Abstract
Substantial evidence exists that in addition to the well-known complications of diabetes, increased fracture risk is an important morbidity. This risk is probably due to altered bone properties in diabetes. Circulating biochemical markers of bone turnover have been found to be decreased in type 2 diabetes (T2D) and may be predictive of fractures independently of bone mineral density (BMD). Serum sclerostin levels have been found to be increased in T2D and appear to be predictive of fracture risk independent of BMD. Bone imaging technologies, including trabecular bone score (TBS) and quantitative CT testing have revealed differences in diabetic bone as compared to non-diabetic individuals. Specifically, high resolution peripheral quantitative CT (HRpQCT) imaging has demonstrated increased cortical porosity in diabetic postmenopausal women. Other factors such as bone marrow fat saturation and advanced glycation endproduct (AGE) accumulation might also relate to bone cell function and fracture risk in diabetes. These data have increased our understanding of how T2D adversely impacts both bone metabolism and fracture risk.
Introduction
Type 2 diabetes mellitus (T2D) is an exceedingly common chronic metabolic disorder that has an enormous impact on public health. Currently, diabetes affects over 387 million adults worldwide and is projected to reach 592 million by 2035.1 Until recently, the list of target organs affected by T2D did not include the skeleton. Yet it is now well-established that T2D is an independent risk factor for fractures, which is not attributable to increased body mass index (BMI) nor other classical osteoporosis risk factors.2 New data from epidemiologic and pathophysiologic reports, as well as from studies employing state-of the-art investigational tools, have recently increased our understanding of how T2D adversely impacts both bone metabolism and fracture risk.
Epidemiological data indicate that older adults with T2D have a higher risk of fractures, with a 50%–80% increased extremity fracture risk.3,4 A meta-analysis of 12 studies reported a relative risk of 1.7 (95% confidence interval: 1.3–2.2) for hip fracture in both men and women with T2D.5 The risk of all clinical fractures was also increased, with a summary relative risk of 1.2 (95% confidence interval: 1.0–1.5).5 Other studies have reported similar results,6 with a direct association between the duration of diabetes and increased fracture risk.7 Given this increased fracture risk, it is perhaps surprising that bone mineral density (BMD) is generally higher in those with T2D compared with those without.8 In a meta-analysis, Vestergaard et al8 reported an increased Z-score of +0.41 at the spine and +0.27 at the hip associated with T2D. In addition, a large prospective study has shown that patients with T2D have a higher fracture risk for a given femoral neck BMD T-score.9 Although dual-energy x-ray absorptiometry (DXA) is the gold standard method for the quantitative assessment of BMD, providing areal BMD of the hip, spine, radius, and total body (including body composition), it has limitations in patients with complex metabolic bone diseases such as chronic kidney disease or diabetic bone disease. It has therefore been suggested that fragility fractures in T2D may result from diabetes-related alterations in skeletal properties not captured by DXA.2 Similarly, patients with T2D have a higher fracture risk for a given FRAX probability (https://www.shef.ac.uk/FRAX/).9 The FRAX algorithm provides the 10-year probability of major osteoporotic fractures per individual tested. A recent study has demonstrated that diabetes mellitus does not modify the effects of risk factors incorporated into FRAX.10 Nevertheless, the study has shown that diabetes has stronger effects on hip fracture risk in younger than older individuals which warrants special consideration for diabetic fracture prevention in clinical practise.
The paradox of higher BMD in association with increased fractures might be attributed to more frequent trauma, as diabetes is associated with an increased frequency of falls.11 However, in studies of diabetes and fracture that controlled for fall frequency, diabetes still remained independently associated with increased fracture risk.3,4 Thiazolidinediones use might also be considered as an explanation, since it has been proposed that these agents divert mesenchymal stem cells from the osteogenic to the adipocytic lineage and are associated with bone loss and increased fracture risk, particularly in women.12 However, thiazolidinedione use cannot fully account for the increased risk of fracture observed with diabetes, since most studies included substantial observation time prior to the widespread use of these medications. Rather, it appears that other bone properties, which are undetectable by DXA, are probably contributing to fracture risk in diabetes.
PTH and biochemical markers of bone turnover in T2D
Decreased bone remodeling in T2D has been demonstrated by a number of lines of evidence. Levels of parathyroid hormone (PTH) tend to be 20%–50% lower in T2D subjects than in controls, even in the setting of reduced eGFR, suggesting a state of reduced PTH secretion in T2D.13–15 Circulating biochemical markers of bone formation, including P1NP, osteocalcin (OCN)14,15 and bone specific alkaline phosphatase16 have been found to be decreased in T2D. These decreases in formation measures are associated with reductions in the bone resorption marker serum CTx.13–16 The decrease in bone remodeling in T2D appears to be predictive of fracture risk regardless of BMD. In a study of 255 T2D women and 240 controls, T2D women with the combination of the lowest PTH and OCN levels had nearly a fivefold increased risk of vertebral fractures independent of lumbar spine BMD.15
DYNAMIC histomorphometry in T2D
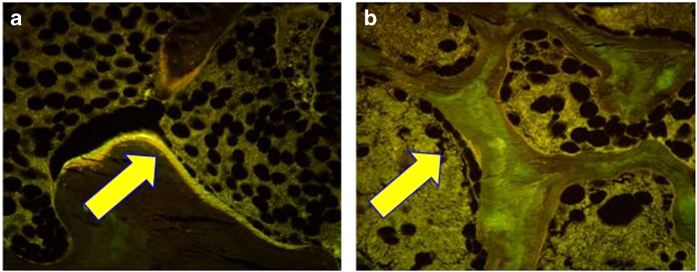
Lower bone formation in T2D on biopsy was reported in one study, but the numbers were very small (n=6 T2D patients; 2 female), and the results were confounded by selecting for low BMD and a problematical control group.17 In a more recent pilot study, low-bone formation was observed in six T2D postmenopausal women and six postmenopausal age-matched non-diabetic controls, where tetracycline double-labeled iliac crest bone biopsies showed virtually no uptake of label in diabetic subjects (Figure 1), with reduced mineralizing surface, osteoid surface, and osteoblast surface.18 Interestingly, corresponding reductions in bone resorption indices were not present, perhaps suggesting a disproportionate reduction in bone formation in diabetes as compared with bone resorption.
