Abstract
Background
In comparison to warfarin, non–vitamin K antagonist oral anticoagulants (NOACs) have the advantages of ease of dosing, fewer drug interactions, and lack of need for ongoing monitoring. We sought to evaluate whether these advantages translate to improved adherence and whether adherence is associated with improved outcomes in patients with atrial fibrillation.
Methods and Results
We performed a retrospective cohort analysis by using a large US commercial insurance database to identify 64 661 patients with atrial fibrillation who initiated warfarin, dabigatran, rivaroxaban, or apixaban treatment between November 1, 2010, and December 31, 2014. During a median of 1.1 y of follow‐up, 47.5% of NOAC patients had a proportion of days covered of ≥80%, compared with 40.2% in warfarin patients (P<0.001). Patients with CHA 2 DS 2‐VASc (risk based on the presence of congestive heart failure, hypertension age 65–74 y, age ≥75 y, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, sex category) score ≥4 were at increased risk of stroke when they were not taking anticoagulation ≥1 month versus <1 week (1–3 months: hazard ratio [HR] 1.96, 3–6 months: HR 2.64, ≥6 months: HR 3.66; all P<0.001). Patients with CHA 2 DS 2‐VASc score 2 or 3 were at increased risk of stroke when they were not taking anticoagulation ≥6 months (HR 2.73, P<0.001). In these patients with CHA 2 DS 2‐VASc score ≥2, nonadherence was not associated with intracranial hemorrhage. Among patients with CHA 2 DS 2‐VASc score 0 or 1, time not taking anticoagulation was not associated with stroke, but not taking anticoagulation ≥3 months was associated with a significant reduction of bleeding.
Conclusions
Adherence to anticoagulation is poor in practice and may be modestly improved with NOACs. Adherence to therapy appears to be most important in patients with CHA 2 DS 2‐VASc score ≥2, whereas the benefits of anticoagulation may not outweigh the harms in patients with CHA 2 DS 2‐VASc score 0 or 1.
Keywords: adherence, bleeding, CHA2DS2‐VASc, non–vitamin K antagonist oral anticoagulants, stroke
Subject Categories: Arrhythmias, Atrial Fibrillation, Intracranial Hemorrhage, Ischemic Stroke, Secondary Prevention
Introduction
Oral anticoagulants are highly effective for stroke prevention in patients with atrial fibrillation,1, 2 but strict adherence to medication is crucial for maximizing treatment benefits. There is hope that non–vitamin K antagonist oral anticoagulants (NOACs) may improve adherence, because of less burden of treatment compared with warfarin.3, 4, 5 This is uncertain, however, as warfarin users may incur lower out‐of‐pocket medication costs and have frequent contact with the healthcare system. Expected adherence to therapy is often an important consideration in clinical decision‐making; data on adherence rates can help physicians and patients choose between medications.
Although poor adherence is a barrier to effective stroke prevention, it can provide researchers with a window into the risk‐benefit balance of the therapy. Given the well‐established efficacy for anticoagulation in stroke prevention, it is unlikely that a clinical trial would randomize patients at increased stroke risk to no anticoagulation, but pharmacy‐linked administrative data may provide a critical tool to assess outcomes among candidates for anticoagulation who are taking and those who are not taking therapy. In this way, studying variation in adherence may contribute to the evidence of safety and efficacy of oral anticoagulants. Because not all patients benefit equally and, in some, the risks of therapy may outweigh the potential benefits,6, 7 examining the impact of nonadherence across the range of risk is important to help guide therapy, particularly among patients with anticipated low incidence of cardioembolism and potential for bleeding.
There are no studies comparing the relative adherence of NOACs in contemporary practice. Further, the impact of nonadherence on important safety and effectiveness outcomes has not been assessed in the NOAC era. Therefore, we sought to characterize (1) the adherence to NOACs (including dabigatran, rivaroxaban, and apixaban) and (2) the association between temporary or permanent discontinuation of therapy, measured by the cumulative time not taking anticoagulation, and the risk of stroke and major bleeding in patients at different baseline risks of stroke.
Methods
Data Source
We conducted a retrospective analysis of administrative claims data from a large US commercial insurance database, Optum Labs Data Warehouse, which includes privately insured and Medicare Advantage enrollees. The database contains longitudinal health information for >100 million enrollees during the past 20 years, from geographically diverse regions across the United States, with greatest representation from the south and midwest.8 The included health plans provide claims for professional (eg, physician), facility (eg, hospital), and outpatient prescription medication services.9 Because this study involved analysis of preexisting, deidentified data, it was exempt from institutional review board approval.
Study Population
We identified adult patients with atrial fibrillation who initiated treatment with warfarin, dabigatran, rivaroxaban, or apixaban between November 1, 2010, and December 31, 2014. We defined patients’ first oral anticoagulant as their index medication and the date the prescription was filled as the index date. We defined the 12 months before the index date as the baseline period and required that patients have continuous enrollment in a medical and pharmacy plan during this time. We also required that patients have a diagnosis of atrial fibrillation (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] diagnosis code 427.31) at baseline or on the index date. We excluded patients with any dispensed prescription for oral anticoagulants during the baseline period. We also excluded patients with ICD‐9‐CM code V58.61 (Long‐term [current] use of anticoagulants) during 3 to 12 months before the index date as this might be an indication of either ongoing or prior oral anticoagulant use.
Patient Baseline Characteristics
For each patient, we assessed demographic and socioeconomic characteristics at the time of the index date, including age, sex, race, household income, and residence region. We used the Charlson–Deyo comorbidity index to assess the patient's overall burden of comorbidities10 and the HAS‐BLED [ie, risk stratification scheme to estimate baseline risk of major hemorrhage based on the presence of hypertension, abnormal renal function, abnormal liver function, stroke, bleeding history or predisposition, age > 65 years, antiplatelet or nonsteroidal anti‐inflammatory drug use and alcoholism] score to measure the baseline bleeding risk. One element of HAS‐BLED, the labile international normalized ratios (INRs), is not available before the initiation of warfarin and is not applicable to NOACs; therefore, the range of HAS‐BLED score in this study was 0 to 8 instead of 0 to 9 in the original HAS‐BLED score.11
We used CHA2DS2‐VASc ((risk based on the presence of congestive heart failure, hypertension, age 65–74 years, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack [TIA], vascular disease, sex category) score to determine patients’ risk of stroke at baseline, based on 9 possible points with higher scores indicating higher risk of stroke. We grouped patients with CHA2DS2‐VASc score of 0 or 1 because the guideline from 2014 American College of Cardiology/American Heart Association/Heart Rhythm Society (AHA/ACC/HRS) recommends oral anticoagulants among patients with CHA2DS2‐VASc score ≥2.12 We further divided patients into those with CHA2DS2‐VASc score 2 or 3 and score ≥4 because previous studies suggested the use of oral anticoagulant decreased in high‐risk patients with elevated CHA2DS2‐VASc score.13 All the conditions in the Charlson–Deyo comorbidity index, HAS‐BLED score, and CHA2DS2‐VASc score were identified by using ICD‐9‐CM codes in the primary or secondary position on any claim during the baseline period.
Primary End Points
The primary end points are the first inpatient admission for (1) stroke, including ischemic stroke and systemic embolism, and (2) major bleeding, including gastrointestinal (GI) bleeding, intracranial hemorrhage, and bleeding from other sites. Published claims‐based algorithms were used in defining outcomes and validated in previous studies.3, 14, 15, 16, 17 ICD‐9‐CM codes are given in Table 1.
Table 1.
