Abstract
Metabolic syndrome, which includes hypertension, hyperglycemia, obesity, insulin resistance, and dyslipidemia, has a negative impact on cognitive health. Endoplasmic reticulum (ER) stress is activated during metabolic syndrome, however it is not known which factor associated with metabolic syndrome contributes to this stress. ER stress has been reported to play a role in the development of insulin resistance in peripheral tissues. The role of ER stress in the development of insulin resistance in hippocampal neurons is not known. In the current study, we investigated ER stress in the hippocampus of 3 different mouse models of metabolic syndrome: the C57BL6 mouse on a high fat (HF) diet; apolipoprotein E, leptin, and apolipoprotein B-48 deficient (ApoE 3KO) mice; and the low density lipoprotein receptor, leptin, and apolipoprotein B-48 deficient (LDLR 3KO) mice. We demonstrate that ER stress is activated in the hippocampus of HF mice, and for the first time, in ApoE 3KO mice, but not LDLR 3KO mice. The HF and ApoE 3KO mice are hyperglycemic; however, the LDLR 3KO mice have normal glycemia. This suggests that hyperglycemia may play a role in the activation of ER stress in the hippocampus. Similarly, we also demonstrate that impaired insulin signaling is only present in the HF and ApoE 3KO mice, which suggests that ER stress may play a role in insulin resistance in the hippocampus. To confirm this we pharmacologically induced ER stress with thapsigargin in human hippocampal neurons. We demonstrate for the first time that thapsigargin leads to ER stress and impaired insulin signaling in human hippocampal neurons. Our results may provide a potential mechanism that links metabolic syndrome and cognitive health.
Keywords: metabolic syndrome, brain, endoplasmic reticulum stress, insulin, hippocampus, apolipoprotein
Introduction
Metabolic syndrome is the term used for the presence of multiple conditions including hypertension, hyperglycemia, obesity, insulin resistance, and dyslipidemia. Metabolic syndrome is a risk factor for neurological disorders including stroke, dementia, and depression (Baker et al., 2010; Biessels et al., 2006; Correia et al., 2011; de la Monte, 2009; Haan, 2006; Neumann et al., 2008; Profenno et al., 2010; Zhao and Townsend, 2009). Several studies have demonstrated that factors associated with metabolic syndrome have a negative impact on cognitive health (Dahl and Hassing, 2013; Farr et al., 2008; Luchsinger et al., 2007; Novak and Hajjar, 2010; Takahashi et al., 2011). Given the prevalence of metabolic syndrome and the devastation associated with neurological disorders, it is imperative to understand the impact of metabolic syndrome on the brain and to uncover the molecular mechanisms that may drive these changes.
It is generally believed that hyperglycemia and impaired glucose tolerance are the major factors that play a role in stress activation and insulin resistance (Back and Kaufman, 2012; Boden et al., 2014; Malhotra and Kaufman, 2007). The endoplasmic reticulum (ER) plays a major role in the response to stress. ER stress is the consequence of either a physiological or pathological disturbance in protein folding, the function of intracellular calcium stores, or the synthesis of fatty acids, sterols, or phospholipids (Schroder, 2008). The increase in misfolded proteins in the ER lumen during ER stress leads to the activation of the unfolded protein response (UPR) (Lin et al., 2008; Ron and Walter, 2007; Schroder, 2008). The purpose of the UPR is to relieve the burden on the ER by increasing ER folding capacity, decreasing protein synthesis (translation), and/or degrading unfolded proteins through the ubiquitin-proteasome system or autophagy, otherwise apoptosis is activated (Eizirik et al., 2008; Lin et al., 2008; Schroder, 2008). The UPR is generated through the action of three transmembrane proteins, one of which is the inositol-requiring enzyme 1 (IRE1), the most conserved branch of the UPR (Lin et al., 2008). Activation of IRE1 signals x-box binding protein 1 (Xbp1) mRNA. Xbp1 is a transcription factor and one of the master regulators of the ER folding capacity (Sriburi et al., 2004). An intron is excised during the UPR, yielding spiced (s) Xbp1mRNA which encodes XBP1s protein (Ron and Walter, 2007). XBP1s is translocated into the nucleus to induce the expression of ER chaperones—heat shock protein 5 (also known as BiP) and heat shock protein 90 beta member 1. These heat shock proteins increase the folding capacity of the ER (Schroder, 2008).
