Abstract
Babesia is known to be prevalent in the Eastern United States and other temperate countries but the prevalence of babesia is not well known in the tropical malaria-endemic countries because of the higher prevalence of malaria.
A 72-year-old Hispanic male from Ecuador presenting with increasing left lower quadrant abdominal pain and distention for one year. He experienced nausea, vomiting, diarrhea, fever, chill, and myalgias. He reported 9 kg weight loss over the last two months. Patient moved to Chicago recently from Ecuador where he worked at a banana plantation and had frequent exposure to many insects and animals.
Vital signs were normal but patient appeared chronically ill. Mild tenderness to palpation over the left side of the abdomen with marked splenomegaly, measuring 16 cm below the costal margin.
Laboratory results with no leukocytosis hemoglobin 7.8 × 109/L; and platelet count, 55 × 109/L. Sodium was 128 mmol/L. Labs showed elevated LDH, ESR and ferritin values. The haptoglobin was low with a positive Combs test. CT abdomen showed moderate splenomegaly with large patchy, wedge-shaped hypodense area in posterior mid and upper spleen suggesting splenic infarction. Rapid malaria screening was negative, but a peripheral smear identified plasmodium species in more than 0.5% of red blood cells. Treatment with atovaquone and proguanil started.
Two weeks later, molecular testing revealed Babesia DNA.
This report details a case of babesiosis in a patient coming from a malaria-endemic region. The initial workup and blood work highly suggested a plasmodium infection. However the polymerase chain reaction confirmed the diagnosis of a Babesia microti.
Learning objectives: We report the first case of human Babesiosis in previously healthy individual from Ecuador.
Keyword: Babesiosis
Introduction
This report details a case of babesiosis in a patient coming from Ecuador, where no babesiosis has been reported from this malaria-endemic region. The initial presentation and blood work highly suggested Plasmodium infection. However, the polymerase chain reaction confirmed the diagnosis of Babesia microti.
Case presentation
A 72-year-old Hispanic male from Ecuador with a history of hypertension was admitted to the hospital for increasing right lower quadrant abdominal pain and distention which started a year before but worsened in the past two months. He had been experiencing nausea, vomiting, diarrhea, dry cough, fever, chill, headaches and myalgias. He reported a 9 kg weight loss over the last two months, which he attributed to decreased appetite. He denied rash, jaundice, or hematochezia. Patient moved to Chicago two months previously from Ecuador where he worked at a banana plantation and had frequent exposure to many insects and animals.
On physical examination, vital signs were normal but patient appeared chronically ill. Mild tenderness to palpation of the abdomen was noted on the left side with marked splenomegaly, measuring 16 cm below the costal margin. There was no evidence of hepatomegaly or ascites, and no palpable peripheral lymph nodes.
Laboratory results included the following (reference ranges provided parenthetically): hemoglobin, 12.1 g/dL (13.5–17.5 g/dL); mean corpuscular volume, 84.0 fL (81.2–95.1 fL); white blood cell count, 7.8 × 109/L (3.5–10.5 × 109/L); and platelet count, 55 × 109/L (150–450 × 109/L). An electrolyte panel yielded normal findings except for a sodium level of 128 mmol/L (135–145 mmol/L). Labs were also notable for elevated LDH, ESR and ferritin values. Total bilirubin was 1.3 with a direct bilirubin of 0.4. The haptoglobin was low with a positive Combs test. Moreover, blood culture had been negative for 3 days.
