Abstract
One of the most important features of the nervous system is memory: the ability to represent and store experiences, in a manner that alters behavior and cognition at future times when the original stimulus is no longer present. However, the brain is not always an anatomically stable structure: many animal species regenerate all or part of the brain after severe injury, or remodel their CNS toward a new configuration as part of their life cycle. This raises a fascinating question: what are the dynamics of memories during brain regeneration? Can stable memories remain intact when cellular turnover and spatial rearrangement modify the biological hardware within which experiences are stored? What can we learn from model species that exhibit both, regeneration and memory, with respect to robustness and stability requirements for long-term memories encoded in living tissues? In this Perspective, we discuss relevant data in regenerating planaria, metamorphosing insects, and hibernating ground squirrels. While much remains to be done to understand this remarkable process, molecular-level insight will have important implications for cognitive science, regenerative medicine of the brain, and the development of non-traditional computational media in synthetic bioengineering.
Keywords: brain, learning, memory, metamorphosis, regeneration, remodeling
Introduction
Biological structures can exhibit considerable morphological plasticity. Many animals can regenerate their original anatomical structure after severe injury,1 and to reconfigure toward the correct morphology after various grafting operations.2 For example, salamanders can regenerate their limbs, eyes, jaws, hearts, and tails after amputation,3,4 and tails grafted to the flank become slowly turned into limbs.5 Some remodel their bodies toward a new configuration as a normal part of their life cycle.6-8 In some species, this drastic remodeling also involves the nervous system, regenerating or massively re-wiring the brain.9 For example, planaria regenerate complete new heads after amputation,10 and salamanders also regenerate their brain after region-specific ablation.11-13 Insects tear down their existing CNS and produce one with a different architecture, in the journey from a caterpillar to a butterfly.14,15
However, a central feature of the nervous system is the ability to represent and store memories, allowing animals to adaptively alter behavior and cognition in light of past experience. The presence of memory and recall is revealed when experience alters behavior and cognition at future times when the original stimulus is no longer present. The brain, as the accepted seat of episodic memory, is often thought to be a stable, unchanging structure, which may seem to be a necessary property to ensure long-term stability of encoded information. Thus, animal model species that exhibit both, brain regeneration and learning, confront us with a fascinating set of questions. Could stable memories persist when cellular turnover and spatial rearrangement modify the hardware within which experiences are stored? What are the dynamics of memories when old cells apoptose, and new cells arise from progenitors? What are the mechanistic robustness and stability requirements for encoding of long-term memories in morphologically-dynamic living tissues? Answering these questions (at both, a molecular and computational level) would have numerous profound implications.
First, these issues directly target the fundamental question of the engram 16,17: how is information encoded in living tissue and what is the relationship between memory and the spatial location of specific physical modifications in the brain? If specific memories remain stable as cells are replaced and moved, we can learn much about the physico-chemical representations of mental content.18 Importantly, this is still a major area of debate. Many in the field believe memories to be stored as changes in synaptic connectivity.19,20 Nevertheless, new data suggest that other cell properties (such as transcriptional or epigenetic alterations) may be a crucial significant component.21,22
Second, the future of regenerative therapies for brain injury and degeneration 23,24 depends on our ability to modify brain structure without radically distorting the decades of memory content and personality of the patient. Given recent data implicating non-neuronal cell types in intelligence,25,26 a wide range of potential regenerative therapies may be expected to have effects on the rich memory set of adult human brains. It becomes imperative to understand how recall of established memories fares when novel cell types are introduced into the brain, altering network connectivity and perhaps replacing endogenous cell groups.
Third, could we exploit such mechanisms of memory in our efforts to bioengineer artificial hybrid biobots, which would have not only desired structural and physiological properties, but also useful behaviors that are robust to cellular turnover 27-29? A related issue is that the discovery of such systems would significantly inform efforts to explore non-traditional computational architectures in information science and computer engineering,30,31 distinct from the current models of information inextricably dependent on a specific physical location within the digital memory.
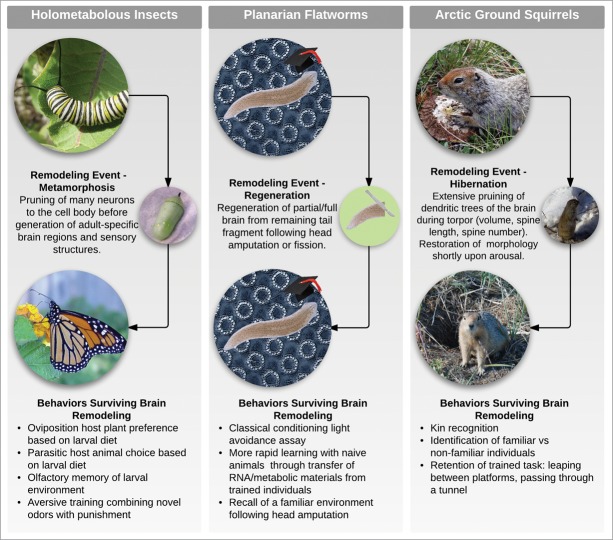
Tantalizing findings have already shown that memories can indeed survive extensive remodeling of the brain, indicating transformative opportunities for both fundamental issues in cognitive science and biomedical engineering. In this Perspective, we discuss relevant data in regenerating planaria, metamorphosing insects, and hibernating ground squirrels (Fig. 1). While much remains to be done, this fascinating intersection of regenerative cell biology and cognitive neuroscience represents a fertile ground for future work of not only deep basic interest but numerous practical applications.
Figure 1.
Animal models of memories that survive brain remodeling Holometabolous insects reorganize their brains during pupation in the transition from larva to adult, with many neurons of the central nervous system pruning to the cell body before the generation of adult specific structures. Planarian species are capable of regenerating their entire brain from a tail fragment in the event of fission or amputation, with new tissue arising from a neoblast stem cell population. Arctic ground squirrels demonstrate a drastic reduction in brain volume during hibernation at near freezing temperatures, which is corrected within hours of arousal. In all 3 of these animal groups, learned behaviors have been observed to survive the striking reorganization of the brain. Photos courtesy of Jerry Friedman (Monarch caterpillar), Ianaré Sévi, Linda Mahoney, and Bering Land Bridge National reserve (top, center, and bottom ground squirrel images respectively), used with permission.