Figure 1.
Histomorphometric changes in bone formation. (a and b) Tetracycline double-labeled bone biopsies in a 57-year-old Caucasian female control (a) and a 58-year-old T2D Caucasian woman (b) bone formation is decreased in T2D with reduced mineralizing surface. The arrows highlight tetracycline uptake in the control subject and the absence of uptake in the diabetic subject. Adapted with permission from ref. 18.
Other bone markers in T2D
Insulin-like growth factor (IGF)-1, an anabolic factor which stimulates osteoblast proliferation, has been inversely associated with the risk and number of vertebral fractures in diabetic women independent of BMD.14,19 Another marker which might reflect bone formation is that of circulating osteogenic precursor cells,20 which have been reported to be decreased in patients with T2D (Figure 2). Circulating osteogenic precursor cells can be detected in the peripheral blood by flow cytometry using antibodies specific for the osteoblast matrix protein OCN.21 Peripheral blood mononuclear cells that were positive for OCN were lower in postmenopausal women with T2D as compared with non-diabetic controls.18 Moreover, within the decreased pool of overall OCN+ cells, the T2D subjects had an increased subpopulation of immature OCN+ cells, that is, cells that also had early markers CD146 and CD34, subpopulations which diminish when osteoblasts mature.20 An additional novel bone marker in T2D may be sphingosine 1-phosphate (S1P), a lipid mediator which increases osteoclastogenesis by increasing RANKL.22 S1P was found to be increased in T2D women (n=482) as compared with controls and was associated with vertebral fractures. Interestingly, this marker suggests an elevation in bone resorption in T2D, in contrast to the reports of decreased s-CTx levels.13–16 It is possible that s-CTx underestimates the level of bone resorption in diabetes because enzymatic cross-linking of bone collagen by lysyl oxidase is reduced in diabetes,23,24 such that less cross-linked telopeptides might be released in diabetes during bone resorption.
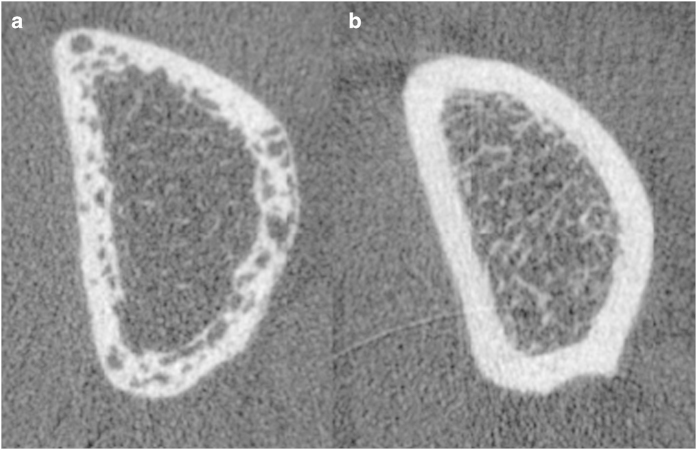
Figure 2.
Cortical porosity in diabetic bone disease with fractures. High-resolution peripheral quantitative computed tomography (HR-pQCT) of the distal radius in type 2 diabetic women with (a) and without (b) fragility fractures.64 Image courtesy: Thomas M. Link, Department of Radiology and Biomedical Imaging, The University of California, San Francisco.
