Abstract
To gain further insights into the effect of elevated cysteine levels on energy metabolism and the possible mechanisms by which cysteine may have these effects, we conducted studies in cysteine dioxygenase (Cdo1)–null mice. Cysteine dioxygenase (CDO) catalyzes the first step of the major pathway for cysteine catabolism. When CDO is absent, tissue and plasma cysteine levels are elevated, resulting in enhanced flux of cysteine through desulfhydration reactions. When Cdo1-null mice were fed a high-fat diet, they gained more weight than their wild-type controls, regardless of whether the diet was supplemented with taurine. Cdo1-null mice had markedly lower leptin levels, higher feed intakes, and markedly higher abundance of hepatic stearoyl-CoA desaturase 1 (SCD1) compared to wild-type control mice, and these differences were not affected by the fat or taurine content of the diet. Thus, reported associations of elevated cysteine levels with greater weight gain and with elevated hepatic Scd1 expression holds in the Cdo1-null mouse model. Hepatic accumulation of acylcarnitines suggested impaired mitochondrial β-oxidation of fatty acids in Cdo1-null mice. The strong associations of elevated cysteine levels with excess H2S production and impairments in energy metabolism suggest that H2S signaling could be involved.
Keywords: cysteine, stearoyl-CoA desaturase, leptin, hydrogen sulfide, taurine
The association between plasma total cysteine (tCys) levels and obesity and insulin resistance has intrigued investigators over the past two decades. A strong association between tCys levels and body mass index (BMI), as well as between changes in tCys and changes in BMI or fat mass over a 6-year period, was identified in the Hordaland Homocysteine Study cohort by El-Khairy et al.1,2 and Elshorbagy et al.3 Elshorbagy et al. also observed a strong positive association of tCys and BMI and showed that the association was mediated by changes in fat mass but not by changes in lean mass.3 These associations have been validated by studies in other population groups. Associations of tCys with obesity and insulin resistance were reported for a group of Hispanic children and adolescents4 and for a cohort comprising 376 vascular disease cases and 478 controls from Ireland, Sweden, and Portugal.5 The strength of tCys as a determinant of BMI in the statistical models led Elshorbagy et al. to propose that tCys may be a causal factor for obesity, although a mechanism has not been established for such a relationship.6
Studies in rodent models have also suggested an association of cysteine status with BMI. The most convincing of these are studies of rodents fed methionine-restricted diets (i.e., low-methionine diets that are not supplemented with cysteine). In general, dietary methionine restriction in rodent models results in decreases in plasma tCys that are associated with decreased weight gain and/or fat mass, increased metabolic rate, and decreased plasma glucose and insulin concentrations.7–12 To address the question of whether the consequences of methionine restriction are mediated by decreased cysteine availability, Elshorbagy et al. fed Fischer-344 rats semi-purified diets in which protein was replaced by a mixture of amino acids to allow modification of methionine and cysteine content.13 The various diets were fed to the rats from age 4 weeks to age 16 weeks. Compared to rats fed a cysteine-supplemented, methionine-restricted diet (0.17% L-methionine + 0.5% L-cysteine) or a control diet (0.86% L-methionine), rats fed the methionine-restricted diet (0.17% L-methionine) had lower plasma tCys concentration, higher energy expenditure per gram of body weight, higher food intake per gram of body weight, lower weight gain, and lower fat-pad mass/body weight percentage (FM/BW%), as well as lower serum levels of insulin, leptin, and triglycerides and a higher serum level of adiponectin. These results indicated that the antiobesity effects of methionine restriction are mediated by the low cysteine levels that result from feeding low-methionine diets without supplemental cysteine.
A role of elevated hepatic stearoyl-CoA desaturase 1 (SCD1) in the mediation of the effects of cysteine on lipid metabolism has been suggested by several studies.12,14,15 Methionine-restricted rats show low levels of hepatic expression (mRNA and protein) of SCD1. They also show low SCD1 activity indices (16:1n-7/16:0; 18:1n-9/18:0) as calculated from the serum fatty acid profiles.15 As a follow-up on the association of cysteine levels with SCD1 expression and activity reported in rodent studies, Vinknes et al. examined the cross-sectional associations of plasma tCys with an index of SCD1 activity (i.e., 16:1n-7/16:0, which was estimated from fatty acid profiles of total plasma or serum lipids) in two independent cohorts of older adults who were part of the Hordaland Health Study (i.e., HUSK, Norway, n = 2021; and Hoorn, the Netherlands, n = 686).16 Of all of the sulfur-containing compounds that were measured, only tCys was positively associated with plasma SCD-16 index and with 16:1n-7 concentration in both populations after adjustment for other lipid, dietary, and lifestyle variables.
To gain further insight into the effect of elevated cysteine levels on adiposity and lipid metabolism and to help elucidate possible mechanisms by which cysteine may have these effects, we conducted a series of studies in cysteine dioxygenase (Cdo1)-null mice. Cysteine dioxygenase is the initial enzyme catalyzing the major pathway for cysteine catabolism. When this pathway is blocked, tissue and plasma cysteine levels are elevated owing to the block in cysteine degradation, but methionine status remains normal, making this model useful for evaluating metabolic effects of elevated cysteine levels.
Materials and methods
Animal procedures
Experimental procedures involving live animals were conducted with the approval of the Cornell University Institutional Animal Care and Use Committee (#2009-0138). C57BL/6 Cdo1+/− male and female mice were crossed to generate mice that were homozygous for the null Cdo1 gene (Cdo1−/−) as well as their wild-type (Cdo1+/+) and heterozygous (Cdo1+/−) littermates. Animals were genotyped at postnatal day 14 (PD14) by polymerase chain reaction (PCR) analysis of genomic DNA samples obtained from tail snips, as described previously.17 Female and male Cdo1+/+and Cdo1−/− mice were used in this study. Mice were housed in a conventional, non-barrier vivarium maintained at 23°C and 45–50% humidity, with a 10-h dark (8:00 PM to 6:00 AM) /14-h light cycle. Pups and their dam had unrestricted access to a semi-purified modified AIN-93G diet (basal diet) from birth until PD21, when mice were removed from their dams. After weaning, mice assigned to the same diet and of the same sex and genotype were housed together with 2–4 mice per cage. Cage assignments were made at the time of weaning to permit collection of feed-intake data during the period in which the experimental diets were fed, which began about 3 weeks later. All mice were maintained on the basal diet from weaning until the start of the experiment, and thereafter were fed either the basal diet, a high-fat diet (HiFat), or a high-fat, taurine-supplemented diet (HiFat+Tau). On average, animals started the assigned experimental diet at PD42 (6 weeks), with a range of PD38 to PD45. The mice were fed the assigned treatment diet for a total duration of 3.3 weeks, from PD38-45 to PD60-69, when the experiment was terminated and tissue and blood samples were collected
The experimental diets were modifications of the AIN-93G formulation18 and were prepared and pelleted by Dyets, Inc. The basal diet was modified to contain less supplemental cystine (1.5 g/kg diet) than the standard AIN93G diet. This lower-cystine diet is the diet we routinely use in our studies with Cdo1−/− mice.19 The high-fat diets were formulated by replacing cornstarch with lard and adjusting other components (including soybean oil, proteins, minerals, and vitamins) to maintain equivalent ratios of protein and micronutrients to total energy as were present in the basal diet. The percent of energy from fat was 16.8% for the basal diet and 41.6% for the two high-fat diets. The caloric densities were 3759 kcal/kg for the basal diet and 4433 kcal/kg for the high-fat diet. For the taurine-supplemented, high-fat diet, 5 g of taurine was added to each kg of diet.
Measurement of body weight and feed intake
Each mouse was individually weighed every 2–3 days, between 9:00 and 11:00 AM. Feed intake was determined by cage for 3- to 4-day intervals over the first two weeks of the 3.3-week treatment period by weighing feed remaining in the hopper plus fragments of pellets collected from the floor of the cage and subtracting the weight of the remaining food from the amount present in the hopper at the beginning of the 3–4 day interval. The feed intake per mouse or per gram mouse was calculated by taking the total feed consumed by the mice in a given cage and dividing it by the number of mice in that cage or by the body weight of all mice in the cage, respectively.
Oxymax/CLAMS metabolic trials
After 2 weeks of dietary treatment, at which time the mice were 8 weeks of age (PD52-59), mice were monitored for O2 uptake, CO2 production, and activity using the Oxymas/CLAMS (Comprehensive Lab Monitoring System) system manufactured by Columbus Instruments. Data were collected for three to five individual mice from each genotype/sex/dietary treatment group (total of 47 mice). Mouse weights were taken before their placement into the metabolic cages. Mice were placed in the cages 2 h before the start of the dark cycle for the first 24-h period and remained in the cages until after the start of the dark cycle of the fourth 24-h period. This allowed collection of a full 3-day (72 h) set of data for each mouse, with results being reported as the average for the 3 days. Gas meters were calibrated before the start of each trial. While in the CLAMS, mice had access to water and their assigned experimental diet, but the diet was provided in powder form as necessitated by the nature of the CLAMS feed hoppers. Although feed intake was measured during the time that mice were in the CLAMS, the data were not used because excess spillage, especially of the high-fat diet, resulted in unusable data. Activity measurements included X-axis motion (along the length of the cage) and Z-axis motion (along the vertical axis of the cage) that were measured by tracking the number of beam breaks. X-axis beams were spaced 1.27 cm apart, while Z-axis beams were 4.0 cm apart. Data were exported and analyzed according to the Windows Oxymax Software Manual (0246-104M) by Columbus Instruments. Energy expenditure and respiratory exchange ratio (RER) were calculated using the following equations:
Upon completion of the trial, the mice, along with any of their original cage mates that were not part of the subset monitored in the CLAMS, were transferred to new cages to mitigate any scent-based territorial effects.
