Abstract
Background
Percutaneous closure of patent ductus arteriosus (PDA) in term neonates is established, but data regarding outcomes in infants born very preterm (<32 weeks of gestation) are minimal, and no published criteria exist establishing a minimal weight of 4 kg as a suitable cutoff. We sought to analyze outcomes of percutaneous PDA occlusion in infants born very preterm and referred for PDA closure at weights <4 kg.
Methods and Results
Retrospective analysis (January 2005–January 2014) was done at a single pediatric center. Procedural successes and adverse events were recorded. Markers of respiratory status (need for mechanical ventilation) were determined, with comparisons made before and after catheterization. A total of 52 very preterm infants with a median procedural weight of 2.9 kg (range 1.2–3.9 kg) underwent attempted PDA closure. Twenty‐five percent (13/52) of infants were <2.5 kg. Successful device placement was achieved in 46/52 (88%) of infants. An adverse event occurred in 33% of cases, with an acute arterial injury the most common complication. We observed no association between weight at time of procedure and the risk of an adverse event. No deaths were attributable to the PDA closure. Compared to precatheterization trends, percutaneous PDA closure resulted in improved respiratory status, including less exposure to mechanical ventilation (mixed effects logistic model, P<0.01).
Conclusions
Among infants born very preterm, percutaneous PDA closure at weights <4 kg is generally safe and may improve respiratory health, but risk of arterial injury is noteworthy. Randomized clinical trials are needed to assess clinically relevant differences in outcomes following percutaneous PDA closure versus alternative (surgical ligation) management strategies.
Keywords: arterial thrombosis; catheterization; complications; ductus arteriosus, patent; neonatal; pediatrics
Subject Categories: Pediatrics, Congenital Heart Disease, Treatment, Quality and Outcomes
Introduction
Patent ductus arteriosus (PDA) is associated with morbidity1 and mortality.2 Closure of the ductus occurs within hours in the majority (>95%) of term infants (≥37 weeks of gestation).3 In contrast, a persistent PDA, defined as failure of the ductus to close within 72 hours postnatal age, is seen in ≈50% of very preterm infants (born at <32 weeks of gestation).2 Although a cause‐and‐effect relationship between PDAs and adverse outcomes has not been established,4 odds for death are sixfold higher in premature infants with a persistent PDA (failure of spontaneous or pharmacological closure) than with a closed ductus.5 Moreover, PDAs are considered precursors to respiratory morbidity, including prolonged ventilator dependency.2, 6
Treatment of PDAs remains a subject of tremendous controversy and debate.4 General consensus is that a PDA should be closed by 2 years of age to avoid the risks of pulmonary hypertension or bacterial endocarditis,7 but disagreement remains about the thresholds and strategies for PDA closure among premature infants earlier in life.8 Until recently, the only alternatives to close a PDA were drug therapy or surgical ligation. However, recent data showing drug therapy and surgical ligation to be independent risk factors for moderate‐to‐severe functional disability, developmental delay, and motor impairment9 have led healthcare providers to consider alternative strategies of PDA closure.
Catheter‐based closure of the PDA is among the safest of interventional cardiac procedures and is considered the procedure of choice among infants ≥4 kg.10 Although an exact lower weight limit for the safe closure of a PDA has not been established, previous studies have excluded preterm infants <4 kg or reported no cases of percutaneous PDA closure below this weight threshold.11, 12, 13, 14 While recent case reports suggest catheter‐based PDA closure is feasible among infants <4 kg,15, 16 data on the safety of the procedure, including adverse events beyond the immediate catheterization period (eg, posthospitalization), are lacking.17 Since very premature infants <4 kg are more complex and medically fragile than are their more mature counterparts,18 a separate consideration of the short‐ and long‐term risks and benefits of percutaneous PDA closure in this unique subgroup is needed.
Specific Aims and Objectives
Primary aim
The primary aim of this study was to characterize procedural success and the incidence and nature of adverse events (procedural and postprocedural) among a cohort of infants born very preterm referred for percutaneous PDA closure at weights <4 kg.
Secondary aim
The secondary aim of this study was to compare respiratory status before and after percutaneous PDA closure.
Methods
This study was approved by the Nationwide Children's Hospital Institutional Review Board. A retrospective analysis of very premature infants <4 kg referred for percutaneous PDA closure between January 1, 2005 and January 1, 2014 was performed.
Data Collected
Demographic data and clinical characteristics at the time of intervention were recorded. The indications for the procedure and any associated congenital heart defects were recorded. Adverse events (AEs) during and after catheterization were classified using a standard format.19, 20
The modified Seldinger technique was used to obtain vascular access. Although the dose and timing of systemic heparin was at the discretion of the attending interventional cardiologist, our standard approach was to provide an initial bolus of 100 units/kg of unfractionated heparin. Repeat bolus doses were provided to maintain activated clotting time >250 s. Prophylactic anticoagulation was not routinely provided following the procedure.
Following baseline hemodynamic measurements (eg, mean pulmonary artery pressures), biplane aortic angiography with anatomic assignment of the PDA (Type A‐E)21 based on size, configuration, and relationship to adjacent structures was performed (Figure 1A and 1B). While the angiographic classification system guides percutaneous closure, decisions regarding the type of PDA device (AVP‐II versus ADO) used were at the discretion of the attending cardiac interventionalist. Consistent with previous reports,22 AVP‐II device specifications were chosen to be at least 1 to 2 mm larger than the narrowest portion of the PDA, with the goal of using the smallest device necessary to achieve closure and avoid residual shunting. Following device deployment, angiography through the delivery catheter was used to document the position of the device relative to the pulmonary arteries. In cases where device size or position was not ideal, the device was recollected and a larger device (if necessary) was redeployed. Prior to release of the device, a pullback across the aortic arch and repeat angiography were performed to assess for aortic narrowing (Figure 1C).