Sclerostin in T2D
Sclerostin, an osteocyte product, is a negative regulator of bone formation which competes with the anabolic Wnt β-catenin pathway by binding to LRP5 or 6.25 In healthy adults, sclerostin levels are increased by factors including age, BMI, inactivity, bone mineral content, and possibly fractures.25 It was first reported in 2012 that sclerostin levels were higher in 74 T2D women and men versus 50 non-diabetic controls and that higher levels correlated with age, male gender and BMD.26 This observation was corroborated by another report in which sclerostin levels were found to be twofold higher in T2D than in controls or T1D, after adjusting for age and BMI.27 A correlation between Wnt disruption and decreased osteoblast activity was further observed in 40 T2D postmenopausal women who, as compared with controls, had decreased β-catenin levels which correlated with lower BAP.16 In the largest diabetes sclerostin study, higher sclerostin levels in 321 men and women with T2D were associated with an increased risk of vertebral fractures independent of lumbar spine BMD.28 Interestingly, diabetic postmenopausal women with fragility fractures were shown to demonstrate significantly higher serum sclerostin levels than diabetic postmenopausal women without fragility fractures.29 While BMI, renal function, glycemic control, and diabetes medication (including insulin) were comparable between women with and without fragility fractures, diabetes duration was significantly longer in those that had sustained fractures. It could be posited from these data that the higher sclerostin levels in T2D reflect the presence of more deeply embedded osteocytes in older bone that has accumulated more microscopic damage. Thus prolonged low-bone turnover in diabetes, as a result of Wnt inhibition, may lead to defective microdamage repair and increased bone microcrack accumulation in a manner reminiscent of high-dose bisphosphonate therapy,30 thus contributing to greater bone fragility. Stressing the interplay between bone health and vascular health and considering diabetic fractures to be true diabetic complications, it is also noteworthy that in diabetics higher serum sclerostin levels appear to be associated with higher amounts of vascular calcifications.31
Ages and bone remodeling
Decreased bone formation might occur in part because of increased advanced glycation endproducts (AGEs) in bone collagen. AGEs are a diverse group of compounds that are generated through the non-enzymatic glycation or glycoxidation of proteins, lipids, and nucleic acids32 with the best-studied being carboxymethyl-lysine and pentosidine.33–36 These compounds are markedly increased in patients with diabetes,35 forming non-enzymatic cross-links within and across collagen fibers.37,38 AGEs interfere with normal osteoblast function39 and attachment to the collagen matrix,40 as well as impair osteoblast development.41,42 AGEs also decrease bone resorption by altering the structural integrity of bone matrix proteins and inhibiting the osteoclastic differentiation process.43 This might have long-lasting skeletal effects that are similar to the “hyperglycemic metabolic memory” that has been described with AGE accumulation in other tissues.44 In the Diabetes Control and Complications Trial (DCCT), accumulation of AGEs in skin collagen of type 1 diabetes patients predicted complications decades later, regardless of subsequent improvements in glycemic control.45 In the bone matrix, accumulation of AGEs leads to more biomechanically brittle bone that has lost its toughness and is less able to deform before fracturing.30 Urinary pentosidine, the best-studied AGE, was associated with a 42% increase in clinical fracture incidence in T2D.38 Although the relationship between in vivo bone and circulating levels of AGEs has not been fully elucidated, AGEs are likely related to both low-bone formation and increased bone fragility in T2D.
Assessment of fracture risk in T2D: from current clinical practice to novel imaging biomarkers
Irrespective of diabetes history, in clinical practice fracture risk is routinely assessed by measuring BMD and by determining the presence of clinical risk factors including age, sex, BMI, smoking and drinking habits, history of fragility fractures, parental fractures, rheumatoid arthritis, and known secondary osteoporosis. These factors are part of the aforementioned, questionnaire-based fracture risk assessment tool (FRAX).46
BMD can be measured by DXA and quantitative computed tomography (QCT) at central and peripheral sites.47 Although DXA can predict fracture risk in diabetic patients to a certain extent, experts have suggested the introduction of a “diabetic correction factor” for T-scores because given T-scores were shown to be associated with a higher risk of fracture in older adults with T2D than in those without DM.48 Similar concepts have been suggested for FRAX scores in elderly subjects with T2D.48 The use of trabecular bone score (TBS) has been also suggested to improve the diagnostic performance of lumbar spine DXA in patients with T2D: from an image processing perspective, TBS is based on two-dimensional texture-analysis of DXA images. Applying methodologies originally used in pattern recognition (for example, in geostatistics characterizing landscapes from aerial views), textural variations are quantified between neighboring gray-scale pixels.49 In clinical practise, operators and clinicians are only presented with the end-result of these calculations: A single (TBS) index which is higher in images with fewer inter-pixel variations (that is, indirect measure of better spinal bone microarchitecture) and lower in images with higher inter-pixel variations (that is, indirect measure of worse spinal bone microarchitecture).50 While BMD is typically normal to higher in subjects with diabetes, TBS appears to be lower in diabetics than in non-diabetic subjects.51 In the same publication, Leslie et al.51 also showed that lumbar spine TBS can predict osteoporotic fractures irrespective of the presence or absence of diabetes. In addition, TBS has been shown to be positively associated with good glycemic control.52,53 While these results are of major clinical interest and relevance, it remains to be stressed that—from an imaging standpoint—TBS only provides an indirect measure of bone microarchitecture at low resolution and reduced image quality.