DEXA body-composition analysis
The same mice that were monitored in the Oxymax/CLAMS were also analyzed for percentage of body fat as determined by dual-energy X-ray absorptiometry (DEXA). Mice were anesthetized using an intraperitoneal injection of tribromoethanol (2.5 g tribromoethanol and 5 ml 2-methyl-2-butanol per 200 mL water; 0.25 mg/g body weight). Body density was assessed in vivo using the Lunar PIXImus Densitometer (GE Medical Systems) coupled with the LUNAR PIXImus 2.10 software. The machine was calibrated with a standard phantom control mouse. After the DEXA, mice were returned to their cages, placed on a sheet of paper towel to prevent aspiration of bedding, and monitored until they had fully recovered from anesthesia. At the time of densitometry, mice were PD58–PD65 (~8.8 weeks of age) and had been on the experimental diet for ~20 days. This was performed 2 days after the same mice had been monitored in the Oxymax/CLAMS system.
Tissue collection and preparation
Blood and tissues of mice were harvested when mice were ~9.3 weeks of age (PD60–PD69) after they had been consuming their assigned experimental diet for a total of 3.3 weeks. Between 10:00 and 12:00 AM (i.e., 4–6 h after the end of the dark period), mice were euthanized with an overdose of isoflurane. Blood was drawn from the inferior vena cava and the liver was removed. Fecal pellets were also collected from the colon. The liver was flash-frozen in liquid nitrogen in duplicate screw-cap centrifuge tubes. Blood samples were maintained at 4 °C until a clot had formed so that samples could be centrifuged to collect serum. Liver, fecal pellets, and serum were stored at −80°C until analyses were performed.
Serum hormone, cytokine, and metabolite analysis
Equal volumes of serum from female mice in each genotype/treatment group were pooled to obtain two pooled samples per group (each containing serum from three mice). Frozen serum was shipped on dry ice to the National Mouse Phenotyping Center at the University of Massachusetts Medical Center for analysis. Serum was analyzed for hormones (insulin, leptin, and resistin) using Luminex-multiplex assays and mouse-specific EIA kits from ALPCO Diagnostics; for adiponectin and corticosterone by enzyme-linked immunosorbent assays (ELISAs); for lactate, cholesterol (total), triglyceride, lipase, creatinine, and urea/blood urea nitrogen (BUN) using a Cobas Clinical Chemistry Analyzer; for glucose using an Analox analyzer; and for non-esterified fatty acids by a colorimetric assay (Wako Diagnostics).
Liver taurine, cysteine, and methionine analyses
Liver concentrations of taurine, total cysteine, and methionine were determined as described previously.19
LC-MS analysis of liver metabolites
Frozen liver samples were homogenized in liquid nitrogen. To extract metabolites, a weighed amount of liver was homogenized in ice-cold 80% methanol/water (200 μL per 5 mg liver), followed by dilution with one additional volume of ice-cold 80% methanol/water (200 μL per 5 mg liver), vortexing, sitting on ice for 10 min, and centrifugation at 20,000 × g at 4 °C for 10 min. A 200 μL aliquot of the supernatant was transferred to a microcentrifuge tube and dried in a SpeedVac (Thermo Scientific). Dried extracts were stored at −80 °C until the liquid chromatography–mass spectrometry (LC-MS) analysis was done. For LC-MS, samples were reconstituted into water (30 μL water per 5 mg liver), diluted with an additional 30 μL acetonitrile/methanol (1:1, v/v), and centrifuged at 20,000 × g at 4 °C for 3 min; 4 μL of the final supernatant was injected into the LC-MS system.
The LC-MS system consisted of an Ultimate 3000 UHPLC (Dionex) coupled to a Q Exactive-Mass spectromer (QE-MS, Thermo Scientific). For analysis of acylcarnitines and related metabolites (i.e., carnitine, thiosulfate), hydrophilic interaction LC (HILIC) was run with an Xbridge amide column (100 × 2.1 mm i.d., 3.5 μm; Waters) as described previously.20 The QE-MS was equipped with a HESI probe. Relevant parameters were heater temperature, 120 °C; sheath gas, 30; auxiliary gas, 10; sweep gas, 3; and spray voltage, 3.6 kV for positive mode and 2.5 kV for negative mode. Capillary temperature was set at 320 °C, and S-lens was 55. The QE-MS scan range was 60–900 (m/z). The maximum injection time (max IT) was 200 ms. Automated gain control (AGC) was targeted at 3 × 106 ions. Peak extraction and peak area integration were performed as described by Liu et al.20
Protein assays and western blotting
Frozen liver samples were homogenized in four volumes of lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, and 0.5% Nonidet P-40) supplemented with 1× Complete Protease Inhibitor Cocktail (Roche) and 1× PhosSTOP phosphatase inhibitor (Roche). Homogenates were centrifuged at 18,000 × g for 20 min at 4 °C. Supernatants were collected and stored at −80°C. Protein was determined using the BCA Protein Assay Kit (Thermo Scientific/Pierce) using bovine serum albumin (BSA) as the standard.
For western blotting, aliquots of tissue supernatant containing 40 μg of total protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) (12%, w/v, polyacrylamide), and the protein bands were transferred onto a 0.45-μm Immobilon-FL PVDF membrane (Millipore Corp.). Membranes were treated with blocking buffer for near-infrared fluorescent westerns (LI-COR Biosciences) and then blotted for immunoreactive proteins. Sources and dilutions of primary antibodies were as follows: anti-SCD1, anti-ACC1, anti-FASN, and anti-PDH (1:1000; Cell Signaling); anti-GAPDH, anti-ACOX1, and anti-COX 4 (1:1000; ProteinTech); anti-CPT1a (1:750; Protein Tech); and anti-COX 5b (1:1000; MitoSciences). Infrared fluorescent dye-labeled secondary antibodies (IRDye, LI-COR Biosciences) were used to visualize and quantify the relative abundance of each protein. Protein abundances were normalized by GAPDH abundance. If multiple gels were used, protein abundances were also normalized by a pooled sample.
Liver and feces triglyceride determination
For determination of triglyceride content, liver tissue and lyophilized fecal samples were analyzed for triglyceride fat concentration using the Cayman Triglyceride Colorimetric Assay Kit (Cayman Chemical Company kit # 10010303).
Statistical analysis
Results are reported as the mean ± SEM for each experimental group. Statistical analyses was performed using JMP version 10 (SAS). Data were analyzed separately for male and female mice using a least-squares model to determine main effects (genotype and dietary treatment) and their interaction. Post hoc individual pairwise comparisons of least-squares means in the model were done using Tukey’s comparison, with differences considered significant at P ≤ 0.05.
Results
Because the goal of this study was to investigate the effects of a lack of CDO and the associated elevated cysteine levels and their effects on energy metabolism, we designed the study to minimize the confounding effects of changes in body size and composition as well as to control for the possible confounding effects of taurine depletion in the Cdo1−/− mice. Cdo1−/− and wild-type mice were the offspring of heterozygous parents. Cdo1−/− pups are protected from taurine depletion and to some extent excess cysteine levels during gestation by the relatively normal cysteine metabolism of the Cdo1+/− dam and are partially protected from taurine depletion during suckling by provision of taurine in the dam’s milk.17 From weaning, mice consumed a basal diet that was based on the AIN93G formulation up until 42 days of age; at 6 weeks, pups were assigned to either the basal, high-fat (HiFat), or high-fat plus taurine (HiFat+Tau) diet for a treatment period of 23 days. The short treatment period and use of young mice was a choice made to minimize potential confounding of the results by long-term adverse effects of dietary treatment or genotype and to allow us to more clearly assess changes that are an early or primary consequence of the Cdo1−/− phenotype.
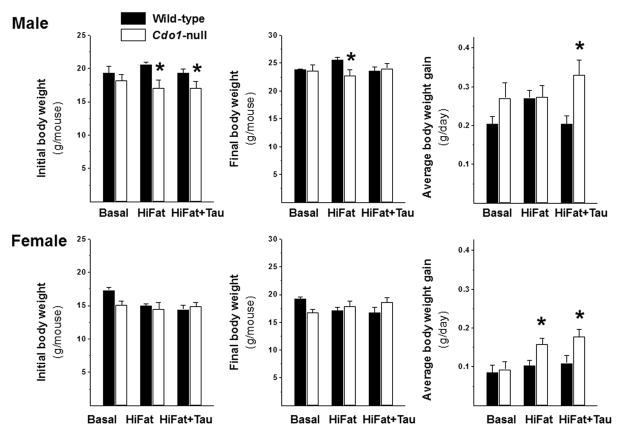
As has been reported previously, young Cdo1−/− mice were smaller than wild-type mice. At the beginning of the dietary treatment period, when mice were ~ 6 weeks of age, the body weights of Cdo1−/− mice were significantly lower (P < 0.05) than those of wild-type mice (17.5 ± 1.9 vs. 19.6 ± 2.0 for male mice and 14.7 ± 1.6 vs. 15.8 ± 1.8 for female mice). However, after random assignment to dietary treatment groups and with the smaller sample size, the initial weights of wild-type and null mice in a given group were significantly different only for male mice fed the HiFat and HiFat+Tau diets, as shown in Figure 1. In contrast, by the end of the dietary treatment period, there was no overall difference in body weights of Cdo1−/− and wild-type mice (P > 0.05), although there was some significant interaction of genotype and diet (P = 0.07), with weights of null male mice fed the HiFat diet remaining lower than those of the wild-type controls. Neither initial nor final body weights were different for female wild-type and null mice in any of the dietary treatments. However, when the gain in body weight over the 3-week period was examined, there was a clear effect of genotype for male mice (P = 0.0020) and a borderline effect for female mice (P = 0.08) with Cdo1−/− mice gaining more weight between weeks 6 and 9 than did the wild-type mice, suggesting that null mice may be experiencing “catch-up” growth during this time period. When the effect of genotype was examined for comparisons of the individual sex/diet groups, the rate of body weight gain was significantly greater only for female null mice fed the HiFat diet and for both male and female mice fed the HiFat+Tau diet.