Figure 1.
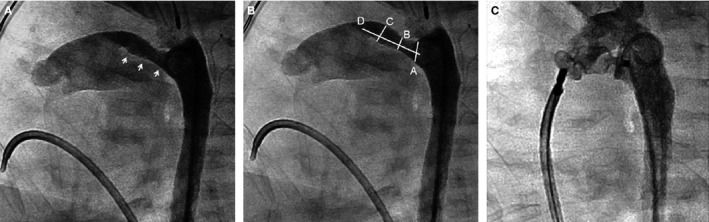
Angiographic still frames in lateral projection illustrating percutaneous closure of PDA in a premature infant. A, Type C PDA in a 1.6‐kg infant. White arrows outline length of the PDA. B, Angiographic parameters used to define PDA classification and guide closure; A, aortic ampulla; B, narrowest dimension; C, PDA size at insertion into pulmonary artery; D, PDA length. C, Aortic angiogram following deployment of a 6‐mm AVP‐II plug. PDA indicates patent ductus arteriosus.
During the first 4 hours following catheterization, pedal pulses (dorsalis pedis and/or tibialis posterior artery) in the cannulated extremity were palpated hourly in the cardiothoracic intensive care unit. Thereafter, pulses were palpated at least every 4 hours until discharge. We defined an acute arterial injury (AAI) as evidence of weak or absent pedal pulse in the cannulated extremity. In cases where an AAI was suspected, infants received unfractionated heparin at a dose (10 units/kg per hour) that did not require activated partial thromboplastin time levels. In all cases of suspected AAI, Doppler ultrasound measurements were performed to exclude arterial thrombus. If no thrombus was identified, and the exam normalized, infants were presumed to have had an arterial spasm. On the other hand, infants with radiological evidence of thrombosis or continued clinical concern for vascular compromise were transitioned to enoxaparin (initial dose of 1.5 to 2 mg/kg every 12 hours).
During hospitalization, enoxaparin was titrated to achieve an anti‐Xa level of 0.5 to 1 unit/mL. Anti‐Xa levels were drawn 4 hours after the second dose and following any dose adjustments. As outpatients, infants on enoxaparin were followed regularly (typically twice per month) by a pediatric hematologist. Follow‐up visits included clinical examination, enoxaparin dosing adjustments, and ultrasound examinations. Enoxaparin was discontinued after 3 months of enoxaparin treatment or demonstration of thrombus resolution on Doppler ultrasound.
Technical success was defined as the infant leaving the catheterization suite with a device in the PDA. Cases in which the device embolized, but was later retrieved and the PDA subsequently closed with a larger device (during the same procedure), were considered technical successes, but also listed as complications. Angiography and postprocedural echocardiograms were reviewed for evidence of a residual shunt or device protrusion.
We used a quantitative measure of pulmonary status (Pulmonary Score) that reflects the spectrum of pulmonary illness as seen in clinical practice.23 The Pulmonary Score is a composite score that uses an arithmetic sum of weighted clinical therapies, including (1) type of respiratory support (mechanical ventilation, continuous positive airway pressure, or nasal cannula); (2) need for supplemental oxygen (FiO2); and (3) pulmonary medications (systemic steroids, diuretics). Pulmonary scores were calculated based on the following: Pulmonary Score=(FiO2) (type of respiratory support)+(medications). The Pulmonary Score assigns more weight (numeric value) to respiratory support that reflects a greater degree of disease (eg, mechanical ventilation receives a 2.5, nasal cannula a 1.0). Over time, lower cumulative scores reflect improving respiratory status. Use of cardiopulmonary medications (eg, furosemide) for the week preceding the calculation of the Pulmonary Score was recorded. FiO2 was calculated as described by Benaron and Benitz.24 To determine a patient's “baseline” status prior to percutaneous closure, we calculated pulmonary scores on a weekly basis 4 weeks before the procedure. In addition, to characterize potential short‐ and longer‐term benefits of PDA closure, the Pulmonary Score was calculated on a weekly basis 4 weeks after the procedure (or until hospital discharge), including immediately (2 days) postprocedure.
Following percutaneous PDA closure, outpatient echocardiograms were reviewed. In addition, outpatient medical records (cardiology clinic, primary care appointments) were reviewed, including duration of cardiology follow‐up and causes of death, if applicable.
Statistical Analysis
A linear mixed effect model with random intercept was used to assess the trend over time (time effect) in the average Pulmonary Score of the cohort before and after catheterization. This approach accounts for the correlation of measurements between different points on the same subject. Given the adverse effects of mechanical ventilation on lung growth and function,25 a second model (mixed effect logistic regression) was used to assess trends over time on the proportion of neonates receiving mechanical ventilation before and after catheterization. Variables are presented as means±SDs or medians with range.
Results
Baseline Patient Characteristics
A total of 52 infants born very preterm were included in the study. Baseline patient characteristics are shown in Table 1. Approximately two thirds of infants (36/52, 69%) underwent medical treatment for PDA closure prior to referral for percutaneous closure. In all cases, the primary reason for referral was respiratory insufficiency, as infants were receiving either mechanical ventilation (41/52, 79%) or noninvasive (continuous positive airway pressure, high‐flow nasal cannula) respiratory support (11/52, 21%) at the time of referral. In addition to the need for respiratory support, infants referred for PDA closure also had evidence of left ventricular volume loading (n=42), failure to thrive (n=4), or concerns for pulmonary hypertension (n=6). Of the 52 patients in the study, 26 (50%) had associated cardiac defects, including atrial septal defect (n=21), ventricular septal defect (n=3), bicuspid aortic valve (n=1), and complete atrioventricular canal (n=1). Ten infants weighed <2.5 kg, and 3 infants weighed <2 kg, at the time of the procedure. All infants were >28 days at the time of catheterization, with more than half (27/52, 52%) >2 months of age. In all cases, the procedure was performed with the infants under general anesthesia.
