Abstract
Background
Skeletal muscle dysfunction and exercise intolerance are clinical hallmarks of patients with heart failure (HF). These have been linked to a progressive catabolic state, skeletal muscle inflammation and impaired oxidative metabolism. Prior studies suggest beneficial effects of ω-3 polyunsaturated fatty acids (PUFAs) and glutamine on exercise performance and muscle protein balance.
Methods and Results
In a randomized double-blind, placebo-controlled trial, 31 HF patients were randomized to either L-alanyl-L-glutamine (8g/d) and PUFA (6.5g/d) or placebo (safflower oil and milk powder) for 3 months. Cardiopulmonary exercise testing, dual-energy X-ray absorptiometry, 6 minute walk test, hand grip strength, functional muscle testing, echocardiography and quality of life and lateral quadriceps muscle biopsy were performed at baseline and at follow-up. Oxidative capacity and metabolic gene expression were analyzed on muscle biopsies. No differences in muscle function, echocardiography, 6 minute walk test or hand grip strength and a non-significant increase in peak VO2 in the treatment group were found. Lean body mass increased and quality-of-life improved in the active treatment group. Molecular analysis revealed no differences in muscle fiber composition, fiber cross sectional area, gene expression of metabolic marker genes (PGC-1α, CPT1, PDK4, GLUT4) and skeletal muscle oxidative capacity.
Conclusions
The combined supplementation of L-alanyl-L-glutamine and PUFA did not improve exercise performance or muscle function but increased lean body mass and quality-of-life in patients with chronic stable HF. These findings suggest potentially beneficial effects of high dose nutritional PUFAs and amino acid supplementations in patients with chronic stable HF.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01534663.
Keywords: heart failure, skeletal muscle, metabolism
Almost 5 million people are affected by heart failure (HF) in the United States with an incidence rate of 10 in 1000 for people over age 65.1 The result of a primary cardiac insult, the syndrome of chronic HF is characterized by clinical symptoms such as dyspnea, edema, and exercise intolerance. In addition, patients exhibit metabolic abnormalities including cytokine activation,2 insulin resistance,3, 4 abnormal oxidative metabolism in skeletal muscle and myocardium3, 5, 6 as well as impaired mitochondrial biogenesis5, 7 leading to muscle atrophy with prognostic significance for HF patients.8
Nutritional supplements including fish oil containing the omega-3 polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been shown to reduce mortality,9 parameters of cardiac function, exercise tolerance and levels of circulating cytokines (TNFα, IL-1, and IL-6) in patients with chronic stable HF.10 Further, PUFAs increase the rate of muscle protein synthesis in older adults.11 In addition, the amino acid glutamine is involved in maintaining a positive nitrogen balance by decreasing muscle proteolysis, as well as the synthesis of glutathione, an antioxidant.12–14 In a recent study, amino acid supplementation in patients with HF improved exercise tolerance.15 While a combination of high protein and PUFA improves muscle function and decreases muscle wasting in murine models,16 the effects of a combined glutamine and PUFA supplementation have not been tested in patients with HF.
We aimed to determine the effects of a combined glutamine and fish oil supplementation on skeletal muscle function and metabolism in patients with HF. For that purpose, we designed a randomized, prospective, double-blind and placebo-controlled at our center. We hypothesized that the combined supplementation of high dose omega-3 PUFAs and the dipeptide L-alanyl-L-glutamine improves both specific muscle functional parameters including muscle strength and fatigability and overall exercise tolerance in patients with HF. Secondary outcome measures included systemic and local markers of inflammation and metabolism including fatty acids and ceramides as well as body anthromorphometry.
Methods
Patient Population and Study Design
In this a single-center, double-blind, placebo-controlled study, all subjects were recruited from the Columbia University Medical Center between October 18, 2011 to October 17, 2012 with follow-up until January 15, 2013. Patients older than 45 years of age with a left ventricular ejection fraction (LVEF) ≤35%, stable on standard HF medications, and on optimal therapy according to the American Heart Association and American College of Cardiology guidelines were eligible for this study.17 Patients were excluded based on the following: major cardiovascular events or procedures in the last six months, dementia or presence of cardiovascular disease that may lead to harm if the patient took part in the study. Patients with congenital heart disease, long QT syndrome, hypertrophic cardiomyopathy or myocarditis were also excluded.
Patients were randomized to either a combination of 8 g/day L-alanyl-L-glutamine and 6.5 g/day fish oil or to a combination of safflower oil and milk powder (placebo) of equivalent caloric intake. The study compounds were provided blinded and randomized by Natural Organic Laboratories, Inc. in cooperation with Barlean’s Organic Oils & Company.
Patients were evaluated at baseline, at 1 and 3 months and clinical and laboratory data collected from electronic medical records. At all study visits, complete clinical evaluations, quality of life questionnaires, a 6 minute walk test (6MWT), handgrip strength test, muscle testing by Biodex testing and blood draw was performed. A cardiopulmonary exercise test (CPET), DXA scan and echocardiography in all subjects and a voluntary muscle biopsy in a subset of patients was performed at study visit 1 and 3.
The primary endpoint of this study was changes in exercise function assessed by CPET, 6MWT and muscle functional testing. Secondary endpoints included skeletal muscle metabolism, echocardiography and inflammatory serum markers. Group size was calculated based on a prior study using omega-3 PUFA supplementation in patients with HF10 and institutional reference data in our cohort of patients with advanced HF. Based on a baseline average VO2max of 15 ml/min/kg (standard deviation of 3 ml/min/kg) and an expected meaningful increase of 20% to 18 ml/min/kg with 80% power and alpha<0.05, a minimal group size of 11 subjects was calculated. This was confirmed by a baseline average 6MWT of 365 m (standard deviation of 45 m) and an expected meaningful increase of at least 10% to 400 m, the minimal group size was calculated as 11 subjects for 80% power and alpha<0.05. To account for attrition, we added a 25% increase and aimed to recruit at least 15 subjects per group.
The study protocol was approved by the Columbia University Medical Center Institutional Review Board and written informed consent was obtained from each patient before study inclusion.
Assessment of Quality-of-Life
The Kansas City Cardiomyopathy Questionnaire (KCCQ)18 and Minnesota Living with HF19 questionnaires were utilized to evaluate all patients.
Echocardiographic Analysis
Conventional echocardiograms were obtained from all patients at study visit 1 and 3 (Sonos-5500® or Sonos-7500®; Philips Healthcare Corp, Andover, MA, USA). The routine standard echocardiographic examination included M-mode, 2D-echocardiogram and Doppler study for measurements of ventricular septal and posterior wall thickness, end-systolic and end-diastolic LV diameters (LVESD and LVEDD). The LV ejection fraction (LVEF) was calculated by biplane Simpson’s method from apical 4- and 2-chamber views. Mitral inflow was obtained by pulsed-wave Doppler echocardiography with the sample volume between mitral leaflet tips during diastole, and peak early (E) and late (A) transmitral filling velocities and their ratio (E/A), and deceleration time of E were measured. The peak positive dP/dt (dP/dtmax, first derivative of LV pressure with respect to time) as an index of contractility was calculated based on continuous wave Doppler determination of the velocities in mitral regurgitant jets. Early diastolic annular velocity (E’) was obtained by placing a tissue Doppler sample volume at the septal and lateral mitral annulus in the apical 4 chamber view and the E/E’ ratio was calculated. Measurements were performed from 5 cardiac cycles and averaged.
Cardiopulmonary Exercise Testing
Patients were subjected to cardiopulmonary exercise testing (CPET) using an incremental biking exercise performed on a bicycle ergometer (Ergometrics 800, SensorMedics Inc., Yorba Linda, CA). Subjects were instructed to maintain a pedal speed of 60 revolutions/minute with linear increase in resistance each minute. Once the workload became too high and the patient could no longer maintain 60 revolutions/minute, the test was stopped and patients subsequently performed 3 minutes of active recovery. Peak effort oxygen consumption (VO2 max), rate of perceived exertion, blood pressure and heart rate were all measured throughout the testing exercise.