Figure 1.
Initial and final body weights and body weight gain of male and female wild-type and Cdo1−/− mice fed the basal, HiFat, or HiFat+Tau diet for 3.3 weeks. Each bar represents the mean ± SEM for the indicated group of mice; n = 6–10 mice per group. Bars denoted by * indicate that the value for Cdo1−/− mice is different from the value for wild-type mice of the same sex and dietary treatment.
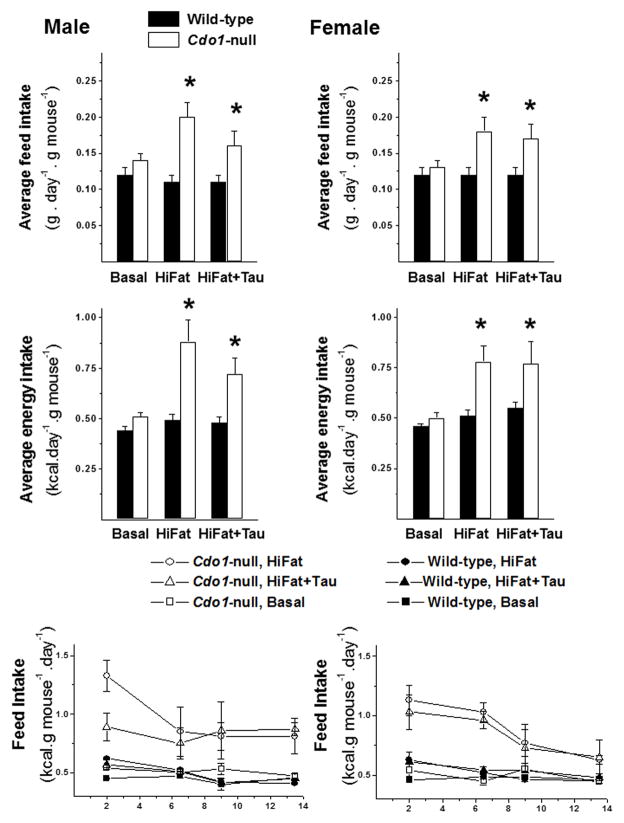
Feed intake of Cdo1−/− mice was significantly higher than that of wild-type mice when mice were fed a high-fat diet (HiFat or HiFat+Tau), as shown in Figure 2. In the male mice fed the HiFat+Tau diet and in female mice fed either the HiFat or HiFat+Tau diet, feed intake was associated with greater weight gain, but this was not true for male null mice fed the HiFat diet, as this group of mice did not exhibit a greater weight gain than wild-type mice that consumed less feed. When energy intake from the diet is plotted as a function of days on the treatment diets, it is clear that wild-type mice immediately adjusted the weight of feed consumed to maintain constant energy intake when they were switched from the basal to the HiFat or HiFat+Tau diets, whereas the Cdo1−/− mice did not initially adjust feed intake during the first few days and were still consuming more energy than their wild-type counterparts during the second week of feeding. This observation suggests that Cdo1−/− mice may have some impairment in their ability to regulate energy balance. Feed intake was not measured during the third week of dietary treatment owing to removal of mice from their cages for calorimetry and activity measurements in CLAMS and for body composition measurements by DEXA.
Figure 2.
Feed intakes and energy intakes of male and female wild-type and Cdo1−/− mice fed the basal, HiFat, or HiFat+Tau diet. Intake data are reported for only the first 2 weeks of the total dietary treatment period owing to movement of mice in and out of cages for CLAMS and DEXA analyses during the final week of treatment. Each bar represents the mean ± SEM for the indicated group of mice; n = average intake of mice in three cages per group. Bars denoted by * indicate that the value for Cdo1−/− mice is different from the value for wild-type mice of same sex and dietary treatment. Line graphs show the average energy intake of mice over the course of food intake–data collection; data points are plotted at the mid-point of each 3- or 4-day interval during which feed intake was measured during the continuous 2-week collection of feed intake data.
Energy expenditure was measured using indirect calorimetry over a period of 3 days. The average daily energy expenditure per gram of mouse is reported in Table 1. Data are shown separately for male and female mice. Energy expenditure was not affected by genotype but was significantly affected by diet in both male (P = 0.0012) and female (P = 0.0166) mice, with energy expenditure tending to be highest in mice fed the HiFat diet. Taurine supplementation of the high-fat diet tended to shift energy expenditure values back toward basal levels. Respiratory exchange ratios (RERs) are also reported and are lower for mice fed the high-fat diets, particularly during the dark period when mice do most of their feeding. This finding is consistent with the higher fat/lower carbohydrate content of the feed. Activity was measured, but none of the activity measurements showed any differences among treatment groups (data not shown).
Table 1.
Energy intake and energy expenditure of wild-type and Cdo1−/− mice fed a basal, high-fat (HiFat), or high-fat plus taurine (HiFat+Tau) diet.
| Genotype–diet groups | Significance level for main effects and 2-way interaction as analyzed by a general linear model† | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild type basal | Cdo1−/− basal | Wild type HiFat | Cdo1−/− HiFat | Wild type HiFat+Tau | Cdo1−/− HiFat+Tau | Genotype | Diet | GxD | |
| Male mice | |||||||||
| Daily energy intake | |||||||||
| Energy intake (kcal/g mouse) 2-week average | 0.44 ± 0.02b | 0.51 ± 0.02b | 0.49 ± 0.03b | 0.88 ± 0.11a | 0.48 ± 0.03b | 0.72 ± 0.0a | P < 0.0001 | P = 0.0016 | P = 0.0073 |
| Energy intake (kcal/g mouse) first week average | 0.46 ± 0.01c | 0.52 ± 0.01c | 0.57 ± 0.01c | 1.10 ± 0.01a | 0.54 ± 0.01c | 0.86 ± 0.10b | P < 0.0001 | P < 0.0001 | P = 0.0035 |
| Energy intake (kcal/g mouse) second week average | 0.43 ± 0.04b | 0.50 ± 0.01b | 0.42 ± 0.01b | 0.74 ± 0.08a | 0.43 ± 0.03b | 0.82 ± 0.1a | P = 0.0004 | P = 0.0823 | P = 0.0707 |
| Daily energy expenditure | |||||||||
| kcal/g mouse (average for days 15–17) | 0.39 ± 0.01b | 0.41 ± 0.02b | 0.44 ± 0.02a,b | 0.49 ± 0.01a | 0.42 ± 0.01b | 0.41 ± 0.01b | P = 0.0012 | ||
| Average daily energy balance | |||||||||
| EE-EI (2-week average intake) (kcal/g mouse/day) | +0.05 | +0.10 | +0.05 | +0.39 | +0.06 | +0.31 | |||
| EE-EI (second week average intake) (kcal/g mouse/day) | +0.04 | +0.09 | –0.02 | +0.35 | +0.01 | +0.41 | |||
| Respiratory exchange ration (RER) | |||||||||
| Average RER during dark periods | 1.04 ± 0.03a | 1.02 ± 0.03a | 0.92 ± 0.01b | 0.91 ± 0.02b | 0.91 ± 0.01b | 0.91 ± 0.01b | P < 0.0001 | ||
| Average RER during light periods | 0.96± 0.01a | 0.91 ± 0.01b | 0.90 ± 0.01b,c | 0.86 ± 0.01c | 0.88 ± 0.01b,c | 0.88± 0.02b,c | P=0.0148 | P = 0.0005 | P = 0.0758 |
| Average RER for total 3 days | 0.99 ± 0.02a | 0.95 ± 0.01b | 0.91 ± 0.01c | 0.88 ± 0.01c | 0.89 ± 0.01c | 0.90 ± 0.01c | P < 0.0001 | ||
| Female mice | |||||||||
| Daily energy intake | |||||||||
| Energy intake (kcal/g mouse) 2-week average | 0.47 ± 0.01b | 0.50 ± 0.03b | 0.51 ± 0.03b | 0.78 ± 0.08a | 0.55 ± 0.03b | 0.77 ± 0.11a | P < 0.0001 | P = 0.0009 | P = 0.0072 |
| Energy intake (kcal/g mouse) first week average | 0.47 ± 0.01b | 0.50 ± 0.02b | 0.58 ± 0.02b | 1.02 ± 0.09a | 0.57 ± 0.01b | 0.99 ± 0.11a | P < 0.0001 | P < 0.0001 | P = 0.0037 |
| Energy intake (kcal/g mouse) second week average | 0.47 ± 0.01b | 0.49 ± 0.01b | 0.45 ± 0.01b | 0.67 ± 0.04a | 0.51 ± 0.03b | 0.69 ± 0.03a | P < 0.0001 | P = 0.0049 | P = 0.0059 |
| Daily energy expenditure | |||||||||
| kcal/g mouse (average for days 15–17) | 0.431 ± 0.021 | 0.454 ± 0.007 | 0.530 ± 0.003 | 0.499 ± 0.014 | 0.439 ± 0.036 | 0.483 ± 0.026 | P = 0.0166 | ||
| Average daily energy balance | |||||||||
| EE-EI (2-week average intake) (kcal/g mouse/day) | –0.00 | +0.05 | −0.02 | +0.28 | +0.11 | +0.29 | |||
| EE-EI (second week average intake) (kcal/g mouse/day) | –0.00 | +0.04 | −0.08 | +0.17 | +0.07 | +0.21 | |||
| RER | |||||||||
| Average RER during dark periods | 1.01 ± 0.02a | 1.03 ± 0.02a | 0.89 ± 0.01b | 0.89 ± 0.01b | 0.89 ± 0.01b | 0.93 ± 0.01b | P < 0.0001 | ||
| Average RER during light periods | 0.93 ± 0.02a | 0.92 ± 0.01a | 0.87 ± 0.01a,b,c | 0.73 ± 0.07c | 0.89 ± 0.02a,b | 0.76 ± 0.09b,c | P = 0.0228 | P = 0.0236 | |
| Average RER for total 3 days | 0.96 ± 0.01a | 0.96 ± 0.02a | 0.88 ± 0.01b | 0.80 ± 0.04b | 0.89 ± 0.02a,b | 0.83 ± 0.06b | P = 0.0005 | ||
Data are expressed as means ± SEM.
