Abstract
In the kinetoplastid parasite Trypanosoma brucei clathrin-mediated endocytosis is essential for survival and aids immune evasion in the mammalian host. The formation of endocytic clathrin coated vesicles in T. brucei is via a unique mechanism owing to an evolutionarily recent loss of the adaptor protein (AP)2 complex, a central hub in endocytic vesicle assembly. Despite this loss, recent studies examining endocytic clathrin coat assembly have highlighted a high degree of conservation between trypanosomes and their mammalian hosts. In particular phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) and its putative effectors, TbCALM and TbEpsinR, are central to clathrin-mediated endocytosis in the trypanosome, just as they are in animal cells. In addition to providing insights into the cell biology of T. brucei, these studies also suggest an ancient, possibly pan-eukaryotic connection between PtdIns(4,5)P2 and endocytosis.
Keywords: AP180; clathrin; CALM; endocytosis; epsin; epsinR; phosphoinositide; PI(4,5)P2; PIP kinase; Trypanosoma; trafficking
Phosphoinositides are derivatives of phosphatidylinositol (PtdIns), reversibly phosphorylated at the 3, 4 or 5 positions of the inositol ring, giving 7 potential differentially phosphorylated species. These lipid species are produced and eliminated through the concerted actions of specific phosphoinositide kinases, phosphatases and phospholipases. Together with small GTPases of the ARF and Rab families, phosphoinositides constitute an organelle identity code on the cytosol-facing membrane leaflet of endomembrane compartments. Local phosphoinositide levels and active small GTPases are sensed by effector proteins that carry phosphoinositide-specific binding domains. In many cases these also function as GTPase coincidence detectors, whereby recruitment to the membrane depends upon both local phosphoinositide levels and the presence of a particular active GTPase.1 It is suggested that this co-incidence detection mechanism of adaptor recruitment allows greater precision in adaptor protein specificity than either system functioning in isolation.1 Thus, spatially and temporally regulated phosphoinositide gradients are central to maintenance of intracellular membrane traffic and hence proper cellular compartmentalization. Well-characterized examples of phosphoinositide regulation of membrane trafficking are endosomal phosphatidylinositol 3-phosphate (PI3P) controlling endosome fusion and maturation.2,3,4 and phosphatidylinositol 4-phosphate (PI4P) controlling peripheral membrane protein recruitment to the trans-Golgi network.1,5,6 The ability of kinases and phosphatases to rapidly modulate the levels of specific phosphoinositides also facilitates swift changes to compartmental address codes and integration with cellular signal transduction pathways important for responses coordinating other biological processes.
Phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) is highly enriched at the plasma membrane of mammalian and yeast cells where, among other roles, it aids the driving of endocytic clathrin coated pit formation through PI(4,5)P2-binding clathrin adaptors, including the central AP2 hub and peripheral adaptors such as CALM/AP180.7,8,9 The kinetoplastid parasite Trypanosoma brucei, and close relatives within the African trypanosome lineage, are unique in having dispensed with the AP2 complex while at the same time relying on extensive clathrin mediated endocytosis to aid in host immune evasion as well as nutrient uptake.10–14 Two recent studies have revealed roles for a phosphoinositide kinase and its product PtdIns(4,5)P2, as well as likely effector molecules bearing phosphoinositide binding domains in clathrin mediated endocytosis in this organism.15,16
All endocytic and exocytic events in trypanosomatids occur in a specialized plasma membrane domain termed the flagellar pocket. This structure is a membrane invagination segregated from the bulk plasma membrane by a tight junction-like assemblage, the flagellar pocket collar that acts as a diffusion barrier for plasma membrane resident proteins and lipids. The recent characterization of TbPIPKA, localized predominantly at the flagellar pocket neck, suggests a mechanism for directing this highly polarized interaction between the endomembrane system and the plasma membrane.15 The authors showed that TbPIPKA acts as a PI4P-5-kinase that is essential in both the insect stage of the parasite life cycle as well as the much more endocytically active mammalian infective stage. Additionally, depletion of this kinase caused severe endocytic defects, similar to those seen following depletion of clathrin heavy chain.10 Furthermore, the same study demonstrated enrichment of PI(4,5)P2, derived from TbPIPKA activity, at the cytosolic face of the flagellar pocket in the insect stage, although attempts to localize these lipid species in mammalian infective forms were surprisingly unsuccessful, given the higher rate of endocytosis in this life stage.