Table 1.
Feasibility Data
| Characteristics | Neonates Analyzed (N=52) |
|---|---|
| Birth weight, g | 1289 (475–1887) |
| Gestational ages at birth | |
| 23 to 25 weeks, n (%) | 17 (33) |
| 26 to 28 weeks, n (%) | 21 (40) |
| 29 to 31 weeks, n (%) | 14 (27) |
| Female, n (%) | 33 (63) |
| Weight (g) at time of procedure | 1602 (1210–3900) |
| Age (d) at time of procedure | 63 (29–91) |
| Additional cardiac defects, n (%) | 26 (50) |
| Genetic/chromosomal anomalies, n (%) | 10 (19) |
Data are presented as median with range unless otherwise noted.
Procedural Data
Femoral arterial and venous access was obtained in all cases. Types of devices used for PDA occlusion, as well as arterial and venous sheath sizes, are shown in Table 2. Following an aortic angiogram, a right and retrograde left heart catheterization was performed in all patients. Classification of ductal morphology included the following: Type A in 9 (17%), Type C in 39 (75%), and Type C/E in 4 (8%). The median minimal PDA diameter by angiography was 3.5 mm (range 2.1–5.8 mm), median PDA length was 9.2 mm (range 4.4–20.6 mm), and median PDA diameter at aortic ampulla was 5.1 mm (range 2.5–8.0 mm). The mean±SD Qp/Qs ratio was 3.4±2.1 (range 1.1–8.2). Systolic pulmonary artery pressures ranged from 22 to 68 mm Hg, and indexed pulmonary vascular resistance from 0.8 to 6.3 Wood Units. At the time of PDA closure, 4 infants had evidence of systemic pulmonary artery pressures. In each case, a significant decrease (>20%) in pulmonary vascular resistance following exposure to oxygen or nitric oxide led the cardiac interventionalist to proceed with device closure.
Table 2.
Devices Used for Attempted Catheterization‐Closure of PDA
| Device Type | Total No. of Cases Utilizing This Device | Arterial Sheath 3 or 3.3 Fr | Arterial Sheath 4 Fr | Venous Sheath 4 Fr | Venous Sheath 5 Fr | Venous Sheath 6 Fr |
|---|---|---|---|---|---|---|
| ADO I | ||||||
| 5/4 | 1 | 0 | 1 | 0 | 1 | 0 |
| 6/4 | 3 | 2 | 1 | 0 | 0 | 3 |
| AVP II | ||||||
| 4 mm | 11 | 7 | 4 | 5 | 5 | 1 |
| 6 mm | 28 | 15 | 13 | 3 | 25 | 0 |
| 8 mm | 7 | 6 | 1 | 0 | 6 | 1 |
| 10 mm | 2 | 0 | 2 | 0 | 2 | 0 |
Devices shown reflect largest device used during attempted closure. ADO 1 indicates Amplatzer Ductal Occluder; AVP II, Amplatzer Vascular Plug II; Fr, [French] F; PDA, patent ductus arteriosus.
Case Characteristics
Case characteristics are shown in Table 3. The majority (75%) of cases were performed in less than 2 hours. We observed no association between patient size and radiation dose or case duration. All devices were delivered using an anterograde approach. Two infants had evidence of small‐to‐moderate residual flows through the PDA device on the final aortic angiogram in the catheterization suite.
Table 3.
Case Characteristics
| Neonates Analyzed (n=52) | |
|---|---|
| Contrast dose, mL/kg | 5.8 (3.2–8.1) |
| Radiation dose, mGy | 117 (16–394) |
| Case duration, h | |
| <1 | 7 (13) |
| ≥1, <2 | 32 (62) |
| ≥2 | 13 (25) |
Data are presented as median with range in parentheses. Case duration=time from attempt at vascular access to sheath removal.
Technical Success
Technical success was achieved in 46 (88%) patients, of which the majority (42/46, 91%) were closed with an AVP‐II device (Table 4). We observed 2 cases where the device embolized but was retrieved in the catheterization suite, and the PDAs subsequently closed using a larger device.
Table 4.
Cases With Successful Catheterization Closure
| PDA Type | N | 4 mm AVP‐II | 6 mm AVP‐II | 8 mm AVP‐II | 10 mm AVP‐II | 5/4 ADO | 6/4 ADO |
|---|---|---|---|---|---|---|---|
| A | 9 | 5 | 0 | 0 | 0 | 1 | 3 |
| C | 33 | 4 | 23 | 4 | 2 | 0 | 0 |
| C/E | 4 | 1 | 1 | 2 | 0 | 0 | 0 |
Devices shown reflect largest device used during closure. ADO indicates Amplatzer Ductal Occluder; AVP II, Amplatzer Vascular Plug II.
Six attempts at PDA closure were unsuccessful and are shown in Table 5. We observed no association between patient size or PDA length and successful device placement. In 5 cases the device appeared in excellent position with complete ductal closure, but the device was not released due to aortic isthmus narrowing (n=4) or left pulmonary artery obstruction (n=1), and the procedure was aborted.
Table 5.