While DXA is a projectional technique, central QCT provides volumetric data on BMD and bone geometry of the spine and the proximal femur. For QCT, a clinical multidetector-CT scanner, a calibration phantom mat, and dedicated software are needed.54 Alterations in volumetric hip BMD—as determined by QCT in diabetic versus non-diabetic subjects—have been confirmed by two independent studies.29,53–55 One of these studies also concluded that patients with T2D appear to have little benefit from elevated BMD due to unchanged load-to-strength ratios.55
High-resolution peripheral QCT
Within the last two decades, dedicated peripheral QCT scanners have been developed to capture volumetric BMD of peripheral sites (that is, the extremities) at varying resolution. In addition to BMD and geometric measures, high-resolution peripheral QCT (HR-pQCT) provides quantitative access to bone microarchitecture, an important surrogate of bone quality and bone strength.56,57 Many micro-architectural parameters quantifiable by HR-pQCT refer to those initially coined by static histomorphometry (for example, trabecular number, trabecular thickness).58 In the first generation HR-pQCT scanner, which has a spatial resolution of ~80 μm, only two microstructural parameters—trabecular number and trabecular heterogeneity—are directly measured by three-dimensional distance transformations.59 Trabecular separation and trabecular thickness are derived from trabecular BMD and trabecular number, which are determined by threshold-based segmentation and a ridge-counting technique.60 Recently, a second generation scanner with shorter scan time and higher spatial resolution has been introduced. Using this second generation scanner, all micro-architectural indices can be calculated directly.61 It is noteworthy that so far, all published HR-pQCT data on bone microarchitecture in T2D have been acquired with scanners of the first generation.62–65
First evidence of associations between impaired cortical strength and T2D originally came from a pQCT study in a cohort of elderly men.66 The first HR-pQCT study investigating bone microarchitecture in T2D then raised the topic of high cortical porosity as a potential predominant structural phenotype:62 while trabecular BMD and trabecular thickness were greater in postmenopausal women with T2D than in those without, high cortical porosity was observed (Figure 2). Based on the unstratified enrollment of diabetic women with and without fractures, this study lead to the formation of a key hypothesis for subsequent research: could cortical porosity serve as a discriminative feature between diabetic women with and without fragility fractures? In line with this hypothesis, Shu et al,63 also reported no differences in peripheral bone microarchitecture between postmenopausal women with and without T2D (without history of fragility fractures). Specifically enrolling postmenopausal diabetic women with and without fragility fractures and comparing them with non-diabetic controls with and without fractures, the importance of high cortical porosity with relatively maintained trabecular properties could be confirmed.64 Interestingly, diabetic women with and without fractures were comparable with respect to clinical characteristics: age, BMI, glycemic control, 25(OH)-vitamin D, kidney function, and PTH levels were similar. The only significant clinical difference was the duration of diabetes: diabetic women with fractures had been suffering from diabetes significantly longer than diabetic women without fractures (13 years versus 8 years). Using finite element analyses, deficits in stiffness, failure load, and cortical load fraction could be detected and attributed to cortical porosity.64 Considering the high prevalence of insulin resistance and T2D in the African-American population, a forth HR-pQCT study specifically focused on the assessment of bone microarchitecture in postmenopausal African-American women: In line with above mentionned studies of mixed racial recruitment, Yu et al. 65 found T2D and unfavorable cortical bone microarchitecture to be similarly associated in African-American women.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) provides an alternative method of depicting and quantifying bone geometry and bone microarchitecture.47 High-resolution MRI has been used to study bone microarchitecture in postmenopausal and age-related osteoporosis,67–69 secondary osteoporosis,70 chronic kidney disease,71 and with respect to treatment effects of anti-osteoporotic drugs.72,73 Regarding diabetic bone disease, there is only a limited number of publications using MRI. Specifically, a Canadian researcher reported poorer bone microarchitecture expressed as greater trabecular heterogeneity in subjects with diabetes mellitus than in healthy controls.74 Although compared with HR-pQCT, MRI has certain disadvantages in studying bone microarchitecture (for example, lower resolution, higher technical complexity in acquisition and postprocessing of image data, no option of measuring BMD, and fewer reference data), the method does hold significant potential for bone research. Specifically, MRI offers the option of imaging the skeleton beyond its geometry and structure. Using techniques such as magnetic resonance-perfusion and MR-spectrocopy, novel imaging surrogates of bone strength (for example, skeletal blood flow and regional biochemical composition of bone marrow) have been introduced.75,76 Recent MR-imaging data have demonstrated that altered bone marrow fat composition is linked with T2D and fragility fractures in postmenopausal women.77 Even after adjustment for age, race, and local (that is, spinal) BMD, lower unsaturated bone marrow fat and higher saturated bone marrow fat as determined by MR-spectroscopy were significantly associated with T2D. Of interest, postmenopausal diabetic women with a history of fragility fractures displayed the lowest unsaturation and the highest saturation levels. The relevance of these findings is supported by epidemiologic data linking low dietary intake of n-3 poly-unsaturated fatty acids (PUFA) with low BMD78–80 and the risk of hip fractures.81 Preclinical data support a potential osteo-protective role of PUFA.82–85 Vice versa, saturated dietary fatty acids appear to be linked with osteoporotic fractures.86 With poor glycemic control also leading to accelerated aging of adipocytes and thus adipocyte dysfunction,87–90 changes in bone marrow fat composition of subjects with T2D are likely to result from a multitude of interacting etiologic factors. It is conceivable that bone marrow adiposity might reflect a shift in stem cell lineage away from osteoblastogenesis toward adipogenesis.91 In the AGEs–Reykjavik cohort (115 men and 134 women; mean age 79; 7% with diabetes), sclerostin levels were positively associated with marrow fat by MRI in men independent of BMD.91 This positive relationship could be explained by a shift in precursor stem cell lineage away from osteoblastogenesis, as reflected by higher sclerostin levels, toward adipogenesis, as reflected by higher bone marrow fat.91
Obesity as a potential confounder
Confounding greater understanding of the mechanisms responsible for increased skeletal fragility in T2D is the frequent concurrence of obesity, which has also recently been shown to be a risk factor for fractures.92–94 Mechanisms proposed to be responsible for increased skeletal fragility in both obesity and T2D include structural, metabolic, material, dynamic, and imaging abnormalities. However, the relative contribution of each condition separately and their combined effects on skeletal fragility remain unclear. The concurrence of obesity and T2D in most individuals has undermined attempts to gain pathogenetic clarity. For example, obese women have been reported to have trabecular and cortical micro-architectural abnormalities, but their diabetic status was not considered.95 Moreover, obese diabetics have lower biochemical and quantitative histomorphometric indices of bone formation,17,96 elevated circulating levels of the osteocyte product sclerostin,27 increased adipocyte markers,97 and abnormal bone marrow fat composition,77 however, these abnormalities are also found in obese individuals without T2D.98–101 Whether T2D has negative effects on specific skeletal parameters that are distinct from the adverse skeletal effects of obesity remains to be clarified.
