $19 959 for pacemaker, $24 885 for implantable cardioverter‐defibrillator, and $46 229 for cardiac resynchronization therapy defibrillator; P=0.048, Kruskal–Wallis test).
Conclusions
These findings establish the first objective assessment of the incidence, risk factors, and economic burden of lead damage following cardiac implantable electronic device replacement in the United States. New care algorithms are warranted to avoid these events, which impose substantial burdens on patients, physicians, and payors.
Keywords: cardiac resynchronization therapy, cost, implantable cardioverter‐defibrillator, pacemaker
Subject Categories: Electrophysiology, Complications
Introduction
Concurrent with the increasing age of the population, the use of cardiac implantable electronic devices (CIEDs) in the United States is growing. A retrospective analysis of the Nationwide Inpatient Sample data set reported that the incidence of CIED implants increased by 96% between 1993 and 2008, with the majority of this growth coming from an exponential increase in implantable cardioverter‐defibrillator (ICD) implantation (504% increase).1 Most of these patients will require a subsequent generator change in their lifetime. Although generator replacements are relatively low‐risk procedures, the development of a pocket hematoma and/or infection are two of the most recognized complications of the procedure.1, 2, 3
Another potential complication during a generator‐replacement procedure is inadvertent damage to of the transvenous leads within the device pocket. Lead damage can occur when a sharp instrument mechanically compromises a lead during dissection of the surrounding fibrous tissue or when an electrosurgical instrument melts or causes a dielectric failure of the silicone, polyurethane, or copolymer lead insulation.4 Prior retrospective studies enrolling a small number of patients have suggested a relationship among lead damage, the use of traditional monopolar electrosurgical instruments, CIED type, number of leads in the pocket, and the material composition of the lead insulation (eg, polyurethane, silicone, or copolymer).4 Because lead failures secondary to damage do not typically present clinically for months following the generator‐change procedure, the incidence and risk factors for occurrence have been poorly characterized to date. In the present study, we sought to determine the incidence of lead damage and the associated total health care utilization costs from clinical management following a CIED‐replacement procedure.
Methods
Data Source
This retrospective database analysis reviewed health care claims data from the Truven Health Analytics MarketScan Commercial Research Database. This data source includes information for >115 million unique patients in the United States, residing in all census regions since 1995.5 Patients are covered with employer‐sponsored private health plans, including employees, early retirees (including those with Medicare Advantage plans), and dependents. The database contains fully deidentified data sets, with randomized identification numbers applied to all data to protect the identities of both the patients and the sites of care. Consequently, institutional review board approval was not required for this study.
The database includes details on billed patient claims for outpatient and inpatient visits and for prescription drug fills. Medical claims list the dates of service; the place of service; the International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis codes; the Current Procedural Terminology (CPT) codes; and total payment. This data source includes only fully adjudicated and paid claims. Total payment for an inpatient or outpatient visit (ie, cost as used throughout this paper) is the sum of amounts paid to all providers by the insurer plus coinsurance, copayments, and deductibles paid by the patient for the same hospital stay or visit. In addition, an eligibility file includes details on patient enrollment, age, sex, and geographic region. This data source does not provide mortality information; however, for a subset of patients, mortality can be identified if the discharge disposition from the inpatient visit was listed as “dead.”
Patient Selection and Study Design
We included patients with a pacemaker (PM), an ICD, or a cardiac resynchronization therapy defibrillator (CRT‐D) who underwent a generator‐replacement procedure. Patients with a CRT‐PM device were excluded from the analysis because they composed only 0.7% of the entire study cohort; therefore, meaningful analysis was not feasible in this cohort.
Patients were eligible for inclusion if there was a claim (on an outpatient or inpatient basis) during the years 2010–2012 for a CIED generator implantation, replacement, or removal procedure (Table S1; Figure 1). The date of this claim served as the index date for analysis. Patients undergoing other major cardiac procedures (diagnostic procedure of the heart and pericardium, percutaneous coronary intervention, coronary artery bypass grafting surgery, catheter ablation, or heart valve surgery) during the same index visit were then excluded from analysis (Table S2), similar to methodology used by Reynolds et al.6
Figure 1.