Cases With Unsuccessful Catheterization Closure
| Case No. | Weight (kg) | PDA Type | PDA Diameter (mm)* | PDA Length (mm) | Device | Complication | Outcome | Follow‐Up Duration (Months) | Late Mortality |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.2 | C | 3.2 | 11.4 | AVP II, 4 mm | Embolized to LPA; unable to be snared | Referred to surgery for device retrieval and ligation | 16 | No |
| 2 | 2.3 | C | 3.2 | 8.0 | AVP II, 6 mm | Aortic Obstruction | Referred for surgical ligation | 36 | Yesa |
| 3 | 2.7 | C | 5.0 | 7.4 | AVP II, 8 mm | LPA obstruction | Referred for surgical ligation | 42 | No |
| 4 | 2.7 | C | 2.5 | 6.5 | AVP II, 6 mm | Aortic obstruction | Referred for surgical ligation | 33 | No |
| 5 | 3.1 | C | 3.0 | 7.0 | AVP II, 6 mm | Aortic obstruction | Referred for surgical ligation | 52 | No |
| 6 | 3.1 | C | 3.0 | 4.4 | AVP II, 6 mm | Aortic obstruction | Referred for surgical ligation | 28 | No |
AVP II indicates AVP II, Amplatzer Vascular Plug II; LPA, left pulmonary artery; PDA, patent ductus arteriosus.
Patient died at 3 years of age due to presumed pulmonary hypertensive crises.
Adverse Events
Adverse events (AEs) are shown in Table 6. We observed 3 cases of device embolization, of which one required surgical removal, and this infant underwent surgical PDA ligation. The most common AE was an AAI in 10 (19%) infants (Table 7) following sheath removal. We observed no association between weight at time of procedure, sheath size, or length of procedure and likelihood of AAI. All patients with AAIs were started on heparin therapy with a median duration of 15.4 hours (range 14–22 hours). Four patients with decreased pulses with no thrombus seen on ultrasound were diagnosed with presumed arterial spasm. Six infants were placed on enoxaparin for long‐term anticoagulation following ultrasound evidence of arterial thrombosis (n=4) or clinical evidence of an absent pulse without ultrasound confirmation of thrombosis (n=2). Three of the 6 infants completed a full treatment course (12 weeks of therapy), while 3 infants had a subsequent ultrasound showing thrombus resolution and treatment was stopped prior to 12 weeks of therapy. Normal perfusion to the extremity (return of distal pulses) was established in all patients. Following initiation of anticoagulation, we observed no evidence of cerebral vascular accidents or worsening intraventricular hemorrhage on head ultrasound or computed tomography scan. No patients died during cardiac catheterization.
Table 6.
Adverse Events (AE)
| Neonates Analyzed (N=52) | |
|---|---|
| Any AE | 17 (33) |
| AE during procedure | 5 (10) |
| Minor airway problema | 1 (2) |
| Blood loss requiring blood transfusion | 1 (2) |
| Embolization retrieved in catheterization suiteb | 2 (4) |
| Embolization not retrieved in catheterization suitec | 1 (2) |
| AE postprocedure | 12 (23) |
| Acute arterial injuryd | 10 (19) |
| Late complicationse | 2 (4) |
Data are presented as n (%).
Endotracheal tube in the right bronchus requiring repositioning.
Cases (n=2) where embolization retrieved in catheterization suite and patent ductus arteriosus (PDA) subsequently closed with a larger device.
Case (n=1) where device embolized but was not able to be retrieved in the catheterization suite and infant subsequently underwent surgical retrieval of the device and closure.
Pulse severely diminished or absent following sheath removal (details provided in Table 7).
Cases (n=2) where PDA device caused left pulmonary artery stenosis.
Table 7.
Arterial Complications
| Case No. | Weight (kg) | Arterial Sheath F | Procedure Time (Min) | Clinical Assessment | Ultrasound Evidence of Thrombosis | Enoxaparin Treatment | Duration of Enoxaparin Treatment (Wk) |
|---|---|---|---|---|---|---|---|
| 1 | 1.2 | 3.3 | 73 | Decreased pulse | Yes | Yes | 12 |
| 2 | 1.9 | 3.3 | 83 | Absent pulse | No | Yes | 8 |
| 3 | 2.2 | 3.3 | 122 | Decreased pulse | No | No | NA |
| 4 | 2.6 | 3.0 | 94 | Absent pulse | No | Yes | 7 |
| 5 | 2.6 | 3.3 | 82 | Absent pulse | Yes | Yes | 12 |
| 6 | 2.7 | 3.0 | 221a | Absent pulse | Yes | Yes | 12 |
| 7 | 3.0 | 4.0 | 131 | Absent pulse | Yes | Yes | 10 |
| 8 | 3.2 | 3.3 | 112 | Decreased pulse | No | No | NA |
| 9 | 3.5 | 3.3 | 119 | Decreased pulse | No | No | NA |
| 10 | 3.8 | 3.5 | 182 | Decreased pulse | No | No | NA |
NA, not applicable. Weight: weight at time of catheterization. Sheath size ([French] F) reflects largest device used during attempted closure. Procedural time: time (min) from percutaneous access to sheath removal. Decreased pulse: diminished pedal (dorsalis pedis/posterior tibialis) pulses compared with pulses in contralateral leg. Absent pulses: absent pedal (dorsalis pedis/posterior tibialis) pulses.
Normal perfusion to the extremity (return of distal pulses) was established in all patients.
Case where device embolized but was subsequently retrieved in the catheterization suite and larger device was placed successfully.
Pulmonary Status
While we observed no change prior to the catheterization, the Pulmonary Score decreased over time after PDA closure (Figure 2A, P<0.01). Similarly, while we observed no change prior to catheterization, the proportion of neonates on mechanical ventilation decreased over time after PDA closure (Figure 2B, P<0.01). Based on the model, the odds of receiving mechanical ventilation decreased, on average, 58% every week following percutaneous PDA closure.
Figure 2.