Columns on the right give P values for the main effects and their interaction as analyzed by a general linear model for the two categorical variables and their interaction using JMP, version 11 (SAS). Values not followed by the same superscript letter are significantly different at P < 0.05 as determined by the Tukey’s post hoc comparison test.
Table 1 also contains a summary of energy intake, calculated both as the average for the entire 2-week period during which feed intake was measured and for each week separately. Regardless of the period considered, there was no difference in energy intake of wild-type and Cdo1−/− mice fed the basal diet. However, Cdo1−/− mice fed the HiFat or HiFat+Tau diet consistently consumed significantly more energy than the wild-type control mice. These values for energy intake were used, along with the energy expenditure values measured during the third week of the study period, to estimate energy balance. Regardless of whether the 2-week average or the 1-week average for the second week was used as the measure of energy intake, it is clear that the Cdo1−/− mice consumed more energy than they expended when fed either the HiFat or the HiFat+Tau diet. This is consistent with their greater gains in body weight.
Thus, at the end of the 3-week dietary treatment period, Cdo1−/− mice were similar in size to their wild-type controls, making this a good end point for examining early metabolic changes in Cdo1−/− mice. Although an excess energy balance would be expected to result in changes in body composition, assessment of body fat content by DEXA and assay of liver triglyceride content did not show any effects of genotype or diet. Percent body fat was 24 ± 1% for male mice and 26 ± 2% for female mice. Hepatic triglyceride content was 40 ± 5 μg/mg protein for male mice and 59 ± 7 μg/mg protein for female mice, and hepatic protein content was 162 ± 12 mg/g liver for male mice and 185 ± 8 mg/g liver for female mice. Thus, there were no dramatic differences in body composition or liver composition between wild-type and Cdo1−/− mice at the time tissue samples were collected. In addition, no triglyceride was detected in the fecal pellets collected from the colons of mice, indicating that there was no fat malabsorption in either wild-type or Cdo1−/− mice.
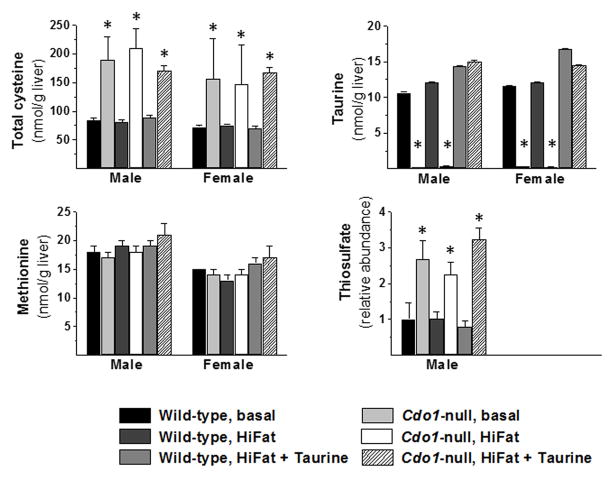
Total cysteine, methionine, and taurine levels in liver are shown in Figure 3. Cdo1−/− mice had elevated hepatic total cysteine levels that ranged from 1.9- to 2.6-fold those of wild-type mice in the same diet/sex group. Hepatic taurine levels in Cdo1−/− mice fed the taurine-free basal or HiFat diet were 2–3% of wild-type levels, but hepatic taurine levels in Cdo1−/− mice fed the HiFat+Tau diet had taurine levels that were similar to wild-type levels, with mean levels being 105% and 87% of wild-type levels for male and female mice, respectively. Hepatic methionine levels in Cdo1−/− mice were not significantly different, ranging from 93–108% of wild-type levels. Also shown in Figure 3 are results of the metabolomics analysis for thiosulfate, which was done only for male mice. Liver thiosulfate was elevated in Cdo1−/− mice, being 2.2- to 3.2-fold the values for wild-type controls. Elevated thiosulfate levels in Cdo1−/− mice have been reported previously19, 21 and are related to the excess flux of cysteine through desulfhydration pathways in the Cdo1−/− mouse.
Figure 3.
Total cysteine, taurine, methionine, and thiosulfate levels in livers of wild-type and Cdo1-null mice fed the basal, HiFat, or HiFat+Tau diet for 3.3 weeks. Each bar represents the mean ± SEM for the indicated group of mice; n = 6–10 mice per group. Bars denoted by * indicate that the value for Cdo1−/− mice is different from the value for wild-type mice of the same sex and dietary treatment.
Pooled serum samples (i.e., two samples per group, each containing an equal volume of serum from three individual mice from that group) were used to assay various hormone and metabolite levels in serum. This was done to contain costs but results in our not being able to make any pairwise comparisons of the results shown in Table 2. Nevertheless, there was an overall effect of genotype on insulin, leptin, and triglyceride levels, with Cdo1−/− mice having higher insulin levels (an average of 160% of wild type), lower leptin levels (an average of 62% of wild type) and higher triglyceride levels (an average of 120% of wild type). Diet also influenced the leptin levels, with leptin levels being higher in mice fed the high-fat diets than in mice fed the basal diet (an average of 170% of those for mice fed the basal diet).
Table 2.
Levels of hormones and metabolites in serum of male wild-type and Cdo1−/− mice fed basal, high-fat, or taurine-supplemented high-fat diets.
| Metabolites | Genotype–diet groups Relative abundance of metabolites |
Significance level for main effects and 2-way interactions as analyzed by a general linear model F(11,30)† |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||||||||||||
| Wild type basal |
Cdo1−/− basal |
Wild type HiFat |
Cdo1−/− HiFat |
Wild type HiFat+ Tau |
Cdo1−/− HiFat+ Tau |
Wild type basal |
Cdo1−/− basal |
Wild type HiFat |
Cdo1−/− HiFat |
Wild type HiFat+ Tau |
Cdo1−/− HiFat+ Tau |
G | D | S | G*D | G*S | D*S | |
| Insulin (ng/mL) | 0.92 | 1.60 | 1.90 | 1.58 | 0.88 | 1.27 | 0.37 | 0.70 | 0.42 | 0.64 | 0.42 | 0.93 | 0.032 | < 0.0001 | 0.056 | |||
| Leptin (ng/mL) | 1.55 | 1.09 | 2.08 | 1.10 | 3.43 | 1.20 | 1.12 | 0.80 | 3.49 | 1.23 | 2.04 | 2.17 | 0.003 | 0.013 | ||||
| Resistin (ng/mL) | 19.7 | 20.4 | 20.0 | 15.8 | 20.9 | 16.1 | 18.1 | 22.9 | 22.6 | 27.5 | 18.7 | 23.1 | 0.036 | 0.022 | ||||
| Adiponectin (μg/mL) | 10.0 | 12.8 | 9.03 | 9.24 | 14.1 | 9.64 | 16.0 | 12.7 | 14.2 | 9.73 | 15.6 | 13.0 | 0.032 | |||||
| NEFA (μM) | 469 | 527 | 384 | 525 | 479 | 505 | 533 | 460 | 503 | 488 | 477 | 499 | 0.012 | |||||
| Corticosterone (ng/mL) | 108 | 126 | 108 | 62.8 | 107 | 118 | 173 | 165 | 105 | 58.7 | 163 | 168 | 0.009 | 0.039 | ||||
| Lactate (mmol/L) | 9.88 | 10.5 | 10.0 | 10.3 | 9.30 | 9.09 | 8.34 | 9.65 | 8.10 | 8.73 | 6.09 | 9.36 | 0.007 | |||||
| Triglyceride (mg/dL) | 112 | 135 | 127 | 140 | 106 | 94.5 | 68.5 | 99.0 | 60.0 | 85.5 | 67.5 | 76.5 | 0.028 | < 0.0001 | ||||
| Total Cholesterol (mg/dL) | 102 | 81.5 | 120 | 109 | 122 | 105 | 67.5 | 65.5 | 83.5 | 82.5 | 81.0 | 104 | 0.002 | < 0.0001 | 0.026 | |||
| Lipase (U/L) | 23.0 | 118 | 93.0 | 20.0 | 24.5 | 20.0 | 22.5 | 15.5 | 19.0 | 19.5 | 19.5 | 22.0 | ||||||
| Creatinine (μmol/L) | 13.2 | 12.5 | 12.2 | 14.3 | 15.0 | 10.5 | 12.0 | 13.4 | 12.0 | 11.4 | 15.0 | 12.0 | ||||||
| Urea/BUN (mg/dL) | 22.0 | 27.5 | 24.5 | 22.0 | 27.0 | 20.5 | 20.5 | 24.5 | 21.5 | 25.0 | 22.0 | 21.5 | 0.006 | 0.062 | ||||
| Glucose (mg/dL) | 545 | 541 | 530 | 601 | 564 | 623 | 593 | 478 | 440 | 591 | 636 | 580 | 0.027 | |||||
Data are the average value for two pooled samples for each group, with each pooled sample taken from three or four mice. Standard deviation values across all groups were as follows: insulin = 0.55; leptin = 0.90; resistin = 4.16; adiponectin = 3.19; NEFA = 47.79; corticosterone = 47.35; lactate = 1.45; triglyceride = 28.97; total cholesterol = 20.27; lipase = 47.23; creatinine = 2.28; urea/BUN = 2.73; Glucose=73.04. Data were analyzed for main effects and their interactions by a full-factorial three-way general linear model using JMP version 10 (SAS).