International Classification of Disease, Ninth Edition, Clinical Modification (ICD 9‐CM) Codes Used to Define Study Outcomes
| Primary Outcome | ICD‐9‐CM Codes |
|---|---|
| Major bleeding | |
| Gastrointestinal bleeding | 456.0, 456.20, 530.21, 530.7, 530.82, 531.0x, 531.2x, 531.4x, 531.6x, 532.0x, 532.2x, 532.4x, 532.6x, 533.0x, 533.2x, 533.4x, 533.6x, 534.0x, 534.2x, 534.4x, 534.6x, 535.01, 535.11, 535.21, 535.31, 535.41, 535.51, 535.61, 535.71, 537.83, 537.84, 562.02, 562.03, 562.12, 562.13, 568.81, 569.3, 569.85, 578.x |
| Nontraumatic intracranial hemorrhage | 430, 431, 432.x |
| Traumatic intracranial hemorrhage | 852.x, 853.x |
| Bleeding from other sites | 423.0, 459.0, 596.7, 599.71, 719.1x, 784.8, 786.3 |
| Stroke | |
| Ischemic stroke | 433.x1, 434.x1, or 436 |
| Transient ischemic attack (TIA) | 435.X |
| Systemic embolism | 444.x |
Outcomes were identified using primary or secondary diagnosis on inpatient claims. When assessing ischemic stroke, TIA, and systemic embolism, we excluded the events that had a primary discharge diagnosis of rehabilitation (ICD‐9‐CM code V57) or any additional diagnoses of subarachnoid hemorrhage (ICD‐9‐CM code 430), intracerebral hemorrhage (ICD‐9‐CM code 431), or trauma (ICD‐9‐CM codes 800–804 and 850–854). When assessing major bleeding, we excluded the events that had a primary discharge diagnosis of rehabilitation (ICD‐9‐CM code V57).
Exposure
Patients were followed from their index date until the earliest date of the occurrence of stroke or bleeding, the end of enrollment in the health insurance plan, or the end of the study period (December 31, 2014). The days covered by oral anticoagulants were determined based on fill date and the days of supply on the pharmacy claims. Some patients switched to a nonindex oral anticoagulant during follow‐up, and they were considered to be taking anticoagulation when using the nonindex oral anticoagulants. We calculated the proportion of days covered (PDC) over a patient's entire follow‐up as a measure of adherence, and we calculated the total number of days when patients were not taking any oral anticoagulation to measure the length of the temporary or permanent discontinuation of anticoagulation therapy.
Statistical Analysis
We conducted descriptive analyses for patients’ baseline characteristics. Categorical variables were summarized as frequencies and percentages and continuous variables as medians and 25th and 75th percentiles.
We used multivariable logistic regression to predict the probability of being adherent (PDC ≥80%) in the entire cohort, as well as for each medication cohort and by underlying stroke risk (CHA2DS2‐VASc score 0 or 1, score 2 or 3, and score ≥4). The main independent variables were index medication and underlying stroke risk (CHA2DS2‐VASc score 0 or 1, score 2 or 3, and score ≥4). We tested the interaction effect between index medication and patients’ baseline risk and included it in the final model. Other covariates included age, sex, race, household income, residence region, individual comorbidities of CHA2DS2‐VASc score and HAS‐BLED score, Charlson–Deyo comorbidity index, and the average out‐of‐pocket costs patients spent on their index medication per 30 days of supply.
We used multivariable Cox proportional hazards models to estimate the effect of time not taking anticoagulation on the risk of stroke and bleeding. The main exposure variable was the cumulative number of days patients were not taking any oral anticoagulants, which was used as a time‐varying covariate in the regression (ie, the number of days patients were not taking any oral anticoagulant changed over time). We categorized time not taking anticoagulation into 5 groups: <1 week, 1 week to <1 month, 1 month to <3 months, 3 months to <6 months, and ≥6 months. We included an interaction term between time not taking oral anticoagulant and baseline risk of stroke. We adjusted for patients’ index medication, age, sex, race, annual household income, residence region, Charlson–Deyo comorbidity index, HAS‐BLED score, and switch to a nonindex medication.
All analyses were conducted by using SAS 9.3 (SAS Institute Inc) and Stata 13.1 (StataCorp).
Sensitivity Analyses
First, apixaban and rivaroxaban had shorter follow‐up times than dabigatran, which could potentially lead to lower PDC in dabigatran patients, because patients often become less adherent over time.18 Therefore, we examined the PDC within the first 6 months of follow‐up.
Second, because the European Society of Cardiology (ESC) recommends oral anticoagulants for men with CHA2DS2‐VASc score ≥1 and women with CHA2DS2‐VASc score ≥2,19 additional analyses were conducted to separate men with CHA2DS2‐VASc score 1 from those without any risk factors in the low‐risk population. However, because the rates of stroke and bleeding were low in the low‐risk population, this was not included in the main results.
Third, the half‐lives of NOACs are shorter than that of warfarin, so the anticoagulation effect of NOACS declines more rapidly when scheduled doses are not taken. Therefore, nonadherence to NOACs might have larger adverse effects than warfarin and the adherence itself may be related to the index medication. To further investigate whether the effects of nonadherence vary by index medication, we conducted additional sensitivity tests by repeating the same regression analyses for each of the medications separately.
Last, in the main analyses, we did not consider TIA as a primary end point, but patients were censored when they had an inpatient admission of TIA. TIA is difficult to validate and may be used as diagnosis for diffuse symptoms or dizziness.20 A sensitivity analysis was conducted to include TIA in the stroke definition.
Results
Patient Characteristics
We identified 64 661 patients who initiated oral anticoagulants between November 1, 2010, and December 31, 2014. The majority of the patients (59.1%) initiated treatment with warfarin, and 6.0% started taking apixaban, 15.8% started taking dabigatran, and 19.1% started taking rivaroxaban. The median age was 73 years (IQR 64–80 years), 56.2% were male, and 79.1% were white. Nearly 90% of patients had CHA2DS2‐VASc score ≥2, and nearly half had a high risk of bleeding (HAS‐BLED score ≥3) (Table 2). The median follow‐up time was 1.1 years (IQR 0.5–2.0 years).
Table 2.
Patient Baseline Characteristics, Stratified by Index Medication (N=64 661)
| Apixaban (n=3900, 6.0%) | Dabigatran (n=10 235, 15.8%) | Rivaroxaban (n=12 336, 19.1%) | Warfarin (n=38 190, 59.1%) | Total (n=64 661) | |
|---|---|---|---|---|---|
| Age, y, median (IQR) | 72 (64–80) | 69 (60–77) | 70 (62–78) | 75 (67–81) | 73 (64–80) |
| Age, n (%) | |||||
| 18–54 y | 331 (8.5) | 1356 (13.2) | 1284 (10.4) | 2136 (5.6) | 5107 (7.9) |
| 55–64 y | 737 (18.9) | 2565 (25.1) | 2667 (21.6) | 5144 (13.5) | 11 113 (17.2) |
| 65–74 y | 1207 (30.9) | 3091 (30.2) | 3955 (32.1) | 11 707 (30.7) | 19 960 (30.9) |
| ≥75 y | 1625 (41.7) | 3223 (31.5) | 4430 (35.9) | 19 203 (50.3) | 28 481 (44.0) |
