Abstract
Background
There are limited data describing sex specificities regarding implantable cardioverter defibrillators (ICDs) in the real‐world European setting.
Methods and Results
Using a large multicenter cohort of consecutive patients referred for ICD implantation for primary prevention (2002–2012), in ischemic and nonischemic cardiomyopathy, we examined the sex differences in subjects' characteristics and outcomes. Of 5539 patients, only 837 (15.1%) were women and 53.8% received cardiac resynchronization therapy. Compared to men, women presented a significantly higher proportion of nonischemic cardiomyopathy (60.2% versus 36.2%, P<0.001), wider QRS complex width (QRS >120 ms: 74.6% versus 68.5%, P=0.003), higher New York Heart Association functional class (≥III in 54.2%♀ versus 47.8%♂, P=0.014), and lower prevalence of atrial fibrillation (18.7% versus 24.9%, P<0.001). During a 16 786 patient‐years follow‐up, overall, fewer appropriate therapies were observed in women (hazard ratio=0.59, 95% CI 0.45–0.76; P<0.001). By contrast, no sex‐specific interaction was observed for inappropriate shocks (odds ratio ♀=0.84, 95% CI 0.50–1.39, P=0.492), early complications (odds ratio=1.00, 95% CI 0.75–1.32, P=0.992), and all‐cause mortality (hazard ratio=0.87 95% CI 0.66–1.15, P=0.324). Analysis of sex‐by‐ cardiac resynchronization therapy interaction shows than female cardiac resynchronization therapy recipients experienced fewer appropriate therapies than men (hazard ratio=0.62, 95% CI 0.50–0.77; P<0.001) and lower mortality (hazard ratio=0.68, 95% CI 0.47–0.97; P=0.034).
Conclusions
In our real‐life registry, women account for the minority of ICD recipients and presented with a different clinical profile. Whereas female cardiac resynchronization therapy recipients had a lower incidence of appropriate ICD therapies and all‐cause death than their male counterparts, the observed rates of inappropriate shocks and early complications in all ICD recipients were comparable.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT01992458.
Keywords: death, sudden; heart failure; mortality; shock
Subject Categories: Catheter Ablation and Implantable Cardioverter-Defibrillator, Epidemiology, Arrhythmias
Introduction
Implantable cardioverter defibrillators (ICDs) are currently an effective and accepted treatment for improving the outcomes of selected patients with ischemic and nonischemic cardiomyopathy with heart failure and severe left ventricular dysfunction.1, 2, 3, 4 However, in the major randomized controlled trials determining guideline recommendations, women were markedly underrepresented.1, 2, 3, 4
Accordingly, the existence of sex‐related differences in outcomes among ICD recipients is still controversial. While in North American registries,5 women seem to experience a lower incidence of appropriate therapies, these data were not confirmed in a large Dutch single‐center prospective cohort study6 and in the recent Nationwide Israeli‐ICD registry.7
Data on sex‐related survival differences are also contradictory. A large North American registry5 and the Israeli‐ICD registry7 have shown no differences in all‐cause death, while a 35% lower mortality was observed in women of the single‐center Dutch cohort.6
Real‐world data from European registries addressing these issues is still absent. In the present article, we aim to determine the proportion of female ICD recipients, as well as differences in terms of characteristics at implant and outcomes (therapies, overall and specific mortalities) in women compared to men.
Methods
We selected 5539 patients from the DAI‐PP study (Défibrillateur Automatique Implantable Prévention Primaire; NCT01992458) for this analysis. To qualify for the study, patients had to be at least 18 years old at the time of ICD implantation. Overall, between 2002 and 2012, all patients with ischemic cardiomyopathy or nonischemic cardiomyopathy, implanted with an ICD (biventricular, single chamber, or dual chamber) in the setting of primary prevention in 12 reference French centers were considered and enrolled in the DAI‐PP follow‐up program. Primary prevention was defined when no prior history of sudden cardiac arrest and/or ventricular tachycardia/fibrillation was documented. Ischemic cardiomyopathy was defined as presence of myocardial dysfunction in the context of previous myocardial infarction and/or history of coronary artery disease with or without revascularization (angioplasty or bypass surgery).
Exclusion criteria included all patients having an ICD implant for secondary prevention purposes or for primary prevention without structural heart disease (including Brugada, long QT syndrome, among others) or structural heart disease other than ischemic or nonischemic cardiomyopathy (hypertrophy cardiomyopathy, noncompaction cardiomyopathy, and arrhythmogenic right ventricular dysplasia).
The study was funded by public sources, including the French Institute of Health and Medical Research (INSERM) and the French Society of Cardiology, and was coordinated by Clinique Pasteur, Toulouse and the Paris Cardiovascular Research Center, European Georges Pompidou Hospital, Paris, in France. The study complied with the Declaration of Helsinki, and the data file of the DAI‐PP study was declared to and authorized by the French data protection committee (Commission Nationale Informatique et Liberté, CNIL). Patients gave informed consent for anonymized use of their data.
Sample Characterization
All variables at the time of the procedure were defined and categorized according to the literature or common practice. In addition to the New York Heart Association (NYHA) functional class, we collected the etiology of the underlying heart disease (ischemic cardiomyopathy or nonischemic cardiomyopathy). Glomerular filtration rate was estimated with the formula of Cockroft–Gault and categorized in 2 categories (≥60 and <60 mL/min); QRS duration was categorized as <120 and ≥120 ms. Atrial fibrillation (AF) was defined as a history of AF (paroxysmal or persistent), documented on standard ECG or 24‐hour Holter monitoring. Comorbidities at the time of ICD implantation were systematically collected from review of medical records: cancer, chronic obstructive pulmonary disease, chronic renal failure, chronic liver disease, history of transient ischemic neurological attack, and others (including diabetes mellitus). The type of ICD device implanted (biventricular, single chamber or dual chamber—no indication on manufacturers) was recorded. Data on device programming was not collected and was left to the discretion of individual investigators, according to each patient's needs. Programming rules were based on high detection windows, as suggested by local guidelines at the time.8 Furthermore, French guidelines did not recommend special programming adjustments, namely, cut‐offs for therapy zones, based on sex. Information on medications at hospital discharge included β‐blockers, amiodarone, Ic class anti‐arrhythmic agents, sotalol, digoxin, calcium blockers, angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker, diuretics, anti‐platelets, and vitamin K antagonists.