Memories survive CNS rearrangements and remodeling in insects
Holometabolous insects (those which undergo complete metamorphosis) pose an interesting system in which to investigate memory storage and retrieval, as the CNS of these animals undergoes dramatic neurogenesis, pruning, and cell death during the transition from larvae to adulthood. Can memory survive pupation? What benefit would such a robust memory storage system provide the animal? These questions were first formalized nearly one hundred years ago by Andrew Delmar Hopkins, the father of forest entomology, who noted a polyphogous species of pine beetle preferentially infested one host even when multiple hosts were available.32 Hopkins postulated that female scolytid beetles chose to lay their eggs on the pine species they themselves consumed as larvae, a phenomenon which was later to become known as ‘Hopkins’ host-selection principle.” This hypothesis, as well as that of cross-metamorphic memory in general, has generated large amounts of controversy with numerous reports claiming both support and opposition of the principle.
In support, a variety of studies using lepidoptera have demonstrated a contribution of larval experience on adult behavior,33-36 with adult oviposition preference being shaped by pre-imaginal (i.e. larval) events. It is possible that adults retain some memory of their larval lives in these studies, however, critics have pointed to an alternate mechanism which could explain the results termed the ‘chemical legacy hypothesis’.37 The chemical legacy model proposes that odor contamination on the pupal case or within the hemolymph could sensitize adults to these odors while emerging, affecting the behavior of the mature insect independent of larval experience. Indeed, numerous studies have directly tested and proven that a chemical legacy can have direct effects on adult behavior. Drosophila pupae intentionally contaminated with methanol change their response to methanol as adults, even in the absence of larval training.38,39 Similarly, parasitic wasps show an oviposition preference for the aphid host in which they themselves develop.40 This behavior was found to be the result of exposure to host cues during eclosion and not from a neutrally-derived larval memory as pupae removed from aphids during pupation fail to show any host preference after emerging as wasps.
However, a number of carefully controlled studies demonstrate memory across metamorphosis while controlling for any chemical contamination. Both weevil and wasp species retain preferences for their larval environments, and contrary to other chemical legacy reports, do not show changes in adult behavior if their pupal cases are intentionally contaminated with odors.41,42 Perhaps most convincingly, aversive associative memory also appears to survive from larvae to adult; in studies pairing electric shock and ethyl acetate, both drosophila and manduca larvae learn to avoid the odor in choice assays and maintain this behavior after emerging as adults.43,44 Presentation of odor or shock alone during larval stages had no effect on adults of either species, ruling out any possibility of chemical sensitization/habituation.
How might this memory be stored during the transition for larvae to adult? Olfactory memory is known to reside in the mushroom bodies, paired lobes of the larval and adult insect brain which receive input from the antennal lobes.45,46 However, during pupation this structure undergoes extensive remodeling, with the γ neurons completely pruning to the main process and the α’/β’ neurons demonstrating partial pruning before establishing adult specific projections.47,48 While it is possible a true synaptic memory may persist within a subset of mushroom body neurons, only indirect evidence exists to support this hypothesis and more research is necessary to conclusively confirm or reject the idea. Perhaps the best support to date comes from research using the Tenebrio genus of grain beetle, in which larvae trained to navigate mazes show more rapid learning rates as adults compared to untrained siblings.49-51 Analysis of total RNA concentration in the brain revealed an enrichment of RNA in the mushroom bodies in the larva which persisted into adulthood.52 However this evidence is still indirect and alternative theories for the survival of memory through metamorphosis are myriad. Non-neural memory, chemoreceptor tuning, and gene silencing have all been suggested but never formally tested. For the few theories that have been tested, little follow-up work exists. The question of trans-metamorphic memory, postulated nearly a century ago, remains unsolved yet ripe for study given the development of molecular tools for model species such as Tribolium.53,54
Memories persist during brain regeneration in planaria
Planaria are bilaterian flatworms, possessing complex internal organs, behavioral repertoires, and remarkable powers of regeneration.55-62 They are a powerful and increasingly popular model for regenerative biology studies, both in terms of understanding stem cell decision-making 63-67 and physiological controls of large-scale pattern formation.68-71 Special emphasis has been placed on understanding the mechanisms guiding CNS regeneration in this model system.10,72,73
Neurons in the planarian brain more closely resemble those of vertebrates than those of advanced invertebrates, exhibiting typical vertebrate features of multipolar shape, dendritic spines with synaptic boutons, a single axon, expression of vertebrate-like neural proteins, and relatively slow spontaneously generated electrical activity.74 Therefore, the planarian is not only the first animal to possess a brain, but may be the ancestor of the vertebrate brain.61,75 Some of the most interesting and controversial data on the persistence of memories in regeneration thus came from studies of this model.76,77
Influenced by the ideas of D. O. Hebb and J. Eccles, who postulated synaptic plasticity as the mechanism of memory encoding,78,79 James McConnell and Robert Thompson explored the implications of the theory in vivo.80 While basal, planarians possess a well-differentiated nervous system and CNS with chemical synapses,74,81 satisfying both requirements for synaptic plasticity according to Hebb's model.80,82 Having first established planaria as a suitable animal model for the studying of associative learning and memory,83 McConnell decided to explore what would happen if conditioned worms were cut in 2 and then tested following regenerating? Which half, if either, would retain the memory? McConnell and his students trained worms using a classical conditioning protocol (paring light with an electric shock) and after the worms demonstrated learning, they cut the worms in half. After fully regenerating some weeks later, the team retrained the regenerated worms using the same protocol. The results showed that head fragment retained the pre-cut training, but also surprisingly, worms that regenerated from the tails (which lost the original brain) required significantly less training trials to learn (“saving” paradigm) compared to untrained animals.77,84
The astonishing results motivated McConnell to examine another theory of memory - the existence of an “engram,” as physical/chemical memory trace which could perhaps be transferred into an untrained animal.85,86 McConnell and colleagues conducted a series of experiments where they showed that naïve worms that were fed on trained worms revealed evidences of memory retrieval in a “saving” paradigm, where the fed worms learned more quickly than naïve animals.82 McConnell and his colleagues fractionated the planarian extracts in an effort to identify the particular molecule that actively allows memory transfer and based on their findings hypothesized that RNA was the active agent allowing memory transfer.86 Further, naïve worms injected with the RNA extract from trained worms demonstrated the “saving” effect, while RNAse treatment abolished it.80 This finding was extremely surprising since at the time it was not known that RNA has multiple regulatory functions.87 These effects were not limited to just one species. A Russian group conditioned another species of planarians' motor responses to shock paired with vibratory stimuli.88 When animals were transected, retention could be observed in both halves some 14 days after sectioning. These experiments pointed to the retention of experientially induced modifications in regenerating tissues. The posterior segments of the worms regenerated heads that retained the conditioned responses. Their results supported those of Corning and John's (1961) with RNase, suggesting on the possibility that RNA inheritance could be involved.89-91
