Abstract
The oral cavity is a unique niche where Candida albicans infections occur in immunocompetent as well as immunosuppressed individuals. Here we critically review the significance of human innate immune response in preventing oral candidiasis. One important line of defense against oropharyngeal candidiasis is the oral microbiota that prevents infection by competing for space and nutrients as well as by secreting antagonistic molecules and triggering local inflammatory responses. C. albicans is able to induce mucosal defenses through activation of immune cells and production of cytokines. Also, saliva contains various proteins that affect C. albicans growth positively by promoting mucosal adherence and negatively through immune exclusion and direct fungicidal activity. We further discuss the role of saliva in unifying host innate immune defenses against C. albicans as a communicating medium and how C. albicans overgrowth in the oral cavity may be a result of aberrations ranging from microbial dysbiosis and salivary dysfunction to epithelial damage. Last we underscore select oral diseases in which C. albicans is a contributory microorganism in immune-competent individuals.
Keywords: oropharyngeal candidiasis, salivary dysfunction, antimicrobial proteins, oral microbiota, microbial dysbiosis, mucosal immunity
Introduction
The oral cavity is a complex ecosystem lined by various types of mucosal epithelia, and it is constantly being bathed in saliva secreted by major and minor salivary glands. The mouth harbors an extensive microbiota that includes beneficial microorganisms along with potentially harmful commensals and transient pathogens. Candida albicans is normally a commensal fungus that colonizes saliva and the oral mucosa, which can transition into a pathogen causing oropharyngeal candidiasis (OPC) or oral thrush.
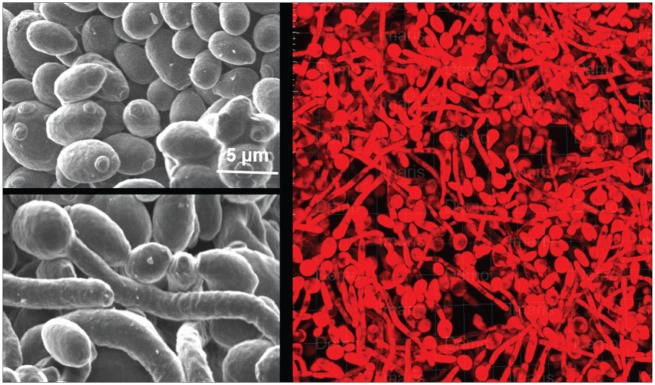
The genus Candida belongs to the kingdom Fungi, which consists of approximately 150 species. Candida species are found in diverse environments, such as air, soil, water, plants, and animals, but only a select few are able to colonize and cause disease in humans. C. albicans, Candida glabrata, Candida parapsilosis, Candida tropicalis, Candida krusei, and Candida dubliniensis are the most common Candida species associated with human disease (Muadcheingka and Tantivitayakul 2015). Most of these Candida species grow as unicellular yeast forms, while C. albicans is also able to form hyphal or pseudohyphal morphologies that allow it to form complex biofilms (Fig. 1) and contribute to its pathogenicity in humans.
Figure 1.
Candida albicans morphologies involved in oropharyngeal candidiasis. C. albicans can exist in the yeast form (left, top) that can transition into a more virulent hyphal form (left, bottom). Both morphologies are present in biofilms (right) that are found on mucosal surfaces in oropharyngeal candidiasis.
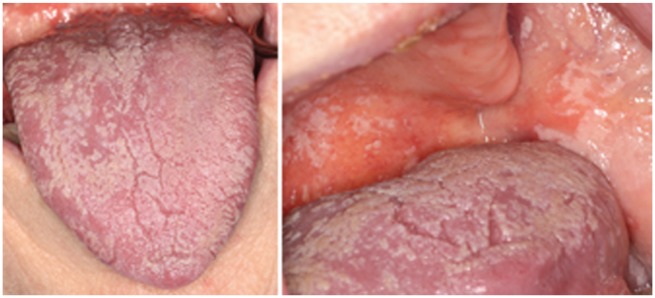
OPC most frequently occurs in individuals whose immune system is compromised by use of immunosuppressant drugs, chemotherapy, or AIDS. The clinical manifestations of OPC are geographically extensive thick white plaques formed on the tongue, buccal mucosa, soft palate, and pharynx (Fig. 2). Although OPC does not typically progress to life-threatening fungemia, it can result in significant patient morbidity through dysphagia, dehydration, and malnutrition. HIV patients represent a distinct group of people highly susceptible to OPC, and the severity of oral symptoms as well as the frequency of isolation of C. albicans is positively correlated with AIDS staging (reviewed in de Repentigny et al. 2004). Perturbations in host immune status allow C. albicans to progress from commensalism to virulence; however, our understanding about other predisposing factors that tip this balance is incomplete. These may include disruption of the bacterial component of the oral microbiota, alterations in salivary quantity or quality, or breach of host epithelial barriers (as a result of burns, catheters or ventilation tubes, or use of oral prosthesis). However, C. albicans is also capable of producing disease in an otherwise healthy host when it enters the dental pulp or colonizes tooth surfaces along with Streptococcus mutans in early childhood caries. The focus of this review is to discuss various host salivary and innate immune factors that contribute toward protection from OPC.
