Abstract
The balance between cellular lineages can be controlled by reactive oxygen species (ROS). Cellular differentiation into adipocytes is highly dependent on the production of ROS to initiate the process through activation of multiple interlinked factors that stimulate mitotic clonal expansion and cellular maturation. The signal transducer and activator of transcription family of signaling proteins have accepted roles in adipogenesis and associated lipogenesis. Non-canonical mitochondrial localization of STAT3 and other members of the STAT family however opens up new avenues for investigation of its role in the aforementioned processes. Following recent observations of differences in mitochondrially localized serine 727 phosphorylated STAT3 (mtSTAT3-pS727) in preadipocytes and adipocytes, here, we hypothesize and speculate further on the role of mitochondrial STAT3 in adipogenesis.
KEYWORDS: adipogenesis, mitochondria, signal transducer and activator of transcription 3, reactive oxygen species, STAT3
INTRODUCTION
Adipose tissue (majorly but not solely composed of adipocytes and endothelial cells) as an endocrine organ is vitally important to homeostasis through the release of hormone cytokines like leptin, adiponectin and resistin as well as its involvement in steroid metabolism.1,2 Imbalances in adipose tissue are largely detrimental to human health e.g. obesity.3-5 Understanding the developmental and maintenance factors in adipocyte biology are essential.
Reactive oxygen species (ROS), often maligned as in oxidative stress, are generally accepted as required for normal cellular homeostasis through redox signaling.6-10 The unidirectional process of adipogenesis relies heavily on ROS production to enable a complex cascade mediated by the redox conformation sensitive C/EBPβ to upregulate genes associated with the process.11,12 Mitochondria, the cellular powerhouses, regulate the fine balance of cellular ROS levels through the electron transport chain (ETC) and disproportionation (dismutation).
The Signal Transducers and Activators of Transcription (STAT) family of proteins have well described roles in adipogenesis and the associated process of lipogenesis through canonical activation through phosphorylation, nuclear translocation and gene activation.13-16 Non-canonical mitochondrial localization, in particular serine 727 phosphorylated STAT3 (hereafter referred to as, mtSTAT3-pS727) have been reported in normal and disease phenotypes to influence cellular respiration through the ETC and associated oxidative phosphorylation through apparent direct interaction with the protein complexes that comprise the ETC.17,18 Further to this mtSTAT3-pS727 has been reported to interact directly with the mitochondrial genome.19 Recent evidence provided a causal link between mitochondrial STAT3 levels, total cellular ROS and adipogenesis in the gold standard mammalian adipogenesis model 3T3-L1.20 Here we discuss the potential links and speculate further on the possible mechanisms that mitochondrial STAT3 plays in adipogenesis. in relation to the larger network of the STAT family.21
Adipogenesis – a sequential process
Adipogenesis is a process of differentiation and maturation of progenitor stem cells (e.g., mesenchymal stromal stem cells) and preadipocytes (e.g., isolated fibroblast 3T3-L1) to mature lipid accumulating adipocytes. The seemingly stepwise process is regulated by well-coordinated directional signaling through the CCAAT/enhancer binding proteins (C/EBPs), and in turn peroxisome proliferator activated receptors (PPARs) for induction of differentiation programming; C/EBPα, C/EBPβ, and C/EBPδ exhibit sequential and spatial expression during adipocyte differentiation with C/EBPβ in particular being important by initiating differentiation and required for transcriptional activation of C/EBPα and PPARγ.22-24 The process is highly conserved in mouse and human models allowing for a standard in vitro differentiation cocktail for the differentiation to white adipose tissue (WAT).25 The differentiation cocktail composed of insulin, dexamethasone and 3-isobutyl-1-methylxanthine (IBMX) promotes differentiation through insulin-like growth factor receptor (IGF-1) stimulation with concomitant cyclic adenosine monophosphate (cAMP) accumulation, the effects are further enhanced through the glucocorticoid receptor agonist, dexamethasone.26-28 The cAMP phosphodiesterase inhibitor, 3-isobutyl-1-methylxanthine (IBMX) further increases levels of cAMP resulting in downregulation of Specificity protein 1 (Sp1), a C/EBPα promoter repressor which delays the DNA binding activity of C/EBPβ and C/EBPδ.23 The insulin sensitizer and PPARγ agonist rosiglitazone is often included to increase differentiation efficiency in the first 3 days of differentiation programming.29
Mitochondria, reactive oxygen species and adipogenesis
The electron transport chain and ROS
As the double membraned energy powerhouse of the mammalian cell, the mitochondria maintain energy balance through aerobic respiration and adenosine triphosphate (ATP) synthesis through oxidative phosphorylation coupled to the inner membrane located ETC complexes I-IV. Electrons from nicotinamide adenine dinucleotide hydride (NADH) from the citric acid (TCA) cycle in the mitochondrial matrix are oxidized by the ETC complexes (I: NADH:Ubiquinone oxidoreductase, II: Succinate:Ubiquinone oxidoreductase, III: Ubiquinol:Ferricytochrome c oxidoreductase, and IV: Ferrocytochrome c oxygen oxidoreductase) in the mitochondrial cristae to the final acceptor, molecular oxygen at complex IV (to generate water), and concomitantly a proton gradient (through H+ transfer) is established across the inner membrane; the gradient is used to drive the rotary molecular motor component F0 of complex V (ATP Synthase) thereby effecting phosphorylation of adenosine diphosphate (ADP) to ATP through the F1 component. The ETC complexes I and III leak electrons to oxygen and reduces it to a radical ROS superoxide (•O2−)10,30 (see Fig. 1). Although radical ROS levels are maintained through spontaneous or enzymatic dismutation [though superoxide dismutase] to the non-radical ROS hydrogen peroxide [H2O2], this balance can be disturbed leading to cellular senescence or cell death through oxidative stress.6,9,31 As low molecular weight, easily membrane diffusable reactive species ROS have been described to mediate signal transduction pathways and cell-to-cell communications.8,32
Figure 1.