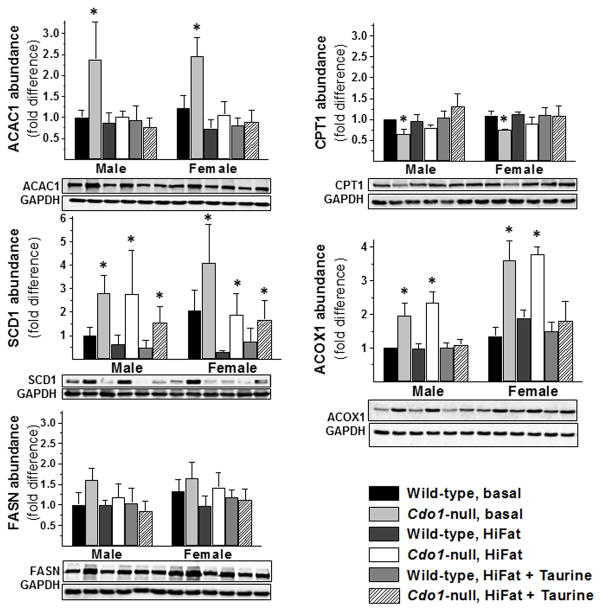
The abundances of several proteins involved in fatty acid metabolism are shown in Figure 4. Of the proteins we screened, the only one that was significantly different in Cdo1−/− mice compared to wild-type mice for all dietary treatment/sex pairwise comparisons was stearoyl CoA desaturase 1 (SCD1). SCD1 was elevated 2- to 3-fold over wild-type levels in livers of Cdo1−/− mice fed the basal or HiFat+Tau diets and 4- to 6-fold over wild-type levels in livers of Cdo1−/− mice fed the HiFat diet. Thus, an elevated abundance of hepatic SCD1 seems to be an early and consistent difference in livers of Cdo1−/− mice compared to wild-type mice.
Figure 4.
Relative abundance of ACAC1, CPT1, SCD1, ACOX1, and FASN in livers of wild-type and Cdo1−/− mice fed the basal, HiFat, or HiFat+Tau diet for 3.3 weeks. Each bar represents the mean ± SEM for the indicated group of mice; n = 6–10 mice per group. Bars denoted by * indicate that the value for Cdo1−/− mice is different from the value for wild-type mice of the same sex and dietary treatment. Representative western blots are shown below the bar graphs for protein abundances.
Acetyl-CoA carboxylase 1 (ACAC1) abundance was elevated and carnitine palmitoyltransferase 1 (CPT1) abundance was low in Cdo1−/− mice fed the basal diet, but both were the same as levels in wild-type mice fed either high-fat diet. This difference would be consistent with favored use of fatty acids for triglyceride synthesis instead of for β-oxidation in the livers of Cdo1−/− mice, but because excess weight gain and energy balance were not observed in Cdo1−/− mice fed the basal diet. Rather, excess weight gain and energy balance were observed in Cdo1−/− mice fed the high-fat diets, in which ACAC1 and CPT1 abundances were not different from wild-type levels. The abundance of acyl-CoA oxidase 1 (ACOX1), a peroxisomal enzyme involved in β-oxidation, was higher in Cdo1−/− mice fed either the basal or HiFat diet compared to wild-type mice fed the same diet, but this difference in ACOX1 abundance was prevented by taurine supplementation. Differences in ACAC1 abundance are unlikely to play a major role in the excess weight gain and energy balance of Cdo1−/− mice, however, because taurine supplementation had no significant effect on weight gain or energy intake of Cdo1−/− mice. Fatty acid synthase (FASN) abundance was not affected by either genotype or dietary treatment.
In the metabolomics profiles run on the liver samples collected from the male mice in each of the genotype/diet groups, a strong effect of genotype on liver acylcarnitine levels was observed. As shown in Table 3, the carnitine esters of C16, C16:1, C17:1, C18, C18:1, and C18:2 were significantly higher in Cdo1−/− mice for all three diet comparison groups, whereas the C3-carnitine levels were significantly lower. Free carnitine levels were not affected by genotype or diet, so there was no evidence of carnitine depletion as a consequence of acylcarnitine accumulation. Levels of most of the shorter acylcarnitines, including C14, C14:1, C10, C10:1, C8, H-C5, and OH-C4, were also significantly higher in Cdo1−/− mice than in wild-type mice when all diet groups were combined, but differences were not significant for all three of the diet group pairwise comparisons. Overall, the elevated acylcarnitine levels suggest a possible impairment in the process of mitochondrial β-oxidation and are consistent with the previously observed elevated blood acylcarnitine levels in Cdo1−/− mice.17 It should be noted that, although SCD1 abundance was consistently higher in livers of Cdo1−/− mice, there was no indication that the relative abundance of C16:1 to C16 or of C18:1 to C18 acylcarnitines was different for Cdo1−/− and wild-type mice. Rather, the fold differences were similar for both the saturated and monounsaturated forms of C16 and C18 fatty acids.
Table 3.
Fold differences in acyl carnitines and carnitine in liver of male wild-type and Cdo1−/− mice fed basal, high-fat, or taurine-supplemented high-fat diets.