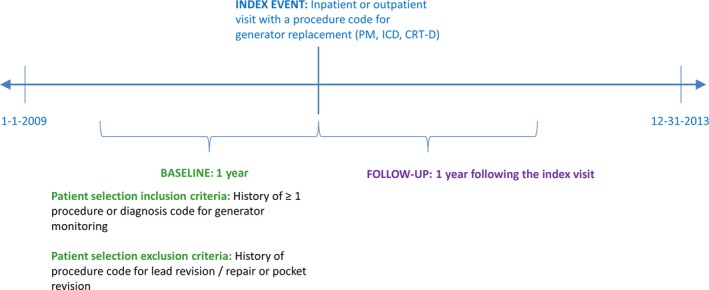
Study period for analysis. CRT‐D indicates cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter‐defibrillator; PM, pacemaker.
Next, a 1‐year baseline period was defined as the 12 months prior to the index visit for device replacement. To ensure that the index CIED procedure was a device replacement and not a de novo procedure, all patients were required to have at least 1 visit with an ICD‐9 diagnosis code for history of cardiac device or an ICD‐9 or CPT procedure code for CIED monitoring during the 1‐year baseline period (Table S3). In addition, to ensure that the index visit for CIED replacement was for a true device replacement due to age of the device and not to complications related to a recent visit, patients were excluded if there was evidence of ≥1 procedure code for lead revision or repair, insertion of a temporary system, or pocket revision during the 1‐year baseline period (Table S2). Finally, all patients were required to be aged ≥18 years, to have continuous health plan enrollment for the duration of follow‐up (with an allowed 1‐month gap in coverage), and not to have a CRT‐PM device type.
The follow‐up period was defined as the 1 year following the CIED‐replacement procedure. The duration of follow‐up was limited to this period to ensure that follow‐up visits with codes for lead damage or repair were likely related to the index device‐replacement procedure (Figure 1).
Study Measures
We summarized information about each patient's age, sex, and race at the index visit (ie, the date of the CIED‐replacement procedure). Comorbidity status was evaluated by calculating a Charlson Comorbidity Index (CCI) score for each patient. The CCI is an objective composite measure of physical health status commonly used in studies of medical claims and chronic disease, principally to predict 10‐year mortality of patients with a variety of comorbid conditions.7, 8 We also summarized information about use of relevant prescription drugs such as antiplatelet agents (with the exception of aspirin, which is an over‐the‐counter medication) and anticoagulants (warfarin, dabigatran, rivaroxaban, apixaban) during the 12 months prior to device replacement.
For each CIED‐replacement procedure, we determined whether the procedure was performed in an outpatient or inpatient setting. Of note, because of the lack of specificity in currently used ICD‐9 and CPT codes, it was not possible to determine whether the CIED‐replacement procedure was a generator change alone or was coupled to a procedure requiring the addition of leads (eg, an upgrade procedure).
Lead damage in the follow‐up period was defined as any outpatient or inpatient visit with an ICD‐9 procedure code for lead revision or repair (00.52, 37.74–37.77, 37.95, 37.97) and with no concurrent ICD‐9 diagnosis code for infection during the same visit (infection and inflammatory reaction due to cardiac device, implant, and graft [996.61], septicemia [038.xx], or endocarditis [421.xx]). Patients with a diagnosis of infection were excluded to eliminate the likelihood that leads were removed for infection as opposed to lead damage. The overall incidence of lead damage was defined as the total number of patients with a follow‐up visit for lead revision or repair in the first year following a CIED‐replacement procedure divided by the total number of patients in the study cohort. If a patient had both outpatient and inpatient visits for lead damage during follow‐up, the visit was categorized as an inpatient visit only to avoid double counting.