ER stress is implicated as a mechanism underlying insulin resistance in peripheral tissues including liver, pancreas, and adipose (Hotamisligil, 2010; Ozcan et al., 2004). Insulin resistance is characterized by a decreased response to normal circulating insulin levels in a particular tissue. In the periphery, insulin resistance occurs following hyperinsulinemia and subsequent desensitization of insulin receptors, which results in decrease in the expression of the receptor (Draznin, 2006). This desensitization is due to an increase in the serine phosphorylation of the insulin receptor substrate 1 (IRS1). The serine phosphorylation of IRS1 reduces the ability of IRS proteins to attract PI 3-kinase, thereby decreasing its activation and downstream insulin signaling (Aguirre et al., 2002; Birnbaum, 2001; Patti and Kahn, 2004; Qiao et al., 1999; Qiao et al., 2002; Um et al., 2004; White, 2003). ER stress-induced JNK activation leads to the phosphorylation of IRS1 at the serine 307 residue (Belgardt and Bruning, 2010; Ropelle et al., 2010). While insulin resistance is reported in both neurons and in the brain of a type 2 mouse model of diabetes (Kim et al., 2011a; Kim et al., 2011b), the mechanism underlying insulin resistance in the brain is not known. Given the fact that brain insulin plays a role in strengthening synapses, enhancing memory, and may play a role in glucose uptake in certain regions under pathologic conditions (Bingham et al., 2002; Figlewicz et al., 1993a; Figlewicz et al., 1993b; Skeberdis et al., 2001), understanding the mechanism may provide a new therapeutic target to prevent neurological complications (Sims-Robinson et al., 2010).
It is not known which aspect of the metabolic syndrome contributes to the activation of ER stress and whether or not this stress can impair insulin signaling in neurons of the hippocampus. It is generally believed that hyperglycemia and impaired glucose tolerance are the major factors that play a role in stress activation and insulin resistance (Back and Kaufman, 2012; Boden et al., 2014; Malhotra and Kaufman, 2007). Therefore, the purpose of this study was to understand which factors associated with the metabolic syndrome contribute to ER stress and brain insulin resistance. To address this we used three different animal models of the metabolic syndrome, the B6 mouse fed a HF diet, the ApoE 3KO and the LDLR 3KO mouse models. The high fat diet (HF) mouse model encompasses many of the facets of metabolic syndrome, including hyperinsulinemia, hyperglycemia, glucose intolerance, hypercholesterolemia, and hypertension (Buettner et al., 2007; Oakes et al., 1997; Tschop and Heiman, 2001; Vincent et al., 2009; Williams et al., 2003). The one major disadvantage of the model is that the major lipid carrying molecule in the B6 HF diet-induced mouse is the high-density lipoprotein (HDL), whereas the low density lipoprotein (LDL) is the major lipid carrying molecule in man (Kennedy et al., 2010). Triple knockout mice deficient in ApoB-48, leptin (ob/ob), and either Apolipoprotein E (ApoE) or LDL receptor (LDLR) have a lipoprotein profile similar to man; these models are known as the ApoE triple knockout (3KO) or the LDLR 3KO, respectively (Lloyd et al., 2008). The ApoE 3KO mice are obese, hyperinsulinemic, hyperglycemic, glucose intolerant, hypercholesterolemic, and hyperlipidemic (Lloyd et al., 2008). The LDLR 3KO mice have a similar phenotype, however, the LDLR 3KO mice are not hyperglycemic or glucose intolerant (Lloyd et al., 2008). Furthermore, to evaluate the role of ER stress in brain insulin resistance, we performed in vitro studies in human hippocampal neurons to pharmacologically induce ER stress using thapsigargin, an agent that disrupts calcium homeostasis (Samali et al., 2010).
Materials and methods
Animals
B6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME) at 4 weeks of age and fed either a standard 10% fat chow (Research diet #D12450B; New Brunswick, NJ) or a 54% fat chow (Research diets #D05090701) ad libitum. Triple knockout ApoE−/− ApoB100only/ob/+ and LDLR−/− ApoB100only/ob/+ mice were generated by Dr. Murielle Veniant and colleagues (Lloyd et al., 2008) and bred to obtain ApoE−/− ApoB100only/ob/+ (ApoE/ob+), ApoE−/− ApoB100only/ob/ob (ApoE 3KO), LDLR−/− ApoB100only/ob/+ (LDLR/ob+), and LDLR−/− ApoB100only/ob/ob (LDLR 3KO) littermates. Mice were weaned at 4 weeks of age and fed standard chow. Male mice were used in all experiments. All data were collected at 20–24 weeks of age.
Mice were housed in a pathogen-free environment. All protocols and procedures were approved by the University of Michigan Committee on the Use and Care of Animals (UCUCA; approval number PRO00003694), and are in compliance with University guidelines, State and Federal regulations, and the standards of the “Guide for the Care and Use of Laboratory Animals.” The University’s Animal Welfare Assurance Number on file with NIH Office of Laboratory Animal Welfare (OLAW) is A3114-01, and the University is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC, Intl.).
Phenotypic measures
Body weights were monitored monthly using a standard laboratory scale. Fasting blood glucose levels were obtained using a standard Glucometer (One Touch Ultra, Milpitas, CA) per our previously published protocol (Vincent et al., 2009). Plasma insulin levels were also measured per our previously published protocol (Hinder et al., 2013; Vincent et al., 2009).