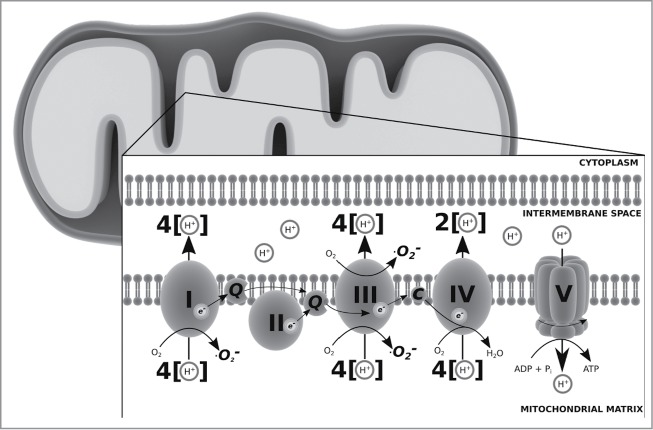
The mitochondrial electron transport chain, oxidative phosphorylation and sites of superoxide (•O2−) ROS production.10,21 Legend: e−, electron; H+, proton; Q, co-enzyme Q10; c, cytochrome c. See text for details.
ROS and initiation of adipogenesis
Reactive oxygen species seemingly act as a linchpin of adipogenesis through the redox sensitive CCAAT/enhancer binding protein β (C/EBPβ) which appears to develop a DNA binding conformation in the presence of increased levels of ROS in the gold standard adipogenesis model 3T3-L1 (mouse).11,12 This results in the transcriptional activation of peroxisome proliferator-activated receptor γ (PPARγ) which is required for mitotic clonal expansion and terminal adipocyte differentiation.33 It should be noted that mitotic clonal expansion is not required for adipogenesis in mesenchymal stromal stem cells. High levels of ROS during onset of adipogenesis in mouse and humans have been reported, naturally this should be regulated as fat accumulation is correlated with oxidative stress in both mice and humans.34 Tormos and co-workers35 has found that complex III produced ROS regulates adipogenesis in human mesenchymal stem cells through targeted antioxidant inhibition of adipocyte differentiation; the differentiation phenotype was rescued by exogenous hydrogen peroxide. Furthermore knockdown analysis of complex III components confirmed the inhibitory studies. This requirement for ROS in adipogenesis was echoed by Kanda et al. in rat bone marrow derived mesenchymal stromal stem cells.36 Taken together these studies indicate a strong role for in the activation of C/EBPβ DNA binding and the induction of adipogenic events through the mediation of PPARγ expression; PPARγ regulates glucose and fatty acid metabolism.33
STAT3 and adipogenesis
An interplay of STAT1, 3, 5A and 5B and 6 have been described to be functionally expressed in both preadipocytes and adipocytes and seemingly function to enhance or inhibit adipogenesis through up-, downregulation or constitutive expression.13,21,37 This is discussed in detail elsewhere21,37 and because of the growing body of knowledge on mitochondrial STAT3,38 discussed here later, we would like to briefly highlight STAT3 activity in adipogenesis through its canonical localization and activity. However, of particular interest in Stephens et al. are the 2 bands (92 and 84 kDa) observed for STAT3 during differentiation.(in the presence and absence of adipogenesis inhibitor tumor necrosis factor α)13 which is described as the result of cross reaction with STAT1. The major isoforms STAT3 α and β have molecular weights of 93kDa and 84kDa, respectively, and exhibit highly specific functions with STAT3β exhibiting longer nuclear retention times39,40; the effect of the individual isoforms on adipogenesis is still forthcoming. Deng et al. described high levels of tyrosine 705 phosphorylated STAT3 (STAT3-pY705) in proliferating 3T3-L1 preadipocytes with significantly lower levels observed in growth arrested preadipocytes and adipocytes.14 Interestingly, the group reported a delayed increase in STAT3-pY705 during mitotic clonal expansion following stimulation with the differentiation cocktail 3-isobutyl-1-methylxanthine, dexamethasone and insulin; taken together the data pointed to STAT3-pY705 being important in proliferative preadipocytes but not in differentiation. Interestingly, the protein inhibitor of activated STAT3 (PIAS3) was later shown to inhibit adipogenic gene expression and it was shown later that STAT3-pY705 regulated the expression of PPARγ through selective inhibition with the JAK2/STAT3 inhibitor AG490.16,41 This is most likely through STAT3 binding to and activating transcription of CEBP/β through direct interaction with the distal region of the CEBP/β promoter.15 The importance of phosphorylation of STAT3 at serine 727 (STAT3-pS727) in adipogenesis is for the most part undescribed; interestingly serine and tyrosine phosphorylation have been reported to negatively regulate each other and it is known that insulin reduces tyrosine phosphorylation and increases serine phosphorylation of STAT3 thereby reducing nuclear targeting of STAT3 in 3T3-.42,43
The mitochondrial STAT family – non-canonical localization/function