McConnell and his colleagues performed a number of essential controls for learning specificity. For example, they tested for pseudoconditioning and sensitization effects due to non-training variables such as the dissection and regeneration processes, and demonstrated that the head is the organ which executes the particular learned behavior which was assayed (2 headed worms learn faster and tail fragments without brain cannot be the conditioned 80,82). Later, several labs replicated the results (transfer of memory through brain extracts), in rats, mice and fish.85 Cowley and Griesel (1966) found that the male grand-offspring of female rats that were prenatally malnourished performed more poorly than controls on the Hebb-Williams maze, despite the fact that their mothers had been on a standard diet from conception through weaning. That is, the effects of a low protein diet lingered across subsequent, well-fed, generations. These findings shifted the field of memory transfer from planaria to rat as the primary model species.85 Following a string of studies with rats and mice create, the field became mired in controversy the entire line of research was abandoned and forgotten.85,92
Given the technical limitations of the time, the mechanisms underlying the results were never fully identified. However, labs are beginning to reinvestigate this fascinating work using modern molecular advances in the understanding of epigenetic processes 93-96 that may allow the transfer of environmentally-imprinted information transgenerationally.97,98 In addition, discoveries of RNAi regulation87 and accumulating evidence that links inheritance via noncoding RNAs99 to memory formation and behavior100 support the feasibility of the McConnell's findings. Yet it is unclear whether those epigenetic processes have pivotal roles in acquisition of specific memories and behaviors or whether they function as general cofactors for learning and memory. Additional materials on this fascinating controversy are found in.101-109
During the last decade, planaria have re-emerged as a leading model in the field of molecular biology. The genome of a freshwater planarian (Schmidtea mediterranea) has been fully sequenced and molecular techniques including gene-specific RNA interference and in situ hybridization are fully functional with the species.110,111 Today, planaria are perhaps the only molecularly-tractable system in which memory and complete brain regeneration can be studied in the same animal. To re-examine this issue, our lab developed an automated training and testing device designed to overcome some of the limitations of older work.112 Prior efforts performed manually suffered from 1) the time consuming nature of the experiments which only allowed the experimenter to spend a short time each day training any one worm (thus lowering overall N and providing weak memories), 2) the difficulty of reproducing precisely the same protocol across labs (given the worms' sensitivity to even subtle environmental differences), and 3) the challenge of using human observation of behavior to support highly surprising findings (the need to avoid any chance of subjectivity during scoring and to provide a complete behavioral dataset that can be analyzed by others).
Our device provides 24*7 environmental training to each individual animal in parallel, and uses objective criteria for scoring, while recording all the movements for future analysis. Using this platform, our initial study confirmed the ability of worms to recognize a surface etch pattern in a place learning task, and the persistence of this information across head regeneration.113 While numerous subsequent studies must be done to improve the protocol and ask questions about the mechanism and specificity of the memory, this early result establishes the planarian as a tractable model within which we can next ask questions like: where is the information encoding learned behavior stored? How many and what kind of cells are needed in order to keep the memory? How is it imprinted on the developing new brain? How does encoding and decoding by naïve tissues work? What is the memory capacity of this system? The plethora of molecular tools, cellular-level analytic methods, and automated behavior analyses enables a rich program of investigation using the basic amputation assay to identify the location and main properties of information storage during brain regeneration.
One possible locus for the cellular basis of memory are the planarian neoblasts (stem cells114), which could be modified through epigenetic changes induced by learning.22,115,116 When the new brain develops, neoblasts could potentially imprint the CNS through self-organization mechanisms.117-119 A second possibility is that non-coding RNAs implement inheritance.99,100 Regardless of the molecular mechanisms required for this process, a complete answer to this question will also require an understanding of the mapping of cognitive content to specific molecular states (encoding and decoding of learned information within RNA, protein, cell networks, or some other mechanism). It is clear that modern techniques and recent findings show great potential for the planarian as an animal model in learning and memory research. Investigating this unique animal, which displays complex behavior and can regenerate its entire brain in only a few days, may provide answers to the enigma of acquisition, storage, and retrieval of memories.
Memory and brain repair in mammals
What happens to memories in mammals whose brains incorporate progeny of (perhaps naïve) stem cells? These are crucial issues because transplants of stem cells into brains is a major medical strategy for stroke and degenerative disease.120-122 The answer to this kind of clinical scenario is unknown. Indeed, even the amount of brain tissue needed for specific cognitive functions is not well-understood, given the (rare) cases of hydrocephaly and greatly reduced brain size with normal cognition.123,124 One model system that provides an entry point into addressing the biomedical aspects of this question is what occurs during hibernation.125
While there are various examples of neural reorganization in vertebrates, perhaps none are as remarkable as that of the European and arctic ground squirrel family. During the winter months these animals go into a state of torpor, where their metabolism and brain activity slows considerably. Direct measurements during this period reveal body temperatures hovering around 0 degrees Celsius and Na+ K+-ATPase muscle activity decreases up to 60 percent.126,127 Under these conditions, massive changes occur within the central nervous system of the animals, with extensive pruning of the dendritic trees within the brain,128 including areas necessary for long term memory such as the hippocampus.129 Neurons of this region demonstrate multiple changes including spine morphology/number, reduced branching as well as shorter dendritic length, and changes in microtubule assembly/disassembly protein abundance.128-132 In addition to the extensive pruning during torpor, perhaps equally impressive is the fact the brain completely reverses this loss during the first few hours of arousal restoring the original branching density present prior to hibernation.131,132
Given this reorganization it has long been questioned whether memory can survive the severe changes in both temperature and dendritic morphology associated with torpor. Early research into this question examined whether hibernating ground squirrels could identify kin and familiar individuals following 9 months of separation. Interestingly, animals appeared to remember kin but demonstrated no recognition of previously familiar non-kin individuals,133 suggesting that only memories of siblings persist through hibernation (perhaps serving to avoid possible inbreeding the following season). However, further work determined this recognition was not due to true memory, but was in fact a result of self-reference, or similarity of kin to the subject animals own odor.134 While these results did not provide evidence of long-term memory surviving torpor, a similar study proved more promising. Groups of ground squirrels were tested in one of 3 tasks; maze navigation, an operant feeding machine, and the ability to discriminate housemates from strangers. While recall performance was weak on both of the associative learning assays, the animals were successfully able to discriminate familiar from unfamiliar individuals.135 It is unclear why the animals could demonstrate memory in one assay but not others, though the authors speculate it could be the result of the complexity of the task or the brain region responsible for the memories. Perhaps most convincingly, in more recent work a number of animals were trained in 2 operant conditioning tasks, jumping between a pair of boxes or crossing through a tube, and found that performance in these tasks was unimpaired following 6 months of hibernation.136 This result was the clearest to date, and provided strong evidence that some memories could survive the dendritic pruning associated with torpor.