Follow‐Up and Outcomes
Follow‐up information was obtained from appointments every 4 to 6 months for device evaluation, according to French guidelines.8 The different end points were occurrence of appropriate therapies, early complications, inappropriate shocks, as well as overall and specific mortalities.
Device interrogation printouts were checked by the local investigator for appropriate and inappropriate ICD therapy. Appropriate ICD therapy was defined as an episode of ventricular tachycardia/ventricular fibrillation resulting in a single or multiple shocks or/and anti‐tachycardia pacing for arrhythmia termination. The date of the first appropriate ICD therapy was recorded, and the overall cumulative number of appropriate therapies was considered. The cause of inappropriate shock(s) was collected as well. Adjudication as appropriate or inappropriate therapy was undertaken by the local electrophysiology (EP) investigator.
Early complications (defined as those that appeared throughout the first 30 days after device implantation) included lead‐related complications (eg, failure of coronary sinus lead placement, rise of threshold, lead dislodgment with or without need of reintervention, phrenic nerve stimulation), bleeding (ecchymosis, hematoma, other bleeds requiring transfusion, and procedure‐related anemia), sepsis, cardiac tamponade, pneumothorax, and death.
Vital status data were obtained from the hospital or the general practitioner, and were systematically controlled through the National Institute of Statistics Economical Studies (INSEE). Causes of death were obtained from the investigators and/or by the French Center on Medical Causes of Death (CépiDc–INSERM). The CépiDc–INSERM is an academic public institution focused on the analysis of circumstances and causes of death based on death certificate and medical records. Causes of deaths were classified according to the International Classification of Diseases (10th Revision). This information was reviewed by 2 investigators and causes of death were adjudicated after consideration of all the available information, and according to the following prespecified groups: cardiovascular (including progressive heart failure death, stroke), noncardiovascular, ICD‐unresponsive sudden cardiac death (arrhythmic or not arrhythmic whenever the assessment was possible), ICD‐related death, as well as unknown when the quality of the information could not allow the investigators to appropriately identify cause of death. Overall, cause of death assessment was possible among 682 patients (out of 826 deceased, 82.6%).
Statistical Analysis
Preparation of this report was in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for reporting of observational studies.9
Comparisons were performed between men and women. Chi‐square was used for comparison of nominal variables and Student t test for continuous variables; the Levene's test was used to check the homogeneity of variance; when appropriate, nonparametric equivalent, Mann–Whitney test, was employed. When baseline differences were present, adjustment was performed using multivariate analysis with binary logistic regression. Results with P<0.05 were regarded as significant.
We compared the adjusted outcomes of appropriate shock and death in men and women by using Cox proportional hazards regression analysis. Hazard curves were traced for comparison of men and women after adjustment for baseline differences.
Data were filled into a predefined data introduction electronic sheet made available to all participant Centers. After completion of follow‐up, data from all Centers were merged and analyzed at the Paris Cardiovascular Research Center (Inserm U970, Cardiovascular Epidemiology Unit) using SAS program v9.3 (SAS Institute Inc, Cary, NC).
Results
The mean age of the sample was 62.5±11.2 years. Among the 5539 patients included in the study, most (60.2%) had an ischemic cardiomyopathy and AF was present in 24.0%. Mean left ventricular ejection fraction and NYHA class were 26.7±7.2% and 2.4±0.7%, respectively. Cardiac resynchronization therapy with defibrillator (CRT‐D) was used in half of patients (53.8%). More information on baseline sample data and medical treatment at the time of implant can be found in Table 1.
Table 1.
Baseline Sample Characteristics and Medication
| Parameter | Global Sample (n=5539) | Male Sex (n=4702) | Female Sex (n=837) | P Value |
|---|---|---|---|---|
| Baseline data | ||||
| Age, y | 62.5±11.2 | 62.6±11.0 | 62.0±12.4 | 0.245 |
| Female sex | 837 (15.1%) | — | — | — |
| NYHA I | 10.6% (484) | 11.0% (426) | 8.2% (57) | 0.014 |
| NYHA II | 40.7% (1861) | 41.2% (1600) | 37.6% (261) | |
| NYHA III | 44.9% (2055) | 43.9% (1703) | 50.6% (352) | |
| NYHA IV | 3.8% (176) | 3.9% (151) | 3.6% (25) | |
| Atrial fibrillation | 1134 (24.0%) | 1002 (24.9%) | 132 (18.7%) | <0.001 |
| Ischemic cardiomyopathy | 3304 (60.2%) | 2976 (63.8%) | 328 (39.8%) | <0.001 |
| Mean LVEF | 26.7±7.2 | 26.7±6.9 | 27.0±8.5 | 0.343 |
| QRS width <120 ms | 1183 (30.5%) | 1025 (31.5%) | 158 (25.4%) | 0.003 |
| GFR <60 mL/min | 1281 (39.6%) | 1111 (40.1%) | 170 (36.2%) | 0.104 |
| Number of comorbiditiesa | 0.9±0.8 | 0.9±0.8 | 0.9±0.7 | 0.165 |
| Single‐chamber ICD | 1258 (22.9%) | 1095 (23.5%) | 163 (19.6%) | <0.001 |
| Double‐chamber ICD | 1280 (23.3%) | 1119 (24.0%) | 161 (19.4%) | |
| CRT‐D | 2952 (53.8%) | 2446 (52.5%) | 506 (61.0%) | |
| Medical therapy at the time of the implant | ||||
| ACEi or ARB | 3263 (82.0%) | 2780 (82.3%) | 483 (80.0%) | 0.161 |
| Furosemide | 2709 (68.1%) | 2294 (68.0%) | 415 (68.7%) | 0.713 |
| Spironolactone | 1284 (32.3%) | 1050 (31.1%) | 234 (38.7%) | <0.001 |
| Calcium channel blockers | 79 (2.0%) | 73 (2.2%) | 6 (1.0%) | 0.058 |
| β‐Blockers | 3378 (84.9%) | 2848 (84.4%) | 530 (87.7%) | 0.032 |
| Amiodarone | 902 (22.7%) | 787 (23.3%) | 115 (19.0%) | 0.021 |
| Digoxin | 225 (5.7%) | 189 (5.6%) | 36 (6.0%) | 0.723 |
| Sotalol | 21 (0.5%) | 18 (0.5%) | 3 (0.5%) | 0.909 |
| Class IC AA | 4 (0.1%) | 4 (0.1%) | 0 (0%) | 0.397 |
| Antiplatelet agents | 2278 (57.2%) | 2007 (59.4%) | 271 (44.9%) | <0.001 |
| Vitamin K antagonists | 1404 (35.3%) | 1226 (36.2%) | 178 (29.5%) | 0.001 |
Comparison among sexes. AA indicates antiarrhythmic agent; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐II receptor blocker; CRT‐D, cardiac resynchronization therapy with defibrillator; GFR, glomerular filtration rate; ICD, implantable cardioverter defibrillators; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Number of comorbidities among the following: cancer, chronic kidney disease, chronic lung disease, hepatic failure, diabetes mellitus, and previous stroke.