The seven genes that encode the STAT family (STAT1, STAT2, STAT3 STAT4, STAT5a, STAT5b and STAT6) mediate cellular differentiation, proliferation, survival and metabolism through well described specific receptor stimulation i.e. Janus Kinase mediated phosphorylation followed by nuclear targeting in canonical JAK-STAT signaling. Mitochondrial localization of STAT2, STAT3, STAT5 and STAT6 have been described in literature; with STAT1 seemingly following suit.38 Mitochondrial STAT proteins are still hotly debated with respect to whether it is an actual phenomenon with respect to translocation, DNA binding, and effect on the ETC. While Wegzryn et al.,17 described a wide tissue distribution in mice for mitochondrial STAT3, Chueh and coworkers' description of STAT5 translocation (through sub-cellular fractionation and fluorescence microscopy) to the mitochondria in cytokine stimulated mammalian cells showed no presence of STAT1 or STAT3.44 Khan et al. used quantitative confocal laser scanning microscopy to identify STAT6 mitochondrial localization (co-localized with Cytochome Oxidase IV, COXIV) but not STAT3 in Hep3B hepatocytes.45 Work by our group provided evidence of STAT3-pS727 in mouse 3T3-L1 mitochondria by quantitative confocal laser scanning microscopy and subcellular fractionation which indicated no cytoplasmic or endosomal contamination.20 Mitochondrial STAT3 remains the best described with respect to mitochondrial localization and associated function in regulation of mitochondrial activity specifically in cellular respiration and associated production of ROS.
Mitochondrial STAT3 – a modulatory cog in the wheel
Beyond the canonical nuclear targeting of activated STAT3, the reports of endosomal targeting46 as well as the reported nuclear activity of unphosphorylated and therefore traditionally unactivated STAT3,47 have further highlighted the complexity of this protein beyond being a simple transcriptional activator. Wegrzyn et al. were the first to describe the strong role in mitochondrial metabolism through the maintenance of the electron transport chain basal activity by maintenance of mitochondrial membrane potential17 through modulation of complex I and II activity; this was subsequently confirmed in multiple cellular phenotypes and appears that up or down modulation of activity is related to stoichiometric ratios of mitochondrial STAT3 to ETC proteins.38 It should be noted that STAT3 does not have a clear mitochondrial targeting sequence/signal. Furthermore it has been suggested that it may be a negative regulator of the Mitochondrial Permeability Transition Pore (MPTP).48 STAT3 activity in the mitochondria appears to be mediated by the complex I subunit GRIM-19 (Genes associated with Retinoid-IFN-induced Mortality-19).49 GRIM-19 has previously been reported to interact with and act as a negative regulator of STAT350,51 and appears to act as a chaperone to recruit STAT3 into mitochondria and enhance STAT3 association with complex I39. Mitochondrial STAT3 has also been shown to play an important role in ischemic pre- and post- conditioning in myocardial cells. Boengler et al. showed the presence of STAT3 in cardiomyocyte mitochondria found a positive correlation with reduced cell death after ischemia through the regulation of MPTP.48 As mentioned, it is still unknown how STAT3 is targeted to the mitochondria but Boengler and co-workers have suggested following co-immunoprecipiation that STAT3 may be imported into the mitochondria via the mitochondrial presequence receptor Tom20.48 Gough et al., have described that serine 727 phosphorylation of STAT3 was required for mitochondrial localization this was evident in the promotion of cellular transformation by the H-Ras oncogene by mitochondrial STAT3 by sustaining altered glycolysis and oxidative phosphorylation (through increased complex V activity), further strengthening the fact that STAT3 regulates mitochondrial metabolism.18 The importance of the phosphorylation at serine 727 was reiterated by Zhang et al. through site directed mutagenesis [SDM] and consequently the group showed the effects of mtSTAT3-pS727 on promoting breast cancer tumor growth, the increased activity of complex I and the accumulation of ROS in the cell.52 In addition to this, site directed mutagenesis experiments conducted by Tammineni et al. showed that a S727A mutation reduced the import and assembly into complex I of STAT3 by GRIM-19.49 Combined these data further highlight the role of mtSTAT3-pS727 in ETC activity and consequently ROS production.