Finally, we sought to determine health care utilization related to lead damage. In >90% of instances, lead damage resulted in care provided in the inpatient setting; therefore, we had insufficient data to assess outpatient costs (only 12 PM, 23 ICD, and 3 CRT‐D patients presented with lead damage in an outpatient setting). Given that sample sizes were sufficient to summarize total visit cost for patients presenting with lead damage in the inpatient setting, we calculated the total hospitalization cost by summing the amount paid to the hospital by the insurer plus coinsurance, copayments, and deductibles paid by the patient. Given the distribution of total hospitalization costs observed, we excluded from cost analyses 19 patients whose total costs fell into the top 5% of the cohort (greater than $73 573 for the PM cohort, $191 042 for ICD, and $296 320 for the CRT‐D cohort).9, 10, 11, 12 We hypothesized that these patients may have had other major procedures besides just lead revision or repair, resulting in the extreme variance in cost. Following exclusion of these patients, the data remained rightward‐skewed; therefore, a nonparametric test of statistical significance (Kruskal–Wallis test) was used for cost comparisons. All costs were adjusted to 2013 US dollars using the Medical Care Component of the Consumer Price Index.13
Data Analyses
Descriptive analyses were conducted for all study measures outlined above, including mean, median, and standard deviation for continuous measures and proportions for binary measures. Statistical significance testing comparing outcomes by CIED type (PM, ICD, CRT‐D) were conducted with the chi‐square test for categorical variables and the Kruskal–Wallis test for continuous variables (the nonparametric equivalent of a 1‐way ANOVA test to account for nonnormality). Cox proportional hazards models were run to identify factors associated with risk of lead‐related complications emerging during follow‐up, with patients followed from the date of discharge to a lead‐damage event, death during an inpatient hospitalization, or the end of follow‐up, whichever occurred first. Covariates included patient demographics (age group, sex, CCI, antiplatelet or anticoagulant therapy history), index procedure setting (inpatient versus outpatient), and CIED type. Patients were censored in the model at their date of death (if an inpatient visit with discharge disposition of “dead” was observed during follow‐up) or at end of follow‐up, whichever occurred first. A P value of <0.05 was considered statistically significant. All analyses were performed using the Instant Health Data suite (Boston Health Economics, Inc) and SAS software (version 9.2; SAS Institute).
Results
The study cohort included 45 252 patients (Figure 2). Their mean age was 72.2±13.8 years, and 64% of the cohort was male (Table 1). The generator‐change procedure was performed in an outpatient setting in 66.4% of patients. The median CCI was 2, with nearly 38% of the cohort having a CCI >3. The 5 most prevalent comorbidities in the study cohort used in the CCI calculation were congestive heart failure, diabetes without chronic complications, chronic pulmonary disease, cerebrovascular disease, and renal disease. An anticoagulant (most commonly warfarin) was used by 26.5% of patients and an antiplatelet drug by 16.7%.
Figure 2.
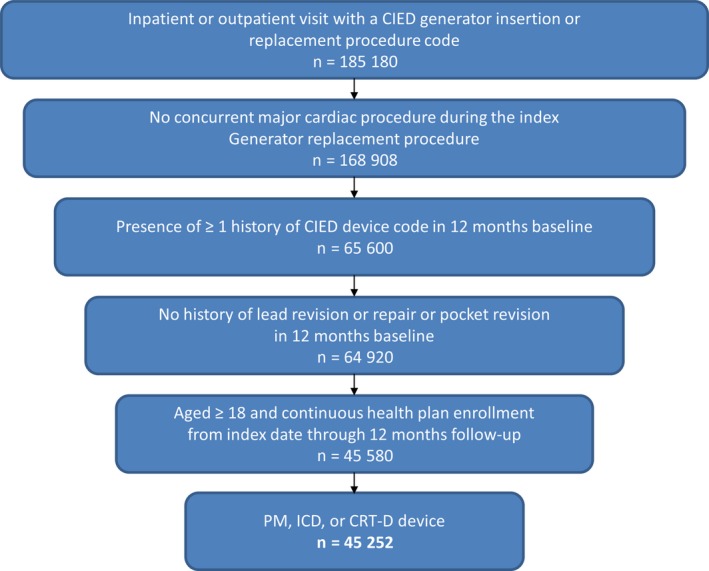
Patient selection. CIED indicates cardiac implantable electronic device; CRT‐D, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter‐defibrillator; PM, pacemaker.