Sex‐Related Differences in Clinical Characteristics at Implant
Women accounted for 15.1% (n=837) of the whole study sample and had higher prevalence of patients in higher NYHA classes (NYHA ≥III: 54.2%♀ versus 47.8%♂, P=0.014). Men presented a higher prevalence of AF, ischemic cardiomyopathy, and had a higher prevalence of narrow QRS complex width (QRS <120 ms). No significant differences were found regarding age, mean left ventricular ejection fraction, and number of comorbidities.
On univariate analysis, women were more frequently implanted with CRT‐D devices (61.0% versus 52.5%; P<0.001). However, after adjustment for baseline differences, this became no longer significant (odds ratio=1.28, 95% CI 0.98–1.66; P=0.07).
Women were more frequently treated with spironolactone and β‐blockers. Conversely, men received amiodarone, vitamin K antagonists, and antiplatelet agents more frequently and had a trend for higher use of calcium channel blockers. However, after adjustment for baseline differences, only spironolactone was used more frequently among women (odds ratio=1.30, 95% CI 1.06–1.59; P=0.014), whereas all other drugs presented similar use among men and women (Table 1).
Appropriate and Inappropriate Therapies and Early Complications
No significant differences were observed in the occurrence of early complications (♂12.8% versus ♀13.3%; P=0.789; adjusted odds ratio=1.00, 95% CI 0.75–1.32; P=0.992) (Figure 1). Except for a higher incidence of pneumothorax in women (♂0.6% versus ♀1.9%, P<0.001), the remaining complication types were evenly distributed between sexes: bleeding (♂4.8% versus ♀3.2%), cardiac tamponade (0% in both groups), infection (♂1.0% versus 0.8%), lead‐related (♂3.4% versus ♀4.2%), and death (♂0.2% versus ♀0.1%) (all P=NS).
Figure 1.
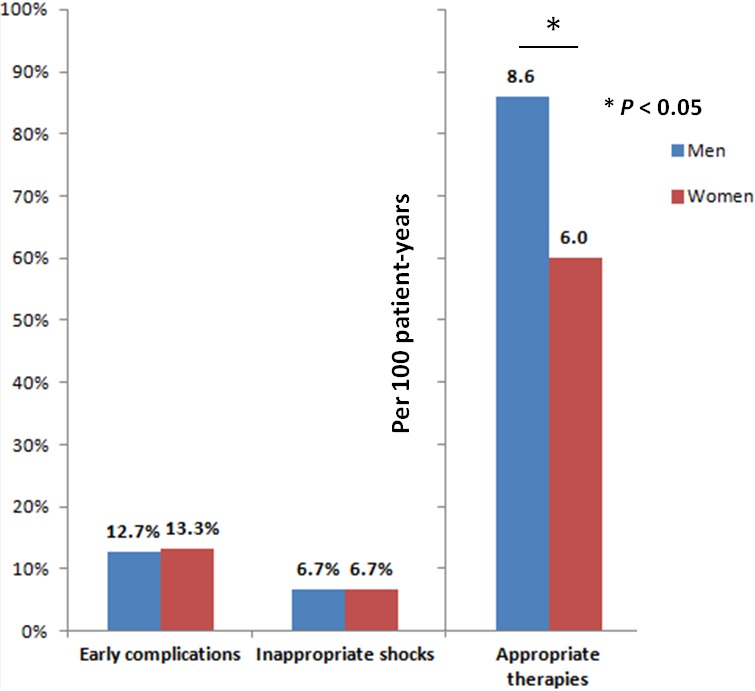
Overall view of outcomes in the male and female sex.
During 16 786 patient‐years of follow‐up, corresponding to a median of 994 days (95% CI 51–2623 without differences between sexes, P=0.997), appropriate therapies were documented in 1181 (22.3%) patients, corresponding to an annual incidence of 8.2 per 100 patient‐years (95% CI 7.8–8.7) (Table 2). On univariate analysis, women had a significantly lower likelihood of receiving appropriate therapies (23.0%♂ versus 17.4%♀; P<0.001). After adjustment for all baseline intersex differences, on multivariate Cox‐regression female sex remained an independent predictor of lower incidence of appropriate therapies (hazard ratio [HR]=0.59, 95% CI 0.45–0.76; P<0.001) (Figure 2). Even though the presence of CRT was not significantly associated with appropriate therapies (HR=0.978, 95% CI 0.80–1.20, P=0.830), analysis of different device strata suggests a larger effect size and more pronounced reduction in the incidence of appropriate therapies in female CRT‐D recipients versus men (HR=0.49, 95% CI 0.34–0.70; P<0.001), than in their single‐ and dual‐chamber ICD counterparts (HR=0.76, 95% CI 0.52–1.11; P=0.159) (Table 2).
Table 2.