Tissue Preparation
The mice were euthanized according to our published protocols (Kim et al., 2011b) with an overdose of sodium pentobarbital. The hippocampus was dissected from each hemisphere and flash frozen in liquid nitrogen and stored at −80°C until use for western immunoblotting.
Human Hippocampal Neurons
Primary Human Hippocampal Neurons (NEURALSTEM INC., Rockville, MD) are a human neural stem cell line of hippocampal origin that has been conditionally immortalized with cMyc-ER. Hippocampal neurons are maintained in N2b medium containing 1 μg/ml fibronectin and 10 μg/ml bFGF growth supplement, with fresh media given every other day. To exclude the effect of insulin, the culture medium is changed to treatment media (NSDM-1 without insulin) 24 hr before experimentation. ER stress is pharmacologically induced in the hippocampal neurons with the addition of 5 nM thapsigargin (Sigma Aldrich, St. Louis, MO) in DMSO for 2–48 hr. Hippocampal neurons were lysed in RIPA buffer (Pierce, Rockford, IL) containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) and stored at −80°C until use for western blotting.
Western Immunoblotting
Western immunoblotting was performed as previously described (Kim et al., 2011a; Kim et al., 2011b). The hippocampus was homogenized in tissue protein extraction reagent (Pierce, Rockford, IL) containing a protease inhibitor cocktail (Calbiochem, San Diego, CA). The lysates were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Tris-buffered saline with Tween-20 supplemented with either 3% bovine serum albumin (BSA; Sigma Aldrich, St. Louis, MO) or 5% milk was used to block the membrane and to dilute the antibodies. Polyclonal antibodies against phospho-JNK, XBP1 (Santa Cruz Biotechnology, INC., Santa Cruz, CA), BiP (Abcam, Cambridge, MA), pIRS1 serine 307, IRS1 (Cell Signaling, Danvers, MA), and glyceraldehyde 3-phosphoate dehydrogenase (GAPDH; EMD Millipore, Billerica, Massachusetts) were used for western immunoblotting. The signal was visualized using LumiGLO enhanced chemiluminescence reagent (Cell Signaling Technology, Danvers, MA). Images were captured using the Chemidoc XRS system and analyzed by Quantity One software (Bio-Rad Laboratory, Hercules, CA).
Statistical Analysis
Data analyses were performed using Prism, version 6 (GraphPad Software, Inc.). Assumptions about Gaussian distribution of data were made using the D’Agostino & Pearson omnibus normality test. At least 8 mice per group were used for metabolic studies. For all other measures at least 5 mice per group were used. T-test was used to compare control versus HF, ApoE/ob+ versus ApoE 3KO, or LDLR/ob+ versus LDLR 3KO. For all experiments, *p<0.05, **p<0.01, #p<0.001, Φp<0.0001, and bar graphs illustrate the mean ± standard error of the mean (SEM).
Results
High-fat diet leads to stress activation and impaired insulin signaling in the hippocampus
To evaluate ER stress and impaired insulin signaling during metabolic syndrome, western immunoblotting was performed on the hippocampus of the B6-HF mouse model. Densitometry analysis of western blotting revealed a 55% increase in the spliced (s) variant of XBP1, 40% increase in BiP, and at least 100% increase in JNK activation (phosphorylation; p) in the hippocampus of HF mice compared with ctrl mice after 20 wks of diet (Figure 1A,B). This is accompanied by at least a 100% increase in the serine phosphorylation of IRS1 at residue 307 (pIRS1 s307) in the HF mice compared with the control mice (Figure 1C,D). The total protein levels of IRS1 are not different between the HF and control mice (Figure 1C,D). The total protein levels of InsR decreased by 38% in the HF mice compared with control mice after 20 weeks of diet (Figure 1C,D).
Figure 1. Stress activation and impaired insulin signaling in the hippocampus of a diet-induced mouse model of metabolic syndrome.
A) Representative immunoblots and B) densitometry of spliced x-box binding protein 1 (XBP1s), heat shock protein 5 (BiP), and phosphorylated c-Jun N-terminal kinase (p-JNK) to evaluate the response to stress. C) Representative immunoblots and D) densitometry of phosphorylated insulin receptor substrate at serine residue 307 (pIRS1 s307), IRS1, and insulin receptor (InsR) to assess insulin signaling. C57BL6 mice on a standard control diet (CTRL) are represented by white bars and mice on the high-fat diet (HF) are represented by dotted bars. For all groups n≥5; *p<0.05, **p<0.01,***p<0.001, #p<0.0001 compared with CTRL.