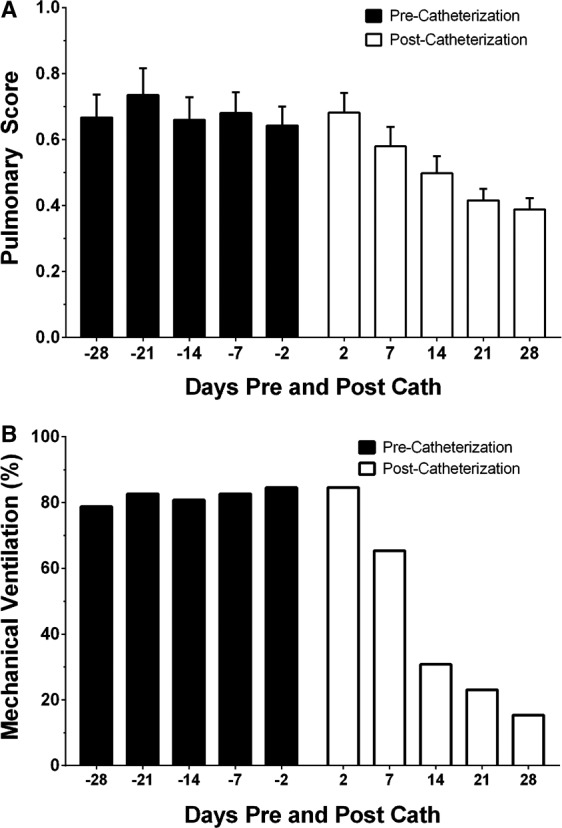
A, Pulmonary Score pre‐ and post–cardiac catheterization. X‐axis represents time (days), with negative values denoting days prior to catheterization. Y‐axis designates Pulmonary Scores (mean±SD) compared to precatheterization trends; Pulmonary Scores decreased following PDA closure (linear mixed‐effect model, P<0.01). B, Proportion of neonates on mechanical ventilation pre‐ and postcatheterization. X‐axis represents time (days), with negative values denoting days prior to catheterization. Y‐axis designates proportion (%) of neonates on mechanical ventilation. Compared to precatheterization trends, the likelihood to receive mechanical ventilation following PDA closure decreased (mixed‐effects logistic model, P<0.01). PDA indicates patent ductus arteriosus.
Outpatient Echocardiographic Follow‐Up
Outpatient echocardiographic data were available in all patients who underwent device implantation. All infants had at least 3 echocardiograms post‐PDA closure (range 3–7). Two infants with residual shunting on angiography continued to have evidence of residual shunting by color flow Doppler through the device on the initial postdischarge echocardiogram (≈1 month). The last outpatient echocardiograms in these patients at 3 and 4 years of age, respectively, revealed no residual shunting. No additional cases of residual PDA shunting were observed in the outpatient setting following discharge.
Mild flow acceleration in the descending aorta (2.1 m/s) on initial outpatient echocardiography (≈1 month) was observed in one patient who received a 6‐mm AVP‐II device at 2.7 kg. At 3‐year follow‐up, no evidence of flow acceleration was observed. We observed 5 patients with mild flow acceleration (1.9–2.8 m/s) in the left pulmonary artery (LPA) on echocardiogram on the initial outpatient echocardiograms. On subsequent outpatient follow‐up 6 to 12 months later, we observed no evidence of residual LPA obstruction in 3 patients, while 2 patients continue to show evidence of mild LPA stenosis, with peak velocities of 2.7 and 2.9 m/s, respectively, at 23 and 26 months. Both patients have undergone ventilation/perfusion scans showing asymmetric split lung perfusion (≈30% to left lung, ≈70% to right lung), consistent with underlying LPA stenosis, but have not undergone re‐intervention to address the LPA stenosis.
Long‐Term Clinical Outcomes
Outpatient clinical data were available in 96% (50/52) of the cohort (successful and unsuccessful cases). Two patients lost to follow‐up had their PDAs closed prior to 2006 and returned to their native countries. The median follow‐up time was 4 years (range 1–9 years). We observed no evidence of long‐term vascular compromise (leg‐length discrepancy, claudication). We observed 3 infants followed in the cardiology clinic for pulmonary hypertension (PH). Among these infants, one was weaned off anti‐PH medications at 22 months corrected gestational age; one infant remains on 2 anti‐PH medications (sildenafil and bosentan) at 14 months corrected gestational age; and one infant died at 3 years of age due to presumed PH crisis. This infant, who underwent unsuccessful device closure, was born at 23.3 weeks gestation and had multiple complications of preterm birth, including tracheostomy, intraventricular hemorrhage, and cerebral palsy. Additionally, we observed another death in a patient at 2 years of age due to infectious complications related to chronic lung disease. While neither death was attributable to the procedure or was device related, autopsy was not performed in either setting.
Discussion
Evidence on the risks of drug therapy or surgical PDA ligation26, 27 has led to growing interest in the use of percutaneous closure in premature infants.10, 15, 16, 28, 29, 30 The main finding of our 10‐year study is that, among a large cohort of infants born very premature and referred for percutaneous closure at weights <4 kg, the majority are successfully closed in the catheterization suite with evidence of improved pulmonary status following closure, but risk of arterial injury in these infants is noteworthy. The present study adds to a growing body of evidence on the potential value of percutaneous PDA closure in preterm infants.10, 16, 31 However, to address adequately the central question of whether the benefits of catheter‐based PDA closure outweigh the risks,4, 32 randomized, controlled trials comparing percutaneous PDA closure versus alternative treatment strategies (surgical ligation, nonintervention/conservative management approaches) are needed.