A continuing quest for novel non-invasive biomarkers reflecting high fracture risk in patients with T2D
Minimal invasive testing of bone strength (“micro-indentation”) is nowadays possible in the setting of clinical research. After local anesthesia at the mid-shaft point of the anterior tibia, a handheld device is inserted through soft tissue and periosteum, to touch the bone surface. Using triggered, pre-defined impacts small bony indentations (that is, local superficial microfractures) are created (<350 μm). The deeper the mean indentation distance, the weaker bone material properties are at the testing site of an individual subject.102,103 Using the most recent generation in vivo testing device, Farr et al.104 have shown that postmenopausal diabetic women had significantly lower bone material strength than non-diabetic controls. Poor bone material strength correlated with poor long-term glycemic control over the past 10 years.104 In vivo micro-indentation has been used to quantify reduced bone strength in patients with osteoporotic hip fractures and atypical femoral fractures.105,106 To the best of our knowledge, in vivo micro-indentation data are still lacking for patients with T1D, men, and patients with fragility fractures.
Given significant advances in knowledge within the last decade, dedicated research in diabetic bone disease with and without fractures and development of novel patient-oriented biomarkers continue to be warranted.
Summary and conclusion
There is growing evidence that the skeleton is an important target organ for complications of T2D. Many large prospective studies have established that T2D is associated with an increased risk of fractures. Yet it remains unknown how to identify which patients with T2D are at increased risk because it is uncertain why bone fragility is increased when aBMD is normal. Available evidence suggests that compromised bone quality, not deficits in aBMD, is the underlying basis for fragility fractures in patients with T2D. Clues supporting this hypothesis include alterations in bone remodeling, increased cortical porosity, and decreased bone material properties. More information is needed about the pathogenesis of these abnormalities and their relationship to the increased fracture risk observed in T2D. Whether a circulating marker, such as an AGE or sclerostin, would be predictive of alterations in bone material properties or cortical porosity remains to be determined. Further investigation of skeletal parameters would shed light on the issue of greater bone fragility in T2D and potentially offset serious challenges in this population as they age.
References
- IDF Diabetes Atlas 2014. Available at http://www.idf.org/diabetesatlas/update-2014.
- Leslie WD , Rubin MR , Schwartz AV et al. Type 2 diabetes and bone. J Bone Miner Res 2012; 27: 2231–2237. [DOI] [PubMed] [Google Scholar]
- Schwartz AV , Sellmeyer DE , Ensrud KE et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab 2001; 86: 32–38. [DOI] [PubMed] [Google Scholar]
- Bonds DE , Larson JC , Schwartz AV et al. Risk of fracture in women with type 2 diabetes: the Women's Health Initiative Observational Study. J Clin Endocrinol Metab 2006; 91: 3404–3410. [DOI] [PubMed] [Google Scholar]
- Janghorbani M , Van Dam RM , Willett WC et al. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007; 166: 495–505. [DOI] [PubMed] [Google Scholar]
- Leslie WD , Lix LM , Prior HJ et al. Biphasic fracture risk in diabetes: a population-based study. Bone 2007; 40: 1595–1601. [DOI] [PubMed] [Google Scholar]
- de Liefde II , van der Klift M , de Laet CE et al. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam study. Osteoporos Int 2005; 16: 1713–1720. [DOI] [PubMed] [Google Scholar]
- Vestergaard P . Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int 2007; 18: 427–444. [DOI] [PubMed] [Google Scholar]
- Giangregorio LM , Leslie WD , Lix LM et al. FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res 2012; 27: 301–308. [DOI] [PubMed] [Google Scholar]
- Leslie WD , Morin SN , Lix LM et al. Does diabetes modify the effect of FRAX risk factors for predicting major osteoporotic and hip fracture? Osteoporos Int 2014; 25: 2817–2824. [DOI] [PubMed] [Google Scholar]
- Schwartz AV , Hillier TA , Sellmeyer DE et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25: 1749–1754. [DOI] [PubMed] [Google Scholar]
- Loke YK , Singh S , Furberg CD . Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ 2009; 180: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobnig H , Piswanger-Solkner JC , Roth M et al. Type 2 diabetes mellitus in nursing home patients: effects on bone turnover, bone mass, and fracture risk. J Clin Endocrinol Metab 2006; 91: 3355–3363. [DOI] [PubMed] [Google Scholar]
- Ardawi MS , Akhbar DH , Alshaikh A et al. Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone 2013; 56: 355–362. [DOI] [PubMed] [Google Scholar]
- Yamamoto M , Yamaguchi T , Nawata K et al. Decreased PTH levels accompanied by low-bone formation are associated with vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 2012; 97: 1277–1284. [DOI] [PubMed] [Google Scholar]
- Gaudio A , Privitera F , Battaglia K et al. Sclerostin levels associated with inhibition of the WNT/beta-catenin signaling and reduced bone turnover in type 2 diabetes mellitus. J Clin Endocrinol Metab 2012; 97: 3744–3750. [DOI] [PubMed] [Google Scholar]
- Krakauer JC , McKenna MJ , Buderer NF et al. Bone loss and bone turnover in diabetes. Diabetes 1995; 44: 775–782. [DOI] [PubMed] [Google Scholar]