What mechanism may be responsible for memory persistence? While such studies present many challenges (not the least of which is the 6–12 month time periods needed for hibernation and arousal) a number of interesting observations have been reported. Prior to torpor, adipose tissue of the ground squirrels shows a significant increase in antioxidant levels which are predicted to reduce reactive oxygen damage during the severe shift in metabolic activity associated with arousal.26 While these levels were measured in the liver and plasma, it is possible that the same mechanism could protect persistent neurons in the brain from reactive oxygen damage following hibernation. In addition, a number of mitotically active immature neurons have been identified within the hippocampus of ground squirrels. The purpose of these cells is not currently known, but they may act as a renewable source of new neurons during the rearrangements associated with hibernation (although it is not clear how this cell population may or may not relate to memory).137 Perhaps most compelling is recent work which identified changes in the phosphorylation of tau across torpor, a microtubule associated protein known to play a role in neural plasticity. Phosphorylation of tau increased during the onset of torpor and decreased with arousal, suggesting a possible mechanism for stabilizing neural connections required for long term memory.138 What makes this finding particularly provocative is the fact that tau protein shows high phosphorylation in patients suffering from Alzheimer's disease, perhaps positioning the ground squirrel as a model to examine the tau signaling pathways with the hope of identifying new targets for therapeutics.
What mechanisms underlie memory?
The question of memory persistence though brain remodeling is an extreme version of a more basic puzzle: how are memories encoded so that they can be reliably decoded within the lifetime of even an un-altered brain? The current paradigm holds that memories are encoded and stored through modification of synaptic connections and the resulting patterns of activation within neuronal networks. This conception is termed “The Synaptic Plasticity Hypothesis.”139 The hypothesis was supported by discoveries of activity-dependent plasticity mechanisms such as long-term potentiation (LTP),140 long term depression (LTD),141 and spike-timing-dependent plasticity (STDP),78 which are all mechanisms that clearly correlate to learning and memory behaviors.139,142,143 However, the synaptic plasticity hypothesis alone has difficulties in explaining the long-life persistency of memories, since the above-mentioned mechanisms are inherently unstable.144,145 In addition, the discovery of memory reconsolidation process146 and the possible requirement for constant activation of protein PKMzeta for memory maintenance 147 argue against the concept of memory storage as a hard-wired neural network.
Memories can chemically unwire, and are highly dynamic and sensitive to disruption, even long after consolidation. How can memories survive for a lifetime in a dynamic neural-network? It is also hard to explain how memory transfers offline, from the acquisition organ to the storage regions in the brain (in mammals: from the hippocampus to the neocortex), long after the remembered episode occurred, (96 see 148 for a detailed discussion). A similar process of memory transfer in the brain has been found in octopus149,150 indicating that this is a fundamental biological mechanism. Finally, recent work showed that memory traces exist even without the complex activation thought to be determined by the specific synaptic weight19,151 and even after erasing the synaptic connectivity related to specific memory.21
These recent findings are challenging the conservative point of view and require new ideas. It is likely that although memory is encoded through synaptic plasticity and recalled by activation of the neural network, there is a “blueprint” of the memories that may be kept safely in place by a mechanism other than the synaptic connections themselves. Thus, future studies of the models of survival of memory traces through brain regeneration has the potential to reveal the mechanism of long-term memory maintenance.
Memory in aneural systems
It is possible that encoding in the CNS is such that it is robust to massive rearrangements of the underlying substrate. On the other hand, it is possible that additional mechanisms can support robust memory by providing a “backup” storage medium during brain rearrangement. For example, in planaria, it is possible that memory can be stored outside the brain and imprinted on the nascent brain during regeneration. Feedback between behavioral (brain-mediated) and other cellular memory mechanisms has been demonstrated in a number of interesting contexts. For example, injecting frog eggs with various substances (orange or citral) results in animals that prefer to feed on material containing those substances.152 Similarly, exposing eggs to a novel odor together with an endogenous alarm chemical results in larvae that produce escape behavior when presented to the odor alone.153 In both of those cases, a novel and as yet unknown mechanism must be at play since the original “learning” takes place in very early embryonic stages, long before a CNS exists. For example, from the Hepper and Waldman study, we must conclude that the intracellular milieu of the frog egg has mechanisms for recognizing various molecules and transmitting this information as a stable memory to the nervous system when it is subsequently formed. This kind of functional coupling between intracellular signaling mechanisms and neural network-mediated behavior suggests that much remains to be discovered about the information sources available to the brain during repair and perhaps even in normal cognition. A more detailed treatment of the parallels between information-processing mechanisms in the CNS and morphogenetic signaling is given in.154
Interestingly, neural-like computation, decision-making, and memory have been reported well beyond the traditional CNS, including sperm,155 amoebae,156 yeast,157 and plants.158-164 These appear to be mediated by well-conserved, ubiquitous mechanisms that appear to be also involved in neural information processing, such as cytoskeleton 165 and electrical networks.166,167 Single somatic cells perform subtraction, addition, low- and band-pass filtering, normalization, gain control, saturation, amplification, multiplication, and thresholding.168 It is becoming clear that neural networks have no monopoly on such functions, and indeed fascinating examples of memory and neural-like dynamics have been found in the immune system,169,170 bone,171,172 heart,173,174 and physiological disorders such as diabetes.175
Why the Mind is in the Brain (or is it?)
“Why is the mind in the head? Because there, and only there, are hosts of possible connections to be performed as time and circumstance demand it”176
If indeed memory and information processing rely on labile connections within a rich network of signaling activity, it becomes immediately clear (counter McCulloch) than the CNS is not the only game in town. Mechanisms that are responsible for cellular computations necessary to rearrange the body plan during remodeling, regeneration, and metamorphosis include the cytoskeleton, metabolic signaling circuits, and the gene regulatory networks. All of these exhibit (physiological) experience-dependent rewiring and rich feedback loops that can store state information. Indeed, all of the major mechanisms by which nerves function – ion channels, neurotransmitters, and electrical synapses not only exist throughout the body but are now known to be functional drivers of many patterning events during regenerative and developmental pattern regulation.177-180 It is possible that functional linkage between the memory-keeping mechanisms in the CNS and the encoding of target morphologies during pattern regulation is at least in part mediated by the same bioelectric mechanisms.181-184 Because the molecular components of non-neural bioelectric signaling are increasingly well-characterized,185-188 it is now possible to specifically test the hypothesis that the somatic and cognitive memory systems are coupled during the above-described examples in which memories survive drastic cellular turnover and rearrangement.
