Abstract
The use of anti-CD20 monoclonal antibodies (mAbs), such as rituximab, in CD20-positive B-cell malignancies has dramatically improved the outcome of chronic lymphoid leukemia and non-Hodgkin’s lymphomas (NHL). However, the occurrence of relapse and development of rituximab-refractory disease highlight the need to develop novel anti-CD20 mAbs, with improved mechanisms of action. Obinutuzumab is the first humanized type II glycoengineered anti-CD20 mAb. In vitro and in vivo data suggested several differences compared with rituximab, including a low level of complement-dependent cytotoxicity and an increased direct nonapoptotic cell death. Moreover, the glycoengineered Fc-linked nonfucosylated oligosaccharide enhanced the Fc–Fcγ receptor (FcγR) IIIa interaction, resulting in improved antibody-dependent cellular cytotoxicity and phagocytosis. Preclinical models suggested that these differences translate into superior survival in murine lymphoma models. Phase I/II trials in monotherapy in relapsed or refractory B-cell NHL demonstrated that obinutuzumab has an acceptable safety profile, infusion-related reactions being the most common adverse event. In rituximab-refractory indolent NHL, the recent randomized phase III GADOLIN study demonstrated an improved median progression-free survival for patients treated with obinutuzumab plus bendamustine rather than bendamustine alone. Further trials are ongoing to determine the role of obinutuzumab as a first-line agent in the treatment of follicular lymphoma.
Keywords: obinutuzumab, rituximab, anti-CD20 monoclonal antibody, non-Hodgkin’s lymphoma
Introduction
Rituximab (Rituxan, Mabthera: Roche, Basel, Switzerland) is an anti-CD20 monoclonal antibody [mAb; immunoglobulin G1 (IgG1&IgG1κ)] approved more than 15 years ago by American and European authorities. It has dramatically changed the landscape of treatment of CD20-positive lymphoproliferative disorders [Keating, 2010] and autoimmune diseases [Gürcan et al. 2009]. Thus, alone or in combination with chemotherapy, rituximab has dramatically improved clinical outcomes in chronic lymphocytic leukemia (CLL) [Hallek et al. 2010] and B-cell non-Hodgkin’s lymphomas (NHL), for example, diffuse large B-cell lymphoma (DLBCL) [Coiffier et al. 2002] or follicular lymphoma (FL) [Hidde-mann et al. 2005]. However, relapses still occur in the majority of patients with FL or CLL and the mortality rate of patients with DLBCL is more than a third. Moreover, patients may develop rituximab-refractory malignancies [Cartron et al. 2011], usually defined by a disease progression during rituximab monotherapy or rituximab chemotherapy or by the lack of response (partial response or better) to a rituximab-containing regimen. According to the International Working Group criteria, patients who relapse within 6 months of completion of the last dose of treatment are also considered as rituximab refractory.
In contrast, resistance is a biological concept impacting clinical efficacy of a drug and results from a complex array of mechanisms including delivery issues, intracellular metabolism, pharmacokinetics, targets and challenged intracellular pathways. A clearer understanding of the mechanism of rituximab action should therefore allow improvements to be made in the efficacy of anti-CD20 mAbs and thus decrease incidence of both relapsed and refractory diseases. The Fc region of rituximab plays a critical role in triggering the cellular events that lead to B-cell elimination in vivo. The importance of the interaction between the Fc portion of rituximab and FcγR has been clearly demonstrated in patients with FL, highlighting potential ways in which anti-CD20 antibody activity may be increased [Cartron et al. 2002; Weng and Levy, 2003]. Changing the Fc region of anti-CD20 antibodies by genetic engineering or by modifying Fc glycosylation demonstrated considerable improvement of the affinity of new anti-CD20 mAbs for FcγRIIIa, and therefore enhanced antibody-dependent cell cytotoxicity antibody-dependent cell cytotoxicity (ADCC) [Cartron et al. 2004]. Antibodies that have been modified in these ways, such as obinutuzumab (GA101, Gazyva, Gazyvaro: Roche, Basel, Switzerland) [Mössner et al. 2010], ublituximab [de Romeuf et al. 2008] and ocaratuzumab [Forero-Torres et al. 2012], have entered clinical trials.
In this review, we summarize the mechanisms of action of rituximab and review the current data available on obinutuzumab, the first approved glycoengineered anti-CD20 mAb exhibiting increased ADCC. We will then focus on results currently available from clinical trials examining the use of obinutuzumab for relapsed or refractory indolent lymphomas.