| Male, n (%) | 2137 (54.8) | 6337 (61.9) | 7250 (58.8) | 20 586 (53.9) | 36 310 (56.2) |
| Race, n (%) | |||||
| Asian | 89 (2.3) | 259 (2.5) | 304 (2.5) | 776 (2.0) | 1428 (2.2) |
| Black | 351 (9.0) | 854 (8.3) | 1054 (8.5) | 3657 (9.6) | 5916 (9.1) |
| Hispanic | 193 (4.9) | 450 (4.4) | 597 (4.8) | 1827 (4.8) | 3067 (4.7) |
| Unknown | 185 (4.7) | 485 (4.7) | 591 (4.8) | 1831 (4.8) | 3092 (4.8) |
| White | 3082 (79.0) | 8187 (80.0) | 9790 (79.4) | 30 099 (78.8) | 51 158 (79.1) |
| Region, n (%) | |||||
| Midwest | 1083 (27.8) | 2625 (25.6) | 3311 (26.8) | 13 784 (36.1) | 20 803 (32.2) |
| Northeast | 710 (18.2) | 2025 (19.8) | 2324 (18.8) | 7257 (19.0) | 12 316 (19.0) |
| South | 1648 (42.3) | 4491 (43.9) | 5401 (43.8) | 13 036 (34.1) | 24 576 (38.0) |
| West | 459 (11.8) | 1094 (10.7) | 1300 (10.5) | 4113 (10.8) | 6966 (10.8) |
| Comorbidities, n (%) | |||||
| Congestive heart failure | 1110 (28.5) | 2460 (24.0) | 3162 (25.6) | 14 866 (38.9) | 21 598 (33.4) |
| Hypertension | 3321 (85.2) | 8410 (82.2) | 10 219 (82.8) | 33 100 (86.7) | 55 050 (85.1) |
| Diabetes | 1321 (33.9) | 3233 (31.6) | 3907 (31.7) | 14 677 (38.4) | 23 138 (35.8) |
| Ischemic stroke/transient ischemic attack | 501 (12.8) | 1185 (11.6) | 1351 (11.0) | 6250 (16.4) | 9287 (14.4) |
| Vascular disease | 1907 (48.9) | 4488 (43.8) | 5527 (44.8) | 21 067 (55.2) | 32 989 (51.0) |
| Renal disease | 678 (17.4) | 1201 (11.7) | 1764 (14.3) | 10 105 (26.5) | 13 748 (21.3) |
| Liver disease | 139 (3.6) | 335 (3.3) | 452 (3.7) | 1635 (4.3) | 2561 (4.0) |
| Major bleeding or predisposition to bleeding | 459 (11.8) | 1096 (10.7) | 1400 (11.3) | 7013 (18.4) | 9968 (15.4) |
| CHA2DS2‐VASc score, n (%) | |||||
| 0 or 1 | 423 (10.8) | 1847 (18.0) | 1848 (15.0) | 2580 (6.8) | 6698 (10.4) |
| 2 or 3 | 1242 (31.8) | 3608 (35.3) | 4302 (34.9) | 9809 (25.7) | 18 961 (29.3) |
| 4 | 872 (22.4) | 2014 (19.7) | 2545 (20.6) | 8323 (21.8) | 13 754 (21.3) |
| ≥5 | 1363 (34.9) | 2766 (27.0) | 3641 (29.5) | 17 478 (45.8) | 25 248 (39.0) |
| HAS‐BLED, n (%) | |||||
| ≥3 | 1707 (43.8) | 3842 (37.5) | 4933 (40.0) | 20 439 (53.5) | 30 921 (47.8) |
| Charlson–Deyo index, n (%) | |||||
| 0–1 | 1646 (42.2) | 5021 (49.1) | 5722 (46.4) | 11 814 (30.9) | 24 203 (37.4) |
| 2–3 | 1245 (31.9) | 3010 (29.4) | 3681 (29.8) | 11 457 (30.0) | 19 393 (30.0) |
| ≥4 | 1009 (25.9) | 2204 (21.5) | 2933 (23.8) | 14 919 (39.1) | 21 065 (32.6) |
CHA 2 DS 2‐VASc, risk based on the presence of congestive heart failure, hypertension, age 65–74 y, age ≥75 y, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, sex category; HAS‐BLED, risk stratification scheme to estimate baseline risk of major hemorrhage based on the presence of hypertension, abnormal renal function, abnormal liver function, stroke, bleeding history or predisposition, age > 65 y, antiplatelet or nonsteroidal anti‐inflammatory drug use and alcoholism.
Adherence
Fewer than half (47.5%) of the NOAC patients had ≥80% days covered by oral anticoagulants, compared with 40.2% in warfarin patients (P<0.001). Apixaban had the highest unadjusted percentage of adherent patients (61.9%), followed by rivaroxaban (50.5%) and dabigatran (38.5%). A higher percentage of high‐risk patients adhered to treatment than did those with a low stroke risk (CHA2DS2‐VASc score 0 or 1, 30.5%; CHA2DS2‐VASc score 2 or 3, 43.4%; CHA2DS2‐VASc score ≥4, 45.3%). After adjustment for confounders, adherence rates were highest with apixaban (predicted probability of adherence [PP] 52.1%, 95% CI 50.3–53.9%), followed by rivaroxaban (PP 47.6%, 95% CI 46.6–48.7%), dabigatran (PP 45.9%, 95% CI 44.8–47.1%), and warfarin (PP 38.7%, 95% CI 38.1–39.3%). Patients initiating warfarin was associated with lower probability of adhering to anticoagulation therapy compared with those initiating NOACs in all risk strata (P<0.001 for all comparisons) (Table 3).
Table 3.
Adherence to OACs (PDC ≥80%), Stratified by Index Medication (N=64 661)
| Apixaban (n=3900) | Dabigatran (n=10 235) | Rivaroxaban (n=12 336) | All NOACs (n=26 471) | Warfarin (n=38 190) | P Value (All NOACs Pooled vs Warfarin) | |
|---|---|---|---|---|---|---|
| Unadjusted adherencea | ||||||
| All | 61.9% | 38.5% | 50.5% | 47.5% | 40.2% | <0.001 |
| CHA2DS2‐VASc score 0 or 1 | 50.1% | 24.6% | 36.5% | 32.6% | 27.1% | <0.001 |
| CHA2DS2‐VASc score 2 or 3 | 62.0% | 40.3% | 52.8% | 49.1% | 38.1% | <0.001 |
| CHA2DS2‐VASc score ≥4 | 64.0% | 42.4% | 53.2% | 51.1% | 42.3% | <0.001 |
| Adjusted adherence, 95% CIb | ||||||
| All | 52.1% (50.3–53.9) | 45.9% (44.8–47.1) | 47.6% (46.6–48.7) | 47.5% (46.7–48.2) | 38.7% (38.1–39.3) | <0.001 |
| CHA2DS2‐VASc score 0 or 1 | 40.6% (35.8–45.4) | 28.6% (26.3–30.9) | 30.8% (28.7–32.9) | 30.8% (29.3–32.3) | 25.2% (23.4–27.0) | <0.001 |
| CHA2DS2‐VASc score 2 or 3 | 51.9% (48.9–55.0) | 46.9% (45.1–48.6) | 48.8% (47.2–50.5) | 48.3% (47.2–49.5) | 37.3% (36.3–38.4) | <0.001 |
| CHA2DS2‐VASc score ≥4 | 54.1% (51.8–56.5) | 48.7% (47.1–50.3) | 50.1% (48.7–51.5) | 50.1% (49.0–51.1) | 42.0% (41.3–42.7) | <0.001 |
OAC, oral anticoagulant; PDC, proportion of days covered; NOAC, non–vitamin K antagonist oral anticoagulant; CHA 2 DS 2‐VASc, risk based on the presence of congestive heart failure, hypertension, age ≥75 y, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, sex category.
Unadjusted adherence was the percentage of patients with PDC ≥80%.
Adjusted adherence was the predicted probability of PDC ≥80% based on multivariable logistic regression.
Risks of Stroke
During a total of 87 157 person‐years of follow‐up, 1150 patients had an ischemic stroke or a systemic embolism. The incidence rate per 100 person‐years was 1.32 in the whole cohort, 0.33 in patients with CHA2DS2‐VASc score 0 or 1, 0.72 in patients with CHA2DS2‐VASc score 2 or 3, and 1.82 in patients with CHA2DS2‐VASc score ≥4 (Table 4).
Table 4.
Number of Events and Incident Rate per 100 Person‐Years, Stratified by CHA2DS2‐VASc
| CHA2DS2‐VAS score 0, 1 (n=6698) | CHA2DS2‐VAS score 2, 3 (n=18 961) | CHA2DS2‐VAS score ≥4 (n=39 002) | Total (N=64 661) | ||
|---|---|---|---|---|---|
| Ischemic stroke and systemic embolism | No. of events | 33 | 189 | 928 | 1150 |
| Incident rate | 0.33 | 0.72 | 1.82 | 1.32 | |
| Ischemic stroke | No. of events | 28 | 157 | 808 | 993 |
| Incident rate | 0.28 | 0.6 | 1.59 | 1.14 | |
| Systemic embolism | No. of events | 5 | 32 | 120 | 157 |
| Incident rate | 0.05 | 0.12 | 0.24 | 0.18 | |
| Major bleeding | No. of events | 73 | 537 | 2629 | 3239 |
| Incident rate | 0.74 | 2.04 | 5.16 | 3.72 | |
| Gastrointestinal bleeding | No. of events | 47 | 337 | 1838 | 2222 |
| Incident rate | 0.47 | 1.28 | 3.61 | 2.55 | |
| Intracranial hemorrhage | No. of events | 10 | 98 | 479 | 587 |
| Incident rate | 0.10 | 0.37 | 0.90 | 0.67 | |
| Other bleeding | No. of events | 16 | 102 | 312 | 430 |
| Incident rate | 0.16 | 0.39 | 0.61 | 0.49 |
CHA 2 DS 2‐VASc, risk based on the presence of congestive heart failure, hypertension, age ≥75 y, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, sex category.