Although investigators have reported that percutaneous closure of the PDA is the procedure of choice among infants ≥4 kg,10 the lower limit for safe closure remains unknown. While 4 kg as a weight‐cutoff is not evidence based, previous studies have excluded preterm infants <4 kg or reported no cases of percutaneous PDA closure below this weight threshold.11, 12, 13, 14 Since very premature infants <4 kg are more complex and medically fragile than are older counterparts,18 our working premise was that more immature infants would represent a separate population with a unique risk/benefit profile. To address fundamental questions regarding the use of weight‐based thresholds for percutaneous PDA closure, large, multicenter studies with sufficient power to detect clinically meaningful differences in outcomes across a range of weight categories are needed. Furthermore, consideration of patient‐specific factors beyond weight (premature birth, congenital anomalies) that underlie risk is imperative for developing risk‐stratification models to inform the practice of evidence‐based medicine.
Consistent with previous reports,33 we observed that AAIs were the most frequent AEs during cardiac catheterization in infants. Previous work has shown that among children undergoing cardiac catheterization, a weight <4 kg is the strongest predictor of arterial injury, including thrombosis.18 Thus, our cohort represents a “high‐risk” subgroup of patients for vascular‐related injuries, wherein the need to identify strategies that safely reduce vascular complications is magnified. Since arterial access was obtained in all infants in the present study, approaches that limit or avoid arterial access will likely reduce AAIs. Zahn et al recently described an approach to percutaneous PDA closure using fluoroscopy and transthoracic echocardiography to guide transvenous PDA closure with the avoidance of arterial access.16 Other investigators have reported similar success in avoiding arterial access without evidence of device embolization or malposition.30 In settings where arterial access is required, use of antithrombotic prophylaxis may be beneficial.34 While we did not observe an association between arterial sheath size and risk of AAI, ongoing efforts to develop smaller sheaths and catheters will likely reduce thrombotic complications.35
Similar to previous reports,16, 31 the majority of infants in the present cohort underwent percutaneous PDA closure using an AVP‐II device. Potential benefits of the AVP‐II device are that the disks on either end of the device have the same diameter as the central occlusion portion, which may reduce rates of aortic or pulmonary blood flow disturbances more effectively than do other devices.16 However, we identified 5 cases of unsuccessful PDA closure, wherein the terminal ends of the device interfered with aortic or pulmonary artery blood flow. While we observed no association between PDA length and successful device placement, this likely remains an important factor in clinical practice. A number of device modifications in the ADO‐II AS (St Jude Medical, Minneapolis, MN; not available in the United States), including shorter lengths (2–6 mm), may be well suited for infants and improve successful closure rates.36 Also, a new, flexible, self‐shaping device (Occlutech PDA occluder) has recently been designed to address all types of PDA morphology. Early reports on the safety and feasibility using the Occlutech PDA device have been encouraging.37
While our overall AE rate may appear to be higher than rates in previously reported studies,10, 38 several differences in the studies are notable. First, the risk of an AE is inversely related to patient weight at the time of catheterization.39 Considering our study describes AE rates among a unique subgroup of low‐weight infants, including 25% who were <2.5 kg, higher rates of AEs were not unexpected. Second, variability in AE across studies likely reflects differences in the length of follow‐up. While previous studies have described AE rates during the procedure only,17 we describe both procedural and postprocedural complications, including those in the outpatient setting.
Limitations
Even at a single institution with a guideline‐driven approach to PDA management, the decision to refer for percutaneous closure was at the discretion of the attending physician, with marked variability in the timing of referral for percutaneous closure. Thus, questions regarding patient selection and the optimal timing for percutaneous closure remain unanswered. Over the course of the study, devices available for percutaneous PDA occlusion varied; thus, a consistent PDA closure algorithm was not feasible. While we intended to describe AEs attributable to the catheterization procedure, we recognize that complications are intimately tied to a variety of patient‐specific variables and the potential bias involved in retrospective analysis.19 Since the present study was conducted at a major academic center, the risk of referral bias is recognized.
Since previous data have shown that mechanical ventilation increases the risk of chronic lung disease in very preterm infants,40 improved respiratory status following percutaneous closure is encouraging and adds to the growing body of evidence31 on the potential value of this approach. However, it is recognized that the possibility of unmeasured effects, including improvement in respiratory function over time (nutrition, linear growth), may have contributed to clinical improvement. Moreover, it will be important to assess whether these short‐term benefits translate into long‐term improvements in respiratory and other clinically significant outcomes.
Although we observed no association between weight at time of catheterization or arterial sheath size and risk of an AE, our small cohort size may explain the null findings. Evidence of mild aortic or pulmonary artery stenosis in some infants following PDA closure highlights the need for objective, evidence‐based guidelines for optimal device placement.41 Consistently applied surveillance protocols, including routine echocardiography and ventilation–perfusion scans, are necessary to better understand the natural history of device‐related stenosis and need for reintervention. Because of uncertainty involving echocardiogram‐derived definitions of pulmonary hypertension, the relationship between PDA closure and development of early or late PH remains unknown.42 Finally, while we able to describe acute arterial injuries systematically, data on venous complications (hematoma) were not available.
Conclusions
Our study describes, among a cohort of infants born very preterm, the safety and efficacy of percutaneous PDA closure at weights <4 kg. While technical success without adverse events was achieved in the majority of neonates, risks of arterial injury were noteworthy. Evidence‐based data are needed to guide clinicians on the optimal timing of catheter‐based interventions to improve outcomes, while at the same time limiting procedure‐related risks. Randomized, controlled clinical trials with sufficient power are needed to assess clinically relevant differences in outcomes following percutaneous PDA closure versus alternative (surgical ligation) management strategies.
Sources of Funding
Supported by a grant from The American Heart Association (# 10CRP3730033, Backes).