Figure 2.
Oral manifestations of oropharyngeal candidiasis. White plaques comprising Candida albicans yeast and hyphal forms can be seen on the tongue (left) and the buccal mucosa and soft palate (right) during oropharyngeal candidiasis.
Interaction of Oral Microbiota with C. albicans
The oral cavity harbors a complex microbiome consisting of both bacteria and fungi, and as a community, they influence each other’s growth (Krom et al. 2014; Oever and Netea 2014). C. albicans forms polymicrobial biofilms with many oral bacteria, including Lactobacillus, Enterococcus, and Staphylococcus species, as well as oral streptococci (reviewed by Harriott and Noverr 2011). These bacteria can have synergistic or antagonistic influences on C. albicans by multiple mechanisms, such as promoting coadhesion to increase biofilm biomass, releasing quorum-sensing molecules and metabolites to reduce fungal growth, affecting virulence-related gene expression, and modulating mucosal immune inflammatory responses (Oever and Netea 2014; Xu et al. 2014).
Synergistic Interactions
C. albicans is known to coaggregate with itself and with other oral bacteria through expression of cell surface adhesins, particularly the agglutinin-like sequence proteins (Klotz et al. 2007), allowing both C. albicans and its bacterial partners to grow more efficiently. Streptococcus of the viridans group (including Streptococcus gordonii, Streptococcus oralis, and Streptococcus sanguinis) was found to have increased colonization and biofilm formation in the presence of C. albicans, while C. albicans was shown to invade the oral mucosa more efficiently in the presence of S. oralis (Diaz et al. 2012), most likely a result of hyphal induction through Cek1 signaling, which contributes to tissue invasion. This signaling pathway also was activated during C. albicans–S. gordonii physical interactions (Bamford et al. 2009). Coinfection of C. albicans with Streptococci also increased the severity and frequency of oral candidiasis lesions and was mediated in part by amplification of mucosal inflammatory responses—specifically, a result of enhanced signaling through Toll-like receptor 2, resulting in amplification of neutrophilic response (Xu et al. 2014). Furthermore, S. gordonii promoted C. albicans hyphae formation when both species were present in a mixed biofilm, and it was mediated by the interspecies signaling molecule autoinducer 2, which potentially represses farnesol-mediated hyphal inhibition (Bamford et al. 2009).
Antagonistic Interactions
Interestingly, use of broad-spectrum antibiotics is a factor that likely predisposes an otherwise immune-competent host to oral candidiasis (Soysa et al. 2008; Yang et al. 2010), underscoring the antagonistic role of some commensal bacteria in C. albicans proliferation in the oral cavity. However, there is a gap in our knowledge regarding this relationship in the oral environment, since most data about human commensals preventing Candida infection are from nonoral niches, including the vagina, respiratory tract, and gut. For example, Pseudomonas aeruginosa, a human airway pathogen that is also a transient bacterium in the oral cavity, was shown to kill C. albicans hyphae as well as prevent hyphal formation by the secretion of a quorum-sensing molecule, 3-oxo-C12 homoserine lactone (Hogan et al. 2004). Vaginal lactobacilli have been shown to inhibit C. albicans growth by production of H2O2 as well as by lowering pH and production of short chain fatty acids to prevent yeast-to-hypha transition (reviewed in Oever and Netea 2014). Furthermore, there is evidence from murine intestinal models that bacteria induce mucosal immunity to strengthen the epithelial barrier and prevent tissue invasion by C. albicans (Oever and Netea 2014). This is largely mediated by short chain fatty acids secreted by bacteria that in turn induce the production of cathelicidin LL-37 (by intestinal epithelial cells), an antimicrobial peptide with a role in maintaining epithelial integrity (Oever and Netea 2014).
Several oral bacteria markedly reduced the formation of C. albicans biofilms on polystyrene surfaces, suggesting that oral bacteria may also play an inhibitory role in C. albicans colonization in the oral cavity (Thein et al. 2006). It has been shown in vitro that the cariogenic bacterium S. mutans secretes signaling molecules, trans-2-decenoic acid (Vilchez et al. 2010), and competence-stimulating peptide (Jarosz et al. 2009) to suppress yeast-to-hyphae transition in C. albicans. Based on the role of vaginal lactobacilli in preventing candidiasis, a probiotic approach in treatment of oral candidiasis has been tested. Lactobacillus acidophilus decreased oral colonization in mice after 2 d of administration and completely eliminated oral yeast by day 6 (Elahi et al. 2005). It was further demonstrated that probiotic bacteria such as Lactocobacillus species present in cheese could decrease the colonization of C. albicans in an elderly population (Hatakka et al. 2007).