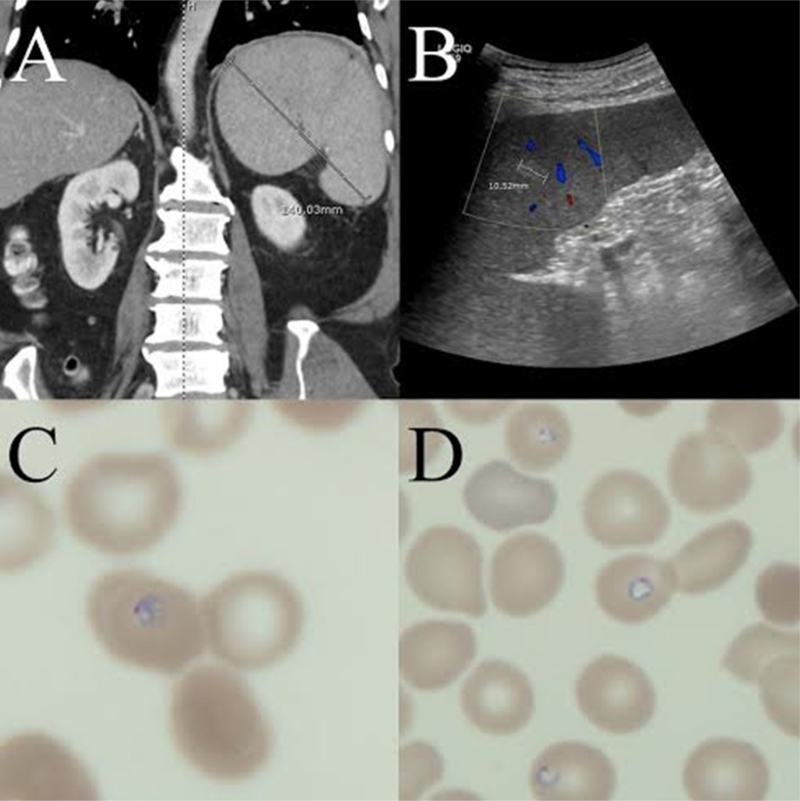
CT chest with contrast to look for possible accessible lymph nodes showed no evidence of acute intrathoracic process and no evidence of mediastinal, hilar or axillary adenopathy. CT of the abdomen and pelvis (Fig. 1A) showed moderate splenomegaly with large patchy, wedge-shaped hypodense area in posterior mid and upper spleen extending toward hilum, suggesting splenic infarction. Abdominal ultrasound (Fig. 1B) showed splenomegaly with an 11 mm hyperechoic lesion on the spleen, which probably represented a small hemangioma. Rapid malaria screening came back negative, but peripheral blood smear (Fig. 1C and D) showed 0.5% of red blood cells containing intraerythrocytic parasites suggestive of plasmodium species. A 3-day treatment course of atovaquone and proguanil was begun for active malaria.
Fig. 1.
(A) CT abdomen and pelvis with contrast. Large heterogeneous spleen and perisplenic thickening. (B) Abdominal ultrasound of the spleen. Focal 11 mm hyperechoic lesion in the spleen probably represents a hemangioma. (C) Peripheral blood smear showing red blood cell containing highly complex rings and fused forms. (D) Peripheral blood smear showing red blood cell containing round to oval shaped ring.
A diagnosis of hyperreactive malarial splenomegaly [1], [2] was made, which is a rare immunologic complication of chronic malarial infection. IgM, IgG Malaria, cryoglobulins, and cold agglutinins were sent. Although the patient failed to meet all the Fakunle criteria to diagnose HMS, the clinical suspicion was high, especially when the malaria IgG came back elevated to 6.44. Blood was also sent to the CDC for diagnostic confirmation. Primaquine was started for treatment of a persistent hypnozoite stage after the G6PD level was found to be normal. Results of HIV, viral hepatitis serology and ANA panel were negative. During the following days, the patient reported feeling well, although with little appetite, his abdominal pain and distention had remarkably improved. This required extensive discussion about the importance of completing the antimalarial medication as well as following up with a primary physician in Ecuador in the case of long term malaria prophylaxis. The patient had to be educated about the importance of the avoidance of physical activity, driving, and horse riding while his spleen remained enlarged, as well as the risks of splenic hemorrhage. The patient was discharged and given a cycle of primaquine to finish in a total course of 14 days.
Two weeks later, we received a call from the CDC stating that molecular testing revealed Babesia microti DNA, although the peripheral blood smear did not demonstrate the classic parasite forms.
Discussion
One of the challenges of clinicians is the diagnosis of two different diseases with similar signs and symptoms. Two diseases that share the same qualities are malaria and babesiosis in where the pathophysiology, symptoms, and physical exam findings are remarkably similar. Both Babesia and Plasmodium are intraerythrocytic protozoa. In the case of babesia, the sporozoite infects the red blood cell, afterwards develops into a trophozoite. It also further develops into merozoite within the red blood cell, which can be seen as the “Maltese cross” pattern on peripheral blood smear. In the case of malaria, the Plasmodium sporozoite is injected into the blood from the bite of the anopheles mosquito. The sporozoite subsequently travels to the liver where it reproduces asexually into multiple merozoites. The merozoites then infect the red blood cells where it will multiply further and be released to infect even more red blood cells. These protozoa have similar pathophysiology, and the diseases caused by them also have similar symptoms such as fever, chills, sweats, fatigue, headache, nausea, vomiting, body aches, and malaise [3], [4], [5]. These symptoms are likely caused by the cytokines released when the infected red blood cell becomes destroyed [6]. Also physical exam findings can also be quite similar such as anemia, jaundice and splenomegaly. Differentiating these diseases out from one another required a good clinical, travel, occupational, and leisure history with lab testing.
The most common form of Babesia is the intraerythrocytic round or oval ring, with a pale blue cytoplasm and one or two red chromatic dots [7]. However, this ring forms may be mistaken for Plasmodium falciparum trophozoites. There are several distinguishing features of babesia from plasmodium. Babesia merozoites are occasionally arranged tetrads (“Maltese Cross”) which is pathognomonic of B. microti and B. duncani [8]. Gametes and schizonts are not observed in babesiosis [9]. Extracellular merozoites, either single or in clumps, may be seen with high levels of parasitemia in babesia. Babesia lacks brownish pigment deposits (hemozoin) in infected RBC, which is typically seen in older rings of P. falciparum infection [9]. A polymerase chain reaction can be required to differentiate the protozoan infections. This advanced testing for babesia is not readily available to many practitioners in malaria endemic countries where babesia is also present.
