ABSTRACT
Arbuscular mycorrhizal (AM) fungi form mutualistic interactions with the majority of land plants, including some of the most important crop species. The fungus takes up nutrients from the soil, and transfers these nutrients to the mycorrhizal interface in the root, where these nutrients are exchanged against carbon from the host. AM fungi form extensive hyphal networks in the soil and connect with their network multiple host plants. These common mycorrhizal networks (CMNs) play a critical role in the long-distance transport of nutrients through soil ecosystems and allow the exchange of signals between the interconnected plants. CMNs affect the survival, fitness, and competitiveness of the fungal and plant species that interact via these networks, but how the resource transport within these CMNs is controlled is largely unknown. We discuss the significance of CMNs for plant communities and for the bargaining power of the fungal partner in the AM symbiosis.
Keywords: arbuscular mycorrhizal symbiosis, biological market, common mycelial networks, defense signals, interplant competition, interfungal competition, nitrogen, nutrient transport, rhizosphere, phosphate
The arbuscular mycorrhizal symbiosis between plants and fungi is formed by approximately 65% of all known land plant species and many plants depend on this symbiosis for their nutrient supply.1 Many fungi also provide non-nutritional benefits to their host that are critical for plant survival or fitness, including protection against pathogens, or improved resistance against drought and salinity.2 AM interactions are therefore essential components of large-scale ecosystem processes and act as ‘ecosystem engineers' of plant communities.3
All AM fungi belong to the phylum Glomeromycota, and are unable to complete their life cycle without the carbon supply from their host.4 The obligate biotrophy of AM fungi and the observation that plants often suppress the AM colonization of their root system when nutrients are readily available, has led to the overall assumption that the host plant is in control of the symbiosis.5 However, this phyto-centric view disregards the long co-evolution of both partners in the AM symbiosis (∼ 450 Million years) that allowed the fungus to develop strategies to improve its bargaining power despite its obligate biotrophic life cycle.6-8
As illustrated in Figure 1, AM fungi and their plant partners form a complex network of many-to-many interactions, in which a single plant host is colonized by multiple fungal species, and fungal ‘individuals’ interact with multiple plant hosts and species simultaneously and interconnect plants by a common mycorrhizal network (CMN). Both partners in the symbiosis can choose among multiple trading partners and do not depend on a single partner for their carbon or nutrient resources. CMNs can connect plants of the same or of different plant species and of different developmental stages, and are involved in the long distance transport of nutrients (carbon, phosphate, nitrogen, or micronutrients), water, stress chemicals, and allelochemicals in soil ecosystems.9-15 Multiple fungal and plant species interact and ‘communicate’ via these CMNs and there is growing evidence that CMNs affect the survival and fitness, behavior and competitiveness of the plants and fungi that are linked via these networks.
Figure 1.
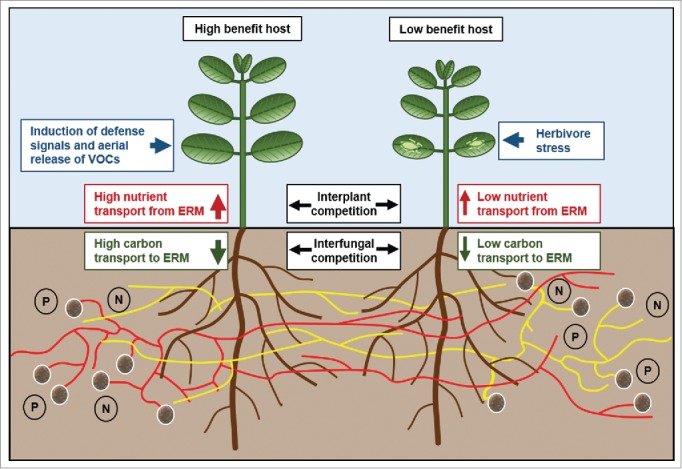
Function of common mycorrhizal networks (CMNs) in soil ecosystems. The roots of plants are connected by CMNs of single or multiple arbuscular mycorrhizal (AM) fungal morphospecies. Plants compete with their carbon resources for nutrients that become available for their CMNs. Plants can differ in their carbon transport to the CMNs and can represent low or high benefit hosts for the AM fungus. Low benefit host plants within a CMN could be for example seedlings that compete with adult plants, or adult plants that transfer less carbon to the CMN due to shading or herbivore damage. AM fungi can discriminate between low and high quality host plants and preferentially transfer resources to high quality hosts what can contribute to the inequalities among plants that have been observed in studies with CMNs. In addition, CMNs can serve as a conduit for the transfer of warning signals or of allelochemicals between plants within one CMN. Warning signals that are formed by donor plants for example in response to herbivore stress can lead in receiver plants to an induction of defense reactions and the release of volatile organic compounds from the leaves (VOCs). Directed transport of allelochemicals to specific plants via CMNs can facilitate the interplant competition and suppress the growth of plant competitors. Fungal CMNs compete for soil nutrients and compete with these nutrients for carbon resources from the different host plants within their CMN.