- Manavalan JS , Cremers S , Dempster DW et al. Circulating osteogenic precursor cells in type 2 diabetes mellitus. J Clin Endocrinol Metab 2012; 97: 3240–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa I , Yamaguchi T , Sugimoto T . Serum insulin-like growth factor-I is a marker for assessing the severity of vertebral fractures in postmenopausal women with type 2 diabetes mellitus. Osteoporos Int 2011; 22: 1191–1198. [DOI] [PubMed] [Google Scholar]
- Eghbali-Fatourechi GZ , Modder UI , Charatcharoenwitthaya N et al. Characterization of circulating osteoblast lineage cells in humans. Bone 2007; 40: 1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin MR , Manavalan JS , Dempster DW et al. Parathyroid hormone stimulates circulating osteogenic cells in hypoparathyroidism. J Clin Endocrinol Metab 2011; 96: 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardawi M , Akbar D , Rouzi A et al. Elevated sphingosine 1-phosphate levels are associated with vertebral fractures in patients with type 2 diabetes mellitus. J Bone Miner Res 2014; 29 Suppl 1. Available at http://www.asbmr.org/education/AbstractDetail?aid=51ef5a65-8072-4369-b2b7-c1381053da59. 11 December 2014. [Google Scholar]
- Saito M , Fujii K , Mori Y et al. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int 2006; 17: 1514–1523. [DOI] [PubMed] [Google Scholar]
- Khosravi R , Sodek KL , Faibish M et al. Collagen advanced glycation inhibits its discoidin domain receptor 2 (DDR2)-mediated induction of lysyl oxidase in osteoblasts. Bone 2014; 58: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E . Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol 2013; 9: 575–583. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin A , Rozas-Moreno P , Reyes-Garcia R et al. Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2012; 97: 234–241. [DOI] [PubMed] [Google Scholar]
- Gennari L , Merlotti D , Valenti R et al. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J Clin Endocrinol Metab 2012; 97: 1737–1744. [DOI] [PubMed] [Google Scholar]
- Yamamoto M , Yamauchi M , Sugimoto T . Elevated sclerostin levels are associated with vertebral fractures in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2013; 98: 4030–4037. [DOI] [PubMed] [Google Scholar]
- Heilmeier U , Carpenter DR , Patsch JM et al. Volumetric femoral BMD, bone geometry, and serum sclerostin levels differ between type 2 diabetic postmenopausal women with and without fragility fractures. Osteoporos Int 2015; 26: 1283–1293. [DOI] [PubMed] [Google Scholar]
- Tang SY , Allen MR , Phipps R et al. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int 2009; 20: 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Santana S , Garcia-Fontana B , Garcia-Martin A et al. Atherosclerotic disease in type 2 diabetes is associated with an increase in sclerostin levels. Diabetes Care 2013; 36: 1667–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye EB , Degenhardt TP , Thorpe SR et al. Role of the Maillard reaction in aging of tissue proteins. Advanced glycation end product-dependent increase in imidazolium cross-links in human lens proteins. J Biol Chem 1998; 273: 18714–18719. [DOI] [PubMed] [Google Scholar]
- Monnier VM , Sell DR , Genuth S . Glycation products as markers and predictors of the progression of diabetic complications. Ann NY Acad Sci 2005; 1043: 567–581. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ , Langborg A , Minhas HS . Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J 1999; 344 Pt 1: 109–116. [PMC free article] [PubMed] [Google Scholar]
- Makita Z , Radoff S , Rayfield EJ et al. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med 1991; 325: 836–842. [DOI] [PubMed] [Google Scholar]
- Brownlee M , Cerami A , Vlassara H . Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 1988; 318: 1315–1321. [DOI] [PubMed] [Google Scholar]
- Vashishth D . The role of the collagen matrix in skeletal fragility. Curr Osteoporos Rep 2007; 5: 62–66. [DOI] [PubMed] [Google Scholar]
- Schwartz AV , Garnero P , Hillier TA et al. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab 2009; 94: 2380–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguineti R , Storace D , Monacelli F et al. Pentosidine effects on human osteoblasts in vitro. Ann NY Acad Sci 2008; 1126: 166–172. [DOI] [PubMed] [Google Scholar]
- McCarthy AD , Uemura T , Etcheverry SB et al. Advanced glycation endproducts interefere with integrin-mediated osteoblastic attachment to a type-I collagen matrix. Int J Biochem Cell Biol 2004; 36: 840–848. [DOI] [PubMed] [Google Scholar]
- McCarthy AD , Etcheverry SB , Cortizo AM . Effect of advanced glycation endproducts on the secretion of insulin-like growth factor-I and its binding proteins: role in osteoblast development. Acta Diabetol 2001; 38: 113–122. [DOI] [PubMed] [Google Scholar]
- Kume S , Kato S , Yamagishi S et al. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res 2005; 20: 1647–1658. [DOI] [PubMed] [Google Scholar]
- Valcourt U , Merle B , Gineyts E et al. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J Biol Chem 2007; 282: 5691–5703. [DOI] [PubMed] [Google Scholar]
- Zhang L , Chen B , Tang L . Metabolic memory: mechanisms and implications for diabetic retinopathy. Diabetes Res Clin Pract 2012; 96: 286–293. [DOI] [PubMed] [Google Scholar]
- Genuth S , Sun W , Cleary P et al. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 2005; 54: 3103–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis JA . Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002; 359: 1929–1936. [DOI] [PubMed] [Google Scholar]
- Link TM . Osteoporosis imaging: state of the art and advanced imaging. Radiology 2012; 263: 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AV , Vittinghoff E , Bauer DC et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 2011; 305: 2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothuaud L , Carceller P , Hans D . Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone 2008; 42: 775–787. [DOI] [PubMed] [Google Scholar]