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002923 doi: 10.1161/JAHA.115.002923)
References
- 1. Dollberg S, Lusky A, Reichman B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population‐based study. J Pediatr Gastroenterol Nutr. 2005;40:184–188. [DOI] [PubMed] [Google Scholar]
- 2. Sellmer A, Bjerre JV, Schmidt MR, McNamara PJ, Hjortdal VE, Host B, Bech BH, Henriksen TB. Morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Arch Dis Child Fetal Neonatal Ed. 2013;98:F505–F510. [DOI] [PubMed] [Google Scholar]
- 3. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. [DOI] [PubMed] [Google Scholar]
- 4. Bose CL, Laughon MM. Patent ductus arteriosus: lack of evidence for common treatments. Arch Dis Child Fetal Neonatal Ed. 2007;92:F498–F502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noori S, McCoy M, Friedlich P, Bright B, Gottipati V, Seri I, Sekar K. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics. 2009;123:e138–e144. [DOI] [PubMed] [Google Scholar]
- 6. Weisz DE, McNamara PJ. Patent ductus arteriosus ligation and adverse outcomes: causality or bias? J Clin Neonatol. 2014;3:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heuchan AM, Clyman RI. Managing the patent ductus arteriosus: current treatment options. Arch Dis Child Fetal Neonatal Ed. 2014;99:F431–F436. [DOI] [PubMed] [Google Scholar]
- 8. Hamrick SE, Hansmann G. Patent ductus arteriosus of the preterm infant. Pediatrics. 2010;125:1020–1030. [DOI] [PubMed] [Google Scholar]
- 9. Janz‐Robinson EM, Badawi N, Walker K, Bajuk B, Abdel‐Latif ME; Neonatal Intensive Care Units N . Neurodevelopmental outcomes of premature infants treated for patent ductus arteriosus: a population‐based cohort study. J Pediatr. 2015;167:1025–1032. [DOI] [PubMed] [Google Scholar]
- 10. Dimas VV, Takao C, Ing FF, Mattamal R, Nugent AW, Grifka RG, Mullins CE, Justino H. Outcomes of transcatheter occlusion of patent ductus arteriosus in infants weighing </= 6 kg. JACC Cardiovasc Interv. 2010;3:1295–1299. [DOI] [PubMed] [Google Scholar]
- 11. Kumar RK, Anil SR, Kannan BR, Philip A, Sivakumar K. Bioptome‐assisted coil occlusion of moderate‐large patent ductus arteriosus in infants and small children. Catheter Cardiovasc Interv. 2004;62:266–271. [DOI] [PubMed] [Google Scholar]
- 12. Bruckheimer E, Godfrey M, Dagan T, Levinzon M, Amir G, Birk E. The Amplatzer Duct Occluder II Additional Sizes device for transcatheter PDA closure: initial experience. Catheter Cardiovasc Interv. 2014;83:1097–1101. [DOI] [PubMed] [Google Scholar]
- 13. Al‐Hamash SM, Wahab HA, Khalid ZH, Nasser IV. Transcatheter closure of patent ductus arteriosus using ado device: retrospective study of 149 patients. Heart Views. 2012;13:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knirsch W, Haas NA, Lewin MA, Dahnert I, Kececioglu D, Berger F, et al. Percutanous closure of patent ductus arteriosus in small infants of less than 8 kg body weight using different devices. Eur J Pediatr. 2004;163:619–621. [DOI] [PubMed] [Google Scholar]
- 15. Roberts P, Adwani S, Archer N, Wilson N. Catheter closure of the arterial duct in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2007;92:F248–F250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zahn EM, Nevin P, Simmons C, Garg R. A novel technique for transcatheter patent ductus arteriosus closure in extremely preterm infants using commercially available technology. Catheter Cardiovasc Interv. 2015;85:240–248. [DOI] [PubMed] [Google Scholar]
- 17. El‐Said HG, Bratincsak A, Foerster SR, Murphy JJ, Vincent J, Holzer R, Porras D, Moore J, Bergersen L. Safety of percutaneous patent ductus arteriosus closure: an unselected multicenter population experience. J Am Heart Assoc. 2013;2:e000424 doi: 10.1161/JAHA.113.000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brotschi B, Hug MI, Kretschmar O, Rizzi M, Albisetti M. Incidence and predictors of cardiac catheterisation‐related arterial thrombosis in children. Heart. 2015;101:948–953. [DOI] [PubMed] [Google Scholar]
- 19. Bergersen L, Gauvreau K, Foerster SR, Marshall AC, McElhinney DB, Beekman RH III, Hirsch R, Kreutzer J, Balzer D, Vincent J, Hellenbrand WE, Holzer R, Cheatham JP, Moore JW, Burch G, Armsby L, Lock JE, Jenkins KJ. Catheterization for congenital heart disease adjustment for risk method (CHARM). JACC Cardiovasc Interv. 2011;4:1037–1046. [DOI] [PubMed] [Google Scholar]
- 20. Bergersen L, Giroud JM, Jacobs JP, Franklin RC, Beland MJ, Krogmann ON, Aiello VD, Colan SD, Elliott MJ, Gaynor JW, Kurosawa H, Maruszewski B, Stellin G, Tchervenkov CI, Walters HL, Weinberg P, Everett AD. Report from the International Society for Nomenclature of Paediatric and Congenital Heart Disease: cardiovascular catheterisation for congenital and paediatric cardiac disease (part 2—nomenclature of complications associated with interventional cardiology). Cardiol Young. 2011;21:260–265. [DOI] [PubMed] [Google Scholar]
- 21. Krichenko A, Benson LN, Burrows P, Moes CAF, Mclaughlin P, Freedom RM. Angiographic classification of the isolated, persistently patent ductus‐arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol. 1989;63:877–880. [DOI] [PubMed] [Google Scholar]