Recently, work by our group showed the defined changes in levels of mtSTAT3-pS727 in relation to total cellular STAT320 and STAT3-pY705 (Unpublished data AH Kramer). The effect on ETC activity by mtSTAT3-pS727 in adipogenesis is however unknown and currently under investigation. The changes in mtSTAT3-pS727 levels observed between preadipocyte and adipocyte mitochondria may contribute to potential modulation of ETC activity and hence the presence of relatively low ROS levels in preadipocytes as opposed to the differentiated progeny. Large stoichiometric shifts may be important when considering that 3T3-L1 derived adipocytes appear to generate a large amount of lactate through aerobic glycolysis (with the concomitant although not shown generation of ATP).53,54 Although argued by the authors to be a non-Warburg effect and a potential mechanism of the cells to dispose of excess glucose, this may be a method of the adipocyte to ensure production of sufficient ATP considering the potential that low mtSTAT3-pS727 may result in decreased ATP production.18,53 Unpublished data by our group further shows that total cellular levels of STAT3-pY705 decrease during differentiation after mitotic clonal expansion, but expression levels remain relatively high in preadipocytes this concurs with previous findings indicating the importance of STAT3-pY705 in proliferating 3T3-L1 cells.13,14 Overall stoichiometric ratios of mtSTAT3-pS727 to cytoplasmic pools of STAT3/STAT3-pY705/STAT3-pS727 in preadipocytes/stem cells and adipocytes need quantitative evaluation to further mechanistic understanding of the contribution of STAT3 to adipogenesis. As mentioned, Andersson et al. described that insulin decreases the total cellular levels of STAT3-pY705 but this results in an increase in STAT3-pS727 levels.42 This results in reduced nuclear targeting and an increased cytoplasmic pool of STAT3-pS727, this was confirmed by our findings.20 Relative to this the levels of serine phosphorylated STAT3 decreased during the progress of differentiation. It may be speculated that insulin plays a key role in signaling the “release” of mtSTAT3-pS727 into the cytoplasm, this however requires quantitative confirmation. As mentioned earlier the importance of complex III ROS in mesenchymal stromal stem cell adipogenesis; as the ETC is sequential it may be possible that mitochondrial STAT3 may play an important role in controlling the overall flux of electrons in the chain through its interaction with complex I, i.e., one effective bottleneck of the ETC.35,36
Mitochondria, the Warburg effect and a potential interplay between STAT3-pY705 and mtSTAT3-pS727 in vivo?
STAT3 has been implicated in activating and maintaining the Warburg effect in cancer through down regulation of mitochondrial activity.55 The Warburg effect, also known as aerobic glycolysis, is when mitochondrial activity is seemingly decreased and glycolytic activity is increased to ensure sufficient ATP production. This has been shown to be essential for survival of cancer cells as well as for stem cells.56-58 In vivo stem cell niches exhibit a propensity for hypoxia resulting in decreased mitochondrial activity, decreased ROS and increased self-renewal (therefore decreased differentiation). The in vivo niche of adipose derived mesenchymal stem cells remains largely unknown but hypoxia culture adipose derived mesenchymal stromal stem cells do exhibit normal phenotypes, undergo self-renewal and the ability to differentiate into adipose tissues indicating the potential activation of the Warburg effect.59,60 This is pure speculation but both canonically active STAT3-pY705, through downregulation of mitochondrial protein expression, and non-canonically active mtSTAT3-pS727, through stabilization of the mitochondrial membrane potential and hence stability as well as decreased ROS production, may play a strong role in the regulation of a Warburg effect in vivo and therefore aid in maintaining a non-differentiated phenotype (Fig. 2).