Besides oral commensal bacteria, the commensal mycobiome has been shown to modulate oral candidiasis. Our recent work (under review) found that prior colonization with C. albicans is essential for infection with C. glabrata, since this yeast does not form hyphae and it utilizes C. albicans for tissue colonization. Also, the synergy between these 2 Candida species has been shown in a reconstituted human vaginal epithelium model in which C. albicans promoted both enhanced C. glabrata colonization and increased expression of virulence genes, together leading to greater tissue invasion and damage (Alves et al. 2014). Also, changes in the oral mycobiome of HIV-infected patients, including a decrease in Pichia (a commensal fungus in healthy patients), occurred simultaneously with an increase in the levels of C. albicans (Mukherjee et al. 2014).
Influence of Salivary Function on C. albicans
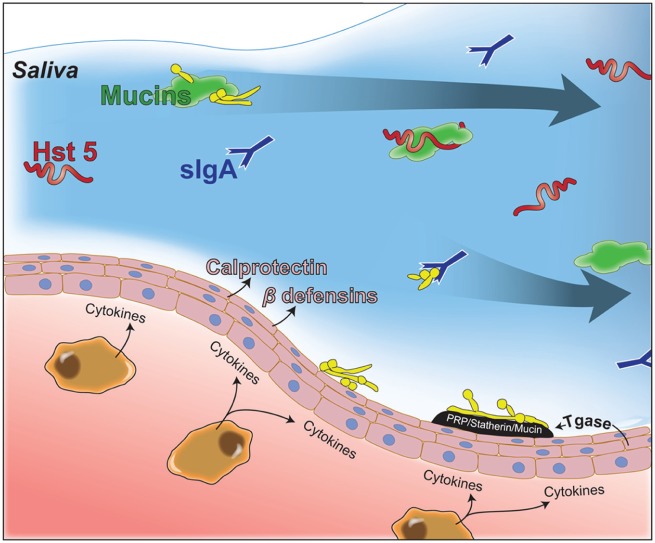
Saliva is the medium that facilitates interactions among the oral microbiome, host innate immune cells, and their secreted immune modulators, and it is a carrier for salivary proteins, thus unifying host defenses against C. albicans (Fig. 3). Salivary gland hypofunction and xerostomia can be a result of autoimmune diseases affecting the salivary glands, such as Sjögren’s syndrome (SS), or as a consequence of medical treatments or medications. Salivary deficiency leads to oral fungal infections as illustrated by SS patients, many of whom suffer from oral candidiasis (Radfar et al. 2003).
Figure 3.
Role of salivary proteins in oropharyngeal candidiasis. Salivary proteins (proline-rich proteins [PRPs], statherins, mucins) and oral epithelial transglutaminase (Tgase) can aid Candida albicans growth by promoting adherence to oral tissues or inhibiting growth through immune exclusion by binding and aggregating fungal cells (mucins and secretory IgA [sIgA]) to facilitate their clearance by swallowing. Antifungal proteins, including salivary histatin 5 (Hst 5) or calprotectin and β-defensins (secreted by oral tissues), prevent C. albicans growth.
Reduction in salivary secretions may cause dysbiosis, favoring C. albicans overgrowth (Leung et al. 2008). One study found that 79 of 103 patients with hyposalivation were positive for C. albicans in oral rinse samples with an inverse correlation of salivary flow rate and Candida load (Radfar et al. 2003). Furthermore, SS patients with oral candidiasis were found to have elevated levels of salivary calprotectin, an antimicrobial peptide, possibly due to mucosal transudation from inflamed mucosa (Sweet et al. 2001). Thus, salivary dysfunction can disarm host defenses in the oral cavity to cause OPC as a result of changes in levels of salivary proteins or due to loss of the protective coating of saliva on mucosal surfaces, reducing epithelial barrier function (Linden et al. 2008).
C. Albicans Adhesion/Aggregation-Promoting Salivary Proteins
Salivary proteins form a salivary-derived pellicle both on tooth surfaces (acquired enamel pellicle; Mann and Dickinson 2006) and on oral epithelia (bound mucosal pellicle; Gibbins et al. 2014). Mucins are large glycoproteins that are major components of this pellicle and cover the mucosal surface with a highly hydrated mucus gel protecting the epithelium against microbially secreted enzymes and mechanical damage (Linden et al. 2008). Pellicle components also include sIgA, cystatin S, basic proline-rich proteins (PRPs), statherins, and carbonic anhydrase (Hannig et al. 2005). It has been shown that circulating salivary mucins and sIgA can aggregate C. albicans cells, and these aggregates are then cleared from the oral cavity by swallowing (Hoffman and Haidaris 1993). However, this aggregation as a part of mucosal pellicle can also promote adherence to oral epithelia (Holmes et al. 2002).
Statherins were also shown to promote C. albicans adherence to salivary pellicles as well as oral epithelial cells, confirming the role of these proteins in promoting oral surface colonization (Johansson et al. 2000). Membrane-bound transglutaminase from buccal oral epithelial cells plays an important role in linking PRPs to the oral epithelia (Bradway and Levine 1993). Interestingly, C. albicans cell surface Hwp1 protein (which is highly expressed on the hyphal wall) has been shown to have homology with PRPs and can therefore function as a transglutaminase substrate (Staab et al. 1999). Thus, by harboring a PRP-like Hwp1 protein on its cell surface, C. albicans has evolved to exploit buccal oral epithelial transglutaminase to promote its adherence to the oral surfaces (Fig. 3). This is of great significance since adhesion to oral epithelia is the primary event that promotes proliferation of fungal cells on mucosal surfaces leading to OPC.