Outlook: implications and future work
Convincing data from insects, planaria, and mammals suggest the ability of memories to survive drastic rearrangement and rebuilding of the CNS. Despite the availability of model systems tractable to both behavioral analysis paradigms and molecular genetics of pattern regulation, this area has not received focused attention and remains fertile ground for new investigation. Suggested lines for investigation in such a research program (currently on-going in our lab) include:
New theory and quantitative in silico analysis in the field of modeling artificial neural networks under topological change, to learn what kinds of encodings might allow the type of memory robustness observed in some species;
Wider surveys of different learning paradigms and kinds of memory persisting in brain-regenerative and metamorphosing organisms, to identify novel and perhaps even more impressive examples of memory persisting through brain remodeling;
Molecular analyses of the mechanisms by which neural networks may exchange information with surrounding tissues (as would be required for the regenerating planarian brain to be imprinted with information by the remaining body fragment). Such studies could test non-neural bioelectrical signaling178,189 and cytoskeletal computation190,191;
Testing the idea that CNS-remodeling operations (regenerative pathways) may function in such a way as to specifically preserve encoded information. If true, this would imply a close relationship between the information-processing algorithms that implement pattern regeneration and those that implement memory.154 One way to approach this hypothesis is to attempt to apply the computational modeling approaches currently used to understand computation in the CNS to the mechanisms regulating pattern formation; existing examples include neural-like models of intracellular signaling pathways192 and information-centered models of regeneration193;
Establishment of additional assays at the intersection of pattern regulation and cognition, such as for example regional brain transplants,12,194-196 and attempts to demonstrate learning in non-neural cellular networks,175 which would drive the formulation of specific models of how non-brain tissue can support memory during brain regeneration.
This novel interdisciplinary area, at the intersection of behavioral neuroscience and molecular developmental biology, raises unique challenges both in terms of novel theory that needs to be developed and new approaches at the bench. The impact of significant progress in this area would be huge, in terms of implications for the basic understanding of how mental content is encoded in cellular structures, the design of new regenerative therapies for radical brain repair in medical contexts, and the engineering of biologically-inspired computational media. Thus, several basic and applied areas of science and biomedicine stand to gain from investigations into a crucial and yet still poorly-understood phenomenon: memory, in its behavioral and morphological aspects.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Daniel Lobo, Vaibhav V. Pai, and Jennifer Hammelman for their helpful suggestions on the manuscript. This work was supported by the Templeton World Charity Foundation (TWCF0089/AB55) and the G. Harold and Leila Y. Mathers Charitable Foundation.
References
- 1.Birnbaum KD, Alvarado AS. Slicing across kingdoms: regeneration in plants and animals. Cell 2008; 132:697-710; PMID:18295584; http://dx.doi.org/ 10.1016/j.cell.2008.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lobo D, Solano M, Bubenik GA, Levin M. A linear-encoding model explains the variability of the target morphology in regeneration. J R Soc Interface 2014; 11:20130918; PMID:24402915; http://dx.doi.org/ 10.1098/rsif.2013.0918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCusker C, Gardiner DM. The axolotl model for regeneration and aging research: a mini-review. Gerontology 2011; 57:565-71; PMID:21372551; http://dx.doi.org/ 10.1159/000323761 [DOI] [PubMed] [Google Scholar]
- 4.Maden M. Axolotl/newt. Methods Mol Biol 2008; 461:467-80; PMID:19030817; http://dx.doi.org/ 10.1007/978-1-60327-483-8_32 [DOI] [PubMed] [Google Scholar]
- 5.Farinella-Ferruzza N. The transformation of a tail into a limb after xenoplastic transformation. Experientia 1956; 15:304-5; http://dx.doi.org/ 10.1007/BF02159624 [DOI] [Google Scholar]
- 6.Tissot M, Stocker RF. Metamorphosis in drosophila and other insects: the fate of neurons throughout the stages. Prog Neurobiol 2000; 62:89-111; PMID:10821983; http://dx.doi.org/ 10.1016/S0301-0082(99)00069-6 [DOI] [PubMed] [Google Scholar]
- 7.Truman JW. Developmental neuroethology of insect metamorphosis. J Neurobiol 1992; 23:1404-22; PMID:1487742; http://dx.doi.org/ 10.1002/neu.480231005 [DOI] [PubMed] [Google Scholar]
- 8.Denver RJ. The molecular basis of thyroid hormone-dependent central nervous system remodeling during amphibian metamorphosis. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 1998; 119:219-28; PMID:9826995; http://dx.doi.org/ 10.1016/S0742-8413(98)00011-5 [DOI] [PubMed] [Google Scholar]
- 9.Arlotta P, Berninger B. Brains in metamorphosis: reprogramming cell identity within the central nervous system. Curr Opin Neurobiol 2014; 27:208-14; PMID:24800935; http://dx.doi.org/ 10.1016/j.conb.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agata K, Umesono Y. Brain regeneration from pluripotent stem cells in planarian. Philos Trans R Soc Lond B Biol Sci 2008; 363:2071-8; PMID:18375378; http://dx.doi.org/ 10.1098/rstb.2008.2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zupanc GK, Zupanc MM. New neurons for the injured brain: mechanisms of neuronal regeneration in adult teleost fish. Regen Med 2006; 1:207-16; PMID:17465804; http://dx.doi.org/ 10.2217/17460751.1.2.207 [DOI] [PubMed] [Google Scholar]