Figure 2.
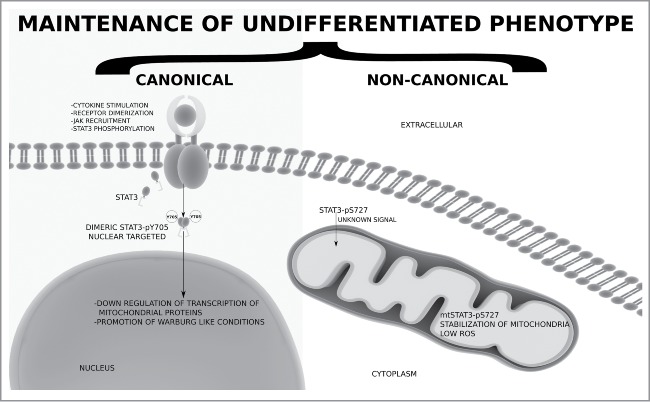
The interplay between canonical and non-canonical pools of STAT3 in maintenance of an undifferentiated (preadipocyte) phenotype. This graphical representation assumes that total STAT3 expression levels remain constant, irrespective of STAT3α or β. We speculate on the potential of the nuclear targeted STAT3-pY705 and mtSTAT3-pS727 maintaining a Warburg like effect with respect to downregulation of mitochondrial biogenesis and activity through STAT3-pY705 activity and stabilization of mitochondrial membrane potential and decreased ROS production through mtSTAT3-pS727. See text for further details.
CONCLUSION
The contribution of STAT3 to mitochondrial metabolism either as directly localized mtSTAT3-S727, nuclear targeted STAT3-pY705 as well as individual isoforms in cellular differentiation requires further investigation and clarification. Furthermore, studies need to be expanded beyond 3T3-L1, where adipogenic variability is well characterized, to other cell and in vivo models before any definitive conclusions may be drawn. However, in light of Phillips and coworkers findings on the stoichiometric ratios of mitochondrial STAT3 to other mitochondrial proteins, and considering the substantial fraction of mtSTAT3-pS727 observed relative to loading controls in mitochondrial isolates from 3T3-L1 cells it be that the preadipocyte phenotype may provide an ideal model to quantitatively investigate mitochondrial STAT3 in vitro. The hormone and cytokine signals that control the molecular switches that regulate the balance between cytoplasmic, nuclear and mitochondrial pools of STAT3 however require elucidation. Further to this the presence and role of mitochondrial STAT1, STAT5a and b and STAT6 in adipogenesis needs to be investigated. Many questions remain regarding the overall role of these proteins in the mitochondria first and foremost being, what the trigger mechanism is that targets the proteins to the organelle? Is there a specific trigger at all? With respect to STAT3 and its role in mitochondrial activity, it should be evident that there must be a signaling event that regulates the levels in the mitochondria. Furthermore, how do these proteins enter the mitochondria? The potential role of molecular chaperones, as discussed by Meier and Larner should be clear in the mediation of import into the mitochondria.38 Beyond the interaction with the ETC, is the question of to what extent the STATs interact with the mitochondrial genome and regulate transcription.19 The exact mechanism, function and activity of the STAT family and notably STAT3 remains to be described in the mitochondria and for example in the case of unphosphorylated nuclear STAT3 in the nucleus as well. The global network of this family however needs description as it is clear that no single event linked to localization (and activity) controls the process of adipogenesis.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed
Acknowledgments
The authors would like to thank www.somersault1824.com for their Library of Science Illustrations
FUNDING
The authors would like to acknowledge Rhodes University and the National Research Foundation of South Africa for funding support.