Anti-CD20 mAbs mechanisms of action
The mechanisms of rituximab action include apoptosis, complement-dependent cytotoxicity (CDC), and FcγR-mediated mechanisms, including ADCC and antibody-dependent cellular phagocytosis (ADCP). Complement-enhanced ADCC (CR3-ADCC) and a vaccinal effect, whereby cell death induced by rituximab results in elicitation of a lymphoma-specific T-cell response, have also been suggested [Hilchey et al. 2009]. Most studies evaluating rituximab’s mechanisms of action have been performed in vitro on CD20-positive lymphoma cell lines and fresh lymphoma cells or in vivo in murine models. The relative contribution of each of these mechanisms in patients is therefore difficult to evaluate, especially as these may vary according to lymphoma subtype. A pioneering work [Cartron et al. 2002] established that the FcγRIIIa-158V/F polymorphism correlates with clinical response in patients who are FcγRIIIa-158V homozygous and have untreated FL demonstrating a superior clinical response to rituximab therapy. Similar results have been obtained in other clinical indications, including DLBCL and rheumatoid arthritis [Kim et al. 2006; Ruyssen-Witrand et al. 2012] with rituximab, but also with other humanized IgG1 antibodies like ofatumumab [Craigen et al. 2009]. These works underline the role of ADCC in the mechanism of action of these antibodies but also prompted the development of therapeutic monoclonal antibodies with an increased affinity for FcγRIIIa. This could be obtained either by mutation of amino acid residues of the Fc portion involved in the Fc–FcγRIIIa interaction [Shields et al. 2001] (Fc-mutated mAbs) or by modification of the oligosaccharide located between the two Fc arms. Thus, it has been demonstrated that mAbs with oligosaccharide expressing low fucose content or Fc-mutated mAbs have an increased affinity for FcγRIIIa, translating into higher ADCC on lymphoma cell lines [Shields et al. 2002; Mizushima et al. 2011].
Other mechanisms of resistance to rituximab are related to the tumor. Several studies have described that histology subtype can negatively influence response rates to anti-CD20 mAbs, such as in CLL [O’Brien et al. 2001] or mantle cell lymphoma [Ghielmini et al. 2005]. The level of CD20 expression, which differs depending on tumor histology, partially explains this phenomenon [van Meerten et al. 2006]. Moreover, some case reports and small series have described the development of CD20-negative phenotypes from CD20+ rituximab-treated lymphomas [Davis et al. 1999; Hiraga et al. 2009]. Indeed, repeated exposure to rituximab can lead to downregulation of CD20 from reduced mRNA levels and post-transcriptional modifications [Czuczman et al. 2008]. Finally, mechanisms of resistance to rituximab can be linked to the anti-CD20 mAb itself and especially its pharmacokinetics [Cartron et al. 2007]. Recently, rituximab internalization mediated by FcγRIIb has been described, resulting in reduction of its clinical efficacy [Lim et al. 2011].
Obinutuzumab, a new class of anti-CD20 mAbs
Obinutuzumab is the first humanized glycoengineered IgG1 anti-CD20 mAb to be tested in clinical trials. Obinutuzumab has been humanized by grafting the complementarity-determining region sequences from the murine antibody B-ly1 into human VH and VL acceptor frameworks [Mössner et al. 2010]. Obinutuzumab was expressed from Chinese hamster ovary (CHO) K1 cell lines engineered to constitutively overexpress the heavy and light chains of obinutuzumab as well as recombinant wild-type β-1,4-N-acetyl-glucosaminyltransferase III and wild-type Golgi α-mannosidase II leading to accumulation of antibody glycoforms containing bisected, complex, nonfucosylated oligosaccharides attached to asparagine 297 in the Fc region.
Such modifications induce increased affinity of obinutuzumab to both FcγRIIIa-158V and -158F compared with rituximab, translating into an increased induction of ADCC relative to rituximab in vitro [Mössner et al. 2010]. FcγRIIIb expressed by neutrophils shares more than 97% amino acid sequence identity with FcγRIIIa in its extracellular ligand-binding domain and the authors also demonstrated higher affinity of obinutuzumab for FcγRIIIb [Golay et al. 2013]. Consequences on neutrophil activation and ADCP induced by obinutuzumab and how this translates into an advantage compared with other anti-CD20 mAbs remain controversial [Golay et al. 2013; Herter et al. 2014]. Glycoengineering also appeared to suppress inhibition by inhibitory killer cell Ig-like receptor [Terszowski et al. 2014].