Figure 1 illustrates the association between nonadherence and the risk of stroke in patients with varying baseline risk. No significant effect was found between nonadherence and the risk of stroke in patients with CHA2DS2‐VASc score 0 or 1. In contrast, not taking oral anticoagulant for ≥6 months was associated with elevated risk of stroke in patients with CHA2DS2‐VASc score 2 or 3 (adjusted hazard ratio [HR] 2.73, 95% CI 1.76–4.23), compared with not taking oral anticoagulants for <1 week. In patients with CHA2DS2‐VASc score ≥4, the association between nonadherence and stroke was more evident: HR was 1.96 (95% CI 1.48–2.60) for not taking oral anticoagulant 1 to 3 months, 2.64 (95% CI 1.93–3.61) for 3 to 6 months, and 3.66 (95% CI 2.68–5.01) for ≥6 months compared with not taking oral anticoagulants <1 week (Figure 1 and Table 5).
Figure 1.
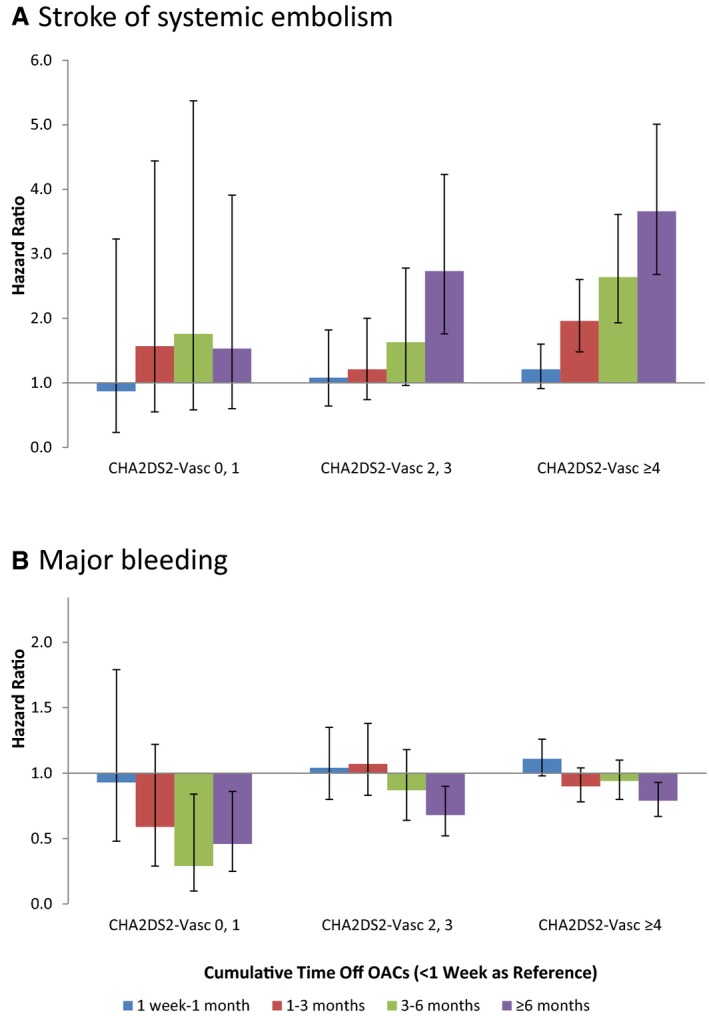
Hazard ratio for primary outcomes stratified by CHA 2 DS 2‐VASc (risk based on the presence of congestive heart failure, hypertension, age 65–74 y, age ≥75 y, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, sex category) score.
Table 5.
Survival Analysis, Ischemic Stroke, and Systemic Embolism as the Outcome
| Time Not Taking OAC | Hazard Ratio (95% CI) |
|---|---|
| CHA2DS2‐VASc score 0 or 1 | |
| <1 wk | Ref |
| 1 wk to 1 mo | 0.87 (0.23–3.23) |
| 1–3 mo | 1.57 (0.55–4.44) |
| 3–6 mo | 1.76 (0.58–.37) |
| ≥6 mo | 1.53 (0.60–3.91) |
| CHA2DS2‐VASc score 2 or 3 | |
| <1 wk | Ref |
| 1 wk to 1 mo | 1.08 (0.64–1.82) |
| 1–3 mo | 1.21 (0.74–2.00) |
| 3–6 mo | 1.63 (0.96–2.78) |
| ≥6 mo | 2.73a (1.76–4.23) |
| CHA2DS2‐VASc score ≥4 | |
| <1 wk | Ref |
| 1 wk to 1 mo | 1.21 (0.91–1.60) |
| 1–3 mo | 1.96a (1.48–2.60) |
| 3–6 mo | 2.64a (1.93–3.61) |
| ≥6 mo | 3.66a (2.68–5.01) |
OAC, oral anticoagulation; CHA 2 DS 2‐VASc, risk based on the presence of congestive heart failure, hypertension, age ≥75 y, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, sex category. Age, sex, race, annual household income, residence region, HAS‐BLED, risk stratification scheme to estimate baseline risk of major hemorrhage based on the presence of hypertension, abnormal renal function, abnormal liver function, stroke, bleeding history or predisposition, age > 65 y, antiplatelet or nonsteroidal anti‐inflammatory drug use and alcoholism, Charlson–Deyo comorbidity index, index medication, and switch to nonindex medication were adjusted.
P<0.001.
Risks of Bleeding
A total of 3239 patients had a major bleeding event, of which nearly 70% were GI bleeds and 18% were intracranial hemorrhages. The incidence rate per 100 person‐years was 3.72 for all major bleeding, 2.55 for GI bleeding, and 0.67 for intracranial hemorrhage.
Nonadherence to oral anticoagulant was related to lower risk of bleeding. HR for not taking oral anticoagulant for ≥6 months was 0.46 (95% CI 0.25–0.86) for patients with CHA2DS2‐VASc score 0 or 1, 0.68 (95% CI 0.52–0.90) for patients with CHA2DS2‐VASc score 2 or 3, and 0.79 (95% CI 0.67–0.93) for patients with CHA2DS2‐VASc score ≥4 (Figure 1 and Table 6). The risks of GI bleeding followed a similar pattern (Figure 2 and Table 6). However, in patients with CHA2DS2‐VASc score ≥2, there was no significant association between nonadherence and the risk of intracranial hemorrhage, whereas there was a significant reduction in intracranial hemorrhage risk in patients with CHA2DS2‐VASc score 0 or 1 (HR 0.23, 95% CI 0.05–0.96 for not taking oral anticoagulant ≥3 months versus <1 week). Because of the small event rate for intracranial hemorrhage, we combined the categories of 3 to 6 months and ≥6 months into a single category: ≥3 months (Figure 2 and Table 6).
Table 6.