REFERENCES
- 1.Li J, Papadopoulos V, Vihma V. Steroid biosynthesis in adipose tissue. Steroids 2015; http://dx.doi.org/ 10.1016/j.steroids.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 2.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89:2548-56; PMID:15181022; http://dx.doi.org/ 10.1210/jc.2004-0395 [DOI] [PubMed] [Google Scholar]
- 3.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796-808; PMID:14679176; http://dx.doi.org/ 10.1172/JCI200319246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995; 95:2409-15; PMID:7738205; http://dx.doi.org/ 10.1172/JCI117936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishimura S, Manabe I, Nagai R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov Med 2009; 8:55-60; PMID:19788868 [PubMed] [Google Scholar]
- 6.Ray PD, Huang B-W, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012; 24:981-90; PMID:22286106; http://dx.doi.org/ 10.1016/j.cellsig.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 2007; 8:813-24; PMID:17848967; http://dx.doi.org/ 10.1038/nrm2256 [DOI] [PubMed] [Google Scholar]
- 8.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 2011; 194:7-15; PMID:21746850; http://dx.doi.org/ 10.1083/jcb.201102095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 2014; 24:R453-62; PMID:24845678; http://dx.doi.org/ 10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 2009; 417:1-13; PMID:19061483; http://dx.doi.org/ 10.1042/BJ20081386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su W-C, Chou HY, Chang CJ, Lee YM, Chen WH, Huang KH, Lee MY, Lee SC. Differential activation of a C/EBP beta isoform by a novel redox switch may confer the lipopolysaccharide-inducible expression of interleukin-6 gene. J Biol Chem 2003; 278:51150-8; PMID:14530280; http://dx.doi.org/ 10.1074/jbc.M305501200 [DOI] [PubMed] [Google Scholar]
- 12.Lee H, Lee YJ, Choi H, Ko EH, Kim J-W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem 2009; 284:10601-9; PMID:19237544; http://dx.doi.org/ 10.1074/jbc.M808742200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stephens JM, Morrison RF, Pilch PF. The expression and regulation of STATs during 3T3-L1 adipocyte differentiation. J Biol Chem 1996; 271:10441-104449; PMID:8631837; http://dx.doi.org/ 10.1074/jbc.271.18.10441 [DOI] [PubMed] [Google Scholar]
- 14.Deng J, Hua K, Lesser SS, Harp JB. Activation of signal transducer and activator of transcription-3 during proliferative phases of 3T3-L1 adipogenesis. Endocrinology 2000; 141:2370-6; PMID:10875236 [DOI] [PubMed] [Google Scholar]
- 15.Zhang K, Guo W, Yang Y, Wu J. JAK2/STAT3 pathway is involved in the early stage of adipogenesis through regulating C/EBPβ transcription. J Cell Biochem 2011; 112:488-97; PMID:21268070; http://dx.doi.org/ 10.1002/jcb.22936 [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Zhou Y, Lei W, Zhang K, Shi J, Hu Y, Shu G, Song J. Signal transducer and activator of transcription 3 (STAT3) regulates adipocyte differentiation via peroxisome-proliferator-activated receptor gamma (PPARgamma). Biol Cell 2010; 102:1-12; http://dx.doi.org/ 10.1042/BC20090070 [DOI] [PubMed] [Google Scholar]
- 17.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, Derecka M, Szczepanek K, Szelag M, Gornicka A, et al.. Function of mitochondrial Stat3 in cellular respiration. Science 2009; 323:793-7; PMID:19131594; http://dx.doi.org/ 10.1126/science.1164551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science 2009; 324:1713-6; PMID:19556508; http://dx.doi.org/ 10.1126/science.1171721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macias E, Rao D, Carbajal S, Kiguchi K, DiGiovanni J. Stat3 binds to mtDNA and regulates mitochondrial gene expression in keratinocytes. J Invest Dermatol 2014; 134:1971-80; PMID:24496235; http://dx.doi.org/ 10.1038/jid.2014.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer AH, Edkins AL, Hoppe HC, Prinsloo E. Dynamic Mitochondrial Localisation of STAT3 in the Cellular Adipogenesis Model 3T3-L1. J Cell Biochem 2015; 116:1232-40; PMID:25565605; http://dx.doi.org/ 10.1002/jcb.25076 [DOI] [PubMed] [Google Scholar]
- 21.Zhao P, Stephens JM. Identification of STAT target genes in adipocytes. JAKSTAT 2013; 1-7; PMID:24058802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev 1998; 78:783-809; PMID:9674695 [DOI] [PubMed] [Google Scholar]
- 23.Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol 2005; 40:229-242; PMID:16126487; http://dx.doi.org/ 10.1080/10409230591008189 [DOI] [PubMed] [Google Scholar]