Only the diet-induced and ApoE 3KO mouse models are hyperglycemic, not the LDLR 3KO
Given the role of hyperglycemia in ER stress activation and impaired insulin signaling, various mouse models with similar metabolic phenotypes except for the presence of hyperglycemia were utilized. The HF (54.0 ± 0.9 g), ApoE 3KO (64.8 ± 1.6 g), and LDLR 3KO (70.3 ± 2. g 2) mice weigh significantly more than mice on the standard chow (control, 32.7 ± 1.0 g), ApoE/ob+, (32.2 ± 1.5 g), and LDLR/ob+, (29.1 ± 0.4 g), respectively (Figure 2A). Fasting blood glucose levels are significantly elevated in the HF (276 ± 12 mg/dl) and ApoE 3KO (446 ± 17 mg/dl) mice compared with the control (207 ± 5 mg/dl) and ApoE/ob+ (197 ± 6 mg/dl), respectively; however, there is no significant difference between the LDLR 3KO (235 ± 19 mg/dl) and the LDLR/ob+ (196 ±4 mg/dl; Figure 2B). This is accompanied by elevated levels of plasma insulin in the HF (9.35 ± 0.75 ng/ml), ApoE 3KO (10.95 ± 2.8 ng/ml), and LDLR 3KO (19.08 ± 3.3 ng/ml) compared with control mice (1.45 ± 0.26 ng/ml), ApoE/ob+ (0.83 ± 0.15 ng/ml), and the LDLR/ob+ (1.03 ± 0.20 ng/ml), respectively (Figure 2C).
Figure 2. Metabolic phenotype of a diet-induced and 3KO mouse models of metabolic syndrome.
A) Weight, B) fasting blood glucose, and C) plasma insulin levels in C57BL6 mice on a standard control (CTRL; white bars) or high-fat (HF; dotted bars) diet, ApoE/ob+ (gray bars), ApoE 3KO (checkered bars), LDLR/ob+ (black bars), and LDLR 3KO (striped bars) at 20–24 wks of age. For all groups n≥5; *p<0.05, **p<0.01, ***p<0.001, #p<0.0001 compared with the respective controls.
Only the ApoE 3KO, not the LDLR 3KO mice have stress activation and impaired insulin signaling
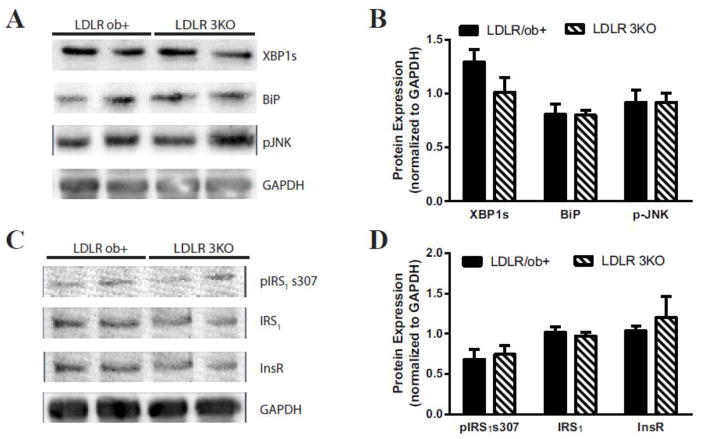
To evaluate the impact of the presence of hyperglycemia on ER stress and impaired insulin signaling in the hippocampus, western immunoblotting was performed in the ApoE and LDLR 3 KO mouse models. Densitometry analysis of western blotting revealed a 79% increase in XBP1s, 52% increase in BiP, and a 79% increase in p-JNK in the hippocampus of ApoE 3KO mice compared with the ApoE/ob+ littermate controls (Figure 3A,B). This is accompanied by a 50% increase in pIRS1 s307 spliced variant of XBP1 in the ApoE 3KO compared with ApoE/ob+ littermate controls; however, the total levels of IRS1 and InsR are not significantly different in the ApoE 3KO compared with ApoE/ob+ littermate controls (Figure 3C,D). On the other hand, the levels of XBP1s, BiP, and p-JNK in the hippocampus of LDLR 3KO mice compared with the LDLR/ob+ littermate controls are not significantly different (Figure 4A,B). Similarly, there is no significant difference in the levels of pIRS1 s307, IRS1, or InsR in the hippocampus of LDLR 3KO mice compared with the LDLR/ob+ littermate controls (Figure 4C,D).
Figure 3. Stress activation and impaired insulin signaling in the hippocampus of the ApoE 3KO mouse models of metabolic syndrome.
A) Representative immunoblots and B) densitometry of XBP1s, BiP, and p-JNK to evaluate the response to stress. C) Representative immunoblots and D) densitometry of pIRS1 s307, IRS1, and InsR to assess insulin signaling. Mice deficient in apolipoprotein E (ApoE−/−) with apolipoprotein B (ApoB) 100 only and normal levels of leptin (ApoE/ob+) are represented by gray bars; whereas, ApoE−/− with ApoB100 only and leptin deficient (ob/ob; ApoE 3KO) mice are represented by checkered bars. For all groups n≥5; *p<0.05, **p<0.01, ***p<0.001, #p<0.0001.