Survival Analysis Using Bleeding as the Outcome, Hazard Ratios, and 95% CIs
| Time Not Taking OAC | All Major Bleeding | Gastrointestinal Bleeding | Intracranial Hemorrhage |
|---|---|---|---|
| CHA2DS2‐VASc score 0 or 1 | |||
| <1 wk | Ref | Ref | Ref |
| 1 wk to 1 mo | 0.93 (0.48–1.79) | 1.13 (0.53–2.44) | 0.33 (0.04–2.78) |
| 1–3 mo | 0.59 (0.29–1.22) | 0.48 (0.18–1.28) | 0.25 (0.03–2.15) |
| 3–6 mo | 0.29c (0.10–0.84) | 0.36 (0.11–1.21) | 0.23c (0.05–0.96) |
| ≥6 mo | 0.46c (0.25–0.86) | 0.46c (0.21–1.00) | NAb |
| CHA2DS2‐VASc score 2 or 3 | |||
| <1 wk | Ref | Ref | Ref |
| 1 wk to 1 mo | 1.04 (0.80–1.35) | 1.03 (0.74–1.43) | 0.99 (0.50–1.96) |
| 1–3 mo | 1.07 (0.83–1.38) | 1.14 (0.83–1.55) | 1.05 (0.55–2.02) |
| 3–6 mo | 0.87 (0.64–1.18) | 0.79 (0.53–1.17) | 1.17 (0.59–2.33) |
| ≥6 mo | 0.68a (0.52–0.90) | 0.64c (0.45–0.92) | 0.90 (0.48–1.69) |
| CHA2DS2‐VASc ≥4 | |||
| <1 wk | Ref | Ref | Ref |
| 1 wk to 1 mo | 1.11 (0.98–1.26) | 1.04 (0.89–1.21) | 1.31 (0.94–1.82) |
| 1–3 mo | 0.90 (0.78–1.04) | 0.84d (0.71–1.00) | 1.23 (0.87–1.74) |
| 3–6 mo | 0.94 (0.80–1.10) | 0.91 (0.76–1.11) | 1.20 (0.82–1.76) |
| ≥6 mo | 0.79d (0.67– 0.93) | 0.75d (0.61–0.91) | 1.06 (0.72–1.56) |
OAC, oral anticoagulant; CHA 2 DS 2‐VASc, risk based on the presence of congestive heart failure, hypertension, age 65–74 y, age ≥75 y, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, sex category.
Age, sex, race, annual household income, residence region, HAS‐BLED, risk stratification scheme to estimate baseline risk of major hemorrhage based on the presence of hypertension, abnormal renal function, abnormal liver function, stroke, bleeding history or predisposition, > 65 years, antiplatelet or nonsteroidal anti‐inflammatory drug use and alcoholism, Charlson‐Deyo comorbidity index, index medication, and switch to non‐index medication were adjusted.
Because of the small number of events, for intracranial hemorrhage, the 3–6 mo and ≥6 mo categories were combined.
P<0.05.
P<0.01.
Figure 2.
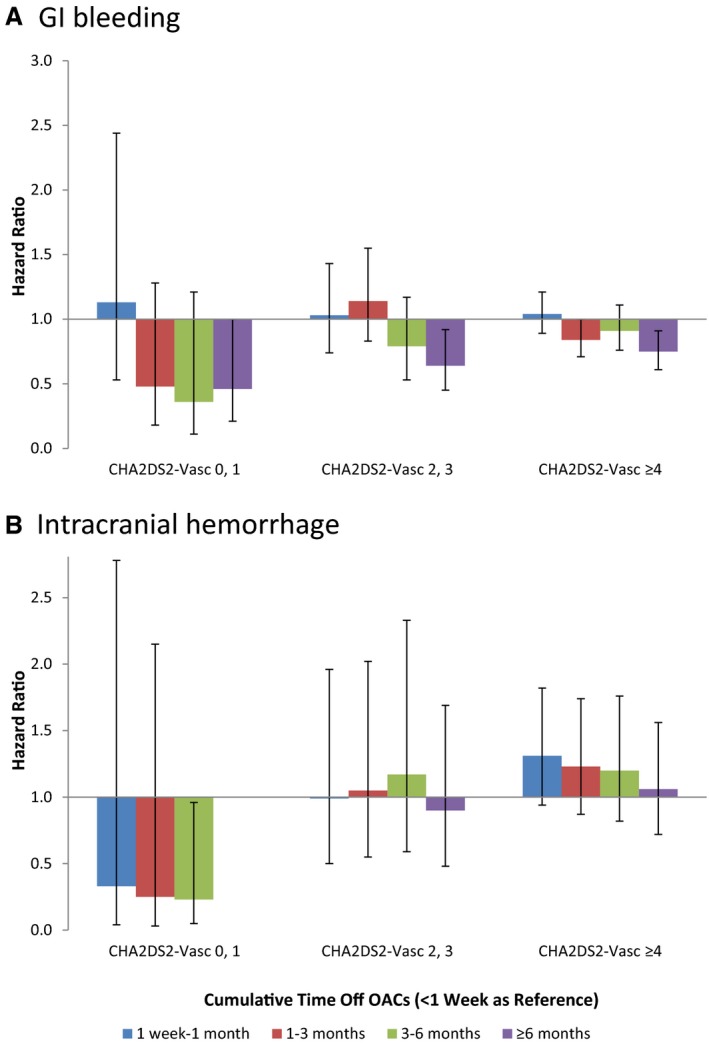
Hazard ratio for secondary outcomes stratified by CHA 2 DS 2‐VASc (risk based on the presence of congestive heart failure, hypertension, age ≥75 y, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, sex category) score.
Sensitivity Analysis
First, we looked at the adherence during the first 6 months. We found better adherence in all patients, but apixaban still had the highest adherence rate (Table 7). Second, among the 6698 low‐risk patients, 39.8% were patients with no risk factors other than sex (10.5% women and 29.3% men), but 60.2% were men with a CHA2DS2‐VASc score of 1. Additional analyses were conducted in these men, and the findings regarding low‐risk patients remained unchanged. Third, findings from stratified analyses by index medication were substantively unchanged, too. Last, we tested including TIA in addition to ischemic stroke and systemic embolism when analyzing the effects on stroke and found similar results.
Table 7.
Adherence to OACs (PDC ≥80%) Within First 6 Months of Follow‐up, Stratified by Index Medication (N=64 661)
| Apixaban (n=3900) | Dabigatran (n=10 235) | Rivaroxaban (n=12 336) | All NOACs (n=26 471) | Warfarin (n=38 190) | P Value (All NOACs Pooled vs Warfarin) | |
|---|---|---|---|---|---|---|
| Unadjusted adherencea | ||||||
| All | 64.5% | 51.2% | 58.4% | 56.5% | 51.6% | <0.001 |
| CHA2DS2‐VASc score 0 or 1 | 53.2% | 37.1% | 45.8% | 42.6% | 40.3% | 0.06 |
| CHA2DS2‐VASc score 2 or 3 | 64.6% | 53.3% | 60.1% | 58.0% | 49.8% | <0.001 |
| CHA2DS2‐VASc score ≥4 | 66.7% | 55.0% | 61.0% | 59.8% | 53.4% | <0.001 |
| Adjusted adherence, 95% CIb | ||||||
| All | 62.5% (60.8–64.2) | 57.3% (56.2–58.4) | 59.5% (58.5–60.5) | 58.9% (58.2–59.7) | 49.9% (49.3–50.5) | <0.001 |
| CHA2DS2‐VASc score 0 or 1 | 51.2% (46.3–56.1) | 41.4% (39.0–43.7) | 44.4% (42.1–46.7) | 43.7% (42.1–45.2) | 37.8% (35.9–39.7) | <0.001 |
| CHA2DS2‐VASc score 2 or 3 | 62.4% (59.5–65.2) | 58.3% (56.6–60.0) | 60.1% (58.6–61.6) | 59.6% (58.5–60.6) | 48.3% (47.3–49.4) | <0.001 |
| CHA2DS2‐VASc score ≥4 | 64.4% (62.2–66.5) | 59.5% (58.0–61.0) | 61.7% (60.3–63.0) | 61.1% (60.2–62.1) | 52.8% (52.1–53.5) | <0.001 |
OAC, oral anticoagulant; PDC, proportion of days covered; NOAC, non–vitamin K antagonist oral anticoagulant; CHA 2 DS 2‐VASc, risk based on the presence of congestive heart failure, hypertension, age 65–74 y, age ≥75 y, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, sex category.
Unadjusted adherence was the percentage of patients with PDC ≥80%.