Salivary Proteins Involved in Clearing or Killing C. Albicans
Salivary sIgA is an abundant antibody produced by salivary glands and secreted in saliva (Brandtzaeg 2013), and it has multifaceted roles in mucosal immunity. Unlike antibodies in systemic sites in which bacteria are opsonized and cleared by immunoglobulin Fc-mediated uptake by macrophages and neutrophils, sIgA functions through immune exclusion by binding and aggregating microorganisms within saliva that are then cleared through swallowing (Brandtzaeg 2013). Furthermore, sIgA inhibits the adhesion of C. albicans to polystyrene by binding fungal cell wall mannoproteins (San Millán et al. 2000), and it reduces C. albicans adherence to epithelial cells in vitro (Holmes et al. 2002). Like salivary sIgA, mucins can also bind and aggregate C. albicans to clear it from the oral cavity by swallowing (de Repentigny et al. 2000; Fig. 3). Mucins can further negatively affect C. albicans growth by suppressing expression of its virulence genes (Kavanaugh et al. 2014).
Besides promoting clearance, saliva itself possesses candidacidal activity, since it contains various antifungal proteins; among these, salivary histatin 5 (Hst 5) is the most potent for killing C. albicans (reviewed previously; Puri and Edgerton 2014). Hst 5 is the main antifungal protein synthesized by human salivary glands and has multiple targets within yeast cells. It is taken up by C. albicans cells through polyamine transporters; it then causes oxidative and osmotic stress and affects mitochondrial functions. However, it ultimately kills by inducing nonlytic release of K+ ions and ATP from the cell. Other antifungal proteins within the oral cavity include LL-37 and the defensins (produced by neutrophils or mucosal tissues) that kill C. albicans by disrupting the cell membrane or by increasing membrane permeability (reviewed in Swidergall and Ernst 2014).
Calprotectin, another antifungal protein, is a Ca2+- and Zn2+-binding protein belonging to the S-100 family of proteins, and it is produced by mucosal keratinocytes as well as a variety of host immune cells, such as PMNs, monocytes, and macrophages (Sohnle et al. 1996). Calprotectin has been shown, in vivo and in vitro, to be a part of neutrophil extracellular traps that may contribute to its antifungal functions (Urban et al. 2009). Reduction in calprotectin levels has been implicated in increased incidence of oral candidiasis among HIV+ patients (Sweet et al. 2001). Calprotectin activity has also been shown to increase synergistically in the presence of lactoferrin (an iron-binding protein ubiquitous in all human exocrine secretions, also produced by neutrophils at the site of infection; Okutomi et al. 1998). Both of these proteins reduce fungal growth by withholding essential metal nutrients from C. albicans. Saliva also contains the proteolytic enzyme lysozyme, produced by oral neutrophil leukocytes, and antileukoprotease, a secretory leukocyte protease inhibitor, both with potential candidacidal activities (reviewed in de Repentigny et al. 2004). Although early workers found conflicting results with regard to changes in salivary antimicrobial proteins (histatins, lactoferrin, and lysozyme) in HIV infection (reviewed in de Repentigny et al. 2004), perhaps as a result of different patient characteristics, these observations underscore the importance of salivary proteins in relationship to oral fungal burden.
Oral Diseases in Immunocompetent Hosts in Which C. albicans Is an Etiologic Microorganism
Denture Stomatitis
Denture stomatitis (DS) is a common mucosal infection of tissues underlying the maxillary denture-bearing surfaces of healthy individuals who wear dentures, with xerostomia also being a risk factor (Campisi et al. 2008). C. albicans was 20-fold more likely to be recovered from the denture surfaces in DS versus non-DS patients (Altarawneh et al. 2013). Differences in the proteome of pooled whole unstimulated saliva from DS patients compared with healthy denture wearers were also found; for example, increased levels of cystatins, immunoglobulins, and lactotransferrin were observed (Byrd et al. 2014). Increased levels of cystatins and immunoglobulins were suggestive of a host defense response to inflammation and presence of Candida in DS patients, while the increase in lactotransferrin, which has both antifungal and antibacterial properties, highlights the microbial component of DS etiology. Plastic denture surfaces or denture liners serve as a “culture plate” for fungal infections; however, bacterial species found within denture biofilms differ in healthy patients versus DS patients (Campos et al. 2008), illustrating the complex interplay between Candida and bacterial species in this polymicrobial disease. Presence or absence of natural dentition in DS patients further modified the associated microbiota and the severity of symptoms (O’Donnell et al. 2015). A rat denture model was developed to study in vivo biofilm formation on dentures placed in immunosuppressed rats (Nett et al. 2010). Interestingly, both bacilli and cocci bacteria were found associated with C. albicans within the denture biofilm and matrix and were often associated with fungal hyphae. Furthermore, C. albicans cells in the denture biofilm showed increased transcription of genes encoding the cell wall adhesion protein Als1 and secreted aspartyl protease Sap5 (Nett et al. 2010).