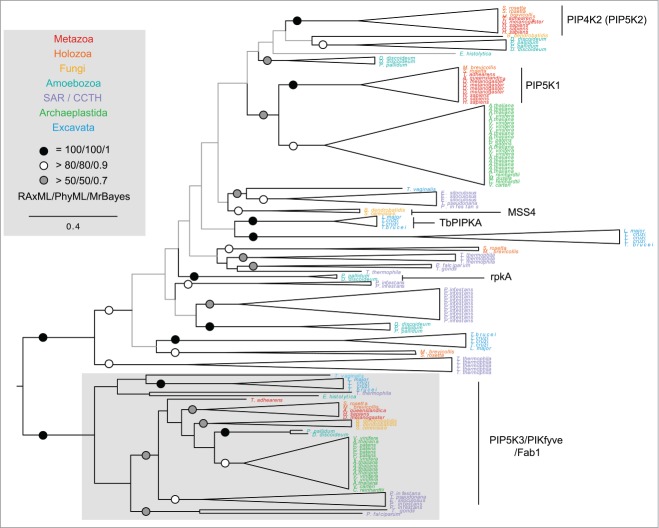
The strong phenotype observed following TbPIPKA depletion suggests that there is little or no redundancy in the generation of flagellar pocket PtdIns(4,5)P2. However the trypanosome genome encodes a total of 4 putative phosphatidylinositol phosphate kinase (PIPK) genes, which suggests that PI(4,5)P2 can be formed by other routes.11,15 While the roles of these other gene products are currently unknown, phylogenetic reconstruction (Fig. 1) demonstrates that one gene likely encodes a PIP5K3 homolog, with the other proteins falling into a poorly resolved clade containing both the PIP5K1 and PIP4K2 metazoan gene families, together with the characterized MSS4 PI4P-5-kinase from Saccharomyces cerevisiae. In agreement with previous studies,17 the only strong support in this clade is within eukaryotic super-groups, suggesting multiple independent PIPK gene family expansions following divergence of the eukaryotic supergroups some one billion years ago. Clearly functional analysis is required to understand the significance of these gene family expansions in T. brucei as simple inference from animals and fungi is unlikely to be insightful.
Figure 1.
Phylogenetic reconstruction of the PIPK family of phosphoinositide kinases. Taxa are colored by eukaryotic super-group according to the key. Unsupported branches are gray; support levels are indicated with discs according to the key. The topology shown is the best scoring ML topology (RaxML). A well-supported clade containing characterized PIPK3 gene products is found across the eukaryotes. The remaining sequences group according to eukaryotic super-group suggesting multiple, independent instances of gene duplication following divergence of the eukaryotic lineages.
The absence of the AP2 complex suggests that other clathrin adaptors likely control endocytic clathrin coated pit formation in T. brucei. To address this question we interrogated the trypanosome genome and identified 2 potential clathrin adaptor proteins orthologous to mammalian CALM/AP180 and EpsinR.8,16,18-21, Indeed, these 2 clathrin adaptors were shown to function together in endocytosis from the flagellar pocket of the bloodstream form parasite, with depletion of both proteins leading to a strong inhibition of endocytic activity.16 Examination of the protein sequence of these 2 adaptors showed that they have highly conserved phosphoinositide-binding domains, suggesting that they may be acting as the link between TbPIPKA derived PtdIns(4,5)P2 at the flagellar pocket membrane and the endocytic clathrin coat. This is supported by the localization of a GFP-fused PI(4,5)P2 binding domain derived from phospholipase C (PLC)δ to the flagellar pocket region of mammalian form parasites (Fig. 2A) in line with the previous report for the insect form. We suggest therefore that PtdIns(4,5)P2 is likely a major and critical signal for TbCALM and TbEpsinR recruitment, which in turn generates membrane curvature22,23 via clathrin recruitment and thereby coated vesicle formation at the flagellar pocket (Fig. 2B). While unprecedented in nature, this minimal arrangement of lipid, monomeric clathrin adaptor and clathrin coat has been shown in vitro to possess clathrin coated bud forming activity.8,24
Figure 2.