Babesiosis is known to be prevalent in the Eastern United States and other temperate countries but the prevalence of babesiosis is not well known in the tropical malaria-endemic countries because of the higher prevalence of malaria. However, it is known that cattle babesiosis is a large problem with significant economic costs [10]. The most prevalent species of babesia that infect cattle and livestock in South America are Babesia bigemina and Babesia bovis which the latter is more pathogenic [10]. The tick vector of cattle babesiosis is primarily Boophilus microplus, which is distributed globally throughout the tropics [10]. This species is not thought to bite humans unlike the Ixodes tick [11]. However, there have been a few rare cases where Babesia spp. that normally infect cattle and other animals to cause disease in humans especially if they are asplenic or immunocompromised [11].
This report details a case of babesiosis in a patient coming from a malaria-endemic region. The initial workup and blood work highly suggested a plasmodium infection. However the polymerase chain reaction confirmed the diagnosis of a Babesia microti infection. Asymptomatic infections with Babesia that infect domestic animals have been reported from Mexico (B. bigemina, B. canis) and Columbia (B. bigemina, B. bovis) [9], [12]. To the best of our knowledge, this is the first case of human babesiosis from Ecuador.
Human babesiosis is a rare but devastating and potentially lethal infectious disease. Timely diagnosis and treatment are important but often delayed. It necessitates increased physician awareness to distinguish from similar infection, such as malaria.
Contributor Information
Moamen Al Zoubi, Email: Moamen.alzoubi@advocatehealth.com.
Tommy Kwak, Email: tommy.kwak@advocatehealth.com.
Jeremy Patel, Email: Jeremy.patel@my.rfums.org.
Mandavi Kulkarni, Email: Mandavikulkarni@gmail.com.
References
- 1.Facer C.A., Crane G.G. Hyperreactive malarious splenomegaly. Lancet (London, England) 1991;338:115–116. doi: 10.1016/0140-6736(91)90107-z. [DOI] [PubMed] [Google Scholar]
- 2.Bates I., Bedu-Addo G. Review of diagnostic criteria of hyper-reactive malarial splenomegaly. Lancet (London, England) 1997;349:1178. doi: 10.1016/S0140-6736(05)63061-9. [DOI] [PubMed] [Google Scholar]
- 3.Hatcher J.C., Greenberg P.D., Antique J., Jimenez-Lucho V.E. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis. 2001;32:1117–1125. doi: 10.1086/319742. [DOI] [PubMed] [Google Scholar]
- 4.Joseph J.T., Roy S.S., Shams N., Visintainer P., Nadelman R.B., Hosur S. Babesiosis in Lower Hudson Valley, New York, USA. Emerg Infect Dis. 2011;17:843–847. doi: 10.3201/eid1705.101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White D.J., Talarico J., Chang H.G., Birkhead G.S., Heimberger T., Morse D.L. Human babesiosis in New York State: review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med. 1998;158:2149–2154. doi: 10.1001/archinte.158.19.2149. [DOI] [PubMed] [Google Scholar]
- 6.Shaio M.F., Lin P.R. A case study of cytokine profiles in acute human babesiosis. Am J Trop Med Hyg. 1998;58:335–337. doi: 10.4269/ajtmh.1998.58.335. [DOI] [PubMed] [Google Scholar]
- 7.Vannier E., Gewurz B.E., Krause P.J. Human babesiosis. Infect Dis Clin North Am. 2008;22 doi: 10.1016/j.idc.2008.03.010. 469–488, viii–ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad P.A., Kjemtrup A.M., Carreno R.A., Thomford J., Wainwright K., Eberhard M. Description of Babesia duncani n.sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol. 2006;36:779–789. doi: 10.1016/j.ijpara.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Bennett J., Dolin R., Blaser M. 2014. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. [Google Scholar]
- 10.Montenegro-James S. Prevalence and control of babesiosis in the Americas. Memórias Do Inst Oswaldo Cruz. 1992;87(Suppl. 3):27–36. doi: 10.1590/s0074-02761992000700003. [DOI] [PubMed] [Google Scholar]
- 11.Hunfeld K-P., Hildebrandt A., Gray J.S. Babesiosis: recent insights into an ancient disease. Int J Parasitol. 2008;38:1219–1237. doi: 10.1016/j.ijpara.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Ríos L., Alvarez G., Blair S. Serological and parasitological study and report of the first case of human babesiosis in Colombia. Rev Soc Bras Med Trop. 2003;36:493–498. doi: 10.1590/s0037-86822003000400010. [DOI] [PubMed] [Google Scholar]