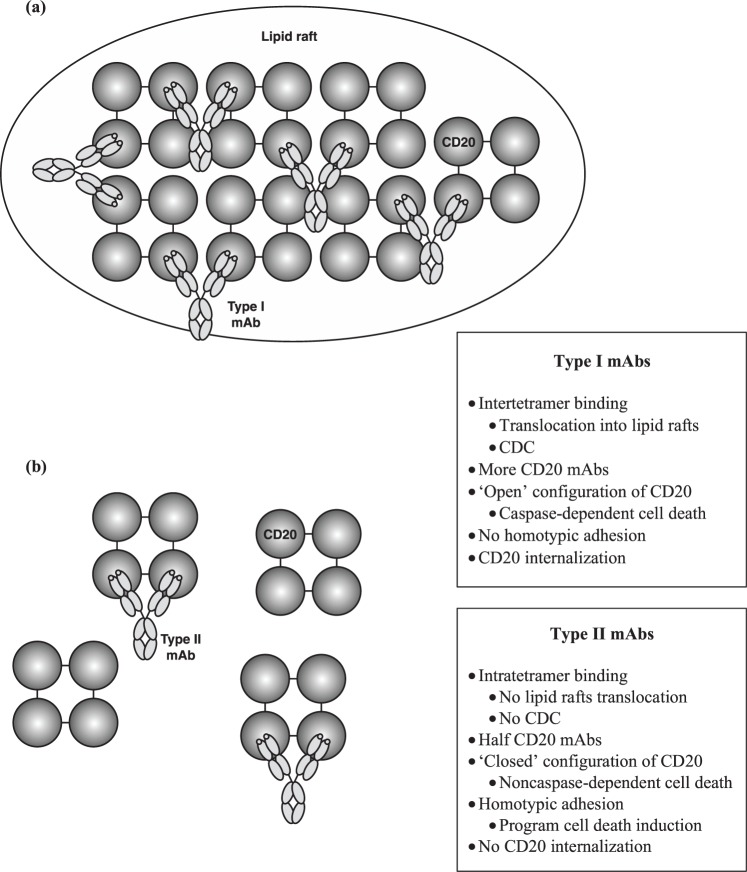
Obinutuzumab like tositumomab is a type II anti-CD20 mAb and thus differs from most anti-CD20 type I mAbs investigated (rituximab, ofatumumab, ublituximab, ocaratuzumab). The seminal difference between type I and type II mAbs is their ability to induce CDC [Cragg et al. 2003] (Table 1). This characteristic is related to the ability of mAbs to induce mAbs–CD20 translocation into lipid rafts. This phenomenon induces aggregation of the Fc portion of mAbs, therefore favoring C1q recruitment and CDC activation [Cragg and Glenny, 2004]. Only type I mAbs are able to induce mAbs–CD20 complex translocation into lipid rafts and thus a significant level of CDC, whereas type II mAbs exhibit a lower level of CDC in vitro. Since this original description, other characteristics of type II mAbs have been described, differentiating these mAbs from type I antibodies: homotypic adhesion resulting in noncaspase-dependent direct cell death, half-maximal CD20 binding at saturating conditions, less or no CD20 modulation. Except for a lack of CD20 modulation, all these in vitro properties have been described for obinutuzumab [Alduaij et al. 2011], and thus enhance direct cell death compared with rituximab [Bologna et al. 2011]. Preclinical development of obinutuzumab elucidated such differences, leading to the proposal of a model of mechanism of action [Dalle et al. 2011; Mössner et al. 2010]. Obinutuzumab conformational structure is different to that of rituximab. First, obinutuzumab binds CD20 in a different overlapping epitope than rituximab, and in a different orientation [Klein et al. 2013; Niederfellner et al. 2011]. In comparison with rituximab, obinutuzumab is rotated 90° around the Fab middle axis and tilts 70° toward the carboxy terminus of the CD20 epitope. Moreover, the elbow angle between VH and CH1 is 30° wider. This characteristic could be related to amino acid substitution at position 11, substituting a leucine for a valine [Mössner et al. 2010]. This results in a new spatial arrangement between CD20 and the antibody, and, unlike rituximab, obinutuzumab can bind its two Fab arms on the same CD20 tetramer. This difference in binding to CD20 antigen (i.e. intra-CD20 tetramer for type II versus inter-CD20 tetramer for type I) led the authors to propose a dynamic model of interaction [Klein et al. 2013], explaining the majority of in vitro observations (Figure 1).
Table 1.
Summary of functional differences between type I and type II mAbs.
| Type I mAbs: rituximab, ofatumumab, ublituximab, veltuzumab | Type II mAbs: obinutuzumab, tositumomab |
|---|---|
| Class I epitope | Class II epitope |
|
|
|
|
|
|
|
|
| Induce ADCC | |
| Induce ADCP |
ADCC, antibody-dependent cell-mediated cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; CDC, complement-dependent cytotoxicity; mAb, monoclonal antibody.
Figure 1.
Hypothetical model explaining dynamic interactions between type I (a) and type II (b) mAbs and CD20 tetramers on the B-cell surface. CDC, complement-dependent cytotoxicity; mAb, monoclonal antibody.