- Harvey NC , Gluer CC , Binkley N et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 2015; 78: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie WD , Aubry-Rozier B , Lamy O et al. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab 2013; 98: 602–609. [DOI] [PubMed] [Google Scholar]
- Dhaliwal R , Cibula D , Ghosh C et al. Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int 2014; 25: 1969–1973. [DOI] [PubMed] [Google Scholar]
- Kim JH , Choi HJ , Ku EJ et al. Trabecular bone score as an indicator for skeletal deterioration in diabetes. J Clin Endocrinol Metab 2015; 100: 475–482. [DOI] [PubMed] [Google Scholar]
- Shepherd JA , Schousboe JT , Broy SB et al. Executive summary of the 2015 ISCD position development conference on advanced measures from DXA and QCT: fracture prediction beyond BMD. J Clin Densitom 2015; 18: 274–286. [DOI] [PubMed] [Google Scholar]
- Melton LJ 3rd , Riggs BL , Leibson CL et al. A bone structural basis for fracture risk in diabetes. J Clin Endocrinol Metab 2008; 93: 4804–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch JM , Burghardt AJ , Kazakia G et al. Noninvasive imaging of bone microarchitecture. Ann NY Acad Sci 2011; 1240: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Consensus Development Panel on Osteoporosis Prevention D, Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001; 285: 785–795. [DOI] [PubMed] [Google Scholar]
- Parfitt AM , Drezner MK , Glorieux FH et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Mineral Res 1987; 2: 595–610. [DOI] [PubMed] [Google Scholar]
- Hildebrand T , Ruegsegger P . A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc 1997; 185: 67–75. [Google Scholar]
- Laib A , Hauselmann HJ , Ruegsegger P . In vivo high resolution 3D-QCT of the human forearm. Technol Health Care 1998; 6: 329–337. [PubMed] [Google Scholar]
- Manske SL , Zhu Y , Sandino C et al. Human trabecular bone microarchitecture can be assessed independently of density with second generation HR-pQCT. Bone 2015; 79: 213–221. [DOI] [PubMed] [Google Scholar]
- Burghardt AJ , Issever AS , Schwartz AV et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2010; 95: 5045–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu A , Yin MT , Stein E et al. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos Int 2011; 23: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch JM , Burghardt AJ , Yap SP et al. Increased cortical porosity in type-2 diabetic postmenopausal women with fragility fractures. J Bone Mineral Res 2012; 28: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu EW , Putman MS , Derrico N et al. Defects in cortical microarchitecture among African-American women with type 2 diabetes. Osteoporos Int 2014; 26: 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit MA , Paudel ML , Taylor BC et al. Bone mass and strength in older men with type 2 diabetes: the osteoporotic fractures in men study. J Bone Miner Res 2010; 25: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S , Genant HK , Grampp S et al. Correlation of trabecular bone structure with age, bone mineral density, and osteoporotic status: in vivo studies in the distal radius using high resolution magnetic resonance imaging. J Bone Miner Res 1997; 12: 111–118. [DOI] [PubMed] [Google Scholar]
- Majumdar S , Link TM , Augat P et al. Trabecular bone architecture in the distal radius using magnetic resonance imaging in subjects with fractures of the proximal femur.. Osteoporosis Int 1999; 10: 231–239. [DOI] [PubMed] [Google Scholar]
- Link TM , Majumdar S , Augat P et al. In vivo high resolution MRI of the calcaneus: differences in trabecular structure in osteoporosis patients. J Bone Mineral Res 1998; 13: 1175–1182. [DOI] [PubMed] [Google Scholar]
- Benito M , Gomberg B , Wehrli FW et al. Deterioration of trabecular architecture in hypogonadal men. J Clin Endocrinol Metab 2003; 88: 1497–1502. [DOI] [PubMed] [Google Scholar]
- Techawiboonwong A , Song HK , Leonard MB et al. Cortical bone water: in vivo quantification with ultrashort echo-time MR imaging. Radiology 2008; 248: 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito M , Vasilic B , Wehrli FW et al. Effect of testosterone replacement on trabecular architecture in hypogonadal men. J Bone Mineral Res 2005; 20: 1785–1791. [DOI] [PubMed] [Google Scholar]
- Chesnut CH 3rd , Majumdar S , Newitt DC et al. Effects of salmon calcitonin on trabecular microarchitecture as determined by magnetic resonance imaging: results from the QUEST study. J Bone Mineral Res 2005; 20: 1548–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JM , Giangregorio LM , Atkinson SA et al. Association of larger holes in the trabecular bone at the distal radius in postmenopausal women with type 2 diabetes mellitus compared to controls. Arthritis Care Res (Hoboken) 2012; 64: 944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JF , Yeung DK , Tsang PH et al. Compromised bone marrow perfusion in osteoporosis. J Bone Mineral Res 2008; 23: 1068–1075. [DOI] [PubMed] [Google Scholar]
- Paccou J , Hardouin P , Cotten A et al. The role of bone marrow fat in skeletal health: usefulness and perspectives for clinicians. J Clin Endocrinol Metab 2015; 100: 3613–3621. [DOI] [PubMed] [Google Scholar]