| Metabolites | Genotype–diet groups Relative abundance of metabolites | Significance level for main effects and 2-way interaction as analyzed by a general linear model F(5,24)† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild type basal | Cdo1−/− basal | Wild type HiFat | Cdo1−/− HiFat | Wild type HiFat+Tau | Cdo1−/− HiFat+Tau | Genotype | Diet | GxD | |
| Propionyl carnitine | 1 ± 0.37a*,†,§ | 0.39 ± 0.04b | 0.81 ± 0.09a | 0.37 ± 0.03b | 0.85 ± 0.11a | 0.62 ± 0.10b | P = 0.0001 | ||
| Butyryl carnitine | 1 ± 0.24a,b | 1.11 ± 0.09a | 0.58 ± 0.08c | 0.63 ± 0.06c | 0.45 ± 0.08c | 0.72 ± 0.08b,c | P = 0.0011 | ||
| Succinyl carnitine | 1 ± 0.35 | 0.68 ± 0.09 | 1.19 ± 0.20 | 0.93 ± 0.16 | 0.97 ± 0.20 | 1.32 ± 0.37 | |||
| Hydroxybutanoyl (OH-C4) carnitine | 1 ± 0.09c | 1.63 ± 0.24b | 0.84± 0.06c | 2.51 ± 0.25a | 0.88 ± 0.14c | 1.46 ± 0.33b,c | P < 0.0001 | P = 0.0246 | |
| Glutaryl carnitine (Lys, Trp) | 1 ± 0.31 | 1.86 ± 0.27 | 1.54 ± 0.21 | 2.11 ± 0.40 | 1.10 ± 0.09 | 1.59 ± 0.48 | |||
| Methylcrotonyl carnitine (Ile) | 1 ± 0.39 | 0.50 ± 0.09 | 0.84± 0.11 | 0.77 ± 0.14 | 0.77 ± 0.11 | 0.98 ± 0.19 | |||
| Hydroxypentanoyl (OH-C5)/3-hydroxy-isovaleryl carnitine (Leu) | 1 ± 0.07c | 1.43 ± 0.08a | 0.58± 0.08b,c | 1.40 ± 0.09a,b | 0.45 ± 0.07c | 1.19 ± 0.15a,b,c | P = 0.0019 | ||
| Octanoyl (C8) carnitine | 1 ± 0.22b,c,d,* | 1.89 ± 0.26a | 0.59± 0.13c,d | 1.08 ± 0.20b,c | 0.97 ± 0.11d | 1.23 ± 0.17b | P < 0.0001 | P = 0.0047 | |
| Octenoyl (C8:1) carnitine | 1 ± 0.37 | 1.18 ± 0.18 | 0.74 ± 0.07 | 0.84 ± 0.08 | 0.84 ± 0.09 | 1.16 ± 0.26 | |||
| Decanoyl (C10) carnitine | 1 ± 0.18 | 2.09 ± 0.49 | 0.81 ± 0.11 | 1.39 ± 0.26 | 0.87 ± 0.14 | 1.93 ± 0.50 | |||
| Decenoyl (C10:1) carnitine | 1 ± 0.17b | 2.12 ± 0.44a | 1.09± 0.18b | 1.62 ± 0.17a,b | 1.08 ± 0.21b | 1.76 ± 0.41a,b | P = 0.0011 | ||
| Tetradecanoyl (C14) carnitine | 1 ± 0.06b | 1.72 ± 0.18a | 0.99± 0.11b | 1.26 ± 0.09a,b | 0.96 ± 0.12b | 1.85 ± 0.42a | P = 0.0004 | ||
| Tetradecenoyl (C14:1) carnitine | 1 ± 0.08b | 2.08 ± 0.29a | 1.04 ± 0.22b | 1.62 ± 0.16a,b | 1.29 ± 0.22a,b | 2.77 ± 1.26a | P = 0.0042 | ||
| Palmitoyl (C16) carnitine | 1 ± 0.14c | 1.95 ± 0.52a | 1.11 ± 0.11c | 1.83 ± 0.20a,b | 1.27 ± 0.17b,c | 2.58 ± 0.55a | P < 0.0001 | ||
| Hexadecenoyl (C16:1) carnitine | 1 ± 0.11b | 2.44 ± 0.11a | 0.94 ± 0.17b | 1.86 ± 0.16a | 0.97 ± 0.15b | 2.89 ± 1.24a | P < 0.0001 | ||
| Hydroxyhexadecanoyl (OH-C16) carnitine | 1 ± 0.11d | 2.51 ± 0.50a | 1.30 ± 0.15b,c,d | 1.90 ± 0.16a,b,c | 1.28 ± 0.25c,d | 2.85 ± 0.96a,b | P = 0.0007 | ||
| Hydroxyhexadecenoyl (OH-C16:1) carnitine | 1 ± 0.18d | 2.76 ± 0.47a | 1.09 ± 0.19c,d | 2.22 ± 0.29a,b | 1.09 ± 0.18b,c,d | 3.36 ± 1.73a,b,c | P = 0.0006 | ||
| Heptadecanoyl (C17:1) carnitine | 1 ± 0.12b | 3.46 ± 0.86a | 1.46 ± 0.21b | 2.56 ± 0.31a | 1.29 ± 0.24b | 2.71 ± 0.54a | P < 0.0001 | ||
| Stearoyl (C18) carnitine | 1 ± 0.20c | 3.33 ± 0.47a | 1.76 ± 0.22b | 2.90 ± 0.26a | 1.89 ± 0.30b | 3.16 ± 0.51a | P < 0.0001 | P = 0.0368 | |
| Oleoly (C18:1)carnitine/ Elaidic carnitine/Vaccenyl carnitine | 1 ± 0.17b | 2.93 ± 0.51a | 1.22 ± 0.21b | 2.94 ± 0.45a | 1.31 ± 0.20b | 4.19 ± 1.42a | P < 0.0001 | ||
| Linoleyl (C18:2) carnitine/Linoelaidyl carnitine | 1 ± 0.13d | 2.16 ± 0.37a,b,c | 1.56 ± 0.31c,d | 2.55 ± 0.35a,b | 1.69 ± 0.15b,c | 3.57 ± 1.13a | P = 0.0002 | P = 0.0486 | |
| Carnitine | 1 ± 0.09 | 0.92 ± 0.07 | 1.01 ± 0.03 | 0.98 ± 0.06 | 0.99 ± 0.07 | 0.97 ± 0.08 | |||
All values for metabolite concentrations are expressed relative to the average value for the wild-type/basal diet group, which was set as 1.0. Data are expressed as means ± SEM.
All values were transformed to log10 values before statistical analysis. Columns on the right give P values for the main effects and their interaction as analyzed by a general linear model for the two categorical variables and their interaction.
Values not followed by the same superscript letter are significantly different at P < 0.05 as determined by the Tukey’s post hoc comparison test. Metabolites indicated by shaded rows were significantly higher in Cdo1−/− mice than in wild-type mice for all three diet groups by Tukey’s post hoc test.
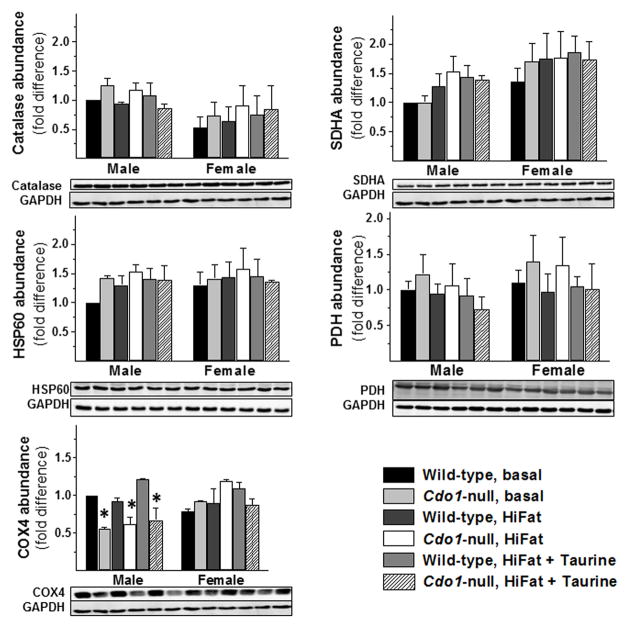
Because excess hydrogen sulfide (H2S) production or accumulation has been shown to impair mitochondrial cytochrome oxidase activity, short-chain fatty acid oxidation, and branched-chain amino acid oxidation in several different experimental models,17,19,22–24 we examined cytochrome c oxidase subunit abundance, mitochondrial and peroxisomal marker protein abundances, and the abundance of enzymes involved in fatty acid metabolism in the liver tissues collected in this study. As shown in Figure 5, neither peroxisomal nor mitochondrial density in mouse liver appeared to be affected by loss of CDO, on the basis of the absence of any significant differences in the abundance of catalase (peroxisomal enzyme) or in the abundances of mitochondrial marker proteins, succinate dehydrogenase subunit A, heat shock protein 60, and pyruvate dehydrogenase catalytic subunit. H2S toxicity inhibits cytochrome c oxidase (COX) and promotes increased degradation of some of its subunits. Loss of COX4 and COX5b subunits was demonstrated in Cdo1−/− mice fed diets enriched in sulfur amino acids (200 g casein + 1.5 g cystine + 4.2 g methionine).19 In the current study, in which mice were fed a diet slightly restricted in sulfur amino acids (200 g casein + 1.5 g cysteine/kg) compared to the standard AIN93G formulation (200 g casein + 3.0 g cysteine/kg), loss of COX4 subunits in liver was observed in the male mice but not in the female mice. COX5b abundance was not significantly affected in either male or female mice (data not shown). Nevertheless, the low COX4 subunit abundance in male Cdo1−/− mice, as well as the elevated hepatic levels of thiosulfate, suggests that, despite the lower sulfur amino acid content of the diet used in this study, Cdo1−/− mice were still being exposed to elevated rates of H2S production.
Figure 5.
Relative abundance of catalase, SDHA, HSP60, PDH, and COX4 in livers of wild-type and Cdo1−/− mice fed the basal, HiFat, or HiFat+Tau diet for 3.3 weeks. Each bar represents the mean ± SEM for the indicated group of mice; n = 6–10 mice per group. Bars denoted by * indicate that the value for Cdo1−/− mice is different from the value for wild-type mice of the same sex and dietary treatment. Representative western blots are shown below the bar graphs for protein abundances.
Discussion
The purpose of this study with Cdo1−/− mice was to investigate early metabolic changes that might underlie the association of elevated cysteine levels with elevated Scd1 expression and obesity. In Cdo1−/− mice, cysteine levels become elevated postnatally at the same time taurine is undergoing depletion. However, dietary taurine supplementation restored tissue taurine levels, permitting the investigation of elevated cysteine levels without the complication of low taurine levels. The Cdo1−/− mice consumed more energy but had similar levels of activity and energy expenditure as wild-type mice. The effect of genotype on energy intake was most pronounced when the mice were switched from a basal diet to a high-fat diet, consistent with the well-established associations of dietary energy density and obesity in both rodents and humans.25,26
When mice were fed the high-fat diet (40% of energy as fat), the Cdo1−/− mice exhibited significantly elevated feed intakes, weight gains, and energy balances. In particular, the feed intakes of mice were dramatically elevated during the first 2–6 days after they were switched from the basal to the high-fat diet. Intakes remained elevated during the second week on the high-fat diet, although intakes were lower than they were during the first week. Together, these results indicate that Cdo1−/− mice have an impaired ability to compensate for the increased caloric density of the diet and consume excess energy when fed a high-fat diet.