In xenograft models, while rituximab could not control the tumor progression, obinutuzumab alone demonstrated good efficiency in controlling disease progression of an aggressive DLBCL model (SUDHL-4 cells) [Mössner et al. 2010]. The same effect has been noticed on FL models [Dalle et al. 2011]. Moreover, obinutuzumab kept the ability to control tumor progression in rituximab-pretreated models of aggressive lymphoma, suggesting the interest of this molecule in second-line treatment for rituximab pretreated B-cell malignancies [Herter et al. 2013]. Other xenograft model studies suggest that the combination of chemotherapy and obinutuzumab could be more effective than obinutuzumab monotherapy or than rituximab and chemotherapy in combination [Dalle et al. 2011; Herting et al. 2014]. Finally, preclinical observations in a cynomolgus monkey model demonstrated that both obinutuzumab and rituximab induced similar peripheral blood B-cell depletion whereas obinutuzumab induced deeper lymphoid and splenic B-cell depletion [Mössner et al. 2010]. In whole blood from healthy donors [Mössner et al. 2010] and patients with CLL [Patz et al. 2011], obinutuzumab also demonstrated greater level of B-cell depletion compared with rituximab.
Clinical trials in indolent non-Hodgkin’s lymphomas
Obinutuzumab is the first type II glycoengineered anti-CD20 mAb to enter into clinical development. Recently, the results of a large phase III trial including rituximab-refractory indolent NHL have been published.
Phase I
The safety of obinutuzumab was first tested in three phase I trials, including relapsed or refractory indolent lymphomas. In the first of these studies [Salles et al. 2012], 21 patients with relapsed or refractory CD20+ indolent NHL received obinutuzumab monotherapy in a dose-escalating fashion. Obinutuzumab was administrated in eight 21-day cycles at different doses ranging from 50/100 to 1200/2000 mg. The overall response rate (ORR) was 43%. Five patients achieved a complete response (CR) and four a partial response (PR), all within the FL subgroup. Two of the nine rituximab-refractory patients responded. The immunotherapy was well tolerated: only seven patients (33%) experienced a grade 3/4 adverse event (AE). The most common AE (48/132) was infusion-related reaction (IRR), and hematopoietic toxicity was the most common severe AE (6/18 grade 3/4) with the occurrence of three lymphopenias, two neutropenias and one anemia. A Japanese trial [Ogura et al. 2013] described the same safety profile in a study including 12 patients with indolent CD20+ B-cell NHL. The ORR was 58% (seven patients) with two CRs and five PRs. Both the CRs were noted in patients with FL. One of the two patients with rituximab-refractory disease achieved PR, but both experienced a reduction in tumor mass. Sehn and colleagues treated 22 patients with relapsed CD20+ non-Hodgkin’s lymphoma or CLL by obinutuzumab monotherapy with a classical dosing regimen (once a week during 4 weeks) [Sehn et al. 2012]. Patients received between 200 and 2000 mg weekly and if CR or PR was achieved, a maintenance treatment was introduced with one dose every 3 months for a maximum of eight doses. The ORR was 32% with six PRs and one CR. For the rituximab-refractory subgroup (13 patients), the authors reported two responders (one CR and one PR). The safety profile was similar to previous trials. IRR was the most common AE (all grades: 73%; grade 3/4: 18%). They also reported the occurrence of five grade 3/4 neutropenias, which resolved with or without growth factor administration. These studies suggest an interesting efficacy and safety profile of obinutuzumab in heavily pretreated patients with CD20+ relapsed NHL, and clinical responses were also observed in patients with rituximab-refractory disease.
Phase II
Several phase II trials tested the efficacy of obinutuzumab in relapsed or refractory indolent B-cell NHL alone or in association with chemotherapy. The phase II of the GAUGUIN study [Salles et al. 2013] evaluated the efficacy and safety of two dose regimens of obinutuzumab: 400 mg on day 1 and 8 of the first cycle, and on day 1 of cycle 2–8 (400/400 mg) or 1600 mg on day 1 and 8 of the first cycle and 800 mg on day 1 of cycle 2–8 (1600/800 mg). Forty patients with relapsed or refractory CD20+ B-cell indolent NHL were randomly assigned to either arm. The end-of-treatment ORR was 55% in the 1600/800 mg regimen (9% of CRs), whereas it was 17% in the 400/400 mg regimen (no CR). In patients with rituximab-refractory disease (for whom the histology was not described), this ORR achieved 50% (5/10) in the 1600/800 mg arm and only 8,3% (1/12) in the alternative arm. The median progression-free survival (PFS) doubled between the two groups: 11.9 months for the 1600/800 mg group versus 6.0 months for the 400/400 mg group. As expected, the most common AE was IRR, noted by almost 75% of patients in both arms. Most of these reactions were grade 1/2, but two patients experienced grade 3/4 IRR, both in the 1600/800 mg arm. In grade 3/4 AEs, the authors also reported four infections and seven hematological toxicities (three lymphopenias, three neutropenias, one anemia). One patient discontinued the treatment due to pancreatitis in the 1600/800 mg arm. This trial demonstrated the superiority of the higher dosing regimen, especially for patients with refractory disease, with acceptable AEs.