Six Minute Walking Distance
Subjects were instructed to walk as fast as possible over a period of six minutes. The test was scored by rounded meters walked in six minutes.
Hand Grip Strength Testing
Hand grip strength (HGS) was measured using a Jamar hand dynamometer (Sammons Preston Inc., Boiling Brook, IL). Subjects were asked to perform a maximal isometric contraction with each hand 3 consecutive times. Each contraction was followed by a 5-second rest period. Averages for each hand were taken. HGS was normalized for total body weight (BW).
Muscle Function Testing
Muscle functional testing was performed by trained staff under the supervision of a physician. Biodex System 4 Pro (Biodex Medical Systems, Shirley, NY) was used to measure both isometric and isokinetic movements. The dominant knee was test first followed by the dominant elbow.
For isometric contractions, subjects contracted against a constant 60-degree angle at the knee and a constant 30 degree angle at the elbow for 6 seconds. Subjects alternated between flexion and extension, 3 repetitions each with a 30 second rest period between each. Data was collected to determine peak torque to body weight (PKTQ/BW).
For isokinetic contractions, subjects performed flexion and extension movements at the knee and elbow for 5 repetitions at a speed of 60 degrees/second. This was followed by a rest period of 2.5 minutes. For the second isokinetic protocol, subjects performed 25 repetitions of flexion and extension movements at the knee and elbow at a speed of 180 degrees/second. Data was collected to determine PKTQ/BW, average power, work to body weight (WK/BW), fatigability, and time to peak torque. Normalization of the data to body weight allowed for a more meaningful comparison of measurements among subjects.
Dual-energy X-ray Absorptiometry
Bone mass, lean mass, fat mass and body composition was measured by dual energy x-ray absorptiometry (DXA) using established protocols.
Blood Collection and Analysis
Venous blood samples were drawn at all study visits. The blood was collected in two 8.5 ml SST tubes and two 7.0ml EDTA tubes. Samples were subsequently spun for 20 minutes at 2500 rpm at 4° Celsius. Aliquots of 500ul serum and plasma were pipetted into cryovials and frozen at −80° Celsius. Levels of TNFα were measured with a commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN).
Liquid chromatography-mass spectrometry (LC/MS)
All solvents for sample extraction and LC/MS were LC/MS grade (or LC grade when LC/MS grade was not available) and were purchased from Fisher Scientific (Pittsburgh, PA, USA). Standards were purchased from Avanti Polar Lipid, Inc. (Alabaster, AL, USA).
Samples were extracted using chloroform:methanol extraction. Briefly, 3 ml of the chloroform:methanol (2/1, v/v) containing 20 µl of a 2 µM internal standard mixture (Avanti LM-6002, containing C12 and C25 ceramides) were added to 100 µl aqueous of tissue or cell homogenate in a clean glass tube. The mixture was vortexed well. 0.5 ml of water was added to the mixture to allow for phase separation. The mixture was vortexed again and centrifuged at 3,000g for 10 minutes. The lower organic phase was transferred to a second clean glass tube using a Pasteur pipette. 2 ml of chloroform were added to the residual aqueous phase, followed by vortex mixing and centrifugation again at 3,000g for 10 min, to extract the remaining lipids. The lower organic phases were pooled and evaporated under nitrogen. The extracted lipids were reconstituted in 30 µl of methanol and transferred to LC/MS autosampler vials (Waters, P/N 600000670CV) for injection.
All experiments were carried out on a Waters Xevo TQ MS ACQUITY UPLC system (Waters, Milford, MA). The system was controlled by Mass Lynx Software version 4.1. The sample was maintained at 4°C in the autosampler and 5 µl was loaded onto a Waters ACQUITY UPLC BEH Phenyl column (3 mm inner diameter × 100 mm with 1.7 µm particles), preceded by a 2.1 × 5 mm guard column containing the same packing. The column was maintained at 40°C throughout analysis. The UPLC flow rate was continuously 300 µl/min in a binary gradient mode with the following mobile phase: initial flow conditions were 20% solvent A (H2O, containing 0.2% formic acid and 1 mM ammonium formate) and 80% solvent B (methanol, containing 0.2% formic acid and 10 mM ammonium formate).20
Analysis of Amino Acids
Plasma amino acids were measured by HPLC-UV (Alliance 2695 HPLC/2487 Dual Absorbance Detector; Waters Corp.). In brief, after the addition of the internal standard (methionine sulfone; Sigma), plasma was stripped of protein by size exclusion filtration (Amicon Ultra 0.5 ml centrifuge filter 10 KD; EMD Millipore), derivatized with an amino-specific chromogen (phenyl isothiocyanate; Sigma) and passed through a C18-HPLC column for amino acid separation (Pico-Tag Column, 60 Å, 4 µm, 3.9 mm × 300 mm) with a detection wavelength 254 nM. Endogenous concentrations of amino acids were calculated relative to a one-point calibrator and normalized to the area of an internal standard.
Muscle Biopsy
Voluntary percutaneous muscle biopsies of the vastus lateralis muscle of the non-dominant leg were performed in a subset of patients using a Bergstrom needle 15 cm above the patella. Specimens were taken before and after the trial period and dissected free of visible connective tissue and fat. The muscle tissue was either immediately frozen in liquid nitrogen and stored at −80°C or fixed in paraformaldehyde.
Histomorphometric Analysis
Muscle fiber cross-sectional area (CSA) were analyzed on muscle biopsies obtained at the beginning and at the end of the study using previously described methods. In short, H&E stained sections (5 µm) were reviewed microscopically at 10× magnification (Nikon Eclipse E 200) and fiber size was analyzed using standard image-processing software (NIH ImageJ). For all slides, the scale was set to 2.84 pixels/unit based on a standardized stage micrometer for 10× magnification. Frequency histograms were constructed based on the number of muscle fibers falling within CSA groups.
Immunohistochemistry was performed for the characterization of muscle fiber oxidative quality and fiber typing. Paraffin-embedded sections (5 µm) were first incubated in 5% goat serum to reduce non-specific immunoreactivity and subsequently with primary anti-myosin antibodies (monoclonal anti-fast twitch skeletal muscle myosin, Sigma, 1:400). Specific immunoreactivity was detected with a secondary rabbit anti-mouse antibody (Pierce, 1:400) followed by conjugation with avidin-biotin-complex (ABC Kit, Pierce). Immunopositive fibers were quantified and expressed as percent of all muscle fibers.
Gene Expression Analysis
Total RNA was extracted using standard methods and the abundance of specific mRNAs was determined by RT-PCR. Sequences of primers and TaqMan probes specific for PGC1α, CPT1, PDK4, GLUT4 were described previously.21–24 Gene expression was normalized using expression of 18S mRNA and expressed as relative expression.
Fatty Acid Oxidation
30 mg of muscle tissue was analyzed as previously described25 with slight modifications. Specimens were placed in flask containing 1 ml low glucose DMEM cell culture medium (Cellgro), 1.5% delipidated BSA (Sigma), 0.2 mM oleic acid, 1 βµCi/ ml [14C] oleic acid, cup with rubber stoppers that had been inserted a cell holding a rolled filter paper and incubated at 37 °C for 2 hours. The filter papers were soaked with 1N KOH and the reaction was stopped injecting 200 ul of 70% perchloric acid and incubating for 1 hour with shaking. At the end of the incubation, the filter papers were transferred to vials containing scintillation liquid and counts per minute (cpm) were measured using a scintillation liquid counter machine. The total fatty acid oxidation reaction rate was evaluated as CO2 produced and analyzed from cpm in the filter papers.