The observation of markedly reduced leptin levels in Cdo1−/− mice is consistent with the observed hyperphagia of these mice. Leptin is part of a well-established physiological circuit for controlling energy homeostasis in mammals. Leptin acts via its receptor in the hypothalamus to activate an anorexigenic pathway mediated by neurons producing α-melanocyte–stimulating hormone and to inhibit an orexigenic pathway mediated by neurons producing neuropeptide Y and agouti-related protein.27–29 These hypothalamic neurons interact with other brain centers to integrate various inputs to affect appetite and regulate metabolism and energy expenditure. Studies in rodent models have clearly shown that leptin administration leads to decreased food intake, increased energy expenditure, and weight loss, whereas low leptin levels initiate an adaptive response to increase food intake and conserve energy, as manifested by hyperphagia and decreased energy expenditure.28–30
The observation of elevated SCD1 abundance in livers of Cdo1−/− mice is consistent with their low leptin levels and with a role of leptin in the suppression of Scd1 gene expression. The inverse association of plasma leptin levels and hepatic SCD1 expression and activity was established by the work of Friedman and coworkers and of Ntambi and coworkers.28,30–32 Cohen et al. examined gene expression arrays in liver from ob/ob (leptin-deficient) or wild-type mice treated with leptin along with free access to feed, with saline along with pair feeding to the intake level of leptin-treated mice, or with saline along with free access to feed.30 When the arrays were analyzed to identify genes whose expression was increased in ob/ob mouse liver relative to wild type and then ranked by the extent to which their expression was corrected upon leptin administration, the gene encoding SCD1 ranked the highest in the analysis. Cohen et al. also assayed SCD (Δ9 desaturase) enzymatic activity in liver and observed levels that were seven times higher than wild type in the ob/ob mice. These levels were normalized by leptin treatment, whereas pair feeding reduced SCD activity to a lesser extent.30 Biddinger et al. determined that leptin exerts its effects on Scd1 gene expression by an insulin- and SREBP-1c–dependent mechanism.32
There is evidence that downregulation of Scd1 expression plays a major role in mediating the actions of leptin to prevent fat accumulation, which would be consistent with elevated SCD1 levels in Cdo1−/− mice playing a role in their excess weight gain. Cohen et al. observed that their leptin-treated ob/ob mice lost significantly more weight and exhibited a much more dramatic correction of their hepatic steatosis compared to pair-fed ob/ob mice, demonstrating that the metabolic actions of leptin involve more than regulation of food intake.30 Ob/ob mice with disrupted expression of Scd1 had much less body fat than did ob/ob mice that expressed Scd1.30 There appears to be a strict requirement for endogenous synthesis of 16:1 and 18:1 by SCD1-mediated desaturation to support triglyceride and cholesteryl ester synthesis, and possibly phospholipid synthesis, in the liver. Studies in mice that do not express Scd1 but still express Scd2 showed that SCD1 deficiency resulted in low levels of 16:1 and 18:1 fatty acids as well as lower levels of hepatic triglycerides and cholesteryl esters and lower rates of very-low-density lipoprotein (VLDL) production.30,33 Diets supplemented with high levels of monounsaturated fats could not restore hepatic lipid and VLDL levels in SCD1-deficient mice to the levels in wild-type controls,34 implicating a coupling of the endogenous pathway for 16:1 and 18:1 fatty acyl-CoA synthesis with the pathway for triglyceride synthesis. Although the Cdo1−/− mice had hepatic SCD1 abundances that were 2- to 6-fold those of wild-type mice, the desaturation ratio for C16 and C18 fatty acids did not appear to be different on the basis of measurement of hepatic acylcarnitines. Thus, it is not clear that SCD1 activity per se is involved in the phenotype of the Cdo1−/− mouse.
Thus, although observations in the Cdo1−/− mouse provide further support for the association of elevated cysteine levels with low leptin levels, high SCD1 levels, and excess energy balance, the data do not indicate that an increased desaturation of C16 and C18 fatty acids is necessarily involved in the mechanism by which leptin promotes excess fat deposition, as has been suggested by investigators working with Scd1 knockout models.30,33,34 Nevertheless, observations in the Cdo1−/−mouse support a general impairment in fatty acid oxidation, which could lead to excess triglyceride synthesis and fat storage.
The basic defect in the Cdo1−/−mouse is a block in cysteine metabolism that blocks the conversion of cysteine to cysteine sulfinic acid, which is the major precursor for taurine biosynthesis, and leads to elevated levels of cysteine that promote cysteine metabolism by desulfhydration, leading to excess production of H2S and thiosulfate.17,19 It is possible that elevated production of H2S underlies both the low leptin levels and the impaired metabolism of fatty acids in the Cdo1−/− mice.
Data from patients with defects in mitochondrial sulfide oxidation due to mutations in ETHE1 (ethylmalonic encephalopathy) as well as studies in Ethe1 knockout mice indicate that elevated levels of H2S lead to impairments in fatty acid and branched-chain amino acid in the mitochondria, along with elevated levels of C4–C6 acylcarnitines in the blood and excretion of ethylmalonic acid and thiosulfate in the urine.23,35,36 Ethe1 encodes a homodimeric persulfide dioxygenase of the mitochondrial matrix that uses molecular oxygen to oxidize the sulfane sulfur of the persulfide generated by action of the inner mitochondrial membrane sulfide:quinone oxidoreductase on H2S, resulting in sulfite formation. ETHE1-deficient patients and mice exhibit a loss of tissue COX activity as well as the loss of holoenzyme and abundance of specific COX subunits, such as COX4 and COX5b, all of which are attributed to the toxicity of H2S on COX.23,37 Short-chain acyl-CoA dehydrogenase and medium-chain acyl-CoA dehydrogenase activities in tissue from ETHE1-deficient patients were in the low range of control values, and may be related to the observed impairments of β-oxidation.24 Although CDO deficiency in mice seems to favor the accumulation of long-chain acylcarnitines more than short-chain acylcarnitines, the fact that H2S levels are elevated in both the ETHE1-deficient and CDO-deficient mouse models and that both exhibit defects in β-oxidation of fatty acids strongly suggests a link between excess H2S and impairments in mitochondrial β-oxidation. The exact nature of the link between excess H2S and impaired mitochondrial metabolism of fatty acids and amino acids remains to be elucidated.
Although the data are limited and H2S measurements are not always reliable, the possibility that H2S can suppress leptin expression is also consistent with observations in Cdo1−/− mice. Leptin levels appear to remain low in Cdo1−/− mice throughout their life spans and regardless of diet. Leptin levels in 12-month old Cdo1−/− mice were 30% and 19% of wild-type levels for mice that had been fed a basal or high-fat diet, respectively, for 5 months (unpublished observations). In cell culture studies, H2S has been found to inhibit glucose-induced activation of p38 mitogen-activated protein kinase (MAPK) and to attenuate the expression levels of leptin and leptin receptors in cardiomyoblasts38 and to produce an increase in phosphatidylinositol-3,4,5 triphosphate (PIP3), Akt phosphorylation, and glucose utilization in glucose-treated 3T3L1 adipocytes.39 In a study of men with type 2 diabetes or overweight and lean control individuals, median plasma H2S levels were lowest in patients with type 2 diabetes, intermediate in overweight participants, and highest in lean subjects; waist circumference or other measures of adiposity were independent predictors of plasma H2S, independent of diabetes.40
In summary, the association of elevated cysteine levels with greater weight gain and with elevated hepatic Scd1 expression holds in the Cdo1−/−mouse model. Furthermore, an association of low leptin levels with high hepatic Scd1 expression holds in the Cdo1−/−mouse, providing further evidence for a role for SCD1 suppression in mediating some of the effects of leptin. Although elevated cysteine levels lead to elevated H2S production, how the elevation of H2S might function to initiate or mediate the effects of elevated cysteine levels is still unclear. In addition, although the mitochondrial β-oxidation of fatty acids and certain amino acid carbon chains are altered in the presence of elevated H2S levels, the metabolic factors that limit the rate of their oxidation remain to be discovered. Nevertheless, the strong associations of elevated cysteine levels with excess H2S production and impairments in energy metabolism suggests that H2S signaling could be involved. Further studies of the possible association of either physiological H2S signaling or H2S toxicity with the regulation of leptin expression and with the regulation of fatty acid oxidation are needed.