Table 1.
Patient Demographic and Clinical Characteristics
| PM | ICD | CRT‐D | P Valuea | Overall | |
|---|---|---|---|---|---|
| Patients (%) | 22 557 (49.8) | 20 632 (45.6) | 2063 (4.6) | 45 252 | |
| Age, y | |||||
| Mean | 75.5 | 68.8 | 69.1 | <0.001 | 72.2 |
| SD | 13.8 | 12.9 | 12.2 | 13.8 | |
| Median | 79.0 | 70.0 | 71.0 | 75.0 | |
| Male (%) | 12 207 (54.1) | 15 374 (74.5) | 1544 (74.8) | <0.001 | 29 125 (64.4) |
| Residence (%) | <0.001 | ||||
| Northeast | 4621 (20.5) | 4223 (20.5) | 480 (23.3) | 9324 (20.6) | |
| South | 7168 (31.8) | 6958 (33.7) | 645 (31.3) | 14 771 (32.6) | |
| Midwest | 6672 (29.6) | 6406 (31.1) | 616 (29.9) | 13 694 (30.3) | |
| West | 3804 (16.9) | 2754 (13.4) | 300 (14.5) | 6858 (15.2) | |
| Missing | 292 (1.3) | 291 (1.4) | 22 (1.1) | 605 (1.3) | |
| Charlson score | |||||
| Mean | 1.80 | 2.42 | 2.86 | <0.001 | 2.13 |
| SD | 1.90 | 2.03 | 2.08 | 2.00 | |
| 25th percentile | 0.00 | 1.00 | 1.00 | 1.00 | |
| Median | 1.00 | 2.00 | 2.00 | 2.00 | |
| 75th percentile | 3.00 | 4.00 | 4.00 | 3.00 | |
| Score breakout (%) | <0.001 | ||||
| 0 | 6960 (30.9) | 3381 (16.4) | 173 (8.4) | 10 514 (23.2) | |
| 1 | 5158 (22.9) | 4788 (23.2) | 452 (21.9) | 10 398 (23.0) | |
| 2 | 4084 (18.1) | 4123 (20.0) | 409 (19.8) | 8616 (19.0) | |
| 3+ | 6355 (28.2) | 8340 (40.4) | 1029 (49.9) | 15 724 (37.8) | |
| Selected Charlson comorbidities (%) | |||||
| Congestive heart failure | 6484 (28.7) | 14 824 (71.9) | 1927 (93.4) | <0.001 | 23 235 (51.4) |
| Diabetes without complications | 6119 (27.1) | 7283 (35.3) | 795 (38.5) | <0.001 | 14 197 (31.4) |
| Diabetes with complications | 1698 (7.5) | 1996 (9.7) | 238 (11.5) | <0.001 | 3932 (8.7) |
| Chronic pulmonary disease | 4728 (21.0) | 4803 (23.3) | 595 (28.8) | <0.001 | 10 126 (22.4) |
| Cerebrovascular disease | 4086 (18.1) | 3338 (16.2) | 352 (17.1) | <0.001 | 7776 (17.2) |
| Renal disease | 2678 (11.9) | 3235 (15.7) | 407 (19.7) | <0.001 | 6320 (14.0) |
| Other selected conditions (%) | |||||
| Bradycardia | 15 090 (66.9) | 3159 (15.3) | 467 (22.6) | <0.001 | 18 716 (41.4) |
| Tachycardia | 2458 (10.9) | 12 572 (60.9) | 1156 (56.0) | <0.001 | 16 186 (35.8) |
| Fibrillation or flutter | 11 953 (53.0) | 10 442 (50.6) | 1239 (60.1) | <0.001 | 23 634 (52.2) |
| Baseline medication use (%) | |||||
| Warfarin | 5672 (25.2) | 5575 (27.0) | 753 (36.5) | <0.001 | 12 000 (26.5) |
| NOAC | 497 (2.2) | 479 (2.3) | 51 (2.5) | 0.583 | 1027 (2.3) |
| Antiplatelet therapy, any | 3004 (13.3) | 4103 (19.9) | 450 (21.8) | <0.001 | 7557 (16.7) |
| CIED replacement setting (%) | |||||
| Inpatient | 5758 (25.5) | 7603 (36.9) | 1844 (89.4) | <0.001 | 15 205 (33.6) |
| Outpatient | 16 799 (74.5) | 13 029 (63.2) | 219 (10.6) | <0.001 | 30 047 (66.4) |
| Mortality over follow‐upb | 38 (0.68) | 56 (0.97) | 10 (1.43) | 0.061 | 104 (0.86) |
CIED indicates cardiac implantable electronic device; CRT‐D, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter‐defibrillator; NOAC, novel oral anticoagulant; PM, pacemaker.