Early Childhood Caries
S. mutans is the major pathogenic organism causing dental caries (Loesche 1986); it is also involved in early childhood caries. Clinical studies found that large numbers of C. albicans were isolated along with S. mutans in plaques from carious lesions in children with early childhood caries (de Carvalho et al. 2006). It was initially surprising to discover this co-colonization, since no evidence of coadhesion between C. albicans and S. mutans was known. However, recent elegant work showed that coculture of these 2 organisms with sucrose resulted in production of the S. mutans exoenzyme (GtfB) that bound to mannans and β-1,3 glucans found on the fungal outer cell wall (Falsetta et al. 2014). In the same study, it was shown that coinfection in rats with both C. albicans and S. mutans increased the severity and number of smooth-surface caries lesions by 2-fold in the presence of sucrose. This striking result was attributed to the synergistic ability of these 2 organisms to form a denser and more polysaccharide-rich biofilm matrix, perhaps creating foci that are more acidic, closely overlaying the enamel.
These caries studies also highlight the importance of co-colonization of C. albicans with other oral microorganisms in the formation of biofilm extracellular matrices. C. albicans secretes insoluble β-1,3-glucans that are delivered to the extracellular matrix by glucan transferases (encoded by BGL2 and PHR1) and an exoglucanase (encoded by XOG1; Taff et al. 2012); these glucans have been shown to be a major factor in contributing to the architecture and stability of the 3-dimensional matrix scaffold (Xiao et al. 2012). While it is known that these matrices inhibit inward diffusion of antifungal drugs protecting fungi from killing (Taff et al. 2012), the matrix also likely serves to protect cells from host innate immune factors by collectively encasing microbial cells in a protective and potentially impenetrable medium.
Periodontal Disease
Despite being an aerobic fungal pathogen, C. albicans has been isolated in periodontal pockets in 15% to 17% of individuals, suggesting that it might have a role in the development and progression of periodontal disease (Reynaud et al. 2001). Patients with chronic periodontitis had an increase in prevalence of subgingival colonization of C. albicans that was correlated with increased severity of periodontal disease (Canabarro et al. 2013). It is unclear whether periodontitis creates an environment favorable for Candida colonization or whether C. albicans colonization itself promotes disease progression. Anaerobic conditions within the periodontal pocket favored higher hemolysin and phospholipase activity of C. albicans recovered from these sites (Sardi et al. 2012); furthermore, invasion of anaerobic bacteria, including P. gingivalis, into epithelial cells and fibroblasts was augmented by the presence of C. albicans, suggesting that C. albicans contributes to the progression of periodontal disease (Tamai et al. 2011). Alternatively, C. albicans may be a passive bystander in periodontal disease when partnered with a periodontal pathogen such as Fusobacterium nucleatum. F. nucleatum has been considered a linker of early- and late-colonizing species in periodontal disease and can establish a physical association with C. albicans facilitating its colonization (Jabra-Rizk et al. 1999). Coaggregation of F. nucleatum and C. albicans hyphae has also been confirmed (Wu et al. 2015).
Endodontic Lesions
The primary cause of pulpal inflammation and endodontic lesions is microorganisms that reach the pulp through dental caries. These infections are usually polymicrobial and are predominantly composed of anaerobic and facultative bacteria that differ from bacteria recovered from enamel caries (Rocas et al. 2015). Although fungi were not commonly recovered in early studies of root canal infections, C. albicans was found in 20% of endodontic lesions in patients with cellulitis or abscesses and periapical radiolucencies (Baumgartner et al. 2000). C. albicans is remarkably resilient in its ability to acquire nutrients so that the hyphae found in dentinal tubes associated with these endodontic lesions may be a marker for its secretion of hyphal-specific proteases that allow digestion of dentinal collagen as a source of nutrition. This may allow C. albicans to have a selective survival advantage over other bacteria in this niche.
Concluding Remarks
Although largely considered a disease of immune-compromised people, oral C. albicans infections are common among denture wearers and individuals with hyposalivation or SS. Thus, there is a large population of immune-competent individuals that are susceptible to oral fungal infections. Maintaining C. albicans at commensal levels in these healthy people likely involves the antagonistic action of healthy oral microbiota and salivary proteins along with appropriate mucosal inflammatory response, and dysfunction or changes in any of these factors (Table) may contribute to C. albicans–associated oral diseases. Although the Centers for Disease Control and Prevention lists broad-spectrum antibiotic usage as a predisposing factor for OPC, extensive epidemiologic studies are needed to gather data for the incidence of OPC caused by antibiotic use in immunocompetent hosts, when encountered in dental clinics and other health care settings. Particularly of interest would be to determine how the oral microbiome is altered in immune-competent people with salivary dysfunction or SS. Such studies would identify crucial groups of microorganisms that might contribute to commensalism or virulence attributes of C. albicans.