The GAUSS phase II trial [Sehn et al. 2015b] randomized 175 patients, with relapsed indolent CD20+ NHL who previously responded to rituximab, to receive either obinutuzumab (1000 mg per week during 4 weeks) or rituximab (375 mg/m2 per week during 4 weeks). There were no patients with refractory indolent NHL in this trial. According to a central independent review, the ORRs for patients with FL (n = 149) were 44.6% and 26.7% for obinutuzumab and rituximab arms, respectively (p = 0.01). However, this difference did not correlate with an improvement in PFS. Moreover, there was no difference in CR or CR unconfirmed (CRu) rate (5.4% in the obinutuzumab arm versus 3% in the rituximab arm, p = 0.34). In terms of safety, no significant difference was found between rituximab and obinutuzumab, except for IRR and cough, which were higher in the obinutuzumab arm. This study prompted interest in the use of obinutuzumab monotherapy for relapsed or refractory indolent NHL, especially in FL.
Two others trials studied the activity of obinutuzumab in combination with chemotherapy or lenalidomide. The GAUDI study [Radford et al. 2013] included 56 patients with relapsed or refractory CD20+ FL to receive obinutuzumab in association with either CHOP (G-CHOP: obinutuzumab plus cyclophosphamide, doxorubicin, vincristine and prednisone every 3 weeks for six to eight cycles) or FC (G-FC: obinutuzumab plus fludarabine and cyclophosphamide every 4 weeks for four to six cycles). In each arm, obinutuzumab was given according to either a 1600/800 mg or a 400/400 mg schedule. Patients who responded to obinutuzumab were eligible for maintenance therapy every 3 months for up to 2 years. At the end of induction therapy, ORR was 96% in the first arm and 93% in the second, with a CR of 64% and 50%, respectively. Interestingly, all patients with rituximab-refractory disease (n = 14) achieved at least PR and four achieved CR (1/4 in the G-CHOP group, and 3/10 in the G-FC group). The most common AEs were IRR (68–82%), with 7% of grade 3/4, and hematological toxicity, with 40–50% of grade 3/4 neutropenia, especially in the G-FC arm. This study highlights that obinutuzumab in association with chemotherapy is a well tolerated and really effective therapy in FL, even in patients with rituximab-refractory disease.
More recently, the LYSA group [Morschhauser et al. 2014] published the phase Ib GALEN trial combining obinutuzumab and lenalidomide for relapsed or refractory CD20+ FL. Nineteen patients received escalating doses of lenalidomide (10, 15, 20, 25 mg) with sequential intravenous obinutuzumab at 1000 mg for six cycles. Sixty-eight percent of them achieved ORR with 53% gaining CR (10/19). The most common AEs were neutropenia (53%), constipation, and asthenia. Few grade 3/4 AEs were reported, of which neutropenia accounted for 42%. One patient in the 10 mg arm died from an unexplained worsening pleural effusion. The authors concluded that this chemotherapy-free association was effective and well tolerated, and they recommended using lenalidomide at 20 mg, due to the significant proportion of neutropenia in the 25 mg arm. A phase II study is ongoing, extended to aggressive NHL [ClinicalTrials.gov identifier: NCT 01582776].
Phase III
Model-based analyses showed that a fixed dose of 1000 mg with an additional C1 infusion resulted in similar serum concentrations to the 1600/800 mg dose [Morschhauser et al. 2011]. Thus, a fixed dose of 1000 mg of obinutuzumab, given on days 1, 8 and 15 of the first 21-day cycle, and only on the first day of the proceeding cycles has been selected for phase III studies.