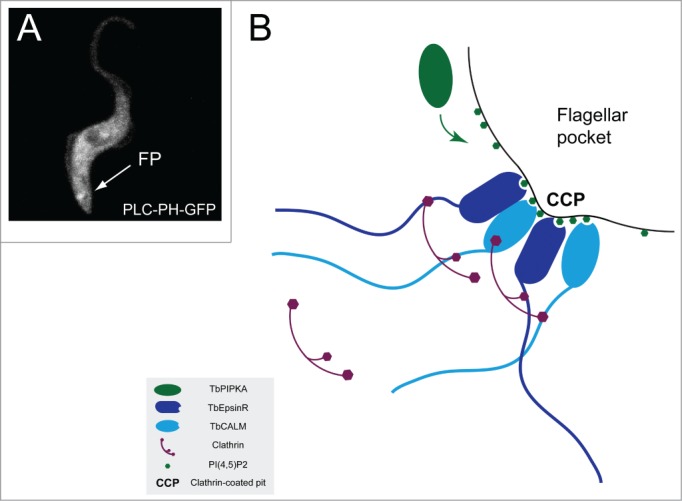
PI(4,5)P2 localization in mammalian life-cycle stage parasites and a model for clathrin coated pit formation. (A) GFP-fused PH domain from PLCδ expressed constitutively in mammalian bloodstream form parasites is enriched in the vicinity of the flagellar pocket (FP). (B) schema for clathrin coated pit formation at the flagellar pocket of T. brucei. TbPIPKA localized to the pocket neck generates a local increase in PtdIns(4,5)P2 levels. This PI(4,5)P2 is free to diffuse within the cytosolic leaflet of the flagellar pocket membrane but is prevented from traveling further by the flagellar pocket collar. Increased PtdIns(4,5)P2 levels are sensed by TbCALM and TbEpsinR which in turn recruit clathrin and impart membrane curvature, thus driving clathrin coated pit assembly.
In summary, a system that at first appeared paradoxical, i.e. high clathrin-mediated endocytic activity in the absence of the AP2 complex, has actually revealed deep conservation in the actions of phosphoinositide lipids, kinases and effectors in clathrin-mediated endocytosis, which likely extends across the extant eukaryotic lineages. We suggest that this may well provide clues as to the ancient core of endocytic trafficking mechanisms, as the phosphoinositide system may well predate adaptin complex origins.25
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature 2005; 438:597-604; PMID:16319879; http://dx.doi.org/ 10.1038/nature04397 [DOI] [PubMed] [Google Scholar]
- 2.Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science 1993; 260:88-91; PMID:8385367; http://dx.doi.org/ 10.1126/science.8385367 [DOI] [PubMed] [Google Scholar]
- 3.Brown WJ, DeWald DB, Emr SD, Plutner H, Balch WE. Role for phosphatidylinositol 3-kinase in the sorting and transport of newly synthesized lysosomal enzymes in mammalian cells. J Cell Biol 1995; 130:781-96; PMID:7642697; http://dx.doi.org/ 10.1083/jcb.130.4.781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raiborg C, Schink KO, Stenmark H. Class III phosphatidylinositol 3-kinase and its catalytic product PtdIns3P in regulation of endocytic membrane traffic. FEBS J 2013; 280:2730-42; PMID:23289851; http://dx.doi.org/ 10.1111/febs.12116 [DOI] [PubMed] [Google Scholar]
- 5.Levine TP, Munro S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr Biol 2002; 12:695-704; PMID:12007412; http://dx.doi.org/ 10.1016/S0960-9822(02)00779-0 [DOI] [PubMed] [Google Scholar]
- 6.Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirchhausen T, Albanesi JP, Roth MG, Yin HL. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 2003; 114:299-310; PMID:12914695; http://dx.doi.org/ 10.1016/S0092-8674(03)00603-2 [DOI] [PubMed] [Google Scholar]
- 7.Jost M, Simpson F, Kavran JM, Lemmon MA, Schmid SL. Phosphatidylinositol-4,5-bisphosphate is required for endocytic coated vesicle formation. Curr Biol 1998; 8:1399-404; PMID:9889104; http://dx.doi.org/ 10.1016/S0960-9822(98)00022-0 [DOI] [PubMed] [Google Scholar]
- 8.Ford MG, Pearse BM, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 2001; 291:1051-5; PMID:11161218; http://dx.doi.org/ 10.1126/science.291.5506.1051 [DOI] [PubMed] [Google Scholar]
- 9.Höning S, Ricotta D, Krauss M, Späte K, Spolaore B, Motley A, Robinson M, Robinson C, Haucke V, Owen DJ. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol Cell 2005; 18:519-31; PMID:15916959 [DOI] [PubMed] [Google Scholar]
- 10.Allen CL, Goulding D, Field MC. Clathrin-mediated endocytosis is essential in Trypanosoma brucei. EMBO J 2003; 22:4991-5002; PMID:14517238; http://dx.doi.org/ 10.1093/emboj/cdg481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, Lennard NJ, Caler E, Hamlin NE, Haas B, et al.. The genome of the African trypanosome Trypanosoma brucei. Science 2005; 309:416-22; PMID:16020726; http://dx.doi.org/ 10.1126/science.1112642 [DOI] [PubMed] [Google Scholar]