The development of CMNs allows the fungus to gain access to multiple trading partners, and ensures a continuous carbon supply for the fungus even when one host plant loses its ability to transfer resources to the fungal partner by e.g. pathogen or herbivore damage or by early senescence. When AM fungi are able to discriminate between host plants within their CMN, the fungus gains bargaining power because the plants within its network are forced to compete. In theory, natural selection should favor those fungi that are able to establish a CMN with many host plants, because inter-plant competition will force the competing plants to transfer more carbon to their fungal partner in order to receive a greater share of nutrients from the CMN.16
In order to better understand how nutrient transport among plants in CMNs is controlled, we examined the fungal phosphate and nitrogen allocation to plants that differed in their ability to provide carbon to their fungal symbiont (low and high quality hosts). The studies demonstrated that fungi were indeed able to discriminate among plants that shared a CMN and preferentially allocated nutrient resources to host plants that were able to provide more carbon benefit.7 Nutrient allocation within the CMN, however, was not controlled on an all-or-none basis, and the fungus also transferred phosphate and nitrogen to low quality hosts, and maintained a high colonization rate in these plants. Host plant quality does not seem to be an important factor for root colonization,7 and AM fungi also invest resources to actively colonize the roots of low quality hosts.17 The strategy to colonize both, low and high quality host plants ensures that the loss of a high quality host is less detrimental for the fungus, and forces also high quality hosts to compete for nutrients from the CMN.
Both partners in the AM symbiosis are able to discriminate between different symbiotic partners, and it has been suggested that the ‘fair trade’ between both partners has contributed to the evolutionary stability of the AM mutualism.6 Carbon to nutrient exchange ratios at the mycorrhizal interface are controlled by resource supply and demand and follow biological market dynamics.6-8 Consistently, we found that in the absence of choice, the fungus transfers more nutrient resources per unit carbon to low quality hosts.7 When the fungus only has access to low quality hosts, the dependency of the fungus for the host plant´s carbon shifts the cost to benefit ratio at the mycorrhizal interface in favor of the host.
When plants invest carbon resources into a fungal network that also benefits their competitors, the preferential nutrient allocation to specific host plants within a CMN will provide the favored host plants with a net benefit to the detriment of the unfavored plants within the CMN.18 Plant species or individuals of one species can differ in their carbon investment into the CMN,19 and CMNs have been shown to amplify inequalities in plant communities,15,20 and between seedlings and established adult plants that are connected by a CMN. While some studies have shown that seedlings can benefit from established CMNs with adult plants,21 other studies demonstrated negative impacts of CMNs on seedling establishment and fitness, and P nutrition.22-24 When AM fungi are able to discriminate among plants within their CMN, the fungal partner should provide more resources to adult plants due to their higher carbon transport to the CMN.
The suppression of plants within CMNs, however, can also be a plant-mediated effect. Allelochemicals, root-secreted secondary metabolites that plants use to regulate the rhizosphere to the detriment of competing neighboring plants have also been shown to be transferred from donor to target plants by CMNs.13,25 It is currently unknown, whether AM fungi are able to control the transfer of allelochemicals within their CMNs, but it is interesting to speculate that AM fungi, by a directed transport of allelochemicals, could suppress specific plants within their CMN, or susceptible fungal competitors. Some plants release allelochemicals with antifungal activities, and it has been shown that some invasive plants use these antifungal allelochemicals to suppress the mycorrhizal colonization of their native plant competitors.26
CMNs also play an important role in the plant-to-plant ‘communication’ by transferring infochemicals and warning signals between plants. Plants that are attacked by herbivores produce volatile organic compounds that act as a repellent for aphids but attract the natural enemies of aphids to the infested leaves. These volatiles are only produced by non-infested plants when they share a CMN with infested plants.9 These warning signals between plants within one CMN are transmitted rapidly, and non-infested plants up-regulated genes of the jasmonate defense pathway shortly after plants within their CMN were attacked by herbivores.27 Herbivore damage can reduce the capability of plants to provide the CMN with carbon, and AM fungi that efficiently share these defense-related signals with other plants within their CMN will be able to reduce the negative impact of herbivore damage on their carbon supply. It is currently not known whether the fungus controls the flow of these defense-related signals within its CMN. The fungus could transfer these warning signals preferentially to host plants that provide more carbon benefit, or to host plants that demonstrate the strongest defense response in order to keep the damage to these plants as small as possible.10 Another strategy could be that the fungus shares these warning signals equally among the plants within its CMN, because the fungus is unable to predict how severely the carbon flow of individual plants will be affected by herbivore damage. Some plants respond to a herbivore attack above-ground with an increased carbon allocation below ground into roots and root exudates. This could increase the carbon transport of these plants into the CMN, and could improve the attractiveness of these plants for fungal colonization and signal transduction.28
Conclusions
AM fungi and their CMNs play a significant role in plant ecosystems and control the fitness and competitiveness of the plant individuals within their CMNs. Our current understanding about resource exchange in the AM symbiosis is primarily based on experiments with root organ cultures or with single plants that are colonized by one AM fungus.6,8 The transferability of these experiments to CMNs, however, is very limited, because in natural ecosystems both partners in the AM symbiosis can choose among multiple trading partners and do not depend on a single partner for their nutrient or carbon supply. Plants play a critical role for the carbon supply of their CMNs and the composition of the plant community within one CMN has been shown to affect the abundance or extension of CMNs in soils.29,30 Very little is known about how AM fungi allocate nutrient resources or infochemicals within their CMN, or how host plants compete with other plants for nutrients that are available for their CMNs. More research is needed to better understand how the costs and benefits of the AM symbiosis are controlled in CMNs, and how fungal networks affect the inter-fungal or inter-plant competitiveness of both partners in natural ecosystems.