The GADOLIN study [Sehn et al. 2015a] is the first phase III trial which tested obinutuzumab in rituximab-refractory CD20+ indolent NHL, according to the International Working Group criteria. Patients (n = 413) were randomly assigned to receive either bendamustine (B arm: 120 mg/m2 on days 1 and 2 for six 28-day cycles) or bendamustine (90 mg/m2 on days 1 and 2 for six 28-day cycles) in combination with obinutuzumab (GB arm: 1000 mg on days 1, 8, 15 for the first cycle and then on day 1 of each cycle). Maintenance therapy with obinutuzumab at a dosage of 1000 mg every 2 months for 2 years or until disease progression was proposed to responder patients in the GB arm. End of induction ORR was 69.2% and 63% for the GB and B arms, respectively. After a median follow up of 21 months, the median progression-free survival (PFS) was 14.9 months in the B arm, and was not reached in the GB arm [hazard ratio: 0.55 (95% CI : 0,4–0,74) p = 0.001]. In a subgroup analysis, the PFS remained statistically significant according to rituximab-refractory type (i.e. rituximab monotherapy, rituximab chemotherapy induction or rituximab maintenance after rituximab chemotherapy induction). The analysis of PFS according to histological type was not available, however 80% of patients included in the study had FL. There was no significant difference in overall survival between the groups, with 34 deaths in the GB arm (22 due to disease progression) and 41 in the B arm (29 due to disease progression). The tolerance profile was similar to previous phase I/II studies. Eleven percent of the patients in the GB arm had grade 3/4 IRRs versus 6% in the B arm. There was no significant difference in hematological grade 3/4 AEs between groups; in the GB arm 33% of patients’ treatment was complicated by neutropenia and 11% by thrombocytopenia versus 26% and 16%, respectively, in the B arm. Other frequent grade 3/4 AEs were anemia, febrile neutropenia, asthenia and digestive disorders. This study does not demonstrate any advantage to adding obinutuzumab to bendamustine in terms of response rate for the treatment of rituximab-refractory indolent lymphoma. Although there was no difference in overall survival, obinutuzumab maintenance seems to improve the PFS.
Conclusion
Obinutuzumab is the first glycoengineered type II anti-CD20 mAb to be tested in clinical trials. Current data about its mechanisms of action suggest several differences compared with rituximab, including improved ADCC and direct cell death, and a weak CDC.
Phase I trials demonstrated that IRRs were the most common side effects and occurred more frequently than with rituximab. A recent phase III trial in refractory indolent lymphomas highlighted the potential interest in obinutuzumab in this setting, with an increased PFS compared with the control arm. However, the lack of advantage in CR in this trial and the absence of PFS improvement in the GAUSS study when randomizing rituximab and obinutuzumab meant it was necessary to await the results of first-line phase III trials before definitively concluding a clinical advantage of obitunuzumab compared with rituximab.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Ludovic Gabellier, Département d’Hématologie Clinique, Centre Hospitalier Régional Universitaire de Montpellier, Montpellier, France.
Guillaume Cartron, Département d’Hématologie Clinique, Centre Hospitalier Régional Universitaire, 80 Avenue Augustin Fliche, 34295 Montpellier Cedex 05, France.
References
- Alduaij W., Ivanov A., Honeychurch J., Cheadle E., Potluri S., Lim S., et al. (2011) Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood 117: 4519–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologna L., Gotti E., Manganini M., Rambaldi A., Intermesoli T., Introna M., et al. (2011) Mechanism of action of type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J Immunol 186: 3762–3769. [DOI] [PubMed] [Google Scholar]
- Cartron G., Blasco H., Paintaud G., Watier H., Le Guellec C. (2007) Pharmacokinetics of rituximab and its clinical use: thought for the best use? Crit Rev Oncol Hematol 62: 43–52. [DOI] [PubMed] [Google Scholar]
- Cartron G., Dacheux L., Salles G., Solal-Celigny P., Bardos P., Colombat P., et al. (2002) Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 99: 754–758. [DOI] [PubMed] [Google Scholar]
- Cartron G., Trappe R., Solal-Céligny P., Hallek M. (2011) Interindividual variability of response to rituximab: from biological origins to individualized therapies. Clin Cancer Res 17: 19–30. [DOI] [PubMed] [Google Scholar]
- Cartron G., Watier H., Golay J., Solal-Celigny P. (2004) From the bench to the bedside: ways to improve rituximab efficacy. Blood 104: 2635–2642. [DOI] [PubMed] [Google Scholar]
- Coiffier B., Lepage E., Briere J., Herbrecht R., Tilly H., Bouabdallah R., et al. (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346: 235–242. [DOI] [PubMed] [Google Scholar]