- 12.Pietsch P, Schneider CW. Brain Transplantation in Salamanders - an Approach to Memory Transfer. Brain Res 1969; 14:707-15; PMID:5822440; http://dx.doi.org/ 10.1016/0006-8993(69)90210-8 [DOI] [PubMed] [Google Scholar]
- 13.Berg DA, Kirkham M, Beljajeva A, Knapp D, Habermann B, Ryge J, Tanaka EM, Simon A. Efficient regeneration by activation of neurogenesis in homeostatically quiescent regions of the adult vertebrate brain. Development 2010; 137:4127-34; PMID:21068061; http://dx.doi.org/ 10.1242/dev.055541 [DOI] [PubMed] [Google Scholar]
- 14.Fahrbach SE. Structure of the mushroom bodies of the insect brain. Ann Rev Entomol 2006; 51:209-32; PMID:16332210; http://dx.doi.org/ 10.1146/annurev.ento.51.110104.150954 [DOI] [PubMed] [Google Scholar]
- 15.Truman JW. Metamorphosis of the central nervous system of Drosophila. J Neurobiol 1990; 21:1072-84; PMID:1979610; http://dx.doi.org/ 10.1002/neu.480210711 [DOI] [PubMed] [Google Scholar]
- 16.Lavond DG, Kim JJ, Thompson RF. Mammalian brain substrates of aversive classical conditioning. Annu Rev Psychol 1993; 44:317-42; PMID:8434892; http://dx.doi.org/ 10.1146/annurev.ps.44.020193.001533 [DOI] [PubMed] [Google Scholar]
- 17.Bruce D. Fifty years since Lashley's In search of the Engram: refutations and conjectures. J Hist Neurosci 2001; 10:308-18; PMID:11770197; http://dx.doi.org/ 10.1076/jhin.10.3.308.9086 [DOI] [PubMed] [Google Scholar]
- 18.Black IB. Information in the brain : a molecular perspective. Cambridge, Mass.: MIT Press, 1991. [Google Scholar]
- 19.Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S. Memory. Engram cells retain memory under retrograde amnesia. Science 2015; 348:1007-13; PMID:26023136; http://dx.doi.org/ 10.1126/science.aaa5542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey CH, Kandel ER. Synaptic remodeling, synaptic growth and the storage of long-term memory in Aplysia. Prog Brain Res 2008; 169:179-98; PMID:18394474; http://dx.doi.org/ 10.1016/S0079-6123(07)00010-6 [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Cai D, Pearce K, Sun PY, Roberts AC, Glanzman DL. Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia. Elife 2014; 3:e03896; PMID:25402831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zovkic IB, Guzman-Karlsson MC, Sweatt JD. Epigenetic regulation of memory formation and maintenance. Learn Mem 2013; 20:61-74; PMID:23322554; http://dx.doi.org/ 10.1101/lm.026575.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chicha L, Smith T, Guzman R. Stem cells for brain repair in neonatal hypoxia-ischemia. Childs Nerv Syst 2014; 30:37-46; PMID:24178233; http://dx.doi.org/ 10.1007/s00381-013-2304-4 [DOI] [PubMed] [Google Scholar]
- 24.Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron 2013; 80:588-601; PMID:24183012; http://dx.doi.org/ 10.1016/j.neuron.2013.10.037 [DOI] [PubMed] [Google Scholar]
- 25.Porto-Pazos AB, Veiguela N, Mesejo P, Navarrete M, Alvarellos A, Ibanez O, Pazos A, Araque A. Artificial astrocytes improve neural network performance. PloS one 2011; 6:e19109; PMID:21526157; http://dx.doi.org/ 10.1371/journal.pone.0019109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman SA, Nedergaard M, Windrem MS. Modeling cognition and disease using human glial chimeric mice. Glia 2015; 63:1483-93; PMID:26010831; http://dx.doi.org/ 10.1002/glia.22862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamm RD, Bashir R. Creating living cellular machines. Ann Biomed Eng 2014; 42:445-59; PMID:24006130; http://dx.doi.org/ 10.1007/s10439-013-0902-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doursat R, Sanchez C. Growing fine-grained multicellular robots. Soft Robotics 2014; 1:110-21; http://dx.doi.org/ 10.1089/soro.2014.0014 [DOI] [Google Scholar]
- 29.Doursat R, Sayama H, Michel O. A review of morphogenetic engineering. Nat Comput 2013; 12:517-35; http://dx.doi.org/ 10.1007/s11047-013-9398-1 [DOI] [Google Scholar]
- 30.Bull L, Budd A, Stone C, Uroukov I, de Lacy Costello B, Adamatzky A. Towards unconventional computing through simulated evolution: control of nonlinear media by a learning classifier system. Artif Life 2008; 14:203-22; PMID:18331191; http://dx.doi.org/ 10.1162/artl.2008.14.2.203 [DOI] [PubMed] [Google Scholar]
- 31.Adamatzky A, Stepney S. Towards theory of unconventional computing. Int J Unconv Comput 2008; 4:I-Ii [Google Scholar]
- 32.Hopkins AD. Economic investigations of the scolytid bark and timber beetles of North America. US Department of Agriculture Program of Work for 1917 1916:353 [Google Scholar]
- 33.Akhtar Y, Isman MB. Larval exposure to oviposition deterrents alters subsequent oviposition behavior in generalist, Trichoplusia ni and specialist, Plutella xylostella moths. J Chem Ecol 2003; 29:1853-70; PMID:12956511; http://dx.doi.org/ 10.1023/A:1024802328458 [DOI] [PubMed] [Google Scholar]
- 34.Chow JK, Akhtar Y, Isman MB. The effects of larval experience with a complex plant latex on subsequent feeding and oviposition by the cabbage looper moth: Trichoplusia ni (Lepidoptera: Noctuidae). Chemoecology 2005; 15:129-33; http://dx.doi.org/ 10.1007/s00049-005-0304-x [DOI] [Google Scholar]
- 35.Olsson POC, Anderbrant C, Lofstedt C. Experience influences oviposition behaviour in two pyralid moths, Ephestia cautella and Plodia interpunctella. Animal Behav 2006; 72:545-51; http://dx.doi.org/ 10.1016/j.anbehav.2005.10.023 [DOI] [Google Scholar]
- 36.Rojas JC, Wyatt TD. The role of pre- and post-imaginal experience in the host-finding and oviposition behaviour of the cabbage moth. Physiol Entomol 1999; 24:83-9; http://dx.doi.org/ 10.1046/j.1365-3032.1999.00117.x [DOI] [Google Scholar]