- 24.Tang Q-Q, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci 2003; 100:44-49; PMID:12502791; http://dx.doi.org/ 10.1073/pnas.0137044100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janderová L, McNeil M, Murrell AN, Mynatt RL, Smith SR. Human mesenchymal stem cells as an in vitro model for human adipogenesis. Obes Res 2003; 11:65-74; PMID:12529487; http://dx.doi.org/ 10.1038/oby.2003.11 [DOI] [PubMed] [Google Scholar]
- 26.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture.II. Factors affecting the adipose conversion. Cell 1975; 5:19-27; PMID:165899; http://dx.doi.org/ 10.1016/0092-8674(75)90087-2 [DOI] [PubMed] [Google Scholar]
- 27.Rubin CS, Hirsch A, Fung C, Rosen OM. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem 1978; 253:7570-7578; PMID:81205 [PubMed] [Google Scholar]
- 28.Russell TR, Ho RJ. Conversion of 3T3 fibroblasts into adipose cells: triggering of differentiation by prostaglandin F2alpha and 1-methyl-3-isobutyl xanthine. Proc Natl Acad Sci 1976; 73:4516-4520; PMID:188043; http://dx.doi.org/ 10.1073/pnas.73.12.4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zebisch K, Voigt V, Wabitsch M, Brandsch M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal Biochem 2012; 425:88-90; PMID:22425542; http://dx.doi.org/ 10.1016/j.ab.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 30.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol 2010; 45, 466-72; PMID:20064600; http://dx.doi.org/ 10.1016/j.exger.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi CI, Suda T. Regulation of reactive oxygen species in stem cells and cancer stem cells. J Cell Physiol 2012; 227:421-30; PMID:21448925; http://dx.doi.org/ 10.1002/jcp.22764 [DOI] [PubMed] [Google Scholar]
- 32.Finkel T. Signal transduction by mitochondrial oxidants. J Biol Chem 2012; 287:4434-40; PMID:21832045; http://dx.doi.org/ 10.1074/jbc.R111.271999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol Metab 2014; 25:293-302; PMID:24793638; http://dx.doi.org/ 10.1016/j.tem.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004; 114:1752-61; PMID:15599400; http://dx.doi.org/ 10.1172/JCI21625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 2011; 14:537-44; PMID:21982713; http://dx.doi.org/ 10.1016/j.cmet.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanda Y, Hinata T, Kang SW, Watanabe Y. Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci 2011; 89:250-8; PMID:21722651; http://dx.doi.org/ 10.1016/j.lfs.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 37.Xu D, Yin C, Wang S, Xiao Y. JAK-STAT in lipid metabolism of adipocytes. Jak-Stat 2013; 2:e27203; PMID:24498541; http://dx.doi.org/ 10.4161/jkst.27203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meier JA, Larner AC. Toward a new STATe: The role of STATs in mitochondrial function. Semin Immunol 2014; 26:20-28; PMID:24434063; http://dx.doi.org/ 10.1016/j.smim.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Y, Qiu J, Dong S, Redell MS, Poli V, Mancini MA, Tweardy D. J Stat3 isoforms, alpha and beta, demonstrate distinct intracellular dynamics with prolonged nuclear retention of Stat3beta mapping to its unique C-terminal end. J Biol Chem 2007; 282:34958-34967; PMID:17855361; http://dx.doi.org/ 10.1074/jbc.M704548200 [DOI] [PubMed] [Google Scholar]
- 40.Ng IHW, Ng DCH, Jans DA, Bogoyevitch MA. Selective STAT3-α or –β expression reveals spliceform-specific phosphorylation kinetics, nuclear retention and distinct gene expression outcomes. Biochem J 2012; 447:125-36; PMID:22799634; http://dx.doi.org/ 10.1042/BJ20120941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng J, Hua K, Caveney EJ, Takahashi N, Harp JB. Protein inhibitor of activated STAT3 inhibits adipogenic gene expression. Biochem Biophys Res Commun 2006; 339:923-31; PMID:16329991; http://dx.doi.org/ 10.1016/j.bbrc.2005.10.217 [DOI] [PubMed] [Google Scholar]
- 42.Andersson CX, Sopasakis VR, Wallerstedt E, Smith U. Insulin antagonizes interleukin-6 signaling and is anti-inflammatory in 3T3-L1 adipocytes. J Biol Chem 2007; 282:9430-5; PMID:17267401; http://dx.doi.org/ 10.1074/jbc.M609980200 [DOI] [PubMed] [Google Scholar]