Study Outcomes in the Global Sample: Analysis by Sex and Device Interaction
| Unadjusted Analysis | ||||
|---|---|---|---|---|
| Outcome | Event Rate, % (n) | P Value | ||
| Global Sample (n=5539) | Male Sex (n=4702) | Female Sex (n=837) | ||
| Appropriate therapies | 8.2 per 100 patient years (95% CI 7.8–8.7) | 8.6 per 100 patient years (95% CI 8.1–9.1) | 6.0 per 100 patient years (95% CI 5.1–7.1) | <0.001 |
| Early complications | 710 (12.8%) | 599 (12.7%) | 111 (13.3%) | 0.785 |
| Inappropriate shocks | 355 (6.7%) | 302 (6.7%) | 53 (6.7%) | 0.997 |
| Mortality | 4.9 per 100 patient years (95% CI 4.6–5.3) | 5.0 per 100 patient years (95% CI 4.7–5.4) | 4.2 per 100 patient years (95% CI 3.5–5.1) | 0.01 |
|
Multivariate‐Adjusted Analysisa
Odds Ratio/Hazard Ratio (95% CI)—All Patients | ||
|---|---|---|
| Appropriate therapies | HR=0.59 (0.45–0.76) | <0.001 |
| Early complications | OR=1.00 (0.75–1.32) | 0.992 |
| Inappropriate shocks | OR=0.84 (0.50–1.39) | 0.492 |
| Mortality | HR=0.87 (0.66–1.15) | 0.324 |
| VVI and DDD‐ICD patients | ||
| Appropriate therapies | HR=0.76 (0.52–1.11) | 0.159 |
| Early complications | OR=0.83 (0.48–1.43) | 0.498 |
| Inappropriate shocks | OR=0.61 (0.28–1.31) | 0.609 |
| Mortality | HR=1.50 (0.96–2.34) | 0.072 |
| CRT‐D patients | ||
| Appropriate therapies | HR=0.49 (0.34–0.70) | <0.001 |
| Early complications | OR=1.10 (0.78–1.55) | 0.574 |
| Inappropriate shocks | OR=1.08 (0.54–2.18) | 0.828 |
| Mortality | HR=0.68 (0.47–0.97) | 0.034 |
CRT‐D indicates cardiac resynchronization therapy with defibrillator; DDD‐ICD, dual chamber device‐implantable cardioverter defibrillators; HR, hazard ratio; OR, odds ratio; VVI, single chamber device.
Adjusted for baseline differences in New York Heart Association class, atrial fibrillation, ischemic cardiomyopathy, QRS width, use of CRT‐D, treatment with β‐blockers, amiodarone, spironolactone, calcium channel blockers, antiplatelet agents, and vitamin K antagonists.
Figure 2.
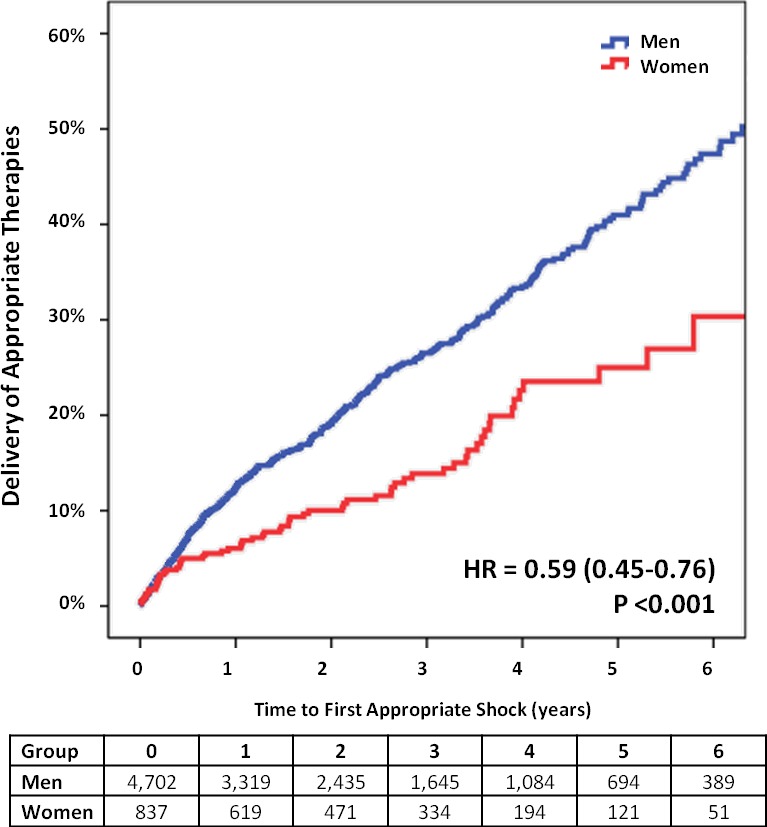
Adjusted event curves for first appropriate therapy. HR indicates hazard ratio. Adjusted for baseline differences in New York Heart Association class, atrial fibrillation, ischemic cardiomyopathy, QRS width, use of cardiac resynchronization therapy with defibrillator, treatment with β‐blockers, amiodarone, spironolactone, calcium channel blockers, antiplatelet agents, and vitamin K antagonists.
Regarding inappropriate shocks, on univariate analysis, no sex‐related benefit was observed (6.7% versus 6.7%; odds ratio=1.00, 95% CI 0.74–1.35, P=0.997). On binary logistic regression, after adjustment for baseline differences, the absence of sex‐specific interaction was confirmed.
Total and Specific Mortalities
Death occurred in 15.2% (6.0 per 100 patient‐years; 95% CI 5.6–6.3). Despite the fact that women experienced a slightly lower annual mortality rate (5.0 per 100 patient years ♂ versus 4.2 per 100 patient years ♀) on univariate Cox‐regression, after adjustments for all baseline differences, this was no longer observed: female sex was not an independent predictor of mortality (HR=0.87, 95% CI 0.66–1.15; P=0.324). By contrast, AF (HR=1.41; 95% CI 1.13–1.77, P=0.003), NYHA ≥III (HR=2.25; 95% CI 1.81–2.79, P<0.001), left ventricular ejection fraction <30% (HR=1.50; 95% CI 1.21–1.86, P<0.001), ischemic heart disease (HR=1.66, 95% CI 1.34–2.00, P<0.001), and glomerular filtration rate ≤60 mL/min (HR=2.31, 95% CI 1.88–2.84, P<0.001) were associated with increased mortality.
On Cox‐regression multivariate analysis, when restricting the analysis only to patients implanted with CRT‐Ds, a significant survival benefit was seen in women implanted with a CRT‐D (HR=0.68, 95% CI 0.47–0.97; P=0.034) (Table 2). In their single‐ and dual‐chamber ICD counterparts, this was not observed (HR=1.50, 95% CI 0.96–2.34; P=0.072). The P‐value for this sex by device interaction was 0.070. Kaplan–Meier curves illustrating this interaction (Figure 3) show that male and female patients implanted with CRT‐Ds diverge right from the beginning. On the other hand, for female and male patients implanted with single‐ and dual‐chamber ICDs, curves are overlapping for the first 4 years, and suddenly diverge after that, at a time where only close to 100 female patients are being followed. These curves also show that male patients with CRT‐Ds do much worse than males implanted with single‐ and dual‐ chamber ICDs. This difference is not as pronounced in female patients.