Figure 4. Stress activation and impaired insulin signaling in the hippocampus of the LDLR 3KO mouse models of metabolic syndrome.
A) Representative immunoblots and B) densitometry of XBP1s, BiP, and p-JNK to evaluate the response to stress. C) Representative immunoblots and D) densitometry of pIRS1 s307, IRS1, and InsR to assess insulin signaling. Mice deficient in low density lipoprotein receptor (LDLR−/−) with apolipoprotein B (ApoB) 100 only and normal levels of leptin (LDLR/ob+) are represented by black bars; whereas, LDLR−/− with ApoB100 only and leptin deficient (ob/ob; LDLR 3KO) mice are represented by striped bars. For all groups n≥5; *p<0.05, **p<0.01, ***p<0.001, #p<0.0001.
Stress activation impairs insulin signaling in human hippocampal neurons in vitro
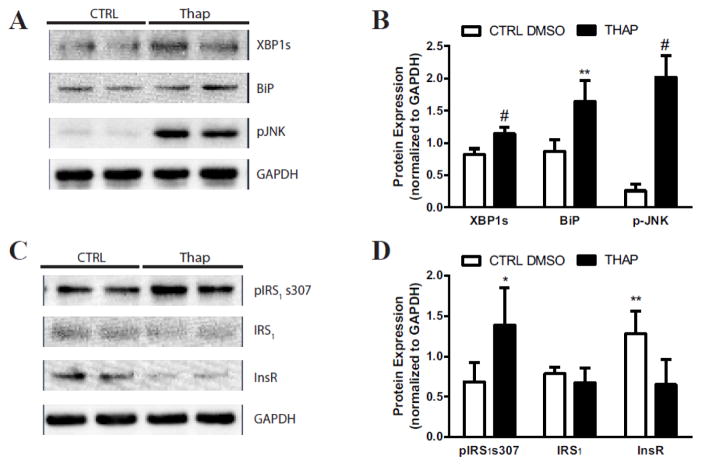
To determine whether the induction of ER stress can culminate in impaired insulin signaling in the hippocampus, ER stress was pharmacologically induced using thapsigargin in human hippocampal neurons in vitro. Densitometry analysis of western blotting revealed a 39% increase in XBP1s, a 91% increase in BiP, and at least a 100% increase p-JNK in human hippocampal neurons treated with thapsigargin compared with the DMSO treated control (Figure 5A & B). Furthermore, at least a 100% increase in pIRS1 s307 was observed in human hippocampal neurons treated with thapsigargin compared with the DMSO treated control; however the total levels of IRS1 were not significantly different in human hippocampal neurons treated with thapsigargin compared with the DMSO treated control (Figure 5C,D). The total protein levels of InsR was decreased by 49% in the in human hippocampal neurons treated with thapsigargin compared with the DMSO treated control (Figure 5C,D).
Figure 5. Thapsigargin treatment induces cellular stress and impairs insulin signaling in human hippocampal neurons.
A) Representative immunoblots and B) densitometry of XBP1s, BiP, and p-JNK to evaluate stress activation. C) Representative immunoblots and D) densitometry of pIRS1 s307, IRS1, and InsR to assess insulin signaling. Human hippocampal neurons treated with DMSO control (CTRL) are represented by white bars; whereas Hhi treated with thapsigargin (THAP) are represented by black bars. For all groups n≥5; *p<0.05, **p<0.01, ***p<0.001, #p<0.0001.
Discussion
In the current study, we investigated the factors associated with the metabolic syndrome that may contribute to cellular stress and insulin resistance in the hippocampus. The ER plays a critical role in the cells response to stress. In fact, previous studies have demonstrated that ER stress leads to the development of insulin resistance by triggering JNK activation (phosphorylation) in the liver and adipose (Nakatani et al., 2005; Ozawa et al., 2005; Ozcan et al., 2004; Ozcan et al., 2006). A recent study reports ER stress, JNK activation, and impaired insulin signaling in the brain of obese rats (Liang et al., 2015). We investigated whether the induction of ER stress can impair insulin signaling in hippocampal neurons. We show for the first time that hyperglycemia correlates with the presence of stress in the hippocampus. We also demonstrate for the first time that pharmacologically inducing ER stress in human hippocampal neurons activates JNK and impairs insulin signaling. These findings suggest that insulin resistance in the hippocampus may be due to the activation of stress induced by hyperglycemia.