- Cragg M., Glenny M. (2004) Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood 103: 2738–2743. [DOI] [PubMed] [Google Scholar]
- Cragg M., Morgan S., Chan H., Morgan B., Filatov A., Johnson P., et al. (2003) Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood 101: 1045–1052. [DOI] [PubMed] [Google Scholar]
- Craigen J., Mackus W., Engleberts P., Miller S., Speller S., Chamberlain L., et al. (2009) Ofatumumab, a human Mab targeting a membrane-proximal small-loop epitope on CD20, induces potent NK cell-medited ADCC. Blood (ASH Ann Meet Abstr) 114: 687. [Google Scholar]
- Czuczman M., Olejniczak S., Gowda A., Kotowski A., Binder A., Kaur H., et al. (2008) Acquirement of rituximab resistance in lymphoma cell lines is associated with both global CD20 gene and protein down-regulation regulated at the pretranscriptional and posttranscriptional levels. Clin Cancer Res 14: 1561–1570. [DOI] [PubMed] [Google Scholar]
- Dalle S., Reslan L., Besseyre de Horts T., Herveau S., Herting F., Plesa A., et al. (2011) Preclinical studies on the mechanism of action and the anti-lymphoma activity of the novel anti-CD20 antibody GA101. Mol Cancer Ther 10: 178–185. [DOI] [PubMed] [Google Scholar]
- Davis T., Czerwinski D., Levy R. (1999) Therapy of B-cell lymphoma with anti-CD20 antibodies can result in the loss of CD20 antigen expression. Clin Cancer Res 5: 611–615. [PubMed] [Google Scholar]
- De Romeuf C., Dutertre C., Le Farff-Tavernier M., Fournier N., Gaucher C., Glacet A., et al. (2008) Chronic lymphocytic leukaemia cells are efficiently killed by an anti-CD20 monoclonal antibody selected for improved engagement of FcgammaRIIIA/CD16. Br J Haematol 140: 635–643. [DOI] [PubMed] [Google Scholar]
- Forero-Torres A., de Vos S., Pohlman B., Pashkevich M., Cronier D., Dang N., et al. (2012) Results of a phase 1 study of AME-133v (LY2469298), an Fc-engineered humanized monoclonal anti-CD20 antibody, in FcγRIIIa-genotyped patients with previously treated follicular lymphoma. Clin Cancer Res 18: 1395–1403. [DOI] [PubMed] [Google Scholar]
- Ghielmini M., Rufibach K., Salles G., Leoncini-Franscini L., Léger-Falandry C., Cogliatti S., et al. (2005) Single agent rituximab in patients with follicular or mantle cell lymphoma: clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: a study of the Swiss Group for Clinical Cancer Research (SAKK). Ann Oncol 16: 1675–1682. [DOI] [PubMed] [Google Scholar]
- Golay J., Da Roit F., Bologna L., Ferrara C., Leusen J., Rambaldi A., et al. (2013) Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood 122: 3482–3491. [DOI] [PubMed] [Google Scholar]
- Gürcan H., Keskin D., Stern J., Nitzberg M., Shekhani H., Ahmed A. (2009) A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol 9: 10–25. [DOI] [PubMed] [Google Scholar]
- Hallek M., Fischer K., Fingerle-Rowson G., Fink A., Busch R., Mayer J., et al. (2010) Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 376: 1164–1174. [DOI] [PubMed] [Google Scholar]
- Herter S., Herting F., Mundigi O., Waldhauer I., Weinzieri T., Fauti T., et al. (2013) Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther 12: 2031–2042. [DOI] [PubMed] [Google Scholar]
- Herter S., Birk M., Klein C., Gerdes C., Umana P., Bacac M. (2014) Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J Immunol 192: 2252–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting F., Friess T., Bader S., Muth G., Hölzlwimmer G., Rieder N., et al. (2014) Enhanced anti-tumor activity of the glycoengineered type II CD20 antibody obinutuzumab (GA101) in combination with chemotherapy in xenograft models of human lymphoma. Leuk Lymphoma 55: 2151–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiddemann W., Kneba M., Dreyling M., Schmitz N., Lengfelder E., Schmits R., et al. (2005) Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German low-grade lymphoma study group. Blood 106: 3725–3732. [DOI] [PubMed] [Google Scholar]
- Hilchey S., Hyrien O., Mosmann T., Livingstone A., Friedberg J., Young F., et al. (2009) Rituximab immunotherapy results in the induction of a lymphoma idiotype-specific T-cell response in patients with follicular lymphoma: support for a ‘vaccinal effect’ of rituximab. Blood 113: 3809–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga J., Tomita A., Sugimoto T., Shimada K., Ito M., Nakamura S., et al. (2009) Down-regulation of CD20 expression in B-cell lymphoma cells after treatment with rituximab-containing combination chemotherapies: its prevalence and clinical significance. Blood 113: 4885–4893. [DOI] [PubMed] [Google Scholar]
- Keating G. (2010) Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma. Drugs 70: 1445–1476. [DOI] [PubMed] [Google Scholar]
- Kim D., Jung H., Kim J., Lee J., Yang D., Park Y., et al. (2006) FCGR3A gene polymorphisms May correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood 108: 2720–2725. [DOI] [PubMed] [Google Scholar]