Adjusted adherence was the predicted probability of PDC ≥80% based on multivariable logistic regression.
Discussion
In a large cohort of patients initiating oral anticoagulants for atrial fibrillation, we found that adherence to anticoagulation therapy was suboptimal. During a median of 1.1 years of follow‐up, fewer than half of the patients took the medications consistently. Adherence was associated with different outcomes in patients at different baseline risks of stroke. Among patients with CHA2DS2‐VASc score ≥2, better adherence was associated with lower stroke risk and a relatively small increase in bleeding risk―in particular, no significant increase in intracranial bleeding. Conversely, among patients with CHA2DS2‐VASc score 0 or 1, better adherence was associated with no significant change in the risk of stroke but an increased risk of bleeding.
The main strength of the study is the statistical power derived from our large cohort of patients treated with anticoagulation in everyday clinical practice. To our knowledge, this study was the first to report adherence rates of all 3 NOACs (dabigatran, rivaroxaban, and apixaban) together and the first to assess the stroke and bleeding risks related to nonadherence in the NOAC era. Previous studies that demonstrated the benefits of oral anticoagulants often compared patients taking anticoagulation with untreated patients or patients treated with antiplatelet agents21, 22 and were therefore subject to confounding; that is, the decision not to anticoagulate may be related to factors that are associated with increased stroke risk. In our study, all the patients were judged by their physicians as reasonable candidates for anticoagulation therapy and the treatment was initiated. We compared patients with similar baseline risk of stroke but different length of time not taking anticoagulation, adjusting for other relevant confounders. Because major bleeding can both prompt treatment interruption and increase the risk of stroke,23, 24 we censored patients at the time of major bleeding to minimize confounding.
Poor adherence is a problem for nearly all long‐term medications, but the need to improve adherence is less appreciated in anticoagulation therapy, because in the past, low prescription rate was responsible for a large percentage of underuse and much effort has been focused on increasing physician adherence to prescribing guidelines, resulting in improving prescription rates during the past several decades.25, 26 However, numerous studies found that even in patients who appropriately initiated warfarin, many struggle to maintain adherence in the long term.27, 28, 29, 30 There has been optimism that NOACs may improve adherence,4, 5 but this is not borne out in our data. Rather, we see that adherence to anticoagulation continues to be a challenge, regardless of the medication used. Our findings of low adherence rates were consistent with previous studies reporting poor adherence to dabigatran.3, 31, 32 Oral anticoagulants are purely preventive and address no symptoms, so they are especially vulnerable to nonadherence. NOACs also have higher out‐of‐pocket costs compared with warfarin, which may be another reason for nonadherence. Lack of routine monitoring has been considered as a major advantage of NOACs in comparison to warfarin. However, our findings suggest that routine follow‐up may still be needed to assess adherence and discuss barriers to the treatment with patients.
The data on adherence provide additional insight into the comparative advantages of NOACs, which may facilitate clinical decision‐making when physicians and patients need to choose between medications. Because of its once‐daily dosing regimen, rivaroxaban is expected to have better adherence than dabigatran and apixaban, which have twice‐daily regimens.21 In a recent Canadian study, patients taking rivaroxaban missed fewer pills than did those taking dabigatran and apixaban.33 In phase 3 clinical trials, rivaroxaban did not demonstrate any superiority in either primary efficacy or safety end points, whereas dabigatran 150 mg had better efficacy, and dabigatran 110 mg, apixaban, and edoxaban all had better safety.34, 35, 36, 37 Current expert opinion regarding which NOAC to choose only recommends rivaroxaban in patients who prefer low pill burden.38 Our data suggested this primary advantage of rivaroxaban may not exist at all—apixaban may actually be associated with better adherence. It may not be wise to prescribe rivaroxaban solely for the purpose of improving adherence. In our study, because of the low adherence rates, it is likely the nonadherence in routine clinical practice was less the result of missing pills but rather failure to refill medications, resulting in discontinuation or long gaps between refills.
Our finding of increased risk of stroke associated with nonadherence among high‐risk patients is consistent with previous studies on temporary or permanent discontinuation of oral anticoagulants.39, 40, 41 There are a few possible explanations for the findings of an association between nonadherence and risk of stroke. Previous studies reported transient elevation of blood markers of thrombin generation or activity to above pretreatment levels soon after stopping warfarin,42, 43 a phenomenon called “rebound hypercoagulability.” However, its impact on the risk of stroke remains largely speculative.24 The ROCKET‐AF (Rivaroxaban–Once‐daily, oral, direct Factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) trial comparing rivaroxaban with warfarin noted an excess risk of stroke in the rivaroxaban arm when transitioning patients to open‐label warfarin at the end of the trial. However, this is unlikely because of rebound phenomenon, because the pattern of events lacked clustering in the days shortly after rivaroxaban cessation.40, 44 Similarly, we did not observe any excess risk shortly after treatment interruption or discontinuation either. In fact, our data showed a dose–response relationship between time not taking treatment and the risk of stroke, and the effect was not significant until patients were not taking OACs for ≥1 month in CHA2DS2‐VASc score ≥4 and 6 months in CHA2DS2‐VASc score 2 or 3. Therefore, the increased risk of stroke associated with nonadherence is most likely the result of prolonged periods of inadequate anticoagulation and a loss of effective protection against stroke.
Interestingly, we found no effect of adherence on intracranial hemorrhage in high‐risk patients, the most fearful and deadly complication of anticoagulation therapy. A reduction in GI bleeding was found when patients were not taking medication for ≥6 months. When weighing the benefits and harms of anticoagulation, not all events are of equal clinical importance. Stroke and intracranial bleeding are associated with greater mortality and morbidity than extracranial bleeding. A recent study suggested GI bleeding with warfarin treatment is no worse and may be less life threatening than those occurring when not taking warfarin, which is in stark contrast to the deleterious effect of warfarin on mortality from intracranial bleeding.45 Collectively, the evidence supports positive net clinical benefit of adhering to oral anticoagulants for most patients with atrial fibrillation.
Our data also shed some light on the efficacy of stroke prevention in low‐risk patients. It has been controversial regarding whether men with CHA2DS2‐VASc score 1 should receive oral anticoagulants. The 2014 AHA/ACC/HRS guidelines recommend oral anticoagulants among patients with CHA2DS2‐VASc score ≥2.12 In contrast, the European guidelines recommend oral anticoagulants among patients with ≥1 stroke risk factors.19 Estimates of baseline untreated stroke risk in this low‐risk population vary in the literature.13, 46, 47 We found that adherence to anticoagulation was not associated with a significant reduction in stroke in patients with CHA2DS2‐VASc score 0 or 1, suggesting that the stroke risk is not easily modified in low‐risk patients and that the bleeding risk is nontrivial. This finding is in contrast with a recent study suggesting oral anticoagulant use was associated with an improved prognosis for stroke, systemic thromboembolism, and death in women with CHA2DS2‐VASc score 2 and men with CHA2DS2‐VASc score 1.22 The difference could be the result of the hospital‐based cohort with high event rates or the inclusion of women with CHA2DS2‐VASc score 2 in the other study. The risk‐benefit balance in low‐risk patients also depends on patients’ specific risk factor, as age 65 to 74 years may be more powerful than the other risk factors weighted as 1 point in the CHA2DS2‐VASc score.48 Thus, blanket policies based on risk thresholds in this group may not be appropriate; the decision may also hinge on the relative value individual patients place on achieving a very small reduction in their risk of stroke and in avoiding the burden and harms of anticoagulation.