Abbreviations
- AM
arbuscular mycorrhizal
- CMN
common mycorrhizal network
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Adam Cloos (South Dakota State University), and Philip E. Pfeffer and Gary Strahan (both USDA, ARS, Wyndmoor) for technical assistance.
Funding
We thank the NSF (IOS award 1051397) for financial support.
References
- [1].Wang B, Qiu YL. Phylogenetic distribution and evolution of mycorrhizae in land plants. Mycorrhiza 2006; 16:299-363; PMID:16845554 [DOI] [PubMed] [Google Scholar]
- [2].Smith SE, Read DJ. Mycorrhizal symbiosis. New York: Academic Press, 2008 [Google Scholar]
- [3].Cameron DD. Arbuscular mycorrhizal fungi as (agro)ecosystem engineers. Plant Soil 2010; 333:1-5 [Google Scholar]
- [4].Redecker D, Raab P. Phylogeny of the Glomeromycota (arbuscular mycorrhizal fungi): recent developments and new gene markers. Mycologia 2006; 98:885-95; PMID:17486965 [DOI] [PubMed] [Google Scholar]
- [5].Smith SE, Smith FA. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 2012; 104:1-13; PMID:21933929 [DOI] [PubMed] [Google Scholar]
- [6].Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A, et al.. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011; 333:880-2; PMID:21836016 [DOI] [PubMed] [Google Scholar]
- [7].Fellbaum CR, Mensah JA, Cloos AJ, Strahan GD, Pfeffer PE, Kiers ET, Bücking H. Fungal nutrient allocation in common mycelia networks is regulated by the carbon source strength of individual host plants. New Phytologist 2014; 203:645-56 [DOI] [PubMed] [Google Scholar]
- [8].Fellbaum CR, Gachomo EW, Beesetty Y, Choudhari S, Strahan GD, Pfeffer PE, Kiers ET, Bücking H. Carbon availability triggers fungal nitrogen uptake and transport in the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci U S A 2012; 109:2666-71; PMID:22308426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Babikova Z, Gilbert L, Bruce TJA, Birkett M, Caulfield JC, Woodcock C, Pickett JA, Johnson D. Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecology Letters 2013; 16:835-43; PMID:23656527 [DOI] [PubMed] [Google Scholar]
- [10].Babikova Z, Johnson D, Bruce TJA, Pickett J, Gilbert L. Underground allies: How and why do mycelial networks help plants defend themselves? Bioassays 2013; 36:21-6 [DOI] [PubMed] [Google Scholar]
- [11].Teste FP, Veneklaas EJ, Dixon KW, Lambers H. Is nitrogen transfer among plants enhanced by contrasting nutrient-acquisition strategies? Plant, Cell Environ 2015; 38:50-60 [DOI] [PubMed] [Google Scholar]
- [12].Gorzelak MA, Asay AK, Pickles BJ, Simard SW. Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities. AOB Plants 2015; http://dx.doi.org/ 10.1093/aobpla/plv050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Barto EK, Hilker M, Muller F, Mohney BK, Weidenhamer JD, Rillig MC. The Fungal Fast Lane: Common Mycorrhizal Networks Extend Bioactive Zones of Allelochemicals in Soils. PLoS One 2011; 6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Voets L, Goubau I, Olsson PA, Merckx R, Declerck S. Absence of carbon transfer between Medicago truncatula plants linked by a mycorrhizal network, demonstrated in an experimental microcosm. FEMS Microbiol Ecol 2008; 65:350-60; PMID:18557940 [DOI] [PubMed] [Google Scholar]
- [15].Weremijewicz J, Janos DP. Common mycorrhizal networks amplify size inequality in Andropogon gerardii monocultures. New Phytologist 2013; 198:203-13; PMID:23356215 [DOI] [PubMed] [Google Scholar]
- [16].Wyatt GAK, Kiers ET, Gardner A, West SA. A biological market analysis of the plant-mycorrhizal symbiosis. Evolution 2014; 68:2603-18; PMID:24909843 [DOI] [PubMed] [Google Scholar]