Table.
Contribution of Host Factors and Microbiome to Oral Diseases Associated with Candida albicans.
| Immune Dysfunction | Loss of Oral Epithelial Integrity | Changes in Salivary Flow and Constituents | Oral Microbial Dysbiosis | |
|---|---|---|---|---|
| Oropharyngeal candidiasis | ✓ | ✓ | ✓ | ? |
| Denture stomatitis | ✓ | ✓ | ? | |
| Childhood caries | ✓ | |||
| Periodontitis | ✓ | |||
| Endodontic lesions | ||||
| Sjögren’s syndrome | ✓ | ✓ | ✓ | ? |
Furthermore, many predisposing factors for oral candidiasis are interconnected, as seen in SS patients where disruption of salivary flow not only reduces amounts of clearing salivary proteins but also alters oral commensal microbiota and promotes disruption in oral epithelia as a result of dry mouth and loss of lubrication. Thus, the overall severity of C. albicans infection is a result of these cumulative effects. Therefore, an ideal treatment strategy should involve utilizing or strengthening multiple immune aspects to bolster the host’s assault on C. albicans. For example, use of probiotics and salivary antifungal proteins such as Hst 5, together with antifungal drugs, could provide an efficient strategy to treat OPC.
Likewise, some recent studies point to a role of interspecies association between endodontic, cariogenic, and periodontal pathogenic bacteria and C. albicans. However, more research is needed to understand whether this identified coassociation of C. albicans with specific bacterial pathogens has an etiologic role in the disease process. This knowledge would be of significance in selection of therapeutic options if C. albicans is indeed a pathogen; for example, addition of antifungal drugs as a part of an antimicrobial regimen for periodontal or endodontic infections might improve outcomes.
Author Contributions
O. Salvatori, S. Puri, S. Tati, M. Edgerton, contributed to conception and design, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
We also thank John Nyquist for the illustrations in this article.
Footnotes
This work was supported by R01DE010641 and R01DE022720 funded by the National Institute of Dental and Craniofacial Research, National Institutes of Health.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Altarawneh S, Bencharit S, Mendoza L, Curran A, Barrow D, Barros S, Preisser J, Loewy ZG, Gendreau L, Offenbacher S. 2013. Clinical and histological findings of denture stomatitis as related to intraoral colonization patterns of Candida albicans, salivary flow, and dry mouth. J Prosthodont. 22(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves CT, Wei XQ, Silva S, Azeredo J, Henriques M, Williams DW. 2014. Candida albicans promotes invasion and colonisation of Candida glabrata in a reconstituted human vaginal epithelium. J Infect. 69(4):396–407. [DOI] [PubMed] [Google Scholar]
- Bamford CV, d’Mello A, Nobbs AH, Dutton LC, Vickerman MM, Jenkinson HF. 2009. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect Immun. 77(9):3696–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner JC, Watts CM, Xia T. 2000. Occurrence of Candida albicans in infections of endodontic origin. J Endod. 26(12):695–698. [DOI] [PubMed] [Google Scholar]
- Bradway SD, Levine MJ. 1993. Do proline-rich proteins modulate a transglutaminase catalyzed mechanism of candidal adhesion? Crit Rev Oral Biol M. 4(3–4):293–299. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. 2013. Secretory IgA: designed for anti-microbial defense. Front Immunol. 4:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd WC, Schwartz-Baxter S, Carlson J, Barros S, Offenbacher S, Bencharit S. 2014. Role of salivary and candidal proteins in denture stomatitis; an exploratory proteomic analysis. Mol Biosyst. 10(9):2299–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi G, Panzarella V, Matranga D, Calvino F, Pizzo G, Lo Muzio L, Porter S. 2008. Risk factors of oral candidosis: a twofold approach of study by fuzzy logic and traditional statistic. Arch Oral Biol. 53(4):388–397. [DOI] [PubMed] [Google Scholar]
- Campos MS, Marchini L, Bernardes LAS, Paulino LC, Nobrega FG. 2008. Biofilm microbial communities of denture stomatitis. Oral Microbiol Immun. 23(5):419–424. [DOI] [PubMed] [Google Scholar]
- Canabarro A, Valle C, Farias MR, Santos FB, Lazera M, Wanke B. 2013. Association of subgingival colonization of Candida albicans and other yeasts with severity of chronic periodontitis. J Periodontal Res. 48(4):428–432. [DOI] [PubMed] [Google Scholar]
- de Carvalho FG, Silva DS, Hebling J, Spolidorio LC, Spolidorio DM. 2006. Presence of mutans streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch Oral Biol. 51(11):1024–1028. [DOI] [PubMed] [Google Scholar]