Statistical Analysis
Data were analyzed using GraphPad Prism, Version 5.0b (San Diego, CA, USA), and are presented as meansSEM. For categorical variables, results are presented as relative frequency. Normality was evaluated for each variable by the Kolmogorov-Smirnov test. Serial values within the two treatment groups were assessed using Student’s paired t-test or Wilcoxon signed rank test. Unpaired analyses were completed when comparing between the two treatment groups using unpaired Student’s t-test or Mann Whitney test. All tests were two-tailed. Differences among three time points (baseline, 1 month and 3 months) were analyzed using One-way Analysis of Variance (ANOVA) (parametric) or the Kruskal-Wallis test due to missing data points because of patient drop outs within the groups at 1 month and 3 months. Tukey and Dunn’s multiple comparison post-hoc tests were used. A p-value of <0.05 was considered statistically significant and no adjustments were made for the multiplicity of comparisons.
Results
Patient demographics and laboratory parameters
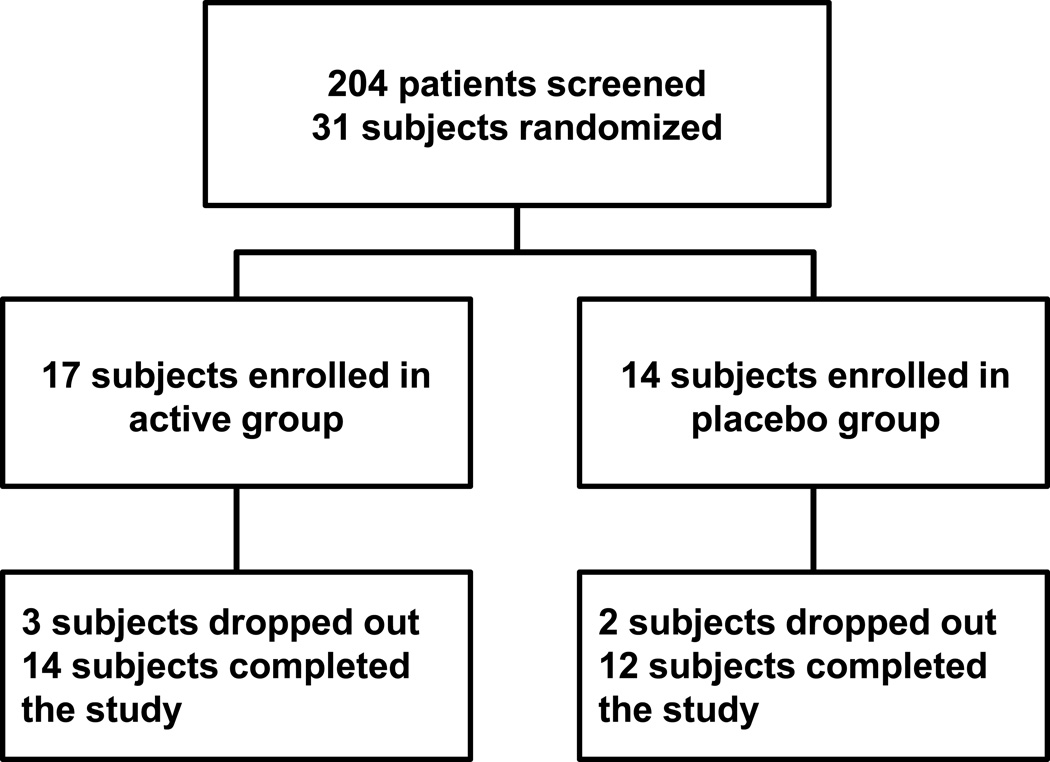
Of 31 patients enrolled in the study, 17 subjects were randomized to the active treatment group and 14 to the placebo group. Of these, 3 subjects dropped out in the active treatment group (one due to diarrhea at 2 weeks and two patients were lost to follow-up) and 2 dropped out of the placebo group (both due to diarrhea at 2 weeks and at 6 weeks). A total of 14 subjects finished the study protocol in the active treatment group and 12 in the placebo group (Figure 1). Clinical characteristics of all patients at baseline and at 3 months follow-up are summarized in Table 1. Laboratory parameters at baseline and at 3 months follow-up are listed in Table 2. No difference in change (baseline to 3 months) in BMI or BP were noted between groups. Supplementation with L-alanyl-L-glutamine and PUFAs showed a trend towards a decrease in triglyceride levels but did not affect circulating levels of TNFα.
Figure 1. Study design and enrollment.
Table 1.
Patient Demographics
| All Subjects (n=31) |
Placebo (n=12) |
Active (n=14) |
|||
|---|---|---|---|---|---|
| Baseline | Baseline | Post | Baseline | Post | |
| Age (yrs) | 59±2 | 56±2 | - | 59±3 | - |
| Gender (% male) | 26 (84) | 9 (82) | - | 12 (86) | - |
| BMI (kg/m2) | 28±1 | 28±2 | 29±2 | 30±1 | 30±1 |
| Percent Fat (%) | 31±1 | 32±2 | 32±3 | 32±2 | 31±1 |
| Heart Rate (bpm) | 70±2 | 68±2 | 72±3 | 71±3 | 71±3 |
| Systolic BP (mmHg) | 114±3 | 114±5 | 115±5 | 114±5 | 110±4 |
| Diastolic BP (mmHg) | 67±2 | 69±3 | 69±3 | 68±3 | 66±2 |
| Etiology (#(%)) | |||||
| Ischemic cardiomyopathy | 16 (52) | 4 (33) | - | 8 (57) | - |
| Dilated cardiomyopathy | 12 (39) | 6 (50) | - | 5 (36) | - |
| Other | 3 (10) | 2 (17) | - | 1 (7) | - |
| Medication (#(%)) | |||||
| Beta Blocker | 28 (90) | 11 (92) | - | 12 (86) | - |
| ACE inhibitors/ARB | 26 84) | 11 (92) | - | 10 (71) | - |
| Spironolactone | 6 (19) | 1 (8) | - | 4 (29) | - |
| Digoxin | 16 (52) | 6 (50) | - | 7 (50) | - |
| Diuretics | 21 (68) | 11 (92) | - | 6 (43) | - |
| Aspirin | 21 (68) | 6 (50) | - | 11 (79) | - |
| Statins | 19 (61) | 7 (58) | - | 8 (57) | - |
| Insulin | 5 (16) | 3 (25) | - | 2 (14) | - |
| Coumadin | 9 (29) | 3 (25) | - | 4 (29) | - |
Post represents 3 months following the intervention. Values expressed at Mean ± SEM
Table 2.