- 37.Corbet SA. Insect chemosensory responses - a chemical legacy hypothesis. Ecolog Entomol 1985; 10:143-53; http://dx.doi.org/ 10.1111/j.1365-2311.1985.tb00543.x [DOI] [Google Scholar]
- 38.Barron AB. The life and death of Hopkins' host-selection principle. J Insect Behav 2001; 14:725-37; http://dx.doi.org/ 10.1023/A:1013033332535 [DOI] [Google Scholar]
- 39.Barron AB, Corbet SA. Preimaginal conditioning in Drosophila revisited. Animal Behav 1999; 58:621-8; PMID:10479377; http://dx.doi.org/ 10.1006/anbe.1999.1169 [DOI] [PubMed] [Google Scholar]
- 40.van Emden HF, Sponagl B, Baker T, Ganguly S, Douloumpaka S. Hopkins ‘host selection principle’, another nail in its coffin. Physiolog Entomol 1996; 21:325-8; http://dx.doi.org/ 10.1111/j.1365-3032.1996.tb00873.x [DOI] [Google Scholar]
- 41.Gandolfi M, Mattiacci L, Dorn S. Preimaginal learning determines adult response to chemical stimuli in a parasitic wasp. Proc Biol Sci 2003; 270:2623-9; http://dx.doi.org/ 10.1098/rspb.2003.2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rietdorf K, Steidle JLM. Was Hopkins right? Influence of larval and early adult experience on the olfactory response in the granary weevil Sitophilus granarius (Coleoptera, Curculionidae). Physiolog Entomol 2002; 27:223-7; http://dx.doi.org/ 10.1046/j.1365-3032.2002.00289.x [DOI] [Google Scholar]
- 43.Tully T, Cambiazo V, Kruse L. Memory through metamorphosis in normal and mutant Drosophila. J Neurosci 1994; 14:68-74; PMID:8283252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackiston DJ, Silva Casey E, Weiss MR. Retention of memory through metamorphosis: can a moth remember what it learned as a caterpillar? PLoS One 2008; 3:e1736; PMID:18320055; http://dx.doi.org/ 10.1371/journal.pone.0001736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Debelle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 1994; 263:692-5; PMID:8303280; http://dx.doi.org/ 10.1126/science.8303280 [DOI] [PubMed] [Google Scholar]
- 46.Wolf R, Wittig T, Liu L, Wustmann G, Eyding D, Heisenberg M. Drosophila mushroom bodies are dispensable for visual, tactile, and motor learning. Learning & Memory 1998; 5:166-78; PMID:10454381 [PMC free article] [PubMed] [Google Scholar]
- 47.Lee T, Lee A, Luo LQ. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development 1999; 126:4065-76; PMID:10457015 [DOI] [PubMed] [Google Scholar]
- 48.Marin EC, Watts RJ, Tanaka NK, Ito K, Luo LQ. Developmentally programmed remodeling of the Drosophila olfactory circuit. Development 2005; 132:725-37; PMID:15659487; http://dx.doi.org/ 10.1242/dev.01614 [DOI] [PubMed] [Google Scholar]
- 49.Sheiman IM, Balobanova EF, Martinovich VP, Polikarpova VP, Slobodchikova LK. Effects of Luliberin and Its Fragments on the Memory of the Beetle Tenebrio-Molitor. J Evol Biochem Phys+ 1984; 20:252-6 [Google Scholar]
- 50.Sheiman IM. Training of Tenebrio Molitor Larvae at Various Stages of Postembryonal Development. Zhurnal Obshchei Biol 1973; 34:470-5 [PubMed] [Google Scholar]
- 51.Alloway T. Retention of learning through metamorphosis in the grain beetle (Tenebrio molitor). Am Zoologist 1971; 12:471-7 [Google Scholar]
- 52.Punzo F, Malatesta RJ. Brain-Rna Synthesis and the Retention of Learning through Metamorphosis in Tenebrio-Obscurus (Insecta, Coleoptera). Comp Biochem Phys A 1988; 91:675-8; http://dx.doi.org/ 10.1016/0300-9629(88)90947-4 [DOI] [PubMed] [Google Scholar]
- 53.Bonneton F. ; When Tribolium complements the genetics of Drosophila. Med Sci (Paris) 2010; 26:297-303; PMID:20346280; http://dx.doi.org/ 10.1051/medsci/2010263297 [DOI] [PubMed] [Google Scholar]
- 54.Peel AD. The evolution of developmental gene networks: lessons from comparative studies on holometabolous insects. Philos Trans R Soc Lond B Biol Sci 2008; 363:1539-47; PMID:18192180; http://dx.doi.org/ 10.1098/rstb.2007.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reddien PW, Sanchez Alvarado A. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol 2004; 20:725-57; PMID:15473858; http://dx.doi.org/ 10.1146/annurev.cellbio.20.010403.095114 [DOI] [PubMed] [Google Scholar]
- 56.Slack JM. Regeneration research today. Dev Dyn 2003; 226:162-6; PMID:12557195; http://dx.doi.org/ 10.1002/dvdy.10232 [DOI] [PubMed] [Google Scholar]
- 57.Sanchez Alvarado A. The freshwater planarian Schmidtea mediterranea: embryogenesis, stem cells and regeneration. Curr Opin Genet Dev 2003; 13:438-44; PMID:12888018; http://dx.doi.org/ 10.1016/S0959-437X(03)00082-0 [DOI] [PubMed] [Google Scholar]
- 58.Salo E, Baguna J. Regeneration in planarians and other worms: New findings, new tools, and new perspectives. J Exp Zool 2002; 292:528-39; PMID:12115936; http://dx.doi.org/ 10.1002/jez.90001 [DOI] [PubMed] [Google Scholar]
- 59.Lobo D, Beane WS, Levin M. Modeling planarian regeneration: a primer for reverse-engineering the worm. PLoS Comput Biol 2012; 8:e1002481; PMID:22570595; http://dx.doi.org/ 10.1371/journal.pcbi.1002481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicolas C, Abramson C, Levin M. Analysis of behavior in the planarian model In: Raffa R, Rawls S, eds. Planaria: A Model for Drug Action and Abuse. Austin: RG Landes Co, 2008:83-94. [Google Scholar]
- 61.Buttarelli FR, Pellicano C, Pontieri FE. Neuropharmacology and behavior in planarians: translations to mammals. Comp Biochem Physiol C Toxicol Pharmacol 2008; 147:399-408; PMID:18294919; http://dx.doi.org/ 10.1016/j.cbpc.2008.01.009 [DOI] [PubMed] [Google Scholar]
- 62.Brown F, Park Y. Seasonal variations in sign and strength of gamma-taxis in planarians. Nature 1964; 202:469-71; PMID:14167828; http://dx.doi.org/ 10.1038/202469a0 [DOI] [PubMed] [Google Scholar]