Kruskal–Wallis test for continuous measures and the chi‐square test for proportions.
Only observed if an inpatient admission over follow‐up had the discharge disposition of “dead”; no Social Security–linked mortality information was available. Among patients with a follow‐up visit for lead damage, only 1 patient died (ICD group).
A PM generator change was performed in 22 557 (50%) patients, an ICD generator change was performed in 20 632 (46%) patients, and a CRT‐D generator change was performed in 2063 (5%) patients. Patients with a CRT‐D were more likely to be younger and male, to have a higher CCI, to be taking an antiplatelet drug and/or warfarin, and to have undergone generator replacement in an inpatient setting (Table 1).
Lead damage occurred in 406 (0.90%) patients at a median of 107 days (interquartile range 35–260 days) following the CIED‐replacement procedure. Patients with a PM had a 0.46% incidence, which increased to 1.27% in ICD patients, and further increased to 1.94% in CRT‐D patients (P<0.001) (Figure 3). Mean time to a visit for lead damage (in either the inpatient or outpatient setting) ranged from 131 to 158 days (Figure 3). Of the 406 patients with lead damage, 368 (91%) presented in an inpatient setting. The median length of stay associated with treatment for lead damage was 3 days and did not significantly differ based on CIED type. The mean cost of the management of lead damage over the first year following generator replacement was $25 797, averaged across all generator types; however, costs were significantly influenced by CIED type. Specifically, mean total hospitalization costs were $19 959 for PM patients, $24 885 for ICD patients, and $46 229 for CRT‐D patients (P=0.048) (Table 2.
Figure 3.

Incidence of lead damage and time to event.
Table 2.
Hospital Length of Stay and Cost for a Follow‐up Visit for Lead Revision or Repair
| PM (n=22 557) | ICD (n=20 632) | CRT‐D (n=2063) | P Valuea | Overall (n=45 252) | |
|---|---|---|---|---|---|
| Patients requiring lead revision or repair, n (%) | 104 (0.46%) | 262 (1.27%) | 40 (1.94%) | 406 (0.90%) | |
| Length of stay (days) | |||||
| Mean±SD | 4.2±3.0 | 4.3±3.8 | 5.7±8.2 | 0.563 | 4.4±4.3 |
| Median | 4.0 | 3.0 | 3.0 | 3.0 | |
| Total visit cost | |||||
| Mean | $19 959 | $24 885 | $46 229 | 0.048 | $25 797 |
| SD | $15 365 | $21 384 | $85 260 | $33 415 | |
| 25th percentile | $8736 | $12 329 | $17 084 | $11 872 | |
| Median | $15 491 | $18 844 | $19 166 | $18 644 | |
| 75th percentile | $30 506 | $28 141 | $41 409 | $28 285 | |
Kruskal–Wallis test for continuous measures and the chi‐square test for proportions.
We examined risk factors for lead damage following a CIED‐replacement procedure in a Cox proportional hazards model (Table 3). Controlling for patient demographic and clinical characteristics, ICD and CRT‐D patients demonstrated double (hazard ratio 2.00, 95% CI 1.57–2.55) and >2.5 times (hazard ratio 2.58, 95% CI 1.73–3.83) the risk of lead damage, respectively, compared with PM patients.
Table 3.
Cox Proportional Hazards Model of Risk Factors for Lead Damage Following CIED Replacement
| Characteristic | HR | 95% CI | P Value |
|---|---|---|---|
| Demographics | |||
| Age, y | |||
| <65a | |||
| 65 to 74 | 0.51 | 0.39–0.67 | <0.0001 |
| 75 to 84 | 0.42 | 0.33–0.54 | <0.0001 |
| ≥85 | 0.23 | 0.15–0.35 | <0.0001 |
| Male (vs female) | 0.95 | 0.77–1.17 | 0.621 |
| History of pharmaceutical therapy | |||
| Warfarin or NOAC | 0.92 | 0.74–1.14 | 0.447 |
| Antiplatelets | 0.82 | 0.63–1.07 | 0.141 |
| Clinical characteristics | |||
| Diabetes without complications | 0.83 | 0.66–1.04 | 0.106 |
| Diabetes with complications | 0.74 | 0.50–1.11 | 0.143 |
| Chronic pulmonary disease | 0.79 | 0.62–0.99 | 0.042 |
| Cerebrovascular disease | 1.20 | 0.93–1.54 | 0.156 |
| Renal disease | 0.84 | 0.63–1.12 | 0.224 |
| Initial CIED replacement procedure setting | |||
| Inpatient (vs outpatient) | 0.97 | 0.79–1.20 | 0.778 |
| Generator type | |||
| PMa | |||
| ICD | 2.00 | 1.57–2.55 | <0.0001 |
| CRT‐D | 2.58 | 1.73–3.83 | <0.0001 |
CIED indicates cardiac implantable electronic device; CRT‐D, cardiac resynchronization therapy defibrillator; HR, hazard ratio; ICD, implantable cardioverter‐defibrillator; NOAC, novel oral anticoagulant; PM, pacemaker.
Reference groups include age 18–64 years, female sex, no warfarin or NOAC use, no antiplatelet use, no comorbid diagnosis, initial outpatient CIED‐replacement setting, generator‐type pacemaker.
Discussion
In this study of commercial claims data, we estimated the incidence of lead damage following CIED‐replacement procedures. Lead damage became clinically manifest at a median of 107 days following the CIED‐replacement procedure. The incidence of follow‐up visits for lead damage increased with device complexity: 0.46% for PMs, 1.27% for ICDs, and 1.94% for CRT‐D devices. This increasing risk of lead damage is likely attributable to the greater number and complexity of leads in patients with ICDs and CRT‐Ds compared with patients with PMs. Furthermore, follow‐up visits for lead repair and revision were associated with substantial mean total inpatient hospitalization costs, ranging from $19 959 for patients with a PM to $46 229 among those with a CRT‐D.
Prior Studies
Prior studies have failed to adequately assess the incidence of lead damage following a CIED‐replacement procedure. In a retrospective analysis of 5918 patients in the Danish Pacemaker and ICD registry, the authors evaluated the incidence of complications within 6 months of a primary or replacement CIED procedure. The incidence of lead‐related reintervention was 1.2% following PM or CRT‐PM procedures and 2.4% following an ICD or CRT‐D procedure14; however, the time to event, associated costs, and the issue necessitating reintervention were all undefined. Separately, the Ontario ICD registry evaluated the incidence of complications occurring within 45 days of an ICD‐replacement procedure in 5176 patients.15 During this short‐term follow‐up, 15 (1.4%) patients required a lead replacement and 9 (0.8%) patients required lead extraction; however, no specific information was provided as to what necessitated lead replacement or extraction.15 Our data show that lead damage typically occurs outside the 45‐day postprocedure window. Two Italian centers reported, in a retrospective chart review study, the incidence of lead failure in 2671 patients undergoing a CIED‐implant procedure.16 Over a mean follow‐up of 27 months, lead failure was observed in only 19 (0.42%) patients16; however, 57% of patients had undergone initial CIED implant, thus the population of patients undergoing a replacement procedure was quite small relative to our study. Finally, in a single‐center Austrian study, the authors evaluated the impact of surgical instrument and technique during pocket dissection on lead damage.17 The authors reported historical incidences of lead damage of 0.59% and 5.11% following PM and ICD replacement, respectively, using a purely sharp dissection technique. The study, however, comprised only 611 patients, nearly three‐fourths of which had a PM, and follow‐up was limited to 6 months.17
Study Limitations