Our study has several limitations that need to be acknowledged. The first, and most important, is that although there are a number of direct and indirect methods to measure adherence, none is considered the gold standard.49 We measured the number and proportion of days covered by anticoagulation based on the fill dates and days of supply provided on a claim, an approach commonly used in claims‐based studies.39, 50 This method provides objective real‐world data and is not susceptible to social desirability and recall bias that exist in other methods, such as self‐reported data or pill counts.49 However, it does not capture whether or when patients took the medications and cannot accurately measure the timing of short interruptions or distinguish interruptions from discontinuation. Therefore, in our study, discontinuation of treatment was considered to be part of nonadherence. In fact, a broad concept of adherence, patients taking medication as prescribed, incorporates persistence of treatment.18 Temporary interruption can be different from permanent discontinuation; however, oral anticoagulation agents, especially NOACs, have very short half‐lives, so even short interruption is associated with elevated risk of stroke and bleeding.40, 41
Additionally, considering the complex dosing of warfarin, the adherence measured by refill data may be inaccurate. Warfarin is dosed by INR testing and continual dose titration, so filled prescriptions may give an incomplete view of medication adherence. A previous study showed that warfarin adherence measured by refill data had good correlation with estimates of time in the therapeutic range.51 Moreover, regarding the relationship between adherence and stroke or bleeding, the significant results were found when patients were not taking medication for at least 1 to 6 months (depending on the patient's baseline risk). It seems most plausible that these long periods of time of not taking medication are more likely the result of to nonadherence than of dose adjustment. In fact, the use of claims data may overestimate adherence, because a patient was only considered not taking medication when we estimated that no tablet was left in this patient's possession based on the fill dates and days supplied per prescription. In reality, patients often interrupt or discontinue treatment while still having medications in possession. Therefore, the true adherence rates may likely to be lower than what we found.
Third, we were not able to explore the reasons for nonadherence, which is a common limitation of the use of administrative claims data.39 Some of the discontinuation or interruption of anticoagulation might be clinically warranted because of the development of contraindications or need for surgery. Anticoagulation may also be indicated for only a short time after ablation or cardioversion.16, 52 In fact, our recent study that used the same database demonstrated similar treatment heterogeneity in patients undergoing catheter ablation: it may be beneficial for high‐risk patients to continue anticoagulation beyond 3 months after ablation, while low‐risk patients may safely discontinue the medication.52 However, in most cases, the indication for oral anticoagulants is lifelong and time not taking of oral anticoagulants likely reflects a patient's considered decision.53 More importantly, regardless of the reasons, temporary or permanent discontinuation of anticoagulation put patients with persistent risk factors at increased risk of stroke. Our findings stress the importance that clinicians need to carefully consider the indication for discontinuing therapy to minimize the loss of protection against stroke.
Fourth, we were not able to validate the diagnoses from administrative claims data against medical records, which likely resulted in misclassification of disease status and outcomes. Nonetheless, these diagnosis codes have been used in numerous studies and shown good performance in validation studies.16, 17, 24, 38, 44 Moreover, although the misclassification may be a problem when estimating the prevalence of diseases or incidence of outcomes, it is in general nondifferential and should not bias the results of comparative effectiveness research in any direction.
Because of the observational nature of our study, despite careful adjustment, residual unmeasured confounding may still exist. Certain clinical and health behavior parameters, such as the type of atrial fibrillation, left ventricular ejection fraction, body mass index, and smoking status, are not available in the claims database. Over‐the‐counter aspirin and nonsteroidal anti‐inflammatory drugs, as well as in‐hospital bridging treatment with heparins, are not available either. Last, our database contains only privately insured and Medicare Advantage enrollees, so the conclusion may not necessarily be generalizable to Medicaid, Medicare fee‐for‐service, or uninsured populations.
Conclusion
Adherence to NOACs is poor in practice. Adherence to therapy appears to be most important in patients with CHA2DS2‐VASc score ≥2, whereas the benefits of anticoagulation may not outweigh the harms in all patients with CHA2DS2‐VASc score 0 or 1. Our results suggest clinicians may need to provide regular follow‐up with patients at elevated risk of stroke to assess and minimize nonadherence after initiating oral anticoagulation therapy. The apparent heterogeneity in treatment effects also underscores the importance of careful and individual consideration of the balance between risk of stroke and bleeding in patients at low risk of stroke.
Sources of Funding
This work was supported by Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
Disclosures
Dr Alexander is chair of the FDA's Peripheral and Central Nervous System Advisory Committee; serves as a paid consultant to PainNavigator, a mobile startup to improve patients’ pain management; serves as a paid consultant to IMS Health; and serves on an IMS Health scientific advisory board. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. All other authors have no relationships to disclose. Dr Gersh serves on Data and Safety Monitoring Board for Mount Sinai St. Lukes, Boston Scientific Corporation, Teva Pharmaceutical Industries Ltd, St. Jude Medical Inc, Janssen Research & Development, Baxter Healthcare Corporation, Cardiovascular Research Foundation, and Thrombosis Research Institute. He also serves on the advisory board for Medtronic Inc, and provides general consulting for Janssen Scientific Affairs (formerly known as Ortho‐McNeil), Xenon Pharmaceuticals, Cipla Limited, and Armetheon Inc.
(J Am Heart Assoc. 2016;5:e003074 doi: 10.1161/JAHA.115.003074)
References
- 1. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, Jensvold NG, Selby JV, Singer DE. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–2692. [DOI] [PubMed] [Google Scholar]
- 3. Zalesak M, Siu K, Francis K, Yu C, Alvrtsyan H, Rao Y, Walker D, Sander S, Miyasato G, Matchar D. Higher persistence in newly diagnosed nonvalvular atrial fibrillation patients treated with dabigatran versus warfarin. Circ Cardiovasc Qual Outcomes. 2013;6:567–574. [DOI] [PubMed] [Google Scholar]
- 4. Shore S, Carey EP, Turakhia MP, Jackevicius CA, Cunningham F, Pilote L, Bradley SM, Maddox TM, Grunwald GK, Barón AE. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the veterans health administration. Am Heart J. 2014;167:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gorst‐Rasmussen A, Skjøth F, Larsen TB, Rasmussen LH, Lip GY, Lane DA. Dabigatran adherence in atrial fibrillation patients during the first year after diagnosis: a nationwide cohort study. J Thromb Haemost. 2015;13:495–504. [DOI] [PubMed] [Google Scholar]
- 6. Abraham NS, Singh S, Alexander GC, Heien H, Haas LR, Crown W, Shah ND. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang H‐Y, Zhou M, Tang W, Alexander GC, Singh S. Risk of gastrointestinal bleeding associated with oral anticoagulants: population based retrospective cohort study. BMJ. 2015;350:h1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum Labs: building a novel node in the learning health care system. Health Aff. 2014;33:1187–1194. [DOI] [PubMed] [Google Scholar]
- 9. Optum . Core US data assets. 2014. Available at: https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf. Accessed October 23, 2015.