- de Repentigny L, Aumont F, Bernard K, Belhumeur P. 2000. Characterization of binding of Candida albicans to small intestinal mucin and its role in adherence to mucosal epithelial cells. Infect Immun. 68(6):3172–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Repentigny L, Lewandowski D, Jolicoeur P. 2004. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin Microbiol Rev. 17(4):729–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz PI, Xie Z, Sobue T, Thompson A, Biyikoglu B, Ricker A, Ikonomou L, Dongari-Bagtzoglou A. 2012. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect Immun. 80(2):620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi S, Pang G, Ashman R, Clancy R. 2005. Enhanced clearance of Candida albicans from the oral cavities of mice following oral administration of Lactobacillus acidophilus. Clin Exp Immunol. 141(1):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai CH, Gonzalez-Begne M, Watson G, Krysan DJ, Bowen WH, et al. 2014. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 82(5):1968–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbins HL, Proctor GB, Yakubov GE, Wilson S, Carpenter GH. 2014. Concentration of salivary protective proteins within the bound oral mucosal pellicle. Oral Dis. 20(7):707–713. [DOI] [PubMed] [Google Scholar]
- Hannig C, Hannig M, Attin T. 2005. Enzymes in the acquired enamel pellicle. Eur J Oral Sci. 113(1):2–13. [DOI] [PubMed] [Google Scholar]
- Harriott MM, Noverr MC. 2011. Importance of Candida-bacterial polymicrobial biofilms in disease. Trends Microbiol. 19(11):557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakka K, Ahola AJ, Yli-Knuuttila H, Richardson M, Poussa T, Meurman JH, Korpela R. 2007. Probiotics reduce the prevalence of oral Candida in the elderly—a randomized controlled trial. J Dent Res. 86(2):125–130. [DOI] [PubMed] [Google Scholar]
- Hoffman MP, Haidaris CG. 1993. Analysis of Candida albicans adhesion to salivary mucin. Infect Immun. 61(5):1940–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DA, Vik A, Kolter R. 2004. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol. 54(5):1212–1223. [DOI] [PubMed] [Google Scholar]
- Holmes AR, Bandara BM, Cannon RD. 2002. Saliva promotes Candida albicans adherence to human epithelial cells. J Dent Res. 81(1):28–32. [DOI] [PubMed] [Google Scholar]
- Jabra-Rizk MA, Falkler WA, Jr, Merz WG, Kelley JI, Baqui AA, Meiller TF. 1999. Coaggregation of Candida dubliniensis with Fusobacterium nucleatum. J Clin Microbiol. 37(5):1464–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz LM, Deng DM, van der Mei HC, Crielaard W, Krom BP. 2009. Streptococcus mutans competence-stimulating peptide inhibits Candida albicans hypha formation. Eukaryot Cell. 8(11):1658–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Bratt P, Hay DI, Schluckebier S, Strömberg N. 2000. Adhesion of Candida albicans, but not Candida krusei, to salivary statherin and mimicking host molecules. Oral Microbiol Immunol. 15(2):112–118. [DOI] [PubMed] [Google Scholar]
- Kavanaugh NL, Zhang AQ, Nobile CJ, Johnson AD, Ribbeck K. 2014. Mucins suppress virulence traits of Candida albicans. mBio. 5(6):e01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz SA, Gaur NK, De Armond R, Sheppard D, Khardori N, Edwards JE, Jr, Lipke PN, El-Azizi M. 2007. Candida albicans Als proteins mediate aggregation with bacteria and yeasts. Med Mycol. 45(4):363–370. [DOI] [PubMed] [Google Scholar]
- Krom BP, Kidwai S, Ten Cate JM. 2014. Candida and other fungal species: forgotten players of healthy oral microbiota. J Dent Res. 93(5):445–451. [DOI] [PubMed] [Google Scholar]
- Leung KC, McMillan AS, Cheung BP, Leung WK. 2008. Sjogren’s syndrome sufferers have increased oral yeast levels despite regular dental care. Oral Dis. 14(2):163–173. [DOI] [PubMed] [Google Scholar]
- Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. 2008. Mucins in the mucosal barrier to infection. Mucosal Immunol. 1(3):183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche WJ. 1986. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 50(4):353–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann AB, Dickinson ME. 2006. Nanomechanics, chemistry and structure at the enamel surface. Monogr Oral Sci. 19:105–131. [DOI] [PubMed] [Google Scholar]
- Muadcheingka T, Tantivitayakul P. 2015. Distribution of Candida albicans and non-albicans Candida species in oral candidiasis patients: correlation between cell surface hydrophobicity and biofilm forming activities. Arch Oral Biol. 60(6):894–901. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, Chandra J, Retuerto M, Sikaroodi M, Brown RE, Jurevic R, Salata RA, Lederman MM, Gillevet PM, Ghannoum MA. 2014. Oral mycobiome analysis of HIV-infected patients: identification of Pichia as an antagonist of opportunistic fungi. PLoS Pathog. 10(3):e1003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett JE, Marchillo K, Spiegel CA, Andes DR. 2010. Development and validation of an in vivo Candida albicans biofilm denture model. Infect Immun. 78(9):3650–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell LE, Robertson D, Nile CJ, Cross LJ, Riggio M, Sherriff A, Bradshaw D, Lambert M, Malcolm J, Buijs MJ, et al. 2015. The oral microbiome of denture wearers is influenced by levels of natural dentition. PLoS One. 10(9):e0137717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oever JT, Netea MG. 2014. The bacteriome-mycobiome interaction and antifungal host defense. Eur J Immunol. 44(11):3182–3191. [DOI] [PubMed] [Google Scholar]