This study had several limitations. First, claims data may contain coding errors and incompletely capture all relevant patient data. Second, given the limitations in existing billing codes, we could not differentiate between generator‐replacement procedures alone versus those in which leads were added. Our study used ICD‐9–based procedure codes; whether ICD‐10 procedure codes can overcome some of the encountered limitations merits further investigation. Third, cost (insurance payment) information in the MarketScan Commercial Research Database analyzed for this study was available only at the visit level, with no detail on line item or cost center costs. Consequently, the cost associated with lead damage outlined in this study includes more than just the cost of a replacement lead itself but rather the total visit cost, which likely includes other provided services. This area is worthy of greater exploration in future cost analysis studies. Further studies may also validate our estimates of lead damage with data sources specifically weighted and designed to be nationally representative, such as the Nationwide Inpatient Sample. Fourth, we had no information about the manufacturer or model of leads, the number of leads in the pocket, the duration during which the leads had been present since initial implantation, or the surgical tools used during the generator‐change procedure. Fifth, because Social Security–linked mortality information was not available in the current analysis, this study is at risk of possible censoring bias for mortality; however, this bias results in the estimated incidence of lead damage being conservative. Mortality was observed only if a patient was rehospitalized in follow‐up and the discharge status was “dead.” The risk of mortality censoring bias is greatest in the CRT‐D cohort (the cohort with the greatest mean CCI and thus the sickest population). Regardless, the highest incidence of lead damage was observed in the CRT‐D population, even with the shorter time of risk for lead damage in follow‐up because of greater mortality risk. Finally, with the advent of ICD‐10 procedure codes in the United States, future studies will be able to assess the location (right versus left) of the damaged lead and the surgical approach (percutaneous endoscopic versus open). There do not, however, appear to be procedure codes that are specific to lead‐damage events, merely codes that identify repair or replacement of leads, similar to what was available in the present study with ICD‐9 procedure code information.
Conclusions
This study estimated the incidence of lead damage following CIED‐replacement procedures using US health care claims data. Incidence ranged from 0.46% following PM replacements to 1.27% for ICDs and 1.94% for CRT‐Ds. Inpatient visits for lead revision or repair were associated with substantial increases in cost to the health care system. These findings suggest that new care algorithms are needed to mitigate these events, which impose substantial burdens on patients, physicians, and payors.
Sources of Funding
This study was funded by Medtronic Advanced Energy. Mittal is a consultant to Medtronic. Nichols and Vose disclose that they are employees of Medtronic Advanced Energy.
Disclosures
This study was funded by Medtronic Advanced Energy, with two of the authors (Nichols and Vose) being full‐time employees. Dr Mittal has received fees from Medtronic for speaking and consulting agreements on various topics; however, he did not receive any fees or other compensation for the analysis reported in this manuscript. Medtronic Advanced Energy owns the PEAK PlasmaBlade®, a novel electrosurgical device; however, specific analysis of this device was not included in the present manuscript as no device‐specific information was available in the Truven health insurance claims dataset.
Supporting information
Table S1. Patient Selection Codes, Cardiac Implantable Electronic Device Generator Procedures
Table S2. Patient Selection Codes, Exclusionary Criteria
Table S3. Patient Selection Codes, Additional Inclusion Criteria to Ensure Index Visit for Device Replacement
Acknowledgments
The authors thank Jeanne McAdara‐Berkowitz PhD for professional assistance with the manuscript.