Figure 3.
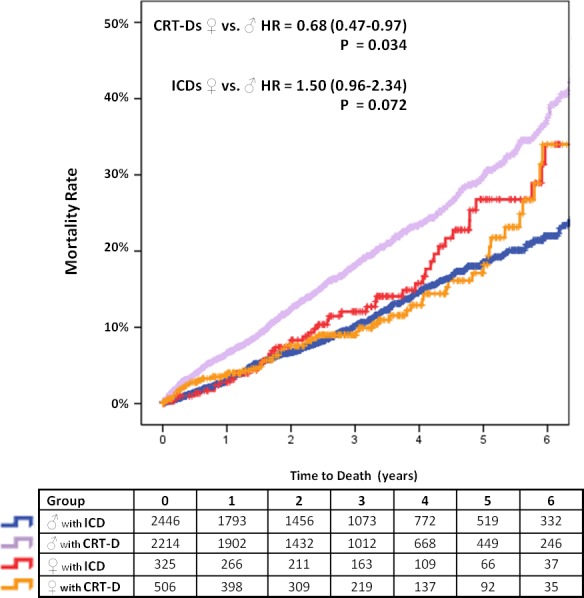
Adjusted event‐curves for overall mortality in men and women. CRT‐D indicates cardiac resynchronization therapy with defibrillator; HR, hazard ratio; ICD, single‐ or dual‐chamber implantable cardioverter defibrillator. Adjusted for baseline differences in New York Heart Association class, atrial fibrillation, ischemic cardiomyopathy, QRS width, use of CRT‐D, treatment with β‐blockers, amiodarone, spironolactone, calcium channel blockers, antiplatelet agents, and vitamin K antagonists.
The specific analysis of causes of death (Table 3), after adjustment for baseline differences, revealed a significant interaction of sex with CRT as regards nonarrhythmic cardiovascular mortality: women with single‐ and dual‐chamber ICD implants fared worse than men (HR=2.49, 95% CI 1.40–4.45, P=0.002), but those implanted with CRT‐Ds were less likely to die of nonarrhythmic cardiovascular causes (HR=0.50, 95% CI 0.35–0.99, P=0.048).
Table 3.
Causes of Death: Analysis by Sex and Device Interaction
| Cause of Death | n (% of Total Deaths) All Patients (n=826) | All Patients, Unadjusted Analysis n (% of Patients) | P Value | |
|---|---|---|---|---|
| Male Sex | Female Sex | |||
| Sudden death | 64 (7.7%) | 54 (1.2%) | 10 (1.2%) | 0.908 |
| ICD‐related | 14 (1.7%) | 14 (0.3%) | 0 (0%) | 0.114 |
| Cardiovascular causes | 407 (49.3%) | 350 (7.6%) | 57 (7.0%) | 0.518 |
| Noncardiovascular | 197 (23.8%) | 181 (3.9%) | 16 (2.0%) | 0.005 |
| Unknown | 144 (17.4%) | 121 (2.6%) | 23 (2.8%) | 0.770 |
| Cause of Death |
n (% of Total Deaths) VVI or DDD‐ICD (n=315) |
VVI or DDD‐ICD Patients, Unadjusted Analysis n (% of Patients) |
P Value | |
|---|---|---|---|---|
| Male Sex | Female Sex | |||
| Sudden death | 28 (8.9%) | 22 (1.0%) | 6 (1.9%) | 0.168 |
| ICD‐related | 4 (1.3%) | 4 (0.2%) | 0 (0%) | 0.444 |
| Cardiovascular causes | 142 (45.1%) | 116 (5.4%) | 26 (8.3%) | 0.042 |
| Noncardiovascular | 83 (26.3%) | 76 (3.5%) | 7 (2.2%) | 0.228 |
| Unknown | 58 (18.4%) | 51 (2.4%) | 7 (2.2%) | 0.871 |
| Cause of Death |
n (% of Total Deaths) CRT‐D Patients (n=500) |
CRT‐D Patients, Unadjusted Analysis n (% of Patients) |
P Value | |
|---|---|---|---|---|
| Male Sex | Female Sex | |||
| Sudden death | 35 (7.0%) | 31 (1.3%) | 4 (0.8%) | 0.372 |
| ICD‐related | 10 (2.0%) | 10 (0.4%) | 0 (0%) | 0.151 |
| Cardiovascular causes | 259 (51.8%) | 229 (9.5%) | 30 (6.0%) | 0.014 |
| Noncardiovascular | 112 (22.4%) | 104 (4.3%) | 8 (1.6%) | 0.004 |
| Unknown | 84 (16.8%) | 68 (2.8%) | 16 (3.2%) | 0.626 |
|
Multivariate‐Adjusted Cox Regressiona
♀ vs ♂—Hazard Ratio (95% CI) | ||
|---|---|---|
|
Death due to noncardiovascular causes All patients |
HR=0.63 (0.30–1.31) | 0.206 |
|
Death due to cardiovascular causes ICD patients |
HR=2.49 (1.40–4.45) | 0.002 |
|
Death due to cardiovascular causes CRT‐D patients |
HR=0.59 (0.35–0.99) | 0.048 |
|
Death due to noncardiovascular causes CRT‐D patients |
HR=0.59 (0.23–1.50) | 0.267 |
CRT‐D indicates cardiac resynchronization therapy with defibrillator; DDD‐ICD, dual chamber device‐implantable cardioverter defibrillators; HR, hazard ratio; ICD, implantable cardioverter defibrillators; NYHA, New York Heart Association; VVI, single chamber device.
Adjusted for baseline differences in NYHA class, atrial fibrillation, ischemic cardiomyopathy, QRS width, use of CRT‐D, treatment with β‐blockers, amiodarone, spironolactone, calcium channel blockers, antiplatelet agents, and vitamin K antagonists.