- Okutomi T, Tanaka T, Yui S, Mikami M, Yamazaki M, Abe S, Yamaguchi H. 1998. Anti-Candida activity of calprotectin in combination with neutrophils or lactoferrin. Microbiol Immunol. 42(11):789–793. [DOI] [PubMed] [Google Scholar]
- Puri S, Edgerton M. 2014. How does it kill? Understanding the candidacidal mechanism of salivary histatin 5. Eukaryot Cell. 13(8):958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radfar L, Shea Y, Fischer SH, Sankar V, Leakan RA, Baum BJ, Pillemer SR. 2003. Fungal load and candidiasis in Sjögren’s syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 96(3):283–287. [DOI] [PubMed] [Google Scholar]
- Reynaud AH, Nygaard-Østby B, Bøygard GK, Eribe ER, Olsen I, Gjermo P. 2001. Yeasts in periodontal pockets. J Clin Periodontol. 28(9):860–864. [DOI] [PubMed] [Google Scholar]
- Rocas IN, Lima KC, Assuncao IV, Gomes PN, Bracks IV, Siqueira JF., Jr. 2015. Advanced caries microbiota in teeth with irreversible pulpitis. J Endod. 41(9):1450–1455. [DOI] [PubMed] [Google Scholar]
- San Millán R, Elguezabal N, Regúlez P, Moragues MaD, Quindós G, Pontón J. 2000. Effect of salivary secretory IgA on the adhesion of Candida albicans to polystyrene. Microbiology. 146(Pt 9):2105–2112. [DOI] [PubMed] [Google Scholar]
- Sardi JC, Duque C, Höfling JF, Gonçalves RB. 2012. Genetic and phenotypic evaluation of Candida albicans strains isolated from subgingival biofilm of diabetic patients with chronic periodontitis. Med Mycol. 50(5):467–475. [DOI] [PubMed] [Google Scholar]
- Sohnle PG, Hahn BL, Santhanagopalan V. 1996. Inhibition of Candida albicans growth by calprotectin in the absence of direct contact with the organisms. J Infect Dis. 174(6):1369–1372. [DOI] [PubMed] [Google Scholar]
- Soysa NS, Samaranayake LP, Ellepola AN. 2008. Antimicrobials as a contributory factor in oral candidosis: a brief overview. Oral Dis. 14(2):138–143. [DOI] [PubMed] [Google Scholar]
- Staab JF, Bradway SD, Fidel PL, Sundstrom P. 1999. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 283(5407):1535–1538. [DOI] [PubMed] [Google Scholar]
- Sweet SP, Denbury AN, Challacombe SJ. 2001. Salivary calprotectin levels are raised in patients with oral candidiasis or Sjögren’s syndrome but decreased by HIV infection. Oral Microbiol Immunol. 16(2):119–123. [DOI] [PubMed] [Google Scholar]
- Swidergall M, Ernst JF. 2014. Interplay between Candida albicans and the antimicrobial peptide armory. Eukaryot Cell. 13(8):950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taff HT, Nett JE, Zarnowski R, Ross KM, Sanchez H, Cain MT, Hamaker J, Mitchell AP, Andes DR. 2012. A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLoS Pathog. 8(8):e1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai R, Sugamata M, Kiyoura Y. 2011. Candida albicans enhances invasion of human gingival epithelial cells and gingival fibroblasts by Porphyromonas gingivalis. Microb Pathog. 51(4):250–254. [DOI] [PubMed] [Google Scholar]
- Thein ZM, Samaranayake YH, Samaranayake LP. 2006. Effect of oral bacteria on growth and survival of Candida albicans biofilms. Arch Oral Biol. 51(8):672–680. [DOI] [PubMed] [Google Scholar]
- Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. 2009. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 5(10):e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez R, Lemme A, Ballhausen B, Thiel V, Schulz S, Jansen R, Sztajer H, Wagner-Dobler I. 2010. Streptococcus mutans inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF). ChemBioChem. 11(11):1552–1562. [DOI] [PubMed] [Google Scholar]
- Wu T, Cen L, Kaplan C, Zhou X, Lux R, Shi W, He X. 2015. Cellular components mediating coadherence of Candida albicans and Fusobacterium nucleatum. J Dent Res. 94(10):1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates JR, III, Heydorn A, Koo H. 2012. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 8(4):e1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Sobue T, Thompson A, Xie Z, Poon K, Ricker A, Cervantes J, Diaz PI, Dongari-Bagtzoglou A. 2014. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell Microbiol. 16(2):214–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Hung CC, Wang AH, Tseng FC, Leaw SN, Tseng YT, Su CL, Chen HT, Lauderdale TL, Lo HJ. 2010. Oropharyngeal colonization of HIV-infected outpatients in Taiwan by yeast pathogens. J Clin Microbiol. 48(7):2609–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]