(J Am Heart Assoc. 2016;5:e002813 doi: 10.1161/JAHA.115.002813)
Accompanying Tables S1 through S3 are available at http://jaha.ahajournals.org/content/5/2/e002813/suppl/DC1
References
- 1. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. 16‐Year trends in the infection burden for pacemakers and implantable cardioverter‐defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58:1001–1006. [DOI] [PubMed] [Google Scholar]
- 2. Prutkin JM, Reynolds MR, Bao H, Curtis JP, Al‐Khatib SM, Aggarwal S, Uslan DZ. Rates of and factors associated with infection in 200 909 Medicare implantable cardioverter‐defibrillator implants: results from the National Cardiovascular Data Registry. Circulation. 2014;130:1037–1043. [DOI] [PubMed] [Google Scholar]
- 3. Sridhar AR, Yarlagadda V, Yeruva MR, Kanmanthareddy A, Vallakati A, Dawn B, Lakkireddy D. Impact of haematoma after pacemaker and CRT device implantation on hospitalization costs, length of stay, and mortality: a population‐based study. Europace. 2015;17:1548–1554. [DOI] [PubMed] [Google Scholar]
- 4. Lim KK, Reddy S, Desai S, Smelley M, Kim SS, Beshai JF, Lin AC, Burke MC, Knight BP. Effects of electrocautery on transvenous lead insulation materials. J Cardiovasc Electrophysiol. 2009;20:429–435. [DOI] [PubMed] [Google Scholar]
- 5. Truven Health MarketScan Commercial Research Database 2009‐2013 . Available at: http://truvenhealth.com/your-healthcare-focus/life-sciences/data-databases-and-online-tools. Accessed January 8, 2015.
- 6. Reynolds MR, Cohen DJ, Kugelmass AD, Brown PP, Becker ER, Culler SD, Simon AW. The frequency and incremental cost of major complications among Medicare beneficiaries receiving implantable cardioverter‐defibrillators. J Am Coll Cardiol. 2006;47:2493–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 8. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 9. Motheral B, Brooks J, Clark MA, Crown WH, Davey P, Hutchins D, Martin BC, Stang P. A checklist for retrospective database studies—report of the ISPOR Task Force on Retrospective Databases. Value Health. 2003;6:90–97. [DOI] [PubMed] [Google Scholar]
- 10. Raebel MA, Malone DC, Conner DA, Xu S, Porter JA, Lanty FA. Health services use and health care costs of obese and nonobese individuals. Arch Intern Med. 2004;164:2135–2140. [DOI] [PubMed] [Google Scholar]
- 11. Pergolizzi JV Jr, Ma L, Foster DR, Overholser BR, Sowinski KM, Taylor R Jr, Summers KH. The prevalence of opioid‐related major potential drug‐drug interactions and their impact on health care costs in chronic pain patients. J Manag Care Spec Pharm. 2014;20:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crawshaw BP, Chien HL, Augestad KM, Delaney CP. Effect of laparoscopic surgery on health care utilization and costs in patients who undergo colectomy. JAMA Surg. 2015;150:410–415. [DOI] [PubMed] [Google Scholar]
- 13. Bureau of Labor Statistics . Consumer Price Index Medical Care Component. Bureau of Labor Statistics; Available at: http://www.bls.gov/cpi/data.htm. Accessed January 8, 2015. [Google Scholar]
- 14. Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krahn AD, Lee DS, Birnie D, Healey JS, Crystal E, Dorian P, Simpson CS, Khaykin Y, Cameron D, Janmohamed A, Yee R, Austin PC, Chen Z, Hardy J, Tu JV. Predictors of short‐term complications after implantable cardioverter‐defibrillator replacement: results from the Ontario ICD Database. Circ Arrhythm Electrophysiol. 2011;4:136–142. [DOI] [PubMed] [Google Scholar]
- 16. Palmisano P, Accogli M, Zaccaria M, Luzzi G, Nacci F, Anaclerio M, Favale S. Rate, causes, and impact on patient outcome of implantable device complications requiring surgical revision: large population survey from two centres in Italy. Europace. 2013;15:531–540. [DOI] [PubMed] [Google Scholar]
- 17. Kypta A, Blessberger H, Saleh K, Honig S, Kammler J, Neeser K, Steinwender C. An electrical plasma surgery tool for device replacement–retrospective evaluation of complications and economic evaluation of costs and resource use. Pacing Clin Electrophysiol. 2015;8:28–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient Selection Codes, Cardiac Implantable Electronic Device Generator Procedures
Table S2. Patient Selection Codes, Exclusionary Criteria
Table S3. Patient Selection Codes, Additional Inclusion Criteria to Ensure Index Visit for Device Replacement