- 63.Handberg-Thorsager M, Fernandez E, Salo E. Stem cells and regeneration in planarians. Front Biosci 2008; 13:6374-94; PMID:18508666; http://dx.doi.org/ 10.2741/3160 [DOI] [PubMed] [Google Scholar]
- 64.Forsthoefel DJ, Park AE, Newmark PA. Stem cell-based growth, regeneration, and remodeling of the planarian intestine. Dev Biol 2011; 356:445-59; PMID:21664348; http://dx.doi.org/ 10.1016/j.ydbio.2011.05.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wagner DE, Wang IE, Reddien PW. Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 2011; 332:811-6; PMID:21566185; http://dx.doi.org/ 10.1126/science.1203983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reddien PW, Oviedo NJ, Jennings JR, Jenkin JC, Sanchez Alvarado A. SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science 2005; 310:1327-30; PMID:16311336; http://dx.doi.org/ 10.1126/science.1116110 [DOI] [PubMed] [Google Scholar]
- 67.Gentile L, Cebria F, Bartscherer K. The planarian flatworm: an in vivo model for stem cell biology and nervous system regeneration. Dis Model Mech 2011; 4:12-9; PMID:21135057; http://dx.doi.org/ 10.1242/dmm.006692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beane WS, Morokuma J, Adams DS, Levin M. A Chemical genetics approach reveals H,K-ATPase-mediated membrane voltage is required for planarian head regeneration. Chem Biol 2011; 18:77-89; PMID:21276941; http://dx.doi.org/ 10.1016/j.chembiol.2010.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oviedo NJ, Morokuma J, Walentek P, Kema IP, Gu MB, Ahn JM, Hwang JS, Gojobori T, Levin M. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol 2010; 339:188-99; PMID:20026026; http://dx.doi.org/ 10.1016/j.ydbio.2009.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beane WS, Morokuma J, Lemire JM, Levin M. Bioelectric signaling regulates head and organ size during planarian regeneration. Development 2013; 140:313-22; PMID:23250205; http://dx.doi.org/ 10.1242/dev.086900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lobo D, Levin M. Inferring regulatory networks from experimental morphological phenotypes: a computational method reverse-engineers planarian regeneration. PLoS Comput Biol 2015; 11:e1004295; PMID:26042810; http://dx.doi.org/ 10.1371/journal.pcbi.1004295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang YF, Ye BP, Wang DY. Molecular actions guiding neural regeneration in planarian. Neurosci Bull 2008; 24:329-37; PMID:18839027; http://dx.doi.org/ 10.1007/s12264-008-0610-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Umesono Y, Agata K. Evolution and regeneration of the planarian central nervous system. Dev Growth Differ 2009; 51:185-95; PMID:19379275; http://dx.doi.org/ 10.1111/j.1440-169X.2009.01099.x [DOI] [PubMed] [Google Scholar]
- 74.Sarnat HB, Netsky MG. The brain of the planarian as the ancestor of the human brain. Can J Neurolog Sci 1985; 12(4):296-302; PMID:4084864 [DOI] [PubMed] [Google Scholar]
- 75.Pagán OR. The first brain : the neuroscience of planarians. 2014. [Google Scholar]
- 76.Brown HM, Beck EC. Does Learning in Planaria Survive Regeneration. Federation Proc 1964; 23:254- [Google Scholar]
- 77.McConnell JV, Jacobson AL, Kimble DP. The effects of regeneration upon retention of a conditioned response in the planarian. J Comparat Physiol Psychol 1959; 52:1-5; http://dx.doi.org/ 10.1037/h0048028 [DOI] [PubMed] [Google Scholar]
- 78.Markram H, Gerstner W, Sjöström PJ. A history of spike-timing-dependent plasticity. Front Synaptic Neurosci 2011; 3:4; PMID:22007168; http://dx.doi.org/ 10.3389/fnsyn.2011.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hebb DO. The organization of behavior; a neuropsychological theory. New York,: Wiley, 1949. [Google Scholar]
- 80.McConnell JV. A Manual of psychological experimentation on planarians Ann Arbor, Mich., 1965. [Google Scholar]
- 81.Sarnat HB, Netsky MG. When does a ganglion become a brain? Evolutionary origin of the central nervous system. Semin Pediatr Neurol 2002; 9:240-53; PMID:12523550; http://dx.doi.org/ 10.1053/spen.2002.32502 [DOI] [PubMed] [Google Scholar]
- 82.McConnell JV. Memory transfer through cannibalism in planarium. J Neuropsychiat 1962; 3 suppl 1:542-8 [Google Scholar]
- 83.Thompson R, McConnell JV. Classical conditioning in the planarian, Dugesia dorotocephala. J Comp Physiol Psychol 1955; 48:65-8; PMID:14354075; http://dx.doi.org/ 10.1037/h0041147 [DOI] [PubMed] [Google Scholar]
- 84.Best J. Protopsychology. Sci Am 1963; 208:54-62 62-; PMID:13967782 [DOI] [PubMed] [Google Scholar]
- 85.Setlow B. Georges Ungar and memory transfer. Journal of the history of the neurosciences 1997; 6:181-92; PMID:11619520; http://dx.doi.org/ 10.1080/09647049709525701 [DOI] [PubMed] [Google Scholar]
- 86.Morange M. What history tells us VI. The transfer of behaviours by macromolecules. J Biosci 2006; 31:323-7; PMID:17006014; http://dx.doi.org/ 10.1007/BF02704104 [DOI] [PubMed] [Google Scholar]
- 87.Smalheiser NR, Manev H, Costa E. RNAi and brain function: was McConnell on the right track? Trends Neurosci 2001; 24:216-8; PMID:11250005; http://dx.doi.org/ 10.1016/S0166-2236(00)01739-2 [DOI] [PubMed] [Google Scholar]
- 88.Cherkashin AN, Sheiman IM. Conditioning inplanarians and RNA content. J Biol Psychol 1967; 9:5-11 [Google Scholar]
- 89.Cherkashin AN, Sheiman IM, Bogorovskaya GI. Uslovniye reflexi y planarii i opiti s regenerazei. Zhurnal Vysshei Nervnoi Deiatelnosti Imeni I P Pavlova 1966; XVI:1110-5. [PubMed] [Google Scholar]
- 90.Cherkashin AN, Sheiman IM. Effect Produced by Ribonucleose on Nervous Activity of Planaria. Dokl Akad Nauk SSSR 1966; 171:996-&; PMID:5994947 [PubMed] [Google Scholar]
- 91.Cherkashin AN, Sheiman IM. O vliianii ribonukleazