There is general consensus that oral anticoagulant (OAC) therapy is recommended for stroke prevention in patients with atrial fibrillation (AF) and stroke risk factors.1, 2, 3 It is important to note that approximately one fourth of patients with AF who initiated OAC therapy (vitamin K antagonists including warfarin) discontinued it within the first year in real‐world clinical practice.4 Since warfarin is a difficult drug for both patients and physicians due to the necessity of frequent monitoring, dietary restrictions, and potential interaction with various drugs, the previous report demonstrating frequent and early discontinuation of OAC (warfarin) is not surprising.5 A warning regarding “rebound increases in thrombogenicity” after discontinuation of warfarin has been indicated because of a potential imbalance between coagulant and anticoagulant activities related to the reduction of the vitamin K–dependent coagulant and coagulation regulatory proteins (such as Protein C and Protein S).6, 7 Despite theoretical cautions, there is no clear clinical evidence concerning increased thrombotic events after warfarin discontinuation.8, 9
Various non–vitamin K oral anticoagulants (NOACs) have been recently developed by demonstrating noninferiority10, 11 or even superiority12, 13 for stroke prevention, and easy‐to‐use drug profiles in comparison with warfarin. For some NOACs, warnings regarding potential increases in stroke/systemic emboli after discontinuation were suggested after obtaining detailed analyses of phase III clinical trial data.14 However, the major purpose of clinical trials is to test a superior/noninferior hypothesis of a new therapy compared with the previous standard of care by primarily considering the first event. Thus, only a little information can be obtained regarding discontinuation of or adherence to NOACs in patients with AF who initiated these drugs in phase III clinical trials. In this issue of JAHA, Yao, et al presented an interesting report based on the contemporary United States insurance claim database, which includes ≈40% of patients treated with NOACs.15 This report addresses the impact of the adherence to OAC on stroke risks and major bleeding problems among patients with AF who were initially treated with OAC. Although the study design is a retrospective cohort analysis, analyzing a large number of patients (64 661) provides us a clue in understanding the importance of adherence/discontinuation issues with OAC use (including NOACs) in patients with AF.
There are a few important points Yao et al has discussed in this study. First, they confirmed that adherence to warfarin therapy at 1.1 year is as low as only 40.2% in real‐world practice. Patients (47.5%) have demonstrated a slightly better adherence to NOACs than warfarin. More than half of the patients who initiated NOACs discontinued them at 1.1 years. This finding is difficult to explain for NOACs with easy‐to‐use drug profiles. As discussed by the authors, pure prevention with no symptomatic recovery with the use of OAC causing expense might be one potential reason for lower adherence to NOACs than expected. Future development of health economic models to show lower occurrence of target events, lower total health cost, lower payment for health insurance with high persistence of OAC is necessary to improve early discontinuation issues. The second and the most important point the authors addressed in their study was that increased stroke risk associated with discontinued OACs was shown in this study only in the high‐risk patients with CHA2DS2‐VASc 2 or more. Obviously, the results of this study suggest that clinicians should emphasize the importance of adherence to OACs for preventing stroke in patients with AF who present with high risks of stroke. We are still uncertain whether the observed increased stroke risk in patients who discontinued OACs is the indication of “rebound increase in thrombogenicity.” As discussed by the authors, increased stroke events in patients with a high risk of stroke who discontinued OACs might only be an indication of a longer “nonprotection period” (Figure).
Figure 1.