Discussion
Our multicentric real‐world data show that similar to randomized controlled trials10 and other registries of ICD recipients in daily clinical practice,11, 12, 13 women account for only a minority of our cohort. Also, they present with a different clinical profile, with higher prevalence of nonischemic cardiomyopathy, more advanced heart failure, broader QRS complex width, and lower prevalence of AF. Women, overall, presented with lower incidence of appropriate ICD therapies and similar mortality compared with men. However, our results suggest that differences in sex‐related outcomes in primary prevention ICDs may be observed among CRT recipients. Whereas female CRT‐D recipients present with lower mortality and incidence of appropriate ICD interventions than their male counterparts, outcomes of single‐ and dual‐chamber ICD recipients seem to be more comparable.
Our findings, suggesting a lower incidence of appropriate ICD therapies among women, have also been reproduced in other studies: the prospective Ontario registry5 and a single‐center North American propensity‐matched observational study.14 However, only the latter has shown that this effect was more pronounced among CRT‐D recipients.14 This is similar to what we observed in our cohort, where the lower incidence of appropriate therapies among women was mostly driven by a significant difference in CRT‐D recipients. We believe these differences may partially result from different clinical risk profiles or ethnic/regional factors in the different samples. Therefore, identifying specific subgroups of primary prevention ICD female recipients who can derive higher benefit from this therapy may be of interest. CRT response, discussed below, may also play a role.
The underlying causes for the more favorable arrhythmic profile of women are not entirely clear but may be related to the lower propensity to sustained ventricular tachycardia in the nonischemic heart failure setting15, 16 but also for ischemic cardiomyopathy–associated sudden cardiac death.17, 18, 19 In our sample, despite having more women with nonischemic cardiomyopathy, the overall reduction in arrhythmic burden was still present even after adjustment for baseline differences. Several mechanisms have been proposed for sex differences regarding the risk of ventricular arrhythmia: higher resting heart rate, different autonomic response to stress, degree of vagal activation, differences in cardiac repolarization, hormonal differences affecting arrhythmic vulnerability, genetic variants influencing QT interval length or adrenergic receptors, and even nutritional factors, adherence to a low‐risk lifestyle, and behavorial and psychological factors.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 Overall, the interplay between vulnerable substrate (underlying structural and electric heart substrate), triggers, and autonomic tone may be slightly different.30
The reason for the sex‐related benefit among women implanted with CRT‐D is still unclear. Arhsad et al have proposed 2 hypothetical explanations32: first, a likely possible greater risk of progression to overt heart failure among women, resulting in a greater preventive benefit of CRT‐D therapy. Second, women may have, on average, a 10 ms shorter QRS duration than men in subjects without heart disease.33 Therefore, on a relative basis, for the same degree of QRS duration, more pronounced conduction disturbances and cardiac dyssynchrony may occur in women, explaining the higher response to cardiac resynchronization therapy. Thus, a higher prevalence of response to CRT and reverse remodeling, as observed in the MADIT‐CRT trial,32 can possibly explain the survival and arrhythmic benefit of our population.
Regarding inappropriate shocks, all existing data have found a similar risk in both men and women,5, 9, 34 as we have observed. Furthermore, we have assessed the underlying reasons for inappropriate therapies and found no intersex differences (namely, a similar incidence of supraventricular tachycardia despite the fact that AF was more frequent among men).
Our data do not reproduce the previously described increased risk of complications in women. MacFadden and colleagues reported a 50% significantly higher (5.4% versus 3.3%) occurrence of any major or minor complications in women at 45‐day follow‐up.5 Recent data from the United States National Cardiovascular Data Registry ICD Registry show that device‐related complications are more common in women (7.2% versus 4.8%; P<0.001).35 Unlike the latter study, we observed no differences regarding 30‐day mortality and observed a much lower rate of cardiac tamponade. Possible explanations for the observed lack of sex‐related differences and the overall higher complication rate in our sample may be the very inclusive definition of end points such as bleeding events and lead‐related complications, and having local investigator‐adjudicated end points instead of using the Centers for Medicare and Medicaid Services inpatient claims. Lastly, the use antiplatelet agents and oral anticoagulants in our sample was more common in males, which may account for the numerically, but not statistically significant, higher rate of bleeding observed in male patients.
As regards mode of death, we have observed that the incidence of specific causes of death in women, namely, nonarrhythmic cardiovascular mortality, may depend on the type of implanted device. In single‐ or dual‐chamber ICD recipients, nonarrhythmic cardiovascular death occurred more commonly in women whereas in those implanted with a CRT‐D it happened less frequently. These findings are contrary to previously published data showing no sex‐related differences in cause of death.6, 36 We believe this may occur because these 2 studies have smaller samples, and therefore are not statistically powered to assess for interactions between sex, device type, and specific mortalities. In patients who are eligible for an ICD, it is known that sudden cardiac death seems to occur less frequently in women.37 Our data seem to suggest that after device implantation, the favorable arrhythmic profile may still exist because, notwithstanding the similar incidence of ICD unresponsive sudden death, women present with fewer appropriate therapies, which can be considered, to a certain level, a sudden cardiac death surrogate.
Limitations
This analysis has a number of limitations. First, the retrospective nature of this registry could have led to information bias. Second, device programming may have been a source of some interindividual variability, but this factor has been minimized by proposed programming rules recommending no differences based on sex.8 Last, no central adjudication for classification of appropriate and inappropriate therapies was used in this registry.
Conclusions
In our real‐life registry, women seem to account for the minority of ICD recipients and present with a different clinical profile. Even though the incidence of early complications and inappropriate shocks was similar, lower all‐cause mortality and incidence of appropriate therapies was observed in female CRT‐D recipients compared with men. Among single‐ and dual‐chamber ICD recipients, the incidence of ICD therapies was comparable but nonarrhythmic cardiovascular death was more common in women.
Appendix
The following investigators and institutions participated in the conception of the registry, and in the organization, collection, storage, and analysis of the data:
Co‐principal Investigators: Serge Boveda, MD, Clinique Pasteur, Toulouse; Eloi Marijon, MD, PhD, Hôpital Européen Georges Pompidou, Paris, France. Conceived, designed and organized the registry in 2009.