- 10. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 11. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 12. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 13. Friberg L, Skeppholm M, Terént A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2‐VASc score of 1. J Am Coll Cardiol. 2015;65:225–232. [DOI] [PubMed] [Google Scholar]
- 14. Gialdini G, Nearing K, Bhave PD, Bonuccelli U, Iadecola C, Healey JS, Kamel H. Perioperative atrial fibrillation and the long‐term risk of ischemic stroke. JAMA. 2014;312:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jun M, James MT, Manns BJ, Quinn RR, Ravani P, Tonelli M, Perkovic V, Winkelmayer WC, Ma Z, Hemmelgarn BR. The association between kidney function and major bleeding in older adults with atrial fibrillation starting warfarin treatment: population based observational study. BMJ. 2015;350:h246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noseworthy PA, Kapa S, Deshmukh AJ, Madhavan M, Van Houten H, Haas LR, Mulpuru SK, McLeod CJ, Asirvatham SJ, Friedman PA, Shah ND, Packer DL. Risk of stroke after catheter ablation versus cardioversion for atrial fibrillation: a propensity‐matched study of 24,244 patients. Heart Rhythm. 2015;12:1154–1161. [DOI] [PubMed] [Google Scholar]
- 17. Tirschwell DL, Longstreth W. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 18. Curtis JP, Krumholz HM. The predicament of comparative effectiveness research using observational data. Ann Intern Med. 2015;163:799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Camm A, Lip G, De Caterina R, Savelieva I, Atar D, Hohnloser S, Hindricks G, Kirchhof P; CPG; Document Reviewers . 2012 Focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation–developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385–1413. [DOI] [PubMed] [Google Scholar]
- 20. Lavallée PC, Meseguer E, Abboud H, Cabrejo L, Olivot J‐M, Simon O, Mazighi M, Nifle C, Niclot P, Lapergue B. A transient ischaemic attack clinic with round‐the‐clock access (SOS‐TIA): feasibility and effects. Lancet Neurol. 2007;6:953–960. [DOI] [PubMed] [Google Scholar]
- 21. Renda G, De Caterina R. The new oral anticoagulants in atrial fibrillation: once daily or twice daily? Vascul Pharmacol. 2013;59:53–62. [DOI] [PubMed] [Google Scholar]
- 22. Fauchier L, Lecoq C, Clementy N, Bernard A, Angoulvant D, Ivanes F, Babuty D, Lip GY. Oral anticoagulation and the risk of stroke or death in patients with atrial fibrillation and one additional stroke risk factor: the Loire Valley Atrial Fibrillation Project. Chest. 2015;???:???–???. Published online October 01, 2015. doi:10.1378/chest.15‐1622 [DOI] [PubMed] [Google Scholar]
- 23. Held C, Hylek EM, Alexander JH, Hanna M, Lopes RD, Wojdyla DM, Thomas L, Al‐Khalidi H, Alings M, Xavier D. Clinical outcomes and management associated with major bleeding in patients with atrial fibrillation treated with apixaban or warfarin: insights from the ARISTOTLE trial. Eur Heart J. 2015;36:1264–1272. [DOI] [PubMed] [Google Scholar]
- 24. Hohnloser SH, Eikelboom JW. The hazards of interrupting anticoagulation therapy in atrial fibrillation. Eur Heart J. 2012;33:1864–1866. [DOI] [PubMed] [Google Scholar]
- 25. Fang MC, Stafford RS, Ruskin JN, Singer DE. National trends in antiarrhythmic and antithrombotic medication use in atrial fibrillation. Arch Intern Med. 2004;164:55–60. [DOI] [PubMed] [Google Scholar]
- 26. Holt TA, Hunter TD, Gunnarsson C, Khan N, Cload P, Lip GY. Risk of stroke and oral anticoagulant use in atrial fibrillation: a cross‐sectional survey. Br J Gen Pract. 2012;62:e710–e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davis NJ, Billett HH, Cohen HW, Arnsten JH. Impact of adherence, knowledge, and quality of life on anticoagulation control. Ann Pharmacother. 2005;39:632–636. [DOI] [PubMed] [Google Scholar]
- 28. Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. Warfarin discontinuation after starting warfarin for atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2010;3:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hylek EM, Evans‐Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–2696. [DOI] [PubMed] [Google Scholar]
- 30. Mant J, Hobbs FR, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, Murray E; Investigators B, Network MRP . Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. [DOI] [PubMed] [Google Scholar]
- 31. Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, Sheu T‐C, Mott K, Goulding MR, Houstoun M. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for non‐valvular atrial fibrillation. Circulation. 2015;131:157–164. [DOI] [PubMed] [Google Scholar]
- 32. Cutler TW, Chuang A, Huynh TD, Witt RG, Branch J, Pon T, White R. A retrospective descriptive analysis of patient adherence to dabigatran at a large academic medical center. J Manag Care Pharm. 2014;20:1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andrade JG, Krahn AD, Skanes AC, Purdham D, Ciaccia A, Connors S. Values and preferences of physicians and patients with non‐valvular atrial fibrillation receiving oral anticoagulation therapy for stroke prevention. Can J Cardiol. 2015;???:???–???. [DOI] [PubMed] [Google Scholar]
- 34. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 35. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 36. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 37. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 38. Shields A, Lip G. Choosing the right drug to fit the patient when selecting oral anticoagulation for stroke prevention in atrial fibrillation. J Intern Med. 2015;278:1–18. Published online: April 15, 2015. doi: 10.1111/joim.12360 [DOI] [PubMed] [Google Scholar]
- 39. Schulman S, Shortt B, Robinson M, Eikelboom J. Adherence to anticoagulant treatment with dabigatran in a real‐world setting. J Thromb Haemost. 2013;11:1295–1299. [DOI] [PubMed] [Google Scholar]
- 40. Patel MR, Hellkamp AS, Lokhnygina Y, Piccini JP, Zhang Z, Mohanty S, Singer DE, Hacke W, Breithardt G, Halperin JL. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (rivaroxaban once‐daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation). J Am Coll Cardiol. 2013;61:651–658. [DOI] [PubMed] [Google Scholar]
- 41. Sherwood MW, Douketis JD, Patel MR, Piccini JP, Hellkamp AS, Lokhnygina Y, Spyropoulos AC, Hankey GJ, Singer DE, Nessel CC. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from ROCKET AF. Circulation. 2014;129:1850–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Palareti G, Legnani C, Guazzaloca G, Frascaro M, Grauso F, De Rosa F, Fortunato G, Coccheri S. Activation of blood coagulation after abrupt or stepwise withdrawal of oral anticoagulants–a prospective study. Thromb Haemost. 1994;72:222–226. [PubMed] [Google Scholar]
- 43. Ascani A, Iorio A, Agnelli G. Withdrawal of warfarin after deep vein thrombosis: effects of a low fixed dose on rebound thrombin generation. Blood Coagul Fibrinolysis. 1999;10:291–296. [PubMed] [Google Scholar]
- 44. Mahaffey KW, Hellkamp AS, Patel MR, Hannan KL, Schwabe K, Nessel CC, Berkowitz SD, Halperin JL, Hankey GJ, Becker RC. End of study transition from study drug to open‐label vitamin K antagonist therapy the ROCKET AF experience. Circ Cardiovasc Qual Outcomes. 2013;6:470–478. [DOI] [PubMed] [Google Scholar]
- 45. Ashburner JM, Go AS, Reynolds K, Chang Y, Fang MC, Fredman L, Applebaum KM, Singer DE. Comparison of frequency and outcome of major gastrointestinal hemorrhage in patients with atrial fibrillation on versus not receiving warfarin therapy (from the ATRIA and ATRIA‐CVRN cohorts). Am J Cardiol. 2015;115:40–46. [DOI] [PubMed] [Google Scholar]
- 46. Chao T‐F, Liu C‐J, Wang K‐L, Lin Y‐J, Chang S‐L, Lo L‐W, Hu Y‐F, Tuan T‐C, Chen T‐J, Lip GY. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2‐VASc score (beyond sex) receive oral anticoagulation? J Am Coll Cardiol. 2015;65:635–642. [DOI] [PubMed] [Google Scholar]
- 47. Lip GY, Skjøth F, Rasmussen LH, Larsen TB. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2‐VASc score. J Am Coll Cardiol. 2015;65:1385–1394. [DOI] [PubMed] [Google Scholar]
- 48. Singer DE, Ezekowitz MD. Adding rigor to stroke risk prediction in atrial fibrillation∗. J Am Coll Cardiol. 2015;65:233–235. [DOI] [PubMed] [Google Scholar]
- 49. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. [DOI] [PubMed] [Google Scholar]
- 50. Nau DP. Proportion of Days Covered (PDC) as a Preferred Method of Measuring Medication Adherence. Springfield, VA: Pharmacy Quality Alliance; 2012. [Google Scholar]
- 51. Wang Y, Kong MC, Ko Y. Comparison of three medication adherence measures in patients taking warfarin. J Thromb Thrombolysis. 2013;36:416–421. [DOI] [PubMed] [Google Scholar]
- 52. Noseworthy PA, Yao X, Deshmukh AJ, Van Houten H, Sangaralingham LR, Siontis KC, Piccini JP, Asirvatham SJ, Friedman PA, Packer DL, Gersh BJ, Shah ND. Patterns of anticoagulation use and cardioembolic risk after catheter ablation for atrial fibrillation. J Am Heart Assoc. 2015;4:e002597 doi: 10.1161/JAHA.115.002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vrijens B, Heidbuchel H. Non‐vitamin K antagonist oral anticoagulants: considerations on once‐vs. twice‐daily regimens and their potential impact on medication adherence. Europace. 2015;17:514–523. [DOI] [PubMed] [Google Scholar]