Laboratory Parameters
| All subjects (n=31) |
Placebo (n=12) |
Active (n=14) |
|||
|---|---|---|---|---|---|
| Baseline | Baseline | Post | Baseline | Post | |
| WBC (×103/µL) | 6.5±0.4 | 6.9±0.7 | 7.2±0.7 | 6.1±0.5 | 6.7±0.6 |
| Hematocrit (%) | 38.6±0.8 | 38.3±1.4 | 38.9±1.3 | 39.1±1.1 | 39.1±1.2 |
| Platelet (×103/µL) | 206.8±9.7 | 205±15.6 | 212.8±17 | 209.3±14.3 | 188±10 |
| Sodium (mEq/L) | 137.9±0.4 | 138.3±0.7 | 138.7±0.7 | 137.5±0.5 | 136.8±1 |
| Potassium (mEq/L) | 4.5±0.08 | 4.5±0.2 | 4.2±0.1 | 4.4±0.06 | 4.2±0.1 |
| Blood urea nitrogen (mg/dL) | 19.9±1.2 | 19±1.9 | 20.5±2.5 | 19.4±1.5 | 24.5±2.8 |
| Creatinine (mg/dL) | 1.1±0.05 | 1.1±0.07 | 1.2±0.1 | 1.1±0.06 | 1.1±0.09 |
| Albumin (mg/dL) | 4.4±0.07 | 4.4±0.1 | 4.4±0.1 | 4.4±0.1 | 4.4±0.09 |
| Total bilirubin (mg/dL) | 0.75±0.07 | 0.73±0.09 | 0.78±0.1 | 0.77±0.1 | 0.71±0.1 |
| Aspartate aminotransferase (U/L) | 23.5±1.4 | 23.1±2.5 | 23.3±2.6 | 23.1±1.7 | 23.2±3.5 |
| Alanine transaminase (U/L) | 22±1.7 | 19.8±1.8 | 20±1.8 | 23.5±2.5 | 21.2±1.5 |
| Cholesterol (mg/dL) | 164.4±8.7 | 164.4±13 | 165.5±12 | 168.6±12 | 170.9±15 |
| HDL (mg/dL) | 43.8±2.3 | 44.5±4.6 | 43.8±3.2 | 43.3±3 | 41.3±3.8 |
| LDL (mg/dL) | 90.9±6.2 | 85.5±9.6 | 84.3±7.5 | 98.3±8 | 100.3±12 |
| Triglycerides (mg/dL) | 184.4±32.9 | 158.8±22 | 186.5±27 | 207.4±57 | 172.3±36 |
| TNFα (pg/mL) | 3.2±0.5 | 3.4±0.6 | 2.5±0.4 | 3.1±0.8 | 3.3±0.5 |
WBC, white blood cell; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TNFα, tumor necrosis factor alpha. Post represents 3 months following the intervention. Values expressed at Mean ± SEM
Exercise parameters
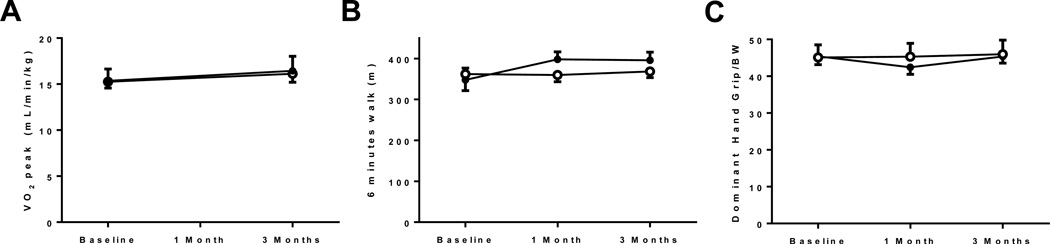
No changes in any of the exercise parameters analyzed in the current study were noted. CPET revealed no differences from baseline to 3 months in VO2max in neither the active treatment nor the placebo group (7.9±4.7 vs. 0.1±0.7% change in VO2max in placebo; p=0.26). 6MWT and handgrip strength corrected for body weight also did not show any changes under treatment (Figure 2). Analysis of specific parameters of isolated leg and arm skeletal muscle function did not reveal differences in response to L-alanyl-L-glutamine and PUFA supplementation compared to placebo (Table 3 and Supplemental Table 1–4).
Figure 2. Exercise parameters.
(A) cardiopulmonary exercise test, (B) 6 minute walk test, (C) Handgrip strength corrected for body weight. Open circles = active treatment, Closed circles = placebo treatment.
Table 3.
Skeletal Muscle Functional Analysis (dominant leg)
| All subjects (n=31) |
Placebo (n=12) |
Active (n=14) |
|||
|---|---|---|---|---|---|
| Baseline | Baseline | Post | Baseline | Post | |
| Isometric | |||||
| Peak torque/BW – extension (%) | 189±9 | 177±18 | 200±20 | 196±10 | 206±11 |
| Peak torque/BW - flexion (%) | 81±4 | 76±6 | 90±8 | 90±5 | 90±5 |
| Isokinetic – 5 repetitions | |||||
| Peak torque/BW – extension (%) | 150±7 | 147±17 | 173±20 | 153±8 | 160±9 |
| Peak torque/BW – flexion (%) | 64±3 | 61±6 | 76±9 | 70±5 | 77±4 |
| Work/BW – extension (%) | 135±7 | 132±15 | 157±17 | 137±8 | 149±9 |
| Work/BW – flexion (%) | 64±5 | 61±9 | 78±12 | 70±6 | 80±5 |
| Total work – extension (J) | 506±36 | 477±64 | 586±69 | 537±50 | 603±51 |
| Total work – flexion (J) | 225±23 | 209±35 | 277±44 | 264±36 | 319±33 |
| Average power – extension (W) | 69±5 | 65±10 | 80±9 | 75±33 | 84±7 |
| Average power – flexion (W) | 28±3 | 26±5 | 37±6 | 33±5 | 41±5 |
| Time to peak – extension (msec) | 507±20 | 526±44 | 404±37 | 494±27 | 456±27 |
| Time to peak – flexion (msec) | 920±67 | 828±104 | 729±45 | 945±84 | 780±93 |
BW, body weight. Post represents 3 months following the intervention. Values expressed at Mean ± SEM
Echocardiography
Comprehensive analysis of markers of both left and right ventricular systolic and diastolic function showed not differences in any of the parameters between both groups (Table 4).
Table 4.
Echocardiographic Parameters
| All subjects (n=31) |
Placebo (n=12) |
Active (n=14) |
|||
|---|---|---|---|---|---|
| Baseline | Baseline | Post | Baseline | Post | |
| LV end-diastolic diameter (mm) | 66 ± 2 | 67 ± 3 | 66 ± 3 | 65 ± 3 | 63 ± 3 |
| Left ventricular ejection fraction (%) | 28 ± 1 | 30 ± 2 | 37 ± 3 | 26 ± 2 | 34 ± 3 |
| Left ventricular dP/dt | 680 ± 16 | 708 ± 25 | 779 ± 27 | 665 ± 24 | 733 ± 31 |
| Mitral valve deceleration time (ms) | 212 ± 9 | 192 ± 16 | 225 ± 12 | 218 ± 13 | 224 ± 9 |
| Pre-A/E | 1.3 ± 0.1 | 1.3 ± 0.2 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 |
| Left ventricular E/E’ | 15 ± 1 | 15 ± 1 | 13 ± 1 | 15± 1 | 14 ± 1 |
| Right ventricular E/E’ | 10.3 ± 0.5 | 9.5± 0.9 | 9.7 ± 1.1 | 11.0 ± 0.6 | 9.3 ± 0.6 |
Post represents 3 months following the intervention. Values expressed at Mean ± SEM
Quality-of-Life
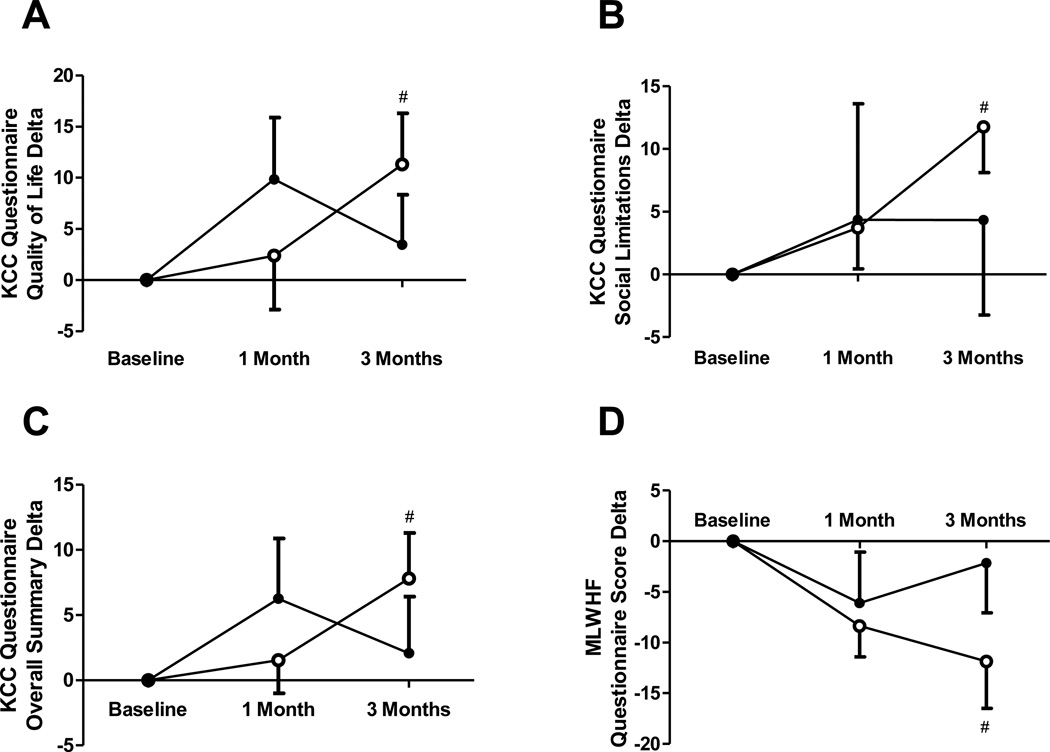
The two quality of life (QoL) questionnaires utilized in this study showed a consistent improvement in QoL of patients with HF in the active treatment group. Using the KCCQ, a significant overall improvement was found (73±19 to 83±12; p=0.04) accompanied by improved social limitation scores (72±24 to 86±15; p=0.006) and overall QoL (62±27 to 75±16; p=0.04) as expressed in absolute values. This was paralleled by improvements in the MLWHF scores in the active treatment group compared to placebo (36±22 to 24±16; p=0.02)(Figure 3).