Chronic hyperglycemia prolongs ER stress and activates the JNK pathway (Hotamisligil, 2005; Malhotra and Kaufman, 2007; Zhong et al., 2012). Our previous study revealed ER stress in the hippocampus of a mouse model of type 2 diabetes and metabolic syndrome, the BKS-db/db mice (Sims-Robinson et al., 2012). Furthermore, studies have reported ER stress in the hypothalamus and hippocampus following HF feeding (Castro et al., 2013; Lu et al., 2011). Activation of the UPR is indicative of the presence of ER stress. The HF mice in the current study demonstrate the activation of the IRE branch, which is the most conserved branch of the UPR (Lin et al., 2008). This is evident by the HF diet-induced increase in the protein expression of XBP1s and BiP.
JNK activation (phosphorylation) can impair insulin signaling via the serine phosphorylation of IRS1 (Draznin, 2006). The activation of JNK and the subsequent serine phosphorylation of IRS1 at the 307 residue has been reported in the hypothalamus and the amygdala during obesity (Belgardt and Bruning, 2010; Castro et al., 2013; Ropelle et al., 2010); however, it is not known whether this mechanism is involved in the hippocampus. The increase in JNK activation and serine phosphorylation of IRS1 observed in the current study suggests this stress activated kinase may indeed play a role in insulin resistance in the hippocampus. Furthermore, insulin resistance is confirmed by the observed decrease in the levels of the InsR in the HF mice (Muoio and Newgard, 2004).
The evaluation of the ApoE 3KO and the LDLR 3KO will provide insight into the role that hyperglycemia may play in stress activation and insulin resistance in the hippocampus. Only the ApoE 3KO mice display stress activation in the hippocampus, evident by the increased protein expression of p-JNK, BiP, and XBP1s. The LDLR 3KO mice levels are comparable to the LDLR ob+ littermate control. This suggests that indeed hyperglycemia plays a role in stress activation in the hippocampus. To our knowledge, this is the first report of ER stress and the UPR in the APOE and LDLR 3KO mouse hippocampus. ER stress and the UPR has been previously reported in the liver, adipose, and muscle of ob/ob mice (Ozcan et al., 2004; Ozcan et al., 2006). Interestingly, the increase in JNK activation and in the serine phosphorylation of IRS1 correlated with presence of ER stress and similarly was only found in the ApoE 3KO mice and not the LDLR 3KO mice. Thus, this implicates ER stress and JNK activation in hippocampal insulin resistance.
To determine whether ER stress can lead to insulin resistance in vitro, human hippocampal neurons were utilized. Treating human hippocampal neurons with thapsigargin induced ER stress, evident by the increase in XBP1s and BiP. Furthermore, an increase in JNK phosphorylation was also observed, suggesting that ER stress can trigger the phosphorylation of JNK in hippocampal neurons. JNK activation has been reported in mouse hippocampal neurons following thapsigargin-induced ER stress (Choi et al., 2010). Thapsigargin-induced ER stress also lead to impaired insulin signaling, evident by an increase in the serine phosphorylation of IRS1 without a change in total IRS1 expression and a decrease in the InsR. These data suggest that ER stress may be a mechanism in insulin resistance in hippocampal neurons. Further studies are needed to determine whether targeting JNK or ER stress may provide beneficial effects therapeutically.
In conclusion, it is likely that multiple pathways are involved in insulin resistance. We demonstrate that hyperglycemia and impaired insulin resistance correlates with ER stress in mouse models of metabolic syndrome. Furthermore, ER stress correlates with JNK activation and an impairment in insulin signaling. We demonstrate in vitro that pharmacologically inducing ER stress leads to JNK activation and impairment of insulin signaling. Future studies will focus on ER stress and JNK pathways as potential therapeutic targets to prevent insulin resistance in the brain during metabolic syndrome and thus, preventing the potential downstream impact on the brain.
Highlights.
Hippocampal ER stress is only present in mouse models with hyperglycemia.
ER stress correlates with impaired insulin signaling in the hippocampus.
Thapsigargin-induced ER stress in hippocampal neurons impairs insulin signaling.
Acknowledgments
The authors wish to acknowledge Carey Backus, Lisa McLean, and Peter Keller for technical assistance and Benjamin Murdock for editorial assistance. This work utilized the Mouse Metabolic Phenotyping Center Core at the University of Washington, Seattle, Washington (U24 DK016126) for plasma lipid measurements and the Vanderbilt Mouse Metabolic Phenotyping Center (U24 DK059637) for insulin measurements.