Co‐investigators in charge of the data collection and analysis at each medical center: Vincent Algalarrondo, MD, PhD, CHU Antoine Béclère, Clamart; Dominique Babuty, MD, PhD, CHU Trousseau, Tours; Pierre Bordachar, MD, PhD, CHU Haut Lévêque, Bordeaux; Abdeslam Bouzeman, MD, Serge Boveda, MD, Rui Providência, MD, PhD, Clinique Pasteur, Toulouse; Pascal Defaye, MD, CHU Michallon, Grenoble; Daniel Gras, MD, Nouvelles Cliniques Nantaises, Nantes; Jean‐Claude Deharo, MD, PhD, CHU La Timone, Marseille; Didier Klug, MD, PhD, CHRU Lille, Lille; Christophe Leclercq, MD, PhD, CHU Pontchaillou, Rennes; Eloi Marijon, MD, PhD; Hôpital Européen Georges Pompidou, Paris; Olivier Piot, MD, Centre Cardiologique du Nord, Saint Denis; Nicolas Sadoul, MD, PhD, CHU Brabois, Nancy.
Data storage, quality control, and statistical analyses: Frankie Beganton, MS, Marie‐Cécile Perier, MPH, Cardiovascular Epidemiology Unit, Paris Cardiovascular Research Center (INSERM Unit 970), Hôpital Européen Georges Pompidou, Paris.
Steering Committee: Serge Boveda, MD, Clinique Pasteur, Toulouse; Pascal Defaye, MD, CHU Michallon, Grenoble; Christophe Leclercq, MD, PhD, CHU Pontchaillou, Rennes; Eloi Marijon, MD, PhD; Hôpital Européen Georges Pompidou, Paris; Nicolas Sadoul, MD, PhD, CHU Brabois, Nancy.
Sources of Funding
This work was supported by the following independent institutions: the Toulouse Association for the Study of Rhythm Disturbances; the French Institute of Health and Medical Research; and the French Society of Cardiology (NCT 01992458).
Disclosures
Dr Providencia has received training grant from Boston Scientific, and Sorin Group and a Research Grant from Medtronic. Dr Babuty has received travel support and clinical study support from Biotronik, Boston Scientific, Medtronic, St. Jude Medical, and Sorin Group. Dr Sadoul has received personal fees from Biotronik, Boston Scientific, Medtronic, Sorin Group, and St. Jude Medical. Dr Piot has received consulting honoraria from Medtronic and St. Jude Medical and research grants from Medtronic and Boston Scientific. Dr Klug has received consultant fees from St. Jude Medical, Medtronic, Sorin Group, Boston Scientific, and Biotronik. Dr Boveda has received consulting fees from Medtronic, Boston Scientific, and Sorin Group. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
(J Am Heart Assoc. 2016;5:e002756 doi: 10.1161/JAHA.115.002756)
References
- 1. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 2. Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–1890. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML; Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 4. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH; Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 5. MacFadden DR, Crystal E, Krahn AD, Mangat I, Healey JS, Dorian P, Birnie D, Simpson CS, Khaykin Y, Pinter A, Nanthakumar K, Calzavara AJ, Austin PC, Tu JV, Lee DS. Sex differences in implantable cardioverter‐defibrillator outcomes: findings from a prospective defibrillator database. Ann Intern Med. 2012;156:195–203. [DOI] [PubMed] [Google Scholar]
- 6. van der Heijden AC, Thijssen J, Borleffs CJ, van Rees JB, Höke U, van der Velde ET, van Erven T, Schalij MJ. Gender‐specific differences in clinical outcome of primary prevention implantable cardioverter defibrillator recipients. Heart. 2013;99:1244–1249. [DOI] [PubMed] [Google Scholar]
- 7. Amit G, Suleiman M, Konstantino Y, Luria D, Kazatsker M, Chetboun I, Haim M, Gavrielov‐Yusim N, Goldenberg I, Glikson M; Israeli Working Group on Pacing and Electrophysiology . Sex differences in implantable cardioverter‐defibrillator implantation indications and outcomes: lessons from the Nationwide Israeli‐ICD Registry. Europace. 2014;16:1175–1180. [DOI] [PubMed] [Google Scholar]
- 8. Chauvin M, Frank R, Le Heuzey JY, Barnay C, Cazeau S, Djiane P, Guenoun M, Leenhardt A, Mabo P, Sadoul N; Groupes de stimulation cardiaque et de rythmologie de la Société française de cardiologie . Recommendations concernant l'implantation et la surveillance des défibrillateurs automatiques implantables. Arch Mal Coeur Vaiss. 2004;97:915–919. [PubMed] [Google Scholar]
- 9. Vandenbroucke JP. The making of strobe. Epidemiology. 2007;18:797–799. [DOI] [PubMed] [Google Scholar]
- 10. Santangeli P, Pelargonio G, Dello Russo A, Casella M, Bisceglia C, Bartoletti S, Santarelli P, Di Biase L, Natale A. Gender differences in clinical outcome and primary prevention defibrillator benefit in patients with severe left ventricular dysfunction: a systematic review and meta‐analysis. Heart Rhythm. 2010;7:876–882. [DOI] [PubMed] [Google Scholar]
- 11. Lin G, Meverden RA, Hodge DO, Uslan DZ, Hayes DL, Brady PA. Age and gender trends in implantable cardioverter defibrillator utilization: a population based study. J Interv Card Electrophysiol. 2008;22:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gauri AJ, Davis A, Hont T, Burke MC, Knight BP. Disparities in the use of primary prevention and defibrillator therapy among blacks and women. Am J Med. 2006;119:116.e17–116.e21. [DOI] [PubMed] [Google Scholar]
- 13. Hernandez AF, Foranow GC, Liang L, Al‐Khatib SM, Curtis LH, LaBresh KA, Yancy CW, Albert NM, Peterson ED. Sex and racial differences in the use of implantable cardioverter‐defibrillators among patients hospitalized with heart failure. JAMA. 2007;298:1525–1532. [DOI] [PubMed] [Google Scholar]
- 14. Bhavnani SP, Pavuluri V, Coleman CI, Guertin D, Yarlagadda RK, Clyne CA, Kluger J. The gender‐paradox among patients with implantable cardioverter‐defibrillators: a propensity‐matched study. Pacing Clin Electrophysiol. 2013;36:878–884. [DOI] [PubMed] [Google Scholar]