Figure 3. Quality-of-life assessment.
(A) quality-of-life characteristics of the Kansas City Cardiomyopathy (KCC) questionnaire, (B) social limitations characteristics of the KCC questionnaire, (C) overall scores of the KCC questionnaire, (D) Minnesota Living With Heart Failure (MLWHF) questionnaire scores. Open circles = active treatment, Closed circles = placebo treatment.
Body anthromorphometry
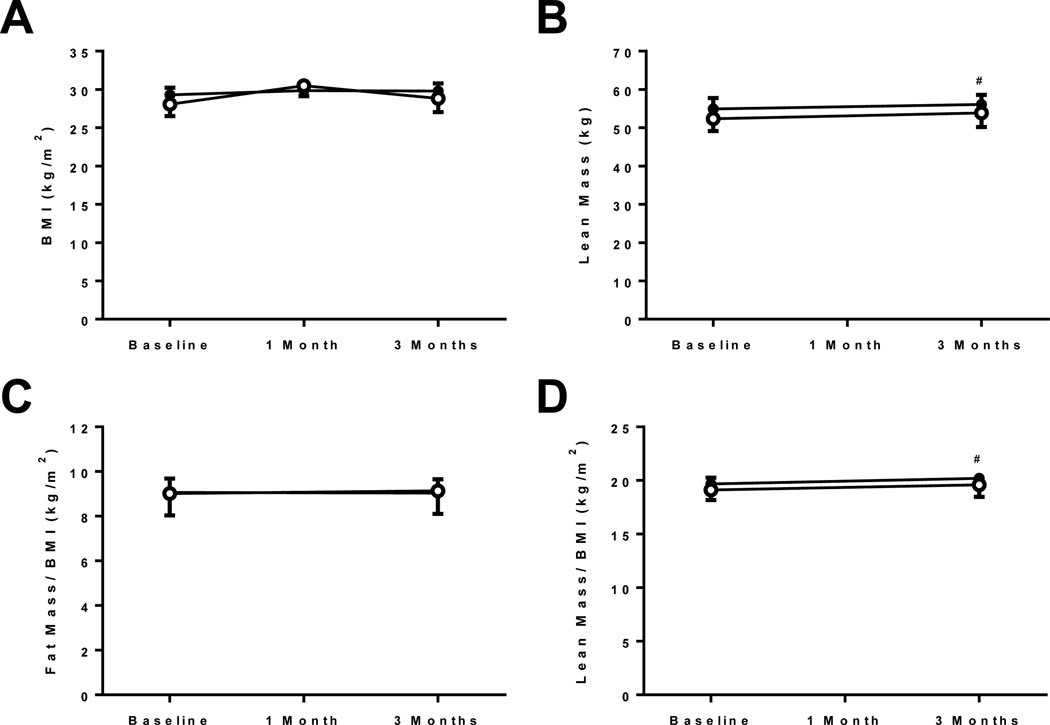
No changes in BMI were noted between the treatment groups. Using DXA scanning, a slight but statistically significant increase in absolute lean body mass (54.4±2.8 to 56.1±2.5 kg at 3 months; p=0.04) and lean body mass corrected for BMI (19.7±0.6 to 20.2±0.5 kg/m2 at 3 months; p=0.04) was noted in the active treatment group compared to placebo (lean body mass: 52.4±3.2 to 53.8±3.7 kg at 3 months; BMI: 19.1±1.0 to 19.6±1.1 kg/m2 at 3 months, respectively). No changes in fat mass were detected (27±2 to 26±2 kg and 25±2 to 26±2 kg at 3 months, respectively) (Figure 4). Further analysis in the extremities did not show significant differences between the groups for fat (right leg active: 4.1±0.4 to 4.0±0.4 kg and placebo: 4.0±0.6 to 4.1±0.6 kg) or lean mass (right leg active: 8.9±0.6 to 8.9±0.6 kg and placebo: 8.1±0.7 to 8.3±0.7 kg).
Figure 4. Body morphometric analysis.
(A) body mass index, (B) lean body mass, (C) fat mass corrected for body mass index, (D) lean mass corrected for body mass index. Open circles = active treatment, Closed circles = placebo treatment.
Analysis of skeletal muscle histomorphometry and metabolism
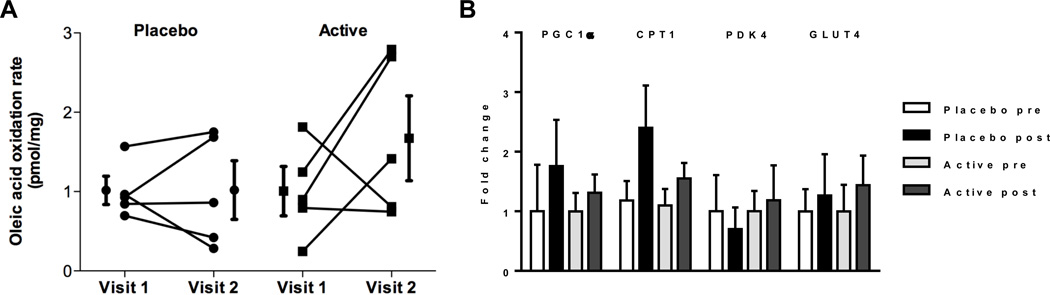
We analyzed skeletal muscle metabolic and histologic parameters in a subset of 9 patients who underwent skeletal muscle biopsy at baseline and at 3 months follow-up (4 patients in the active treatment group and 5 patients in the placebo group). The analysis of muscle fiber CSA, myosin immunohistochemistry and fiber typing did not reveal significant differences between the two groups. Further, no differences in skeletal muscle fatty acid oxidation rates or expression of metabolic marker genes were noted (Figure 5).
Figure 5. Markers of skeletal muscle metabolism.
(A) oleic acid oxidation rates indicating total fatty acid oxidation rates (n=5 paired samples per group), (B) metabolic marker gene expression (n=5 samples per group).