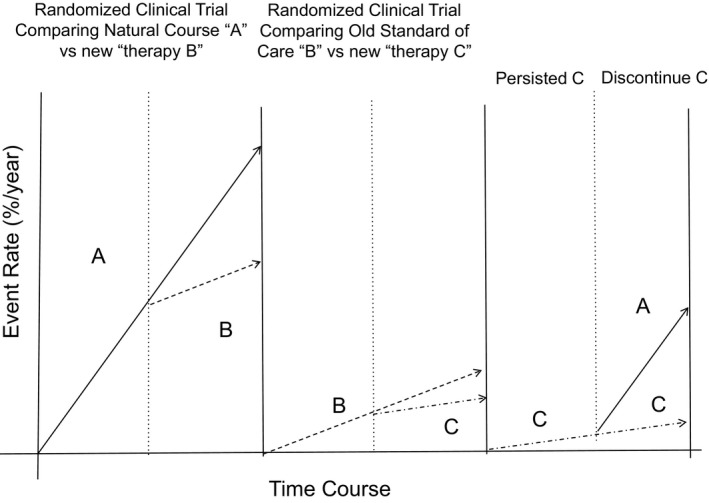
Concept of Systematic Improvement of Clinical Care based on Clinical Evidence and Its Disturbance by “Discontinuation” of the Therapy. Before establishing the “standard of care,” target events such as stroke/systemic embolism occur as a natural course as shown by line A. After completing large enough well‐designed clinical trials demonstrating the reduction of target events with the use of the new “therapy B” compared with natural course “A,” “therapy B” becomes the tentative “standard of care” with the event rate shown as dotted line B. When the next new “therapy C” was proven to be more effective in reducing target events than “therapy B,” “therapy C” becomes the next “standard of care” with the event rate shown as another dotted line C. The patients expect relatively lower target events rate only when they adhered to the “evidence‐based therapy.” Once “therapy C” was discontinued, the rate of the target events returns to the rate of “natural course.” Higher event rate shown in line A compared with the dotted line C represents “rebound,” but only returns to the event rate in a natural course by discontinuing the evidence‐based therapy.
There is a difference between “randomized trials” and actual “clinical settings.” The former uses a highly regulated population in institutes with physicians familiar with clinical trials. Thus, the adherence rate to therapy in the world of “randomized trials” is generally higher than that observed in actual “clinical settings.” Yao et al demonstrated an important difference between the “randomized trials” and actual “clinical settings” with regard to adherence to OAC therapy. In the “world of randomized trials,” the discontinuation rates of both warfarin and NOACs were lower (eg, ARISTOTLE: 27.5% for warfarin and 25.3% apixaban with median follow‐up of 1.8 years,13 RE‐LY: 16.6% for warfarin, 20.7% for 110 mg×2 dabigatran, and 21.2% for 150 mg×2 dabigatran at 2 years,12) than the current report of ≈40%/1.1 year. It is most likely that the prevention effects of OACs revealed in clinical trial results can be expected only when the discontinuation rates of OACs in actual “clinical settings” become similar to the rates revealed in clinical trials.
Early discontinuation of proven drugs is a problem not only restricted to OACs. In patients with myocardial infarction, angiotensin‐converting enzyme inhibitors were proven to prevent left ventricular remodeling and improve outcomes.16 However, the persistent use of angiotensin‐converting enzyme inhibitors in patients receiving angiotensin‐converting enzyme inhibitors after myocardial infarction at 2 years is only 50%.17 Current practice guidelines emphasize the importance of “initiating evidence‐based therapy,” but do not indicate the importance of “persistence of evidence‐based therapy for enough period of time” due to lack of clinical evidence. Figure demonstrates the concept of systemic improvement of clinical care using the logic of “Evidence Based Medicine.” Line A demonstrates the “natural course” of the disease. After completing a nicely designed large‐scale randomized trial demonstrating lower target events in patients treated with “new therapy B” versus “natural course A,” the “new therapy B” becomes the “standard of care” for the next generation. This concept was represented by our experience that aspirin became the “standard of care” in patients with acute myocardial infarction after completing the ISIS‐2 (Second International Study of Infarct Survival Collaborative Group)18 and similar other trials.19 The next randomized trials demonstrated lower target event rates with the use of new “treatment C” versus “treatment B,” and “treatment C” became “standard of care” for the next generation similar to the combination of aspirin and clopidogrel becoming the “standard of care” after completing the CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial) trial.20 In acute care settings, adherence and discontinuation issues are not as many compared with long‐term therapy, such as stroke prevention in AF patients. If patients discontinue the “evidence‐based therapy,” event rates become similar to that of the “natural course” (Figure). Although the increased event rate after discontinuing “evidence‐based therapy” mimics “rebound,” it may simply reflect the return of the event rate observed in the “natural course.” To avoid returning to the “natural course,” it is important to adhere to evidence‐based therapy once the physician/patient has decided to undergo that therapy. We have to keep in mind that the effects of the therapy demonstrated by clinical trials can be achieved only by its long enough persistence.
It is not surprising that serious bleeding complications were lower when OAC was discontinued for more than 3 months. All phase III NOACs clinical trials in patients with AF demonstrated that the patients treated by NOACs run the risk of serious bleeding by ≈2% to 3%/year.10, 11, 12, 13 Recent approval of reversal agents such as idarucizumab for reversing dabigatran anticoagulation may improve adherence rates by decreasing both patients’ and physicians’ fears regarding major bleeding problems. We need long‐term observation data to understand whether the reduction of bleeding events by discontinuing OAC has any meaningful impact on clinical care in the future.