- Klein C., Lammens A., Schafer W., Georges G., Schwaiger M., Mössner E., et al. (2013) Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs 5: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S., Vaughan A., Ashton-Key M., Williams E., Dixon S., Chan H., et al. (2011) Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 118: 2530–2540. [DOI] [PubMed] [Google Scholar]
- Mizushima T., Yagi H., Takemoto E., Shibata-Koyama M., Isoda Y., Iida S., et al. (2011) Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes Cells 16: 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morschhauser F., Salles G., Cartron G., Crump M., Birkett J., Carlile D., et al. (2011) Dose selection for phase III studies of the monoclonal anti-CD20 antibody obinutuzumab (GA101): a rational approach. Haematologica 96: 390. [Google Scholar]
- Morschhauser F., Salles G., Le Gouill S., Tilly H., Thieblemont C., Bouabdallah K., et al. (2014) Phase Ib study of obinutuzumab combined with lenalidomide for relapsed/refractory follicular B-cell lymphoma. Blood (ASH Ann Meet Abstr) 124: 4458. [Google Scholar]
- Mössner E., Brünker P., Moser S., Püntener U., Schmidt C., Herter S., et al. (2010) Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 115: 4393–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederfellner G., Lammens A., Mundigl O., Georges G., Schaefer W., Schwaiger M., et al. (2011) Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/ II distinction of CD20 antibodies. Blood 118: 358–367. [DOI] [PubMed] [Google Scholar]
- O’Brien S., Kantarjian H., Thomas D., Giles F., Freireich E., Cortes J., et al. (2001) Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol 19: 2165–2170. [DOI] [PubMed] [Google Scholar]
- Ogura M., Tobinai K., Hatake K., Uchida T., Suzuki T., Kobayashi Y., et al. (2013) Phase I study of obinutuzumab (GA101) in Japanese patients with relapsed or refractory B-cell non-Hodgkin lymphoma. Cancer Sci 104: 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz M., Isaeva P., Forcob N., Müller B., Frenzel L., Wendtner C., et al. (2011) Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. Br J Haematol 152: 295–306. [DOI] [PubMed] [Google Scholar]
- Radford J., Davies A., Cartron G., Morschhauser F., Salles G., Marcus R., et al. (2013) Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: results of the GAUDI study (BO21000). Blood 122: 1137–1143. [DOI] [PubMed] [Google Scholar]
- Ruyssen-Witrand A., Rouanet S., Combe B., Dougados M., Le Loët X., Sibilia J., et al. (2012) Fcγ receptor type IIIA polymorphism influences treatment outcomes in patients with rheumatoid arthritis treated with rituximab. Ann Rheum Dis 71: 875–877. [DOI] [PubMed] [Google Scholar]
- Salles G., Morschhauser F., Lamy T., Milpied N., Thieblemont C., et al. (2012) Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood 119: 5126–5132. [DOI] [PubMed] [Google Scholar]
- Salles G., Morschhauser F., Solal-Céligny P., Thieblemont C., Lamy T., Tilly H., et al. (2013) Obinutuzumab (GA101) in patients with relapsed/refractory indolent non-Hodgkin lymphoma: results from the phase II GAUGUIN study. J Clin Oncol 31: 2920–2926. [DOI] [PubMed] [Google Scholar]
- Sehn L., Assouline S., Stewart D., Mangel J., Gascoyne R., Fine G., et al. (2012) A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood 119: 5118–5125. [DOI] [PubMed] [Google Scholar]
- Sehn L., Chua N., Mayer J., Dueck G., Trněný M., Bouabdallah K., et al. (2015a) GADOLIN: Primary results from a phase III study of obinutuzumab plus bendamustine compared with bendamustine alone in patients with rituximab-refractory indolent non-Hodgkin lymphoma. J Clin Oncol 33: LBA8502. [Google Scholar]
- Sehn L., Goy A., Offner F., Martinelli G., Caballero M., Gadeberg O., et al. (2015b) Randomized phase II trial comparing obinutuzumab (GA101) with rituximab in patients with relapsed CD20+ indolent B-cell non-Hodgkin lymphoma: final analysis of the GAUSS study. J Clin Oncol 33: 3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields R., Namenuk A., Hong K., Meng Y., Rae J., Briggs J., et al. (2001) High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J Biol Chem 276: 6591–6604. [DOI] [PubMed] [Google Scholar]
- Shields R., Lai J., Keck R., O’Connell L., Hong K., Meng Y., et al. (2002) Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem 277: 26733–26740. [DOI] [PubMed] [Google Scholar]
- Terszowski G., Klein C., Stern M. (2014) KIR/HLA interactions negatively affect rituximab- but not GA101 (obinutuzumab)-induced antibody-dependent cellular cytotoxicity. J Immunol 192: 5618–5624. [DOI] [PubMed] [Google Scholar]
- van Meerten T., van Rijn R., Hol S., Hagenbeek A., Ebeling S. (2006) Complement-induced cell death by rituximab depends on CD20 expression level and acts complementary to antibody-dependent cellular cytotoxicity. Clin Cancer Res 12: 4027–4035. [DOI] [PubMed] [Google Scholar]
- Weng W., Levy R. (2003) Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 21: 3940–3947. [DOI] [PubMed] [Google Scholar]