Funding
This work was supported by the National Institute of Health (NINDS 5K01NS079461, to C. S-R.; NIDDK Supplement to 5-R24-DK-082941, to C.S-R.; NIA Training Grant T32 AG000114, to C. S-R.; NINDS Neurology Training Grant T32 NS007222, to C. S-R.), the A. Alfred Taubman Medical Research Institute, and the Program for Neurology Research and Discovery.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. The Journal of biological chemistry. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annual review of biochemistry. 2012;81:767–793. doi: 10.1146/annurev-biochem-072909-095555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Cholerton BA, Plymate SR, Fishel MA, Watson GS, Duncan GE, Mehta PD, Craft S. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2010;22:569–579. doi: 10.3233/JAD-2010-100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgardt BF, Bruning JC. CNS leptin and insulin action in the control of energy homeostasis. Annals of the New York Academy of Sciences. 2010;1212:97–113. doi: 10.1111/j.1749-6632.2010.05799.x. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Koffeman A, Scheltens P. Diabetes and cognitive impairment. Clinical diagnosis and brain imaging in patients attending a memory clinic. Journal of neurology. 2006;253:477–482. doi: 10.1007/s00415-005-0036-4. [DOI] [PubMed] [Google Scholar]
- Bingham EM, Hopkins D, Smith D, Pernet A, Hallett W, Reed L, Marsden PK, Amiel SA. The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes. 2002;51:3384–3390. doi: 10.2337/diabetes.51.12.3384. [DOI] [PubMed] [Google Scholar]
- Birnbaum MJ. Turning down insulin signaling. The Journal of clinical investigation. 2001;108:655–659. doi: 10.1172/JCI13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Cheung P, Kresge K, Homko C, Powers B, Ferrer L. Insulin resistance is associated with diminished endoplasmic reticulum stress responses in adipose tissue of healthy and diabetic subjects. Diabetes. 2014;63:2977–2983. doi: 10.2337/db14-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity. 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- Castro G, MFCA, Weissmann L, Quaresma PG, Katashima CK, Saad MJ, Prada PO. Diet-induced obesity induces endoplasmic reticulum stress and insulin resistance in the amygdala of rats. FEBS open bio. 2013;3:443–449. doi: 10.1016/j.fob.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Choi AY, Yoon H, Choe W, Yoon KS, Ha J, Yeo EJ, Kang I. Baicalein protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis through inhibition of reactive oxygen species production and CHOP induction. Experimental & molecular medicine. 2010;42:811–822. doi: 10.3858/emm.2010.42.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia CC, Lima F, Junqueira F, Campos MS, Bastos O, Petribu K, Laks J, Galvin JE. AD8-Brazil: cross-cultural validation of the ascertaining dementia interview in Portuguese. Journal of Alzheimer’s disease : JAD. 2011;27:177–185. doi: 10.3233/JAD-2011-100915. [DOI] [PubMed] [Google Scholar]
- Dahl AK, Hassing LB. Obesity and cognitive aging. Epidemiologic reviews. 2013;35:22–32. doi: 10.1093/epirev/mxs002. [DOI] [PubMed] [Google Scholar]
- de la Monte SM. Insulin resistance and Alzheimer’s disease. BMB reports. 2009;42:475–481. doi: 10.5483/bmbrep.2009.42.8.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draznin B. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: the two sides of a coin. Diabetes. 2006;55:2392–2397. doi: 10.2337/db06-0391. [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocrine reviews. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Bentson K, Ocrant I. The effect of insulin on norepinephrine uptake by PC12 cells. Brain research bulletin. 1993a;32:425–431. doi: 10.1016/0361-9230(93)90210-3. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Szot P, Israel PA, Payne C, Dorsa DM. Insulin reduces norepinephrine transporter mRNA in vivo in rat locus coeruleus. Brain research. 1993b;602:161–164. doi: 10.1016/0006-8993(93)90258-o. [DOI] [PubMed] [Google Scholar]
- Haan MN. Therapy Insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nature clinical practice. Neurology. 2006;2:159–166. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- Hinder LM, Vincent AM, Hayes JM, McLean LL, Feldman EL. Apolipoprotein E knockout as the basis for mouse models of dyslipidemia-induced neuropathy. Experimental neurology. 2013;239:102–110. doi: 10.1016/j.expneurol.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54(Suppl 2):S73–78. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AJ, Ellacott KL, King VL, Hasty AH. Mouse models of the metabolic syndrome. Disease models & mechanisms. 2010;3:156–166. doi: 10.1242/dmm.003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, McLean LL, Philip SS, Feldman EL. Hyperinsulinemia induces insulin resistance in dorsal root ganglion neurons. Endocrinology. 2011a;152:3638–3647. doi: 10.1210/en.2011-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Sullivan KA, Backus C, Feldman EL. Cortical neurons develop insulin resistance and blunted Akt signaling: a potential mechanism contributing to enhanced ischemic injury in diabetes. Antioxidants & redox signaling. 2011b;14:1829–1839. doi: 10.1089/ars.2010.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Chen J, Zhan L, Lu X, Sun X, Sui H, Zheng L, Xiang H, Zhang F. Endoplasmic reticulum stress impairs insulin receptor signaling in the brains of obese rats. PloS one. 2015;10:e0126384. doi: 10.1371/journal.pone.0126384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annual review of pathology. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DJ, McCormick J, Helmering J, Kim KW, Wang M, Fordstrom P, Kaufman SA, Lindberg RA, Veniant MM. Generation and characterization of two novel mouse models exhibiting the phenotypes of the metabolic syndrome: Apob48-/-Lepob/ob mice devoid of ApoE or Ldlr. American journal of physiology Endocrinology and metabolism. 2008;294:E496–505. doi: 10.1152/ajpendo.00509.2007. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu DM, Zheng YL, Hu B, Cheng W, Zhang ZF, Shan Q. Ursolic acid improves high fat diet-induced cognitive impairments by blocking endoplasmic reticulum stress and IkappaB kinase beta/nuclear factor-kappaB-mediated inflammatory pathways in mice. Brain, behavior, and immunity. 2011;25:1658–1667. doi: 10.1016/j.bbi.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Archives of neurology. 2007;64:570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxidants & redox signaling. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Newgard CB. Biomedicine. Insulin resistance takes a trip through the ER. Science. 2004;306:425–426. doi: 10.1126/science.1104680. [DOI] [PubMed] [Google Scholar]
- Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. The Journal of biological chemistry. 2005;280:847–851. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- Neumann KF, Rojo L, Navarrete LP, Farias G, Reyes P, Maccioni RB. Insulin resistance and Alzheimer’s disease: molecular links & clinical implications. Current Alzheimer research. 2008;5:438–447. doi: 10.2174/156720508785908919. [DOI] [PubMed] [Google Scholar]
- Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nature reviews Cardiology. 2010;7:686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes ND, Cooney GJ, Camilleri S, Chisholm DJ, Kraegen EW. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes. 1997;46:1768–1774. doi: 10.2337/diab.46.11.1768. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Miyazaki M, Matsuhisa M, Takano K, Nakatani Y, Hatazaki M, Tamatani T, Yamagata K, Miyagawa J, Kitao Y, Hori O, Yamasaki Y, Ogawa S. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54:657–663. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti ME, Kahn BB. Nutrient sensor links obesity with diabetes risk. Nature medicine. 2004;10:1049–1050. doi: 10.1038/nm1004-1049. [DOI] [PubMed] [Google Scholar]
- Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biological psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Goldberg JL, Russell JC, Sun XJ. Identification of enhanced serine kinase activity in insulin resistance. The Journal of biological chemistry. 1999;274:10625–10632. doi: 10.1074/jbc.274.15.10625. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Zhande R, Jetton TL, Zhou G, Sun XJ. In vivo phosphorylation of insulin receptor substrate 1 at serine 789 by a novel serine kinase in insulin-resistant rodents. The Journal of biological chemistry. 2002;277:26530–26539. doi: 10.1074/jbc.M201494200. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature reviews Molecular cell biology. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Ropelle ER, Flores MB, Cintra DE, Rocha GZ, Pauli JR, Morari J, de Souza CT, Moraes JC, Prada PO, Guadagnini D, Marin RM, Oliveira AG, Augusto TM, Carvalho HF, Velloso LA, Saad MJ, Carvalheira JB. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS biology. 2010:8. doi: 10.1371/journal.pbio.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Samali A, Fitzgerald U, Deegan S, Gupta S. Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. International journal of cell biology. 2010;2010:830307. doi: 10.1155/2010/830307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder M. Endoplasmic reticulum stress responses. Cellular and molecular life sciences : CMLS. 2008;65:862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims-Robinson C, Kim B, Rosko A, Feldman EL. How does diabetes accelerate Alzheimer disease pathology? Nature reviews. Neurology. 2010;6:551–559. doi: 10.1038/nrneurol.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims-Robinson C, Zhao S, Hur J, Feldman EL. Central nervous system endoplasmic reticulum stress in a murine model of type 2 diabetes. Diabetologia. 2012;55:2276–2284. doi: 10.1007/s00125-012-2573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. The Journal of cell biology. 2004;167:35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Yamamoto N, Shinoda J, Iwata M, Watanabe T. Characteristics of the stages of change in physical behavior of male workers suffering from impaired glucose tolerance. Sangyo eiseigaku zasshi = Journal of occupational health. 2011;53:153–161. doi: 10.1539/sangyoeisei.b10005. [DOI] [PubMed] [Google Scholar]
- Tschop M, Heiman ML. Rodent obesity models: an overview. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2001;109:307–319. doi: 10.1055/s-2001-17297. [DOI] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009;58:2376–2385. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- Williams TD, Chambers JB, Roberts LM, Henderson RP, Overton JM. Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clinical and experimental pharmacology & physiology. 2003;30:769–778. doi: 10.1046/j.1440-1681.2003.t01-1-03808.x. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochimica et biophysica acta. 2009;1792:482–496. doi: 10.1016/j.bbadis.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Li J, Chen Y, Wang JJ, Ratan R, Zhang SX. Activation of endoplasmic reticulum stress by hyperglycemia is essential for Muller cell-derived inflammatory cytokine production in diabetes. Diabetes. 2012;61:492–504. doi: 10.2337/db11-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]