Lipidomic analysis of circulating fatty acids and ceramides
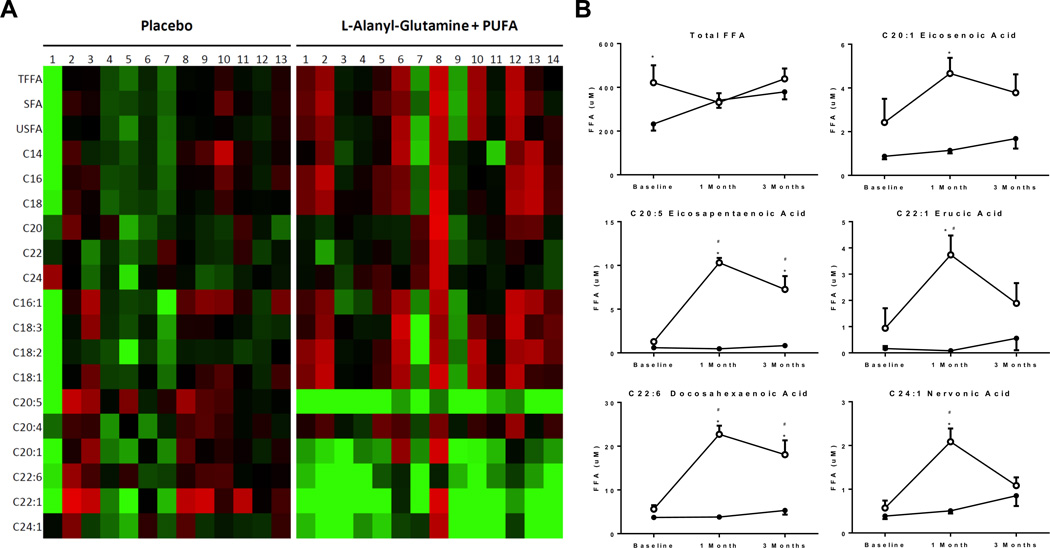
Using LC/MS lipidomics, we analyzed the circulating profile of fatty acids in both groups. No differences in total fatty acids between baseline and follow-up at 3 months between the two groups were noted (Figure 6). Next, this analysis revealed the expected increase in unsaturated fatty acids (C20:1, C20:5, C22:1, C22:6 and C24:1) in the active treatment group (Figure 6A and 6B) confirming the regular uptake of the study compounds by the patients in this group and a distinct treatment effect. No effects were noted on saturated fatty acids in both, the active or the placebo group (Supplemental Figure 1 and Supplemental Table 5).
Figure 6. Dynamics in circulating total, saturated and unsaturated free fatty acid (FFA) levels.
(A) heatmap of individual changes in specific FFA levels following 1 month of supplementation compared to baseline in both groups, green = increase, red = decrease, (B) overall time course of total FFAs and specific mono- and polyunsaturated FFAs in both groups (*p<0.05 vs. placebo, #p<0.05 vs. baseline). Open circles = active treatment, Closed circles = placebo treatment.
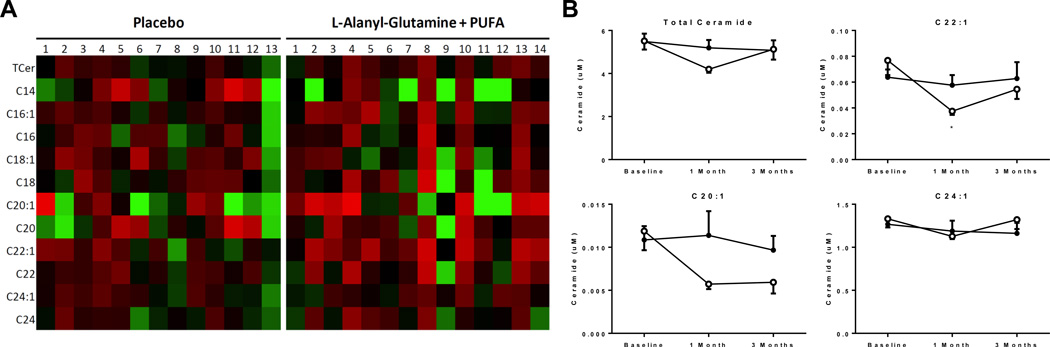
Lipidomic analysis of circulating ceramides, known lipotoxic and pro-inflammatory lipid intermediates, revealed a decrease in circulating levels at 4 weeks in total ceramides (5.5±0.4 to 4.2±0.2 µM at 4 weeks) and two ceramide subspecies (C22:1: 0.077±0.011 to 0.037±0.003 µM; C20:1: 0.012±0.002 to 0.006±0.001 µM at 4 weeks). The effect, however, was not detectable in samples collected at 3 months follow-up (Figure 7A and 7B, Supplemental Figure 2 and Supplemental Table 6).
Figure 7. Dynamics in total and individual very long-chain circulating ceramide species.
(A) heatmap of individual changes in specific ceramide levels following 1 month of supplementation compared to baseline in both groups, green = increase, red = decrease, (B) overall time course of total ceramides and specific very long chain ceramides in both groups (*p<0.05 vs. placebo). Open circles = active treatment, Closed circles = placebo treatment.
Amino Acid Analysis
Using mass spectrometry, we analyzed circulating amino acids in both groups. We did not observe any differences in circulating amino acids between baseline and follow-up within or between the two groups (Supplemental Table 7). Although not significant, the active treatment group did have higher levels of circulating glutamine after 3 months of treatment, while the placebo group did not (511±21 to 530±28 umol/l and 528±16 to 510±36 umol/l, respectively, p=NS). Of note, the downstream metabolites of glutamine metabolism, citrulline and arginine, did not change following 3 months of treatment in either group (citrulline: 39.4±3.9 to 34.0±3.5 umol/l and 28.2±3.1 to 27.1±3.9 umol/l; arginine: 67.5±7.9 to 72.0±8.0 umol/l and 58.0±5.3 to 61.5±7.5 umol/l, respectively. All p=NS).
Discussion
The current study demonstrates that the combined supplementation of L-alanyl-L-glutamine and PUFA does not improve exercise performance or muscle function nor markers of systemic inflammation or peripheral skeletal muscle structure or metabolism in patients with advanced HF. We found, however, an increased lean body mass and improved quality-of-life in patients with advanced HF in the treatment group. These findings suggest that the combined supplementation of L-alanyl-L-glutamine and PUFA may improve subjective parameters of quality-of-life and may improve body composition in patients with advanced HF.
The syndrome of HF is associated with high morbidity and mortality that have been linked to the progressive development of metabolic abnormalities, inflammation, and atrophy in the myocardium and skeletal muscle in these patients. In patients with advanced HF, a distinct metabolic switch from fatty acids to glucose as the principal energy substrate for ATP generation occurs in the myocardium. It is hypothesized that the switch is the result of a regression to the fetal gene program, with decreased expression of carnitine palmitoyl transferase (CPT)-1, medium chain acyl-CoA dehydrogenase (MCAD) and citrate synthase [1]. This may then account for mitochondrial dysfunction and ultimately, a preferential flux through the glycolytic pathway. Interestingly, this metabolic switch is also seen in skeletal muscle with increased type II glycolytic muscle fibers and decreased type I oxidative fibers [2]. This intrinsic metabolic defect in skeletal muscle is thought to contribute to exercise intolerance but therapeutic interventions improving peripheral skeletal muscle function are lacking. Therefore, nutritional approaches with dietary supplementation in addition to current pharmacologic therapies are particularly appealing as they are novel and mechanistically different.
Fish oil contains a large amount of PUFAs and has been shown to be cardioprotective and anti-inflammatory. PUFA supplementation reduced mortality in a large study of patients with HF, has been shown to carry anti-inflammatory properties and improve ventricular function in HF.9, 10 The cellular pathways of PUFA action are still uncertain but it is believed that the double bounds of EPA and DHA may act directly or through metabolites such as eicosanoids as scavengers of ROS.26 Further, PUFAs have been shown to have anti-fibrotic effects in the failing myocardium27 and can reverse lipotoxicity in animal models of human metabolic cardiomyopathies28 proposed to be mediated through membrane stabilization, improved mitochondrial function and higher ATP production.26
Nutritional supplementation with glutamine or its amino acid metabolites is of particular interest in catabolic states such as HF. Glutamine is an amino acid largely produced by the skeletal muscle and liver, and accounts for the majority of carbon and nitrogen metabolism in the cell.29 Glutamine has also been shown to attenuate inflammation, especially in the critically ill.13 Glutamine attenuates skeletal muscle proteolysis, can replete intermediates in the tricarboxylic acid (TCA) cycle through anaplerosis and might activate the suppressed oxidative metabolism in HF.16, 29, 30 The predominant anaplerotic reaction in exercising human muscle is regulated by alanine aminotransferase, an enzyme that catalyzes the conversion between pyruvate and glutamate and alanine and α-ketoglutarate.29, 30 Previous studies found that replenishing TCA cycle intermediates with pyruvate or glutamine acutely increased flux through the TCA cycle and suggests improved oxidative metabolism and ATP production.31 Of note, anaplerotic flux might play a more important role than the total concentration of TCA cycle intermediates in determining the overall TCA flux. Nevertheless, our study did reveal changes in fatty acid oxidation rates or metabolic gene expression in skeletal muscle between the two groups.