- 15. Russo AM, Stamato NJ, Lehmann MH, Hafley GE, Lee KL, Pieper K, Buxton AE; MUSTT Investigators . Influence of gender on arrhythmia characteristics and outcome in the Multicenter UnSustained Tachycardia Trial. J Cardiovasc Electrophysiol. 2004;15:993–998. [DOI] [PubMed] [Google Scholar]
- 16. Aronson D, Burger AJ. The effect of sex on ventricular arrhythmic events in patients with congestive heart failure. Pacing Clin Electrophysiol. 2002;25:1206–1211. [DOI] [PubMed] [Google Scholar]
- 17. Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. [DOI] [PubMed] [Google Scholar]
- 18. Albert CM, McGovern BA, Newell JB, Ruskin JN. Sex differences in cardiac arrest survivors. Circulation. 1996;93:1170–1176. [DOI] [PubMed] [Google Scholar]
- 19. Kannel WB, Wilson PW, D'Agostino RB, Cobb J. Sudden coronary death in women. Am Heart J. 1998;136:205–212. [DOI] [PubMed] [Google Scholar]
- 20. Haigney MC, Zareba W, Nasir JM, McNitt S, McAdams D, Gentlesk PJ, Goldstein RE, Moss AJ; MADIT II Investigators . Gender differences and risk of ventricular tachycardia or ventricular fibrillation. Heart Rhythm. 2009;6:180–186. [DOI] [PubMed] [Google Scholar]
- 21. Dash R, Frank KF, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum handling in failing human myocardium. J Mol Cell Cardiol. 2001;33:1345–1353. [DOI] [PubMed] [Google Scholar]
- 22. Yarnoz MJ, Curtis AB. More reasons why men and women are not the same (gender differences in electrophysiology and arrhythmias). Am J Cardiol. 2008;101:1291–1296. [DOI] [PubMed] [Google Scholar]
- 23. Larsen JA, Kadish AH. Effects of gender on cardiac arrhythmias. J Cardiovasc Electrophysiol. 1998;9:655–664. [DOI] [PubMed] [Google Scholar]
- 24. Airaksinen KE, Ik Heimo MJ, Linnaluoto M, Tahvanainen KU, Huikuri HV. Gender difference in autonomic and hemodynamic reactions to abrupt coronary occlusion. J Am Coll Cardiol. 1998;31:301–306. [DOI] [PubMed] [Google Scholar]
- 25. Albert CM, Nam EG, Rimm EB, Jin HW, Hajjar RJ, Hunter DJ, MacRae CA, Ellinor PT. Cardiac sodium channel gene variants and sudden cardiac death in women. Circulation. 2008;117:16–23. [DOI] [PubMed] [Google Scholar]
- 26. Newton‐Cheh C, Cook NR, VanDenburgh M, Rimm EB, Ridker PM, Albert CM. A common variant at 9p21 is associated with sudden and arrhythmic cardiac death. Circulation. 2009;120:2062–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arking DE, Pfeufer A, Post W, Kao WH, Newton‐Cheh C, Ikeda M, West K, Kashuk C, Akyol M, Perz S, Jalilzadeh S, Illig T, Gieger C, Guo CY, Larson MG, Wichmann HE, Marbán E, O'Donnel CJ, Hirshhorn JN, Kääb S, Spooner PM, Meitinger T, Chakravarti A. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38:644–651. [DOI] [PubMed] [Google Scholar]
- 28. Gavin MC, Newton‐Cheh C, Gaziano JM, Cook NR, VanDenburgh M, Albert CM. A common variant in the β2‐adrenergic receptor and risk of sudden cardiac death. Heart Rhythm. 2011;8:704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marijon E, Bougouin W, Périer MC, Celermajer DS, Jouven X. Incidence of sports‐related sudden death in France by specific sports and sex. JAMA. 2013;310:642–643. [DOI] [PubMed] [Google Scholar]
- 30. Marijon E, Bougouin W, Celermajer DS, Périer MC, Dumas F, Benameur N, Karam N, Lamhaut L, Tafflet M, Mustafic H, de Deus NM, Le Heuzey JY, Desnos M, Avillach P, Spaulding C, Cariou A, Prugger C, Empana JP, Jouven X. Characteristics and outcomes of sudden cardiac arrest during sports in women. Circ Arrhythm Electrophysiol. 2013;6:1185–1191. [DOI] [PubMed] [Google Scholar]
- 31. Albert CM, Oh K, Whang W, Manson JE, Chae CU, Stampfer MJ, Willet WC, Hu FB. Dietary alpha‐linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation. 2005;112:3232–3238. [DOI] [PubMed] [Google Scholar]
- 32. Arshad A, Moss AJ, Foster E, Padeletti L, Barsheshet A, Goldenberg I, Greenberg H, Hall WJ, McNitt S, Zareba W, Solomon S, Steinberg JS; MADIT‐CRT Executive Committee . Cardiac resynchronization therapy is more effective in women than in men: the MADIT‐CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) trial. J Am Coll Cardiol. 2011;57:813–820. [DOI] [PubMed] [Google Scholar]
- 33. Crow RS, Hannan PJ, Folsom AR. Prognostic significance of corrected QT and corrected JT interval for incident coronary heart disease in a general population sample stratified by presence of absence of wide QRS complex: the ARIC study with 12 years of follow‐up. Circulation. 2003;108:1985–1989. [DOI] [PubMed] [Google Scholar]
- 34. Russo AM, Day JD, Stolen K, Mullin CM, Doraiswamy V, Lerew DL, Olshansky B. Implantable cardioverter defibrillators: do women fare worse than men? Gender comparison in the INTRINSIC RV trial. J Cardiovasc Electrophysiol. 2009;20:973–978. [DOI] [PubMed] [Google Scholar]
- 35. Russo AM, Daugherty SL, Masoudi FA, Wang Y, Curtis J, Lampert R. Gender and outcomes after primary prevention implantable cardioverter‐defibrillator implantation: findings from the National Cardiovascular Data Registry (NCDR). Am Heart J. 2015;170:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zabarovskaja S, Gadler F, Braunschweig F, Stählberg M, Hörnsten J, Linde C, Lund LH. Women have better long‐term prognosis than men after cardiac resynchronization therapy. Europace. 2012;14:1148–1155. [DOI] [PubMed] [Google Scholar]
- 37. Rho RW, Patton KK, Poole JE, Cleland JG, Shadman R, Anand I, Maggioni AP, Carson PE, Swedberg K, Levy WC. Important differences in mode of death between men and women with heart failure who would qualify for a primary prevention implantable cardioverter‐defibrillator. Circulation. 2012;126:2402–2407. [DOI] [PubMed] [Google Scholar]