- Patsch JM , Li X , Baum T et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res 2013; 28: 1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina EK , Kiel DP , Roubenoff R et al. Protective effects of fish intake and interactive effects of long-chain polyunsaturated fatty acid intakes on hip bone mineral density in older adults: the Framingham Osteoporosis study. Am J Clin Nutr 2011; 93: 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen R , Tuppurainen M , Erkkila AT et al. Associations of dietary polyunsaturated fatty acids with bone mineral density in elderly women. Eur J Clin Nutr 2012; 66: 496–503. [DOI] [PubMed] [Google Scholar]
- Weiss LA , Barrett-Connor E , von Muhlen D . Ratio of n-6 to n-3 fatty acids and bone mineral density in older adults: the Rancho Bernardo study. Am J Clin Nutr 2005; 81: 934–938. [DOI] [PubMed] [Google Scholar]
- Farina EK , Kiel DP , Roubenoff R et al. Dietary intakes of arachidonic acid and alpha-linolenic acid are associated with reduced risk of hip fracture in older adults. J Nutr 2011; 141: 1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griel AE , Kris-Etherton PM , Hilpert KF et al. An increase in dietary n-3 fatty acids decreases a marker of bone resorption in humans. Nutr J 2007; 6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins BA , Li Y , Seifert MF . Dietary ratio of n-6/n-3 PUFAs and docosahexaenoic acid: actions on bone mineral and serum biomarkers in ovariectomized rats. J Nutr Biochem 2006; 17: 282–289. [DOI] [PubMed] [Google Scholar]
- Coetzer H , Claassen N , van Papendorp DH et al. Calcium transport by isolated brush border and basolateral membrane vesicles: role of essential fatty acid supplementation. Prostaglandins Leukot Essen Fatty Acids 1994; 50: 257–266. [DOI] [PubMed] [Google Scholar]
- Ortiz-Alvarado O , Miyaoka R , Kriedberg C et al. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid in the management of hypercalciuric stone formers. Urology 2012; 79: 282–286. [DOI] [PubMed] [Google Scholar]
- Orchard TS , Cauley JA , Frank GC et al. Fatty acid consumption and risk of fracture in the Women's Health Initiative. Am J Clin Nutr 2010; 92: 1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL , Tchkonia T , Pirtskhalava T et al. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol 2002; 37: 757–767. [DOI] [PubMed] [Google Scholar]
- Monickaraj F , Aravind S , Gokulakrishnan K et al. Accelerated aging as evidenced by increased telomere shortening and mitochondrial DNA depletion in patients with type 2 diabetes. Mol Cell Biochem 2012; 365: 343–350. [DOI] [PubMed] [Google Scholar]
- de Heredia FP , Larque E , Portillo MP et al. Age-related changes in fatty acids from different adipose depots in rat and their association with adiposity and insulin. Nutrition 2008; 24: 1013–1022. [DOI] [PubMed] [Google Scholar]
- Morley JE . Diabetes and aging: epidemiologic overview. Clin Geriatr Med 2008; 24: 395–405. [DOI] [PubMed] [Google Scholar]
- Ma YH , Schwartz AV , Sigurdsson S et al. Circulating sclerostin associated with vertebral bone marrow fat in older men but not women. J Clin Endocrinol Metab 2014; 99: E2584–E2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston JE , Watts NB , Chapurlat R et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med 2011; 124: 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson CM , Marshall LM , Adams AL et al. BMI and fracture risk in older men: the osteoporotic fractures in men study (MrOS). J Bone Miner Res 2011; 26: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H , Kanis JA , Oden A et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res 2014; 29: 223–233. [DOI] [PubMed] [Google Scholar]
- Sornay-Rendu E , Boutroy S , Vilayphiou N et al. In obese postmenopausal women, bone microarchitecture and strength are not commensurate to greater body weight: the Os des Femmes de Lyon (OFELY) study. J Bone Miner Res 2013; 28: 1679–1687. [DOI] [PubMed] [Google Scholar]
- Gerdhem P , Isaksson A , Akesson K et al. Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus. Osteoporos Int 2005; 16: 1506–1512. [DOI] [PubMed] [Google Scholar]
- Weyer C , Funahashi T , Tanaka S et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 2001; 86: 1930–1935. [DOI] [PubMed] [Google Scholar]
- Bredella MA , Torriani M , Ghomi RH et al. Determinants of bone mineral density in obese premenopausal women. Bone 2011; 48: 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsanz V , Chalfant J , Mo AO et al. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 2009; 94: 3387–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A , Dempster DW , Recker RR et al. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab 2013; 98: 2562–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein K , Amrein S , Drexler C et al. Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J Clin Endocrinol Metab 2012; 97: 148–154. [DOI] [PubMed] [Google Scholar]
- Bridges D , Randall C , Hansma PK . A new device for performing reference point indentation without a reference probe. Rev Sci Instrum 2012; 83: 044301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall C , Bridges D , Guerri R et al. Applications of a new handheld reference point indentation instrument measuring bone material strength. J Med Dev 2013; 7: 410051–410056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr JN , Drake MT , Amin S et al. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res 2013; 29: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Perez A , Guerri R , Nogues X et al. Microindentation for in vivo measurement of bone tissue mechanical properties in humans. J Bone Miner Res 2010; 25: 1877–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri-Fernandez RC , Nogues X , Quesada Gomez JM et al. Microindentation for in vivo measurement of bone tissue material properties in atypical femoral fracture patients and controls. J Bone Miner Res 2013; 28: 162–168. [DOI] [PubMed] [Google Scholar]