- 22. Delaney JW, Fletcher SE. Patent ductus arteriosus closure using the Amplatzer (R) vascular plug II for all anatomic variants. Catheter Cardiovasc Interv. 2013;81:820–824. [DOI] [PubMed] [Google Scholar]
- 23. Madan A, Brozanski BS, Cole CH, Oden NL, Cohen G, Phelps DL. A pulmonary score for assessing the severity of neonatal chronic lung disease. Pediatrics. 2005;115:e450–e457. [DOI] [PubMed] [Google Scholar]
- 24. Benaron DA, Benitz WE. Maximizing the stability of oxygen delivered via nasal cannula. Arch Pediatr Adolesc Med. 1994;148:294–300. [DOI] [PubMed] [Google Scholar]
- 25. Hislop AA, Wigglesworth JS, Desai R, Aber V. The effects of preterm delivery and mechanical ventilation on human lung growth. Early Human Dev. 1987;15:147–164. [DOI] [PubMed] [Google Scholar]
- 26. Natarajan G, Chawla S, Aggarwal S. Short‐term outcomes of patent ductus arteriosus ligation in preterm neonates: reason for concern? Am J Perinatol. 2010;27:431–437. [DOI] [PubMed] [Google Scholar]
- 27. Teixeira LS, Shivananda SP, Stephens D, Van Arsdell G, McNamara PJ. Postoperative cardiorespiratory instability following ligation of the preterm ductus arteriosus is related to early need for intervention. J Perinatol. 2008;28:803–810. [DOI] [PubMed] [Google Scholar]
- 28. Bruckheimer E, Godfrey M, Dagan T, Levinzon M, Amir G, Birk E. The Amplatzer Duct Occluder II additional sizes device for transcatheter PDA closure: initial experience. Catheter Cardiovasc Interv. 2014;83:1097–1101. [DOI] [PubMed] [Google Scholar]
- 29. Sungur M, Karakurt C, Ozbarlas N, Baspinar O. Closure of patent ductus arteriosus in children, small infants, and premature babies with Amplatzer Duct Occluder II additional sizes: multicenter study. Catheter Cardiovasc Interv. 2013;82:245–252. [DOI] [PubMed] [Google Scholar]
- 30. Bentham J, Meur S, Hudsmith L, Archer N, Wilson N. Echocardiographically guided catheter closure of arterial ducts in small preterm infants on the neonatal intensive care unit. Catheter Cardiovasc Interv. 2011;77:409–415. [DOI] [PubMed] [Google Scholar]
- 31. Abu Hazeem AA, Gillespie MJ, Thun H, Munson D, Schwartz MC, Dori Y, Rome JJ, Glatz AC. Percutaneous closure of patent ductus arteriosus in small infants with significant lung disease may offer faster recovery of respiratory function when compared to surgical ligation. Catheter Cardiovasc Interv. 2013;82:526–533. [DOI] [PubMed] [Google Scholar]
- 32. Laughon M, Bose C, Benitz WE. Patent ductus arteriosus management: what are the next steps? J Pediatr. 2010;157:355–357. [DOI] [PubMed] [Google Scholar]
- 33. Glatz AC, Shah SS, McCarthy AL, Geisser D, Daniels K, Xie D, Hanna BD, Grundmeier RW, Gillespie MJ, Rome JJ. Prevalence of and risk factors for acute occlusive arterial injury following pediatric cardiac catheterization: a large single‐center cohort study. Catheter Cardiovasc Interv. 2013;82:454–462. [DOI] [PubMed] [Google Scholar]
- 34. Bratincsak A, Moore JW, El‐Said HG. Low dose tissue plasminogen activator treatment for vascular thrombosis following cardiac catheterization in children: a single center experience. Catheter Cardiovasc Interv. 2013;82:782–785. [DOI] [PubMed] [Google Scholar]
- 35. Baruteau AE, Hascoet S, Baruteau J, Boudjemline Y, Lambert V, Angel CY, Belli E, Petit J, Pass R. Transcatheter closure of patent ductus arteriosus: past, present and future. Arch Cardiovasc Dis. 2014;107:122–132. [DOI] [PubMed] [Google Scholar]
- 36. Kenny D, Morgan GJ, Bentham JR, Wilson N, Martin R, Tometzki A, Oslizlok P, Walsh KP. Early clinical experience with a modified Amplatzer Ductal Occluder for transcatheter arterial duct occlusion in infants and small children. Catheter Cardiovasc Interv. 2013;82:534–540. [DOI] [PubMed] [Google Scholar]
- 37. Abdelbasit MA, Alwi M, Kandavello G, Che Mood M, Samion H, Hijazi ZM. The new Occlutech(R) PDA occluder: initial human experience. Catheter Cardiovasc Interv. 2015;86:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang JK, Wu MH, Lin MT, Chiu SN, Chen CA, Chiu HH. Transcatheter closure of moderate‐to‐large patent ductus arteriosus in infants using Amplatzer Duct Occluder. Circ J. 2010;74:361–364. [DOI] [PubMed] [Google Scholar]
- 39. Backes CH, Cua C, Kreutzer J, Armsby L, El‐Said H, Moore JW, Gauvreau K, Bergersen L, Holzer RJ. Low weight as an independent risk factor for adverse events during cardiac catheterization of infants. Catheter Cardiovasc Interv. 2013;82:786–794. [DOI] [PubMed] [Google Scholar]
- 40. Fischer HS, Buhrer C. Avoiding endotracheal ventilation to prevent bronchopulmonary dysplasia: a meta‐analysis. Pediatrics. 2013;132:E1351–E1360. [DOI] [PubMed] [Google Scholar]
- 41. Masri S, El Rassi I, Arabi M, Tabbakh A, Bitar F. Percutaneous closure of patent ductus arteriosus in children using Amplatzer Duct Occluder II: relationship between PDA type and risk of device protrusion into the descending aorta. Catheter Cardiovasc Interv. 2015;86:E66–E72. [DOI] [PubMed] [Google Scholar]
- 42. Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, Poindexter BB, Ingram DA, Abman SH. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;191:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