- [17].Knegt B, Jansa J, Franken O, Engelmoer DJP, Werner GDA, Bücking H, Kiers T. Host plant quality mediates competition between arbuscular mycorrhizal fungi. Fungal Ecol 2014; http://dx.doi.org/ 10.1016/j.funeco.2014.09.011 [DOI] [Google Scholar]
- [18].Selosse MA, Richard F, He S, Simard SW. Mycorrhizal networks: des liaisons dangereuses? Trends Ecol Evol 2006; 21:621-8; PMID:16843567; http://dx.doi.org/ 10.1016/j.tree.2006.07.003 [DOI] [PubMed] [Google Scholar]
- [19].Walder F, Niemann H, Natarajan M, Lehmann MF, Boller T, Wiemken A. Mycorrhizal networks: common goods of plants shared under unequal terms of trade. Plant Physiol 2012; 159:789-97; PMID:22517410; http://dx.doi.org/ 10.1104/pp.112.195727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Booth MG, Hoeksema JD. Mycorrhizal networks counteract competitive effects of canopy trees on seedling survival. Ecology 2010; 91:2294-302; PMID:20836451; http://dx.doi.org/ 10.1890/09-1139.1 [DOI] [PubMed] [Google Scholar]
- [21].van der Heijden MGA, Horton TR. Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J Ecol 2009; 97:1139-50; http://dx.doi.org/ 10.1111/j.1365-2745.2009.01570.x [DOI] [Google Scholar]
- [22].Pietikäinen A, Kytöviita M-M. Defoliation changes mycorrhizal benefit and competitive interactions between seedlings and adult plants. J Ecol 2007; 95:639-47; http://dx.doi.org/ 10.1111/j.1365-2745.2007.01257.x [DOI] [Google Scholar]
- [23].Merrild MP, Ambus P, Rosendahl S, Jakobsen I. Common arbuscular mycorrhizal networks amplify competition for phosphorus between seedlings and established plants. New Phytologist 2013; 200:229-40; PMID:23738787; http://dx.doi.org/ 10.1111/nph.12351 [DOI] [PubMed] [Google Scholar]
- [24].Kytöviita M-M, Vestberg M, Tuomi J. A test of mutual aid in common mycorrhizal networks: established vegetation negates benefit in seedlings. Ecology 2003; 84:898-906; http://dx.doi.org/ 10.1890/0012-9658(2003)084[0898:ATOMAI]2.0.CO;2 [DOI] [Google Scholar]
- [25].Barto EK, Weidenhamer JD, Cipollini D, Rillig MC. Fungal superhighways: do common mycorrhizal networks enhance below ground communication? Trends Plant Sci 2012; 17:633-7; PMID:22818769; http://dx.doi.org/ 10.1016/j.tplants.2012.06.007 [DOI] [PubMed] [Google Scholar]
- [26].Stinson KA, Campbell SA, Powell JR, Wolfe BE, Callaway RM, Thelen GC, Hallett SG, Prati D, Kliromonos JN. Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. Plos Biol 2006; 4:e140; PMID:16623597; http://dx.doi.org/ 10.1371/journal.pbio.0040140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Song YY, Ye ML C., He X, Zhu-Salzman K, Wang RL, Su YJ, Luo SM, Zeng RS. Hijacking common mycorrhizal networks for herbivore-induced defence signal transfer between tomato plants. Sci Rep 2014; 4:3915; PMID:24468912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Holland JN, Cheng W, Crossley DA Jr. Herbivore-induced changes in plant carbon allocation: assessement of below-ground C fluxes using carbon-14. Oecologia 1996; 107:87-94; http://dx.doi.org/ 10.1007/BF00582238 [DOI] [PubMed] [Google Scholar]
- [29].Derelle D, Declerck S, Genet P, Dajoz I, van Aarle I. Association of highly and weakly mycorrhizal seedlings can promote the extra- and intraradical development of a common mycorrhizal network. FEMS Microbiol Ecol 2012; 79:251-9; PMID:22029624; http://dx.doi.org/ 10.1111/j.1574-6941.2011.01214.x [DOI] [PubMed] [Google Scholar]
- [30].Engelmoer DJP, Kiers ET. Host diversity affects the abundance of the extraradical arbuscular mycorrhizal network. New Phytologist 2015; 205:1485-91; PMID:25297948; http://dx.doi.org/ 10.1111/nph.13086 [DOI] [PubMed] [Google Scholar]