Disclosures
Kengo Ayabe has nothing to disclose. Shinichi Goto has nothing to disclose. Shinya Goto received a research grant from Sanofi and Pfeizer within 2 years. Shinya Goto is a consultant for Armethrom for drug development. Shinya Goto received a Grant‐in‐Aid for Scientific Research in Japan (24390202), a grant from Senshin Medical Research Foundation, a grant from Nakatani Foundation for Advancement of Measuring Technologies in Biomedical Engineering, and a grant for the next‐generation supercomputer Research and Development program supported by Riken, and Strategic Program for Innovational Research Field 1 for Super‐computational Life Science.
(J Am Heart Assoc. 2016;5:e003258 doi: 10.1161/JAHA.116.003258)
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; Guidelines‐CPG ESCCfP, Document R . 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation–developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385–1413. [DOI] [PubMed] [Google Scholar]
- 2. Ogawa S, Hori M. Urgent statement on antithrombotic therapy of atrial fibrillation. Circ J. 2011;75:2719–2721. [DOI] [PubMed] [Google Scholar]
- 3. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, Members AATF. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. Warfarin discontinuation after starting warfarin for atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2010;3:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Brien EC, Simon DN, Allen LA, Singer DE, Fonarow GC, Kowey PR, Thomas LE, Ezekowitz MD, Mahaffey KW, Chang P, Piccini JP, Peterson ED. Reasons for warfarin discontinuation in the outcomes registry for better informed treatment of atrial fibrillation (ORBIT‐AF). Am Heart J. 2014;168:487–494. [DOI] [PubMed] [Google Scholar]
- 6. Odegaard OR, Lindahl AK, Try K, Kvalheim G, Sorbo JH. Recurrent venous thrombosis during warfarin treatment related to acquired protein S deficiency. Thromb Res. 1992;66:729–734. [DOI] [PubMed] [Google Scholar]
- 7. Kurt M, Shorbagi A, Aksu S, Haznedaroglu I, Altundag K, Erkin G. Warfarin‐induced skin necrosis and leukocytoclastic vasculitis in a patient with acquired protein C and protein S deficiency. Blood Coagul Fibrinolysis. 2007;18:805–806. [DOI] [PubMed] [Google Scholar]
- 8. Van Cleve R. The rebound phenomenon—fact or fancy? Experience with discontinuation of long‐term anticoagulation therapy after myocardial infarction. Circulation. 1965;32:878–880. [DOI] [PubMed] [Google Scholar]
- 9. Genewein U, Haeberli A, Straub PW, Beer JH. Rebound after cessation of oral anticoagulant therapy: the biochemical evidence. Br J Haematol. 1996;92:479–485. [DOI] [PubMed] [Google Scholar]
- 10. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; Investigators RA . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 11. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; Investigators EA‐T . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 12. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 13. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; Committees A, Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 14. Reynolds MR. Discontinuation of rivaroxaban: filling in the gaps. J Am Coll Cardiol. 2013;61:659–660. [DOI] [PubMed] [Google Scholar]
- 15. Yao X, Abraham NS, Alexander GC, Crown W, Montori VM, Sangaralingham LR, Gersh BJ, Shah ND, Noseworthy PA. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc. 2016;5:e003074 doi: 10.1161/JAHA.115.003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith SC Jr, Blair SN, Bonow RO, Brass LM, Cerqueira MD, Dracup K, Fuster V, Gotto A, Grundy SM, Miller NH, Jacobs A, Jones D, Krauss RM, Mosca L, Ockene I, Pasternak RC, Pearson T, Pfeffer MA, Starke RD, Taubert KA. AHA/ACC scientific statement: AHA/ACC guidelines for preventing heart attack and death in patients with atherosclerotic cardiovascular disease: 2001 update: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 2001;104:1577–1579. [DOI] [PubMed] [Google Scholar]
- 17. Akincigil A, Bowblis JR, Levin C, Jan S, Patel M, Crystal S. Long‐term adherence to evidence based secondary prevention therapies after acute myocardial infarction. J Gen Intern Med. 2008;23:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS‐2. ISIS‐2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2:349–360. [PubMed] [Google Scholar]
- 19. Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial I . Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation. N Engl J Med. 2001;345:494–502. [DOI] [PubMed] [Google Scholar]


