Introduction
Moderate-to-severe atopic dermatitis (AD) often requires systemic treatments with immunomodulatory drugs. Unfortunately, systemic agents currently used for AD have extensive side effects, and many patients experience suboptimal therapeutic responses. Additionally, many of these treatments, such as cyclosporine A, can only be used for a limited time because of potentially deleterious side effects. Novel targeted treatments are now moving into clinical trials for AD, including ustekinumab.1, 2
Case report
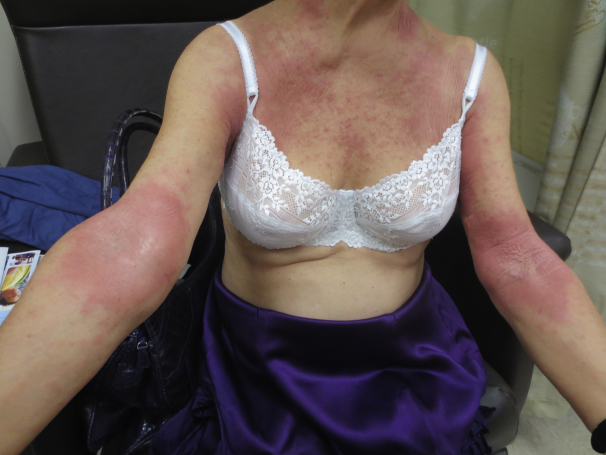
A 70-year-old, 58-kg white woman presented to the clinic in January 2014 with worsening chronic AD. She reported worsening pruritus and scaly, erythematous patches on her neck, trunk, and extremities (Fig 1). Her medical history was also significant for allergic contact dermatitis, with positive patch tests for propylene glycol, formaldehyde-releasers, and tea tree oil. Her recent worsening was not believed to be related to her concomitant allergic contact dermatitis.
Fig 1.
Initial scaly, erythematous patches.
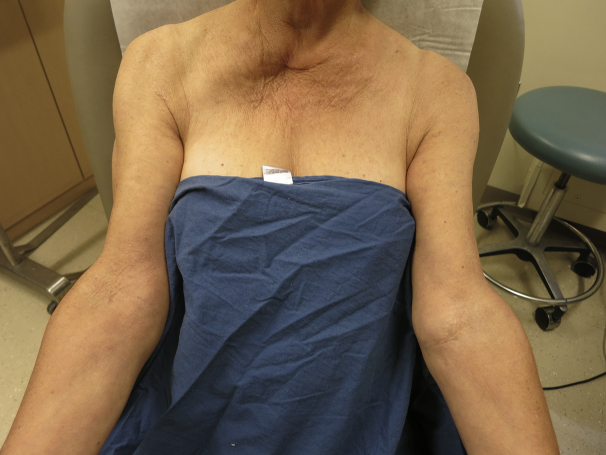
The patient had moderate-to-severe AD for 26 years, had tried numerous topical steroids with minimal benefit, and failed to show sustained improvement on narrow band ultraviolet B therapy. She initially responded to treatment with 300 mg/d of cyclosporine A, but her disease flared after 1 year, and no further improvement was achieved after adding mycophenolate, 2 g/d to her regimen for 1 month. At this time, the patient was started on 45 mg of ustekinumab, with subsequent doses given at 3 weeks, 11 weeks, and 19 weeks from baseline. The patient showed impressive improvement in erythema, lichenification, and pruritus after only 2 treatments and clearance at week 19 (Fig 2). The SCORAD (SCORing Atopic Dermatitis), which accounts for objective and subjective symptoms, was performed as part of the examination at each visit. The patient's scores decreased from 50 to 10 at week 8, remained at 10 at week 11, and decreased to 0 at week 19. After 4 doses of ustekinumab, the patient reached clearance at week 19.
Fig 2.
Drastic improvement at week 19 of ustekinumab treatment.
Discussion
Ustekinumab is a human monoclonal antibody targeting the p40 subunit of interleukin (IL)-12/23, effectively blocking the IL-23/T helper cell 17 (Th17) pathway,3 and is approved by the US Food and Drug Administration for moderate-to-severe psoriasis.
AD is increasingly recognized as a Th2- and Th22-centered disease with some contributions of Th17 and Th1 axes.4 IL-23 is believed to promote expansion of Th17 and Th22 T cells, and Th17 T cells have been reported recently to play a role in acute AD lesions.5 Thus, by blocking IL-23 in AD with ustekinumab, Th17 and Th22 pathways are inhibited.3 Few reports describe beneficial effects of ustekinumab in AD.3 This is the first description of improvement of AD disease activity with ustekinumab given in shorter time intervals in a refractory patient. Based on anecdotal experience at our institution, AD tends to exacerbate 1 to 2 weeks before the expected ustekinumab dose if the standard psoriasis dose is used. Thus, we wanted to evaluate shorter dosing intervals. Our report highlights the possible efficacy of targeting Th17/IL-23 in AD, and, unlike with psoriasis, effective treatment of AD with ustekinumab may require shorter dosing intervals, but this still needs to be evaluated in large clinical trials. The Th17/IL-23 cytokines are promising therapeutic targets, with hopes for further investigation into their possible role in AD therapeutics.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Guttman-Yassky received research grants from Regeneron, Bristol Meyers Squibb, Janssen, Celgene, Dermira, and Leo Pharmaceuticals. She is a consultant for and/or on the advisory board of Regeneron, Sanofi Aventis, GlaxoSmithKline/Steifel, Dermira, Celsus, Bristol Meyers Squibb, Celgene, Anacor, Leo Pharma, and Galderma. Dr Shroff has no direct conflicts of interest to declare.
References
- 1.Beck L.A., Thaçi D., Hamilton J.D. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 2.Rockefeller University. Pilot study of ustekinumab for subjects with chronic atopic dermatitis. Available at: http://clinicaltrials.gov/ct2/show/NCT01806662?term=rockefeller+eczema+stelara&rank=1. Accessed November 6, 2014.
- 3.Puya R., Alvarez-Lopez M., Velez A., Casas Asuncion E., Moreno J.C. Treatment of severe refractory adult atopic dermatitis with ustekinumab. Int J Dermatol. 2012;51(1):115–116. doi: 10.1111/j.1365-4632.2011.05195.x. [DOI] [PubMed] [Google Scholar]
- 4.Nograles K.E., Zaba L.C., Shemer A. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123(6):1244–1252.e2. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gittler J.K., Shemer A., Suárez-Fariñas M. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130(6):1344–1354. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]