- 12.Engstler M, Pfohl T, Herminghaus S, Boshart M, Wiegertjes G, Heddergott N, Overath P. Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. Cell 2007; 131:505-15; PMID:17981118; http://dx.doi.org/ 10.1016/j.cell.2007.08.046 [DOI] [PubMed] [Google Scholar]
- 13.Manna PT, Kelly S, Field MC. Adaptin evolution in kinetoplastids and emergence of the variant surface glycoprotein coat in African trypanosomatids. Mol Phylogenet Evol 2013; 67:123-8; PMID:23337175; http://dx.doi.org/ 10.1016/j.ympev.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manna PT, Boehm C, Leung KF, Natesan SK, Field MC. Life and times: synthesis, trafficking, and evolution of VSG. Trends Parasitol 2014; 30:251-8; PMID:24731931; http://dx.doi.org/ 10.1016/j.pt.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demmel L, Schmidt K, Lucast L. Havlicek K, Zankel A, Koestler T, Reithofer V, de Camilli P, Warren G. The endocytic activity of the flagellar pocket in Trypanosoma brucei is regulated by an adjacent phosphatidylinositol phosphate kinase. J Cell Sci 2014; 127:2351-64; PMID:24639465; http://dx.doi.org/ 10.1242/jcs.146894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manna PT, Gadelha C, Puttick A E, Field MC. ENTH and ANTH domain proteins participate in AP2-independent clathrin-mediated endocytosis. J Cell Sci 2015; 128:2130-42; PMID:25908855; http://dx.doi.org/ 10.1242/jcs.167726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leondaritis G, Siokos J, Skaripa I, Galanopoulou D. Genome-wide analysis of the phosphoinositide kinome from two ciliates reveals novel evolutionary links for phosphoinositide kinases in eukaryotic cells. PLoS One 2013; 2913; 8:e78848; PMID:24244373; http://dx.doi.org/ 10.1371/journal.pone.0078848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalthoff C, Groos S, Kohl R, Mahrhold S, Ungewickell EJ. Clint: a novel clathrin-binding ENTH-domain protein at the Golgi. Mol Biol Cell 2002; 13:4060-73; PMID:12429846; http://dx.doi.org/ 10.1091/mbc.E02-03-0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills IG, Praefcke GJK, Vallis Y, Peter BJ, Olesen LE, Gallop JL, Butler PJG, Evans PR, McMahon HT. EpsinR: an AP1/clathrin interacting protein involved in vesicle trafficking. J Cell Biol 2003; 160:213-22; PMID:12538641; http://dx.doi.org/ 10.1083/jcb.200208023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirst J, Motley A, Harasaki K, Peak Chew SY, Robinson MS. EpsinR: an ENTH domain-containing protein that interacts with AP-1. Mol Biol Cell 2003; 14:625-41; PMID:12589059; http://dx.doi.org/ 10.1091/mbc.E02-09-0552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabernet-Castello C, Dacks JB, Field MC. The single ENTH-domain protein of trypanosomes; endocytic functions and evolutionary relationship with epsin. Traffic 2009; 10:894-911; PMID:19416477; http://dx.doi.org/ 10.1111/j.1600-0854.2009.00910.x [DOI] [PubMed] [Google Scholar]
- 22.Lai C-L, Jao CC, Lyman E, Gallop JL, Peter BJ, McMahon HT, Langen R, Voth GA. Membrane binding and self-association of the epsin N-terminal homology domain. J Mol Biol 2012; 423:800-17; PMID:22922484; http://dx.doi.org/ 10.1016/j.jmb.2012.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller SE, Mathiasen S, Bright NA, Pierre F, Kelly BT, Kladt N, Schauss A, Merrifield CJ, Stamou D, Höning S, et al.. CALM regulates clathrin-coated vesicle size and maturation by directly sensing and driving membrane curvature. Dev Cell 2015; 33:163-75; PMID:25898166; http://dx.doi.org/ 10.1016/j.devcel.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dannhauser PN, Ungewickell EJ. Reconstitution of clathrin-coated bud and vesicle formation with minimal components. Nat Cell Biol 2012; 14:634-9; PMID:22522172; http://dx.doi.org/ 10.1038/ncb2478 [DOI] [PubMed] [Google Scholar]
- 25.Michell RH. Inositol derivatives: evolution and functions. Nat Rev Mol Cell Biol 2008; 9:151-61; PMID:18216771; http://dx.doi.org/ 10.1038/nrm2334 [DOI] [PubMed] [Google Scholar]