Most studies using glutamine supplementation have been completed in acute settings where it is infused and testing is done within minutes or after a bout of exercise. Some authors believe that early metabolic support can improve the myocardial recovery in response to ischemia. When myocardial infarction was induced in rats, those re-perfused with glutamine compared to aspartate and glutamate, showed a significant recovery of cardiac output and no decrease in the ATP/ADP ratio.32 However, ingestion of glutamine before exercise might only augment the pool of TCA cycle intermediates but does not increase TCA cycle flux nor extend endurance capacity. The contribution to anaplerosis in skeletal muscle has not been studied.
Increased amino acid intake in elderly sedentary subjects results in higher ambulatory capacity and maximal isometric muscle strength.33 A 30 day amino acid supplementation (4 g twice daily) in elderly HF patients improved exercise capacity, reduced circulatory dysfunction, and increased peripheral oxygen availability.34 This was associated with increased peak VO2, a shorter recovery time and improved peripheral metabolism suggesting that a high protein and essential amino acid diet may improve markers of nutrition and exercise performance in patients with HF.35 This was confirmed in a randomized single-blind crossover study in patients with Type 2 diabetes mellitus on a 12 week amino acid supplementation (12 g/day) also showing improved exercise performance.36 In our study, however, we did not detect differences in exercise performance or plasma amino acid levels but found a small, but significant, increase in total lean body mass in the active treatment group. This small increase in total lean mass may suggest an increased activity level and thus improved quality of life for the active treatment group, but further analysis is needed.
Our current study utilized a distinct form of glutamine, L-alanyl-L-glutamine, a dipeptide of L-alanine and L-glutamine. This formulation is highly soluble and more stable than glutamine. Further, it increases the enteric uptake of glutamine and increases plasma half-life.37 Prior studies have shown beneficial effects of the use of L-alanyl-L-glutamine compared to glutamine alone in critically ill patients,38 following vascular surgery,39 and in animal models of ischemia and reperfusion40 as well as endurance exercise.41 Due to its high solubility, the dipeptide was administered together with the fishoil formulation as a combined study compound.
Another unique aspect of our study design was the high concentration of fishoil and glutamine in our study. 6.5 g/d of fishoil and 8 g/d of glutamine accounted for around 400 kcal additional nutritional intake per day. To avoid effects of caloric supplementation alone in a patient cohort known to develop a progressive catabolic state, we controlled for equal caloric intake using a placebo formulation based on safflower oil and milk powder. Of note, the placebo formula has similar total fat, carbohydrate and protein content as the active supplementation.
Future studies will analyze the effects of glutamine and fish oil potentially in combination with other micronutrients in patients with HF to better discern the actions of these nutritional supplements and their therapeutic utility in patients with HF. A search for the metabolic mechanisms will provide stronger evidence for our initial hypothesis. The working hypothesis that anti-inflammatory and pro-anabolic interventions will positively affect the clinical status of patients with chronic inflammatory disease states is applicable to other conditions such as rheumatoid arthritis, chronic kidney disease, or cancer.
Our study has several limitations. The analysis represents a single center study with a selected patient cohort. The cohort is predominantly male but had a high proportion of African Americans and Hispanics. Muscle biopsies were taken only from volunteers and represent only a subgroup in this study. We did not analyze amino acid metabolism or pools in circulation or skeletal muscle.
In summary, we show beneficial effects of L-alanyl-L-glutamine and PUFAs on quality of life and body compositions but a lack of effects on exercise performance, markers of inflammation and cardiac structure or performance in this single center, randomized and blinded study. These findings suggest only mild to modest benefits of nutritional supplementation using glutamine and PUFAs in patients with HF.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by grants from the National Heart, Lung and Blood Institute (K23 HL095742-01, P30 HL101272-01, UL1 RR 024156, HL073029) and the Herbert and Florence Irving Scholar Award to Dr. Schulze.
Footnotes
Disclosures
The study compounds were provided by Natural Organic Laboratories, Inc. in cooperation with Barlean’s Organic Oils & Company.
References
- 1.Francis GS, Greenberg BH, Hsu DT, Jaski BE, Jessup M, LeWinter MM, Pagani FD, Pina IL, Semigran MJ, Walsh MN, Wiener DH, Yancy CW., Jr Accf/aha/acp/hfsa/ishlt 2010 clinical competence statement on management of patients with advanced heart failure and cardiac transplant: A report of the accf/aha/acp task force on clinical competence and training. Circulation. 2010;122:644–672. doi: 10.1161/CIR.0b013e3181ecbd97. [DOI] [PubMed] [Google Scholar]
- 2.Levine B, Kalman J, Mayer L, Fillit HM, Packer MP. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. The New England journal of medicine. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 3.Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, Yu S, Kato T, Khan R, Takayama H, Knoll R, Milting H, Chung CS, Jorde U, Naka Y, Mancini DM, Goldberg IJ, Schulze PC. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–2853. doi: 10.1161/CIRCULATIONAHA.111.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doehner W, Rauchhaus M, Ponikowski P, Godsland IF, von Haehling S, Okonko DO, Leyva F, Proudler AJ, Coats AJ, Anker SD. Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. J Am Coll Cardiol. 2005;46:1019–1026. doi: 10.1016/j.jacc.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 5.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 6.Hambrecht R, Schulze PC, Gielen S, Linke A, Mobius-Winkler S, Yu J, Kratzsch JJ, Baldauf G, Busse MW, Schubert A, Adams V, Schuler G. Reduction of insulin-like growth factor-i expression in the skeletal muscle of noncachectic patients with chronic heart failure. J Am Coll Cardiol. 2002;39:1175–1181. doi: 10.1016/s0735-1097(02)01736-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, Lopaschuk GD. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ Heart Fail. 2013;6:1039–1048. doi: 10.1161/CIRCHEARTFAILURE.112.000228. [DOI] [PubMed] [Google Scholar]
- 8.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 9.Gissi HFI, Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the gissi-hf trial): A randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 10.Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, Gheorghiade M, Dei Cas L. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2011;57:870–879. doi: 10.1016/j.jacc.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. The American journal of clinical nutrition. 2011;93:402–412. doi: 10.3945/ajcn.110.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahzad K, Chokshi A, Schulze PC. Supplementation of glutamine and omega-3 polyunsaturated fatty acids as a novel therapeutic intervention targeting metabolic dysfunction and exercise intolerance in patients with heart failure. Current clinical pharmacology. 2011;6:288–294. doi: 10.2174/157488411798375958. [DOI] [PubMed] [Google Scholar]
- 13.Engel JM, Muhling J, Kwapisz M, Heidt M. Glutamine administration in patients undergoing cardiac surgery and the influence on blood glutathione levels. Acta anaesthesiologica Scandinavica. 2009;53:1317–1323. doi: 10.1111/j.1399-6576.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 14.Carubelli V, Castrini AI, Lazzarini V, Gheorghiade M, Metra M, Lombardi C. Amino acids and derivatives, a new treatment of chronic heart failure? Heart Fail Rev. 2015;20:39–51. doi: 10.1007/s10741-014-9436-9. [DOI] [PubMed] [Google Scholar]