Acknowledgments
This project was supported by Grants DK-056649 (MHS) and CA-193256 (JWL) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Julie Niewiadomski, James Zhou, Lawrence L. Hirschberger, and Heather B. Roman each carried out portions of the research and contributed to the writing. Jason W. Locasale and Xiaojing Liu performed the LC-MS analyses. Julie Niewiadomski and Martha H. Stipanuk were responsible for the design of the studies and for writing the manuscript.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.El-Khairy L, Ueland PM, Nygård O, Refsum H, Vollset SE. Lifestyle and cardiovascular disease risk factors as determinants of total cysteine in plasma: the Hordaland Homocysteine Study. Am J Clin Nutr. 1999;70:1016–1024. doi: 10.1093/ajcn/70.6.1016. [DOI] [PubMed] [Google Scholar]
- 2.El-Khairy L, Vollset SE, Refsum H, Ueland PM. Predictors of change in plasma total cysteine: longitudinal findings from the Hordaland homocysteine study. Clin Chem. 2003;49:113–120. doi: 10.1373/49.1.113. [DOI] [PubMed] [Google Scholar]
- 3.Elshorbagy AK, Nurk E, Gjesdal CG, Tell GS, Ueland PM, Nygård O, Tverdal A, Vollset SE, Refsum H. Homocysteine, cysteine, and body composition in the Hordaland Homocysteine Study: does cysteine link amino acid and lipid metabolism? Am J Clin Nutr. 2008;88:738–746. doi: 10.1093/ajcn/88.3.738. [DOI] [PubMed] [Google Scholar]
- 4.Elshorbagy AK, Valdivia-Garcia M, Refsum H, Butte N. The association of cysteine with obesity, inflammatory cytokines and insulin resistance in Hispanic children and adolescents. PLoS One. 2012;7(9):e44166. doi: 10.1371/journal.pone.0044166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elshorbagy AK, Valdivia-Garcia M, Graham IM, Palma Reis R, Sales Luis A, Smith AD, Refsum H. The association of fasting plasma sulfur-containing compounds with BMI, serum lipids and apolipoproteins. Nutr Metab Cardiovasc Dis. 2012;22:1031–1038. doi: 10.1016/j.numecd.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Elshorbagy AK, Refsum H, Smith AD, Graham IM. The association of plasma cysteine and gamma-glutamyltransferase with BMI and obesity. Obesity (Silver Spring) 2009;17:1435–1440. doi: 10.1038/oby.2008.671. [DOI] [PubMed] [Google Scholar]
- 7.Elshorbagy AK, Valdivia-Garcia M, Refsum H, Smith AD, Mattocks DA, Perrone CE. Sulfur amino acids in methionine-restricted rats: hyperhomocysteinemia. Nutrition. 2010;26:1201–1204. doi: 10.1016/j.nut.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Richie JP, Jr, Komninou D, Leutzinger Y, Kleinman W, Orentreich N, Malloy V, Zimmerman JA. Tissue glutathione and cysteine levels in methionine-restricted rats. Nutrition. 2004;20:800–805. doi: 10.1016/j.nut.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell. 2006;5:305–314. doi: 10.1111/j.1474-9726.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 10.Rizki G, Arnaboldi L, Gabrielli B, Yan J, Lee GS, Ng RK, Turner SM, Badger TM, Pitas RE, Maher JJ. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res. 2006;47:2280–2290. doi: 10.1194/jlr.M600198-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Flowers MT, Ntambi JM. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr Opin Lipidol. 2008;19:248–256. doi: 10.1097/MOL.0b013e3282f9b54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elshorbagy AK, Valdivia-Garcia M, Mattocks DA, Plummer JD, Smith AD, Drevon CA, Refsum H, Perrone CE. Cysteine supplementation reverses methionine restriction effects on rat adiposity: significance of stearoyl-coenzyme A desaturase. J Lipid Res. 2011;52:104–112. doi: 10.1194/jlr.M010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrone CE, Mattocks DA, Jarvis-Morar M, Plummer JD, Orentreich N. Methionine restriction effects on mitochondrial biogenesis and aerobic capacity in white adipose tissue, liver, and skeletal muscle of F344 rats. Metabolism. 2010;59:1000–1011. doi: 10.1016/j.metabol.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Elshorbagy AK, Valdivia-Garcia M, Mattocks DA, Plummer JD, Orentreich DS, Orentreich N, Refsum H, Perrone CE. Effect of taurine and N-acetylcysteine on methionine restriction-mediated adiposity resistance. Metabolism. 2013;62:509–517. doi: 10.1016/j.metabol.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Vinknes KJ, Dekker JM, Drevon CA, Refsum H, Nurk E, Nijpels G, Stehouwer CD, Teerlink T, Tell GS, Nygård O, Vollset SE, Ueland PM, Elshorbagy AK. Plasma sulfur amino acids and stearoyl-CoA desaturase activity in two Caucasian populations. Prostaglandins Leukot Essent Fatty Acids. 2013;89:297–303. doi: 10.1016/j.plefa.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Ueki I, Roman HB, Valli A, Fieselmann K, Lam J, Peters R, Hirschberger LL, Stipanuk MH. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am J Physiol Endocrinol Metab. 2011;301:E668–E684. doi: 10.1152/ajpendo.00151.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 19.Roman HB, Hirschberger LL, Krijt J, Valli A, Kožich V, Stipanuk MH. The cysteine dioxgenase knockout mouse: altered cysteine metabolism in nonhepatic tissues leads to excess H2S/HS− production and evidence of pancreatic and lung toxicity. Antioxid Redox Signal. 2013;19:1321–1336. doi: 10.1089/ars.2012.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Ser Z, Locasale JW. Development and quantitative evaluation of a high-resolution metabolomics technology. Anal Chem. 2014;86:2175–2184. doi: 10.1021/ac403845u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurkowska H, Roman HB, Hirschberger LL, Sasakura K, Nagano T, Hanaoka K, Krijt J, Stipanuk MH. Primary hepatocytes from mice lacking cysteine dioxygenase show increased cysteine concentrations and higher rates of metabolism of cysteine to hydrogen sulfide and thiosulfate. Amino Acids. 2014;46:1353–1365. doi: 10.1007/s00726-014-1700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiranti V, Zeviani M. Altered sulfide H2S metabolism in ethylmalonic encephalopathy. Cold Spring Harb Perspect Biol. 2013 Jan 1;5(1):a011437. doi: 10.1101/cshperspect.a011437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiranti V, Viscomi C, Hildebrandt T, Di Meo I, Mineri R, Tiveron C, Levitt MD, Prelle A, Fagiolari G, Rimoldi M, Zeviani M. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med. 2009;15:200–205. doi: 10.1038/nm.1907. [DOI] [PubMed] [Google Scholar]
- 24.Zafeiriou DI, Augoustides-Savvopoulou P, Haas D, Smet J, Triantafyllou P, Vargiami E, Tamiolaki M, Gombakis N, van Coster R, Sewell AC, Vianey-Saban C, Gregersen N. Ethylmalonic encephalopathy: clinical and biochemical observations. Neuropediatrics. 2007;38:78–82. doi: 10.1055/s-2007-984447. [DOI] [PubMed] [Google Scholar]
- 25.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 26.Ledikwe JH, Blanck HM, Kettel Khan L, Serdula MK, Seymour JD, Tohill BC, Rolls BJ. Dietary energy density is associated with energy intake and weight status in US adults. Am J Clin Nutr. 2006;83:1362–1368. doi: 10.1093/ajcn/83.6.1362. [DOI] [PubMed] [Google Scholar]
- 27.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen P, Friedman JM. Leptin and the control of metabolism: role for stearoyl-CoA desaturase-1 (SCD-1) J Nutr. 2004;134:2455S–2463S. doi: 10.1093/jn/134.9.2455S. [DOI] [PubMed] [Google Scholar]
- 29.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 30.Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki M, Jacobson MJ, Man WC, Cohen P, Asilmaz E, Friedman JM, Ntambi JM. Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform regulated by leptin and dietary factors. J Biol Chem. 2003;278:33904–33911. doi: 10.1074/jbc.M304724200. [DOI] [PubMed] [Google Scholar]
- 32.Biddinger SB, Miyazaki M, Boucher J, Ntambi JM, Kahn CR. Leptin suppresses stearoyl-CoA desaturase 1 by mechanisms independent of insulin and sterol regulatory element-binding protein-1c. Diabetes. 2006;55:2032–2041. doi: 10.2337/db05-0742. [DOI] [PubMed] [Google Scholar]
- 33.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;275:30132–3138. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki M, Kim YC, Ntambi JM. A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J Lipid Res. 2001;42:1018–1024. [PubMed] [Google Scholar]
- 35.Barth M, Ottolenghi C, Hubert L, Chrétien D, Serre V, Gobin S, Romano S, Vassault A, Sefiani A, Ricquier D, Boddaert N, Brivet M, de Keyzer Y, Munnich A, Duran M, Rabier D, Valayannopoulos V, de Lonlay P. Multiple sources of metabolic disturbance in ETHE1-related ethylmalonic encephalopathy. J Inherit Metab Dis. 2010;33(Suppl 3):S443–S453. doi: 10.1007/s10545-010-9227-y. [DOI] [PubMed] [Google Scholar]
- 36.Drousiotou A, DiMeo I, Mineri R, Georgiou T, Stylianidou G, Tiranti V. Ethylmalonic encephalopathy: application of improved biochemical and molecular diagnostic approaches. Clin Genet. 2011;79:385–390. doi: 10.1111/j.1399-0004.2010.01457.x. [DOI] [PubMed] [Google Scholar]
- 37.Di Meo I, Fagiolari G, Prelle A, Viscomi C, Zeviani M, Tiranti V. Chronic exposure to sulfide causes accelerated degradation of cytochrome c oxidase in ethylmalonic encephalopathy. Antioxid Redox Signal. 2011;15:353–362. doi: 10.1089/ars.2010.3520. [DOI] [PubMed] [Google Scholar]
- 38.Zhuang XD, Hu X, Long M, Dong XB, Liu DH, Liao XX. Exogenous hydrogen sulfide alleviates high glucose-induced cardiotoxicity via inhibition of leptin signaling in H9c2 cells. Mol Cell Biochem. 2014;391:147–155. doi: 10.1007/s11010-014-1997-3. [DOI] [PubMed] [Google Scholar]
- 39.Manna P, Jain SK. Hydrogen sulfide and L-cysteine increase phosphatidylinositol 3,4,5-trisphosphate (PIP3) and glucose utilization by inhibiting phosphatase and tensin homolog (PTEN) protein and activating phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase (AKT)/protein kinase Cζ/λ (PKCζ/λ) in 3T3l1 adipocytes. J Biol Chem. 2011;286:39848–39859. doi: 10.1074/jbc.M111.270884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiteman M, Gooding KM, Whatmore JL, Ball CI, Mawson D, Skinner K, Tooke JE, Shore AC. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53:1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]