- 43.Chung J, Uchida E, Grammer TC, Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol 1997; 17:6508-16; PMID:9343414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chueh FY, Leong KF, Yu CL. Mitochondrial translocation of signal transducer and activator of transcription 5 (STAT5) in leukemic T cells and cytokine-stimulated cells. Biochem Biophys Res Commun 2010; 402:778-783; PMID:21036145; http://dx.doi.org/ 10.1016/j.bbrc.2010.10.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan R, Lee JE, Yang Y-M, Liang F-X, Sehgal PB. Live-cell imaging of the association of STAT6-GFP with mitochondria. PLoS One 2013; 8:e55426; PMID:23383189; http://dx.doi.org/ 10.1371/journal.pone.0055426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu F, Mukhopadhyay S, Sehgal PB. Live cell imaging of interleukin-6-induced targeting of ‘transcription factor’ STAT3 to sequestering endosomes in the cytoplasm. Am J Physiol Cell Physiol 2007; 293:C1374-82; PMID:17670892; http://dx.doi.org/ 10.1152/ajpcell.00220.2007 [DOI] [PubMed] [Google Scholar]
- 47.Timofeeva OA, Chasovskikh S, Lonskaya I, Tarasova NI, Khavrutskii L, Tarasov SG, Zhang X, Korostyshevskiy VR, Cheema A, Zhang L, et al.. Mechanisms of unphosphorylated STAT3 transcription factor binding to DNA. J Biol Chem 2012; 287:14192-200; PMID:22378781; http://dx.doi.org/ 10.1074/jbc.M111.323899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol 2010; 105:771-85; PMID:20960209; http://dx.doi.org/ 10.1007/s00395-010-0124-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tammineni P, Anugula C, Mohammed F, Anjaneyulu M, Larner AC, Sepuri NB. The import of the transcription factor STAT3 into mitochondria depends on GRIM-19, a component of the electron transport chain. J Biol Chem 2013; 288:4723-32; PMID:23271731; http://dx.doi.org/ 10.1074/jbc.M112.378984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lufei C, Ma J, Huang G, Zhang T, Novotny-Diermayr V, Ong CT, Cao X. GRIM-19, a death-regulatory gene product, suppresses Stat3 activity via functional interaction. EMBO J 2003; 22:1325-35; PMID:12628925; http://dx.doi.org/ 10.1093/emboj/cdg135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Yang J, Roy SK, Tininini S, Hu J, Bromberg JF, Poli V, Stark GR, Kalvakolanu DV. The cell death regulator GRIM-19 is an inhibitor of signal transducer and activator of transcription 3. Proc Natl Acad Sci U S A 2003; 100:9342-7; PMID:12867595; http://dx.doi.org/ 10.1073/pnas.1633516100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Q, Raje V, Yakovlev VA, Yacoub A, Szczepanek K, Meier J, Derecka M, Chen Q, Hu Y, Sisler J, et al.. Mitochondrial localized Stat3 promotes breast cancer growth via phosphorylation of serine 727. J Biol Chem 2013; 288:31280-8; PMID:24019511; http://dx.doi.org/ 10.1074/jbc.M113.505057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phillips D, Reilley MJ, Aponte AM, Wang G, Boja E, Gucek M, Balaban RS. Stoichiometry of STAT3 and mitochondrial proteins: Implications for the regulation of oxidative phosphorylation by protein-protein interactions. J Biol Chem 2010; 285:23532-6; PMID:20558729; http://dx.doi.org/ 10.1074/jbc.C110.152652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabater D, Arriarán S, Romero Mdel M, Agnelli S, Remesar X, Fernández-López JA, Alemany M. Cultured 3T3L1 adipocytes dispose of excess medium glucose as lactate under abundant oxygen availability. Sci Rep 2014; 4:3663; PMID:24413028; http://dx.doi.org/ 10.1038/srep03663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Demaria M, Giorgi C, Lebiedzinska M, Esposito G, D'Angeli L, Bartoli A, Gough DJ, Turkson J, Levy DE, Watson CJ, et al.. A STAT3-mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging (Albany. NY) 2010; 2:823-42; PMID:21084727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.López-Lázaro M. The warburg effect: why and how do cancer cells activate glycolysis in the presence of oxygen? Anticancer Agents Med Chem 2008; 8:305-12; PMID:18393789; http://dx.doi.org/ 10.2174/187152008783961932 [DOI] [PubMed] [Google Scholar]
- 57.Ruckenstuhl C, Büttner S, Carmona-Gutierrez D, Eisenberg T, Kroemer G, Sigrist SJ, Fröhlich KU, Madeo F. The Warburg effect suppresses oxidative stress induced apoptosis in a yeast model for cancer. PLoS One 2009; 4:e4592; PMID:19240798; http://dx.doi.org/ 10.1371/journal.pone.0004592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol 2014; 15:243-56; PMID:24651542; http://dx.doi.org/ 10.1038/nrm3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int 2012; 2012:812693; PMID:22577397; http://dx.doi.org/ 10.1155/2012/812693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng Y, Dangelmajer S, Lee YM, Wijesekera O, Castellanos CX, Denduluri A, Chaichana KL, Li Q, Zhang H, Levchenko A, et al.. Hypoxia-cultured human adipose-derived mesenchymal stem cells are non-oncogenic and have enhanced viability, motility, and tropism to brain cancer. Cell Death Dis 2014; 5:e1567; PMID:25501828; http://dx.doi.org/ 10.1038/cddis.2014.521 [DOI] [PMC free article] [PubMed] [Google Scholar]