- 15.Lombardi C, Carubelli V, Lazzarini V, Vizzardi E, Quinzani F, Guidetti F, Rovetta R, Nodari S, Gheorghiade M, Metra M. Effects of oral amino acid supplements on functional capacity in patients with chronic heart failure. Clinical Medicine Insights. Cardiology. 2014;8:39–44. doi: 10.4137/CMC.S14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Norren K, Kegler D, Argiles JM, Luiking Y, Gorselink M, Laviano A, Arts K, Faber J, Jansen H, van der Beek EM, van Helvoort A. Dietary supplementation with a specific combination of high protein, leucine, and fish oil improves muscle function and daily activity in tumour-bearing cachectic mice. British journal of cancer. 2009;100:713–722. doi: 10.1038/sj.bjc.6604905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heart Failure Society of A. Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. Hfsa 2010 comprehensive heart failure practice guideline. Journal of cardiac failure. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the kansas city cardiomyopathy questionnaire: A new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 19.Rector TS, Cohn JN. Assessment of patient outcome with the minnesota living with heart failure questionnaire: Reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan multicenter research group. Am Heart J. 1992;124:1017–1025. doi: 10.1016/0002-8703(92)90986-6. [DOI] [PubMed] [Google Scholar]
- 20.Clugston RD, Jiang H, Lee MX, Piantedosi R, Yuen JJ, Ramakrishnan R, Lewis MJ, Gottesman ME, Huang LS, Goldberg IJ, Berk PD, Blaner WS. Altered hepatic lipid metabolism in c57bl/6 mice fed alcohol: A targeted lipidomic and gene expression study. J Lipid Res. 2011;52:2021–2031. doi: 10.1194/jlr.M017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang YJ, Liu ZX, Lee HY, Distefano A, Samuel VT, Zhang D, Cline GW, Handschin C, Lin J, Petersen KF, Spiegelman BM, Shulman GI. Paradoxical effects of increased expression of pgc-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19926–19931. doi: 10.1073/pnas.0810339105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Depre C, Shipley GL, Chen W, Han Q, Doenst T, Moore ML, Stepkowski S, Davies PJ, Taegtmeyer H. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nature Medicine. 1998;4:1269–1275. doi: 10.1038/3253. [DOI] [PubMed] [Google Scholar]
- 23.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drosatos K, Bharadwaj KG, Lymperopoulos A, Ikeda S, Khan R, Hu Y, Agarwal R, Yu S, Jiang H, Steinberg SF, Blaner WS, Koch WJ, Goldberg IJ. Cardiomyocyte lipids impair beta-adrenergic receptor function via pkc activation. American journal of physiology. Endocrinology and metabolism. 2011;300:E489–E499. doi: 10.1152/ajpendo.00569.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G, Hernandez-Ono A, Crooke RM, Graham MJ, Ginsberg HN. Effects of antisense-mediated inhibition of 11beta-hydroxysteroid dehydrogenase type 1 on hepatic lipid metabolism. J Lipid Res. 2011;52:971–981. doi: 10.1194/jlr.M013748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jump DB, Depner CM, Tripathy S. Omega-3 fatty acid supplementation and cardiovascular disease. J Lipid Res. 2012;53:2525–2545. doi: 10.1194/jlr.R027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Shearer GC, Chen Q, Healy CL, Beyer AJ, Nareddy VB, Gerdes AM, Harris WS, O'Connell TD, Wang D. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic gmp/protein kinase g signaling in cardiac fibroblasts. Circulation. 2011;123:584–593. doi: 10.1161/CIRCULATIONAHA.110.971853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan RS, Chokshi A, Drosatos K, Jiang H, Yu S, Harris CR, Schulze PC, Homma S, Blaner WS, Shulman GI, Huang LS, Goldberg IJ. Fish oil selectively improves heart function in a mouse model of lipid-induced cardiomyopathy. Journal of cardiovascular pharmacology. 2013;61:345–354. doi: 10.1097/FJC.0b013e318283d845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stumvoll M, Perriello G, Meyer C, Gerich J. Role of glutamine in human carbohydrate metabolism in kidney and other tissues. Kidney international. 1999;55:778–792. doi: 10.1046/j.1523-1755.1999.055003778.x. [DOI] [PubMed] [Google Scholar]
- 30.Bowtell JL, Bruce M. Glutamine: An anaplerotic precursor. Nutrition. 2002;18:222–224. doi: 10.1016/s0899-9007(01)00795-x. [DOI] [PubMed] [Google Scholar]
- 31.Gibala MJ. Regulation of skeletal muscle amino acid metabolism during exercise. International journal of sport nutrition and exercise metabolism. 2001;11:87–108. doi: 10.1123/ijsnem.11.1.87. [DOI] [PubMed] [Google Scholar]
- 32.Khogali SE, Pringle SD, Weryk BV, Rennie MJ. Is glutamine beneficial in ischemic heart disease? Nutrition. 2002;18:123–126. doi: 10.1016/s0899-9007(01)00768-7. [DOI] [PubMed] [Google Scholar]
- 33.Scognamiglio R, Avogaro A, Negut C, Piccolotto R, de Kreutzenberg SV, Tiengo A. The effects of oral amino acid intake on ambulatory capacity in elderly subjects. Aging clinical and experimental research. 2004;16:443–447. doi: 10.1007/BF03327399. [DOI] [PubMed] [Google Scholar]
- 34.Aquilani R, Viglio S, Iadarola P, Opasich C, Testa A, Dioguardi FS, Pasini E. Oral amino acid supplements improve exercise capacities in elderly patients with chronic heart failure. Am J Cardiol. 2008;101:104E–110E. doi: 10.1016/j.amjcard.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Aquilani R, Opasich C, Gualco A, Verri M, Testa A, Pasini E, Viglio S, Iadarola P, Pastoris O, Dossena M, Boschi F. Adequate energy-protein intake is not enough to improve nutritional and metabolic status in muscle-depleted patients with chronic heart failure. European journal of heart failure. 2008;10:1127–1135. doi: 10.1016/j.ejheart.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Scognamiglio R, Negut C, Piccolotto R, Dioguardi FS, Tiengo A, Avogaro A. Effects of oral amino acid supplementation on myocardial function in patients with type 2 diabetes mellitus. Am Heart J. 2004;147:1106–1112. doi: 10.1016/j.ahj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Rogero MM, Tirapegui J, Pedrosa RG, Castro IA, Pires IS. Effect of alanyl-glutamine supplementation on plasma and tissue glutamine concentrations in rats submitted to exhaustive exercise. Nutrition. 2006;22:564–571. doi: 10.1016/j.nut.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Grau T, Bonet A, Minambres E, Pineiro L, Irles JA, Robles A, Acosta J, Herrero I, Palacios V, Lopez J, Blesa A, Martinez P Metabolism NWGSS. The effect of l-alanyl-l-glutamine dipeptide supplemented total parenteral nutrition on infectious morbidity and insulin sensitivity in critically ill patients. Critical care medicine. 2011;39:1263–1268. doi: 10.1097/CCM.0b013e31820eb774. [DOI] [PubMed] [Google Scholar]
- 39.Alves WF, Aguiar EE, Guimaraes SB, da Silva Filho AR, Pinheiro PM, Soares Gdos S, de Vasconcelos PR. L-alanyl-glutamine preoperative infusion in patients with critical limb ischemia subjected to distal revascularization reduces tissue damage and protects from oxidative stress. Annals of vascular surgery. 2010;24:461–467. doi: 10.1016/j.avsg.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Stangl R, Szijarto A, Onody P, Tamas J, Tatrai M, Hegedus V, Blazovics A, Lotz G, Kiss A, Modis K, Gero D, Szabo C, Kupcsulik P, Harsanyi L. Reduction of liver ischemia-reperfusion injury via glutamine pretreatment. The Journal of surgical research. 2011;166:95–103. doi: 10.1016/j.jss.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman JR, Ratamess NA, Kang J, Rashti SL, Kelly N, Gonzalez AM, Stec M, Anderson S, Bailey BL, Yamamoto LM, Hom LL, Kupchak BR, Faigenbaum AD, Maresh CM. Examination of the efficacy of acute l-alanyl-l-glutamine ingestion during hydration stress in endurance exercise. Journal of the International Society of Sports Nutrition. 2010;7:8. doi: 10.1186/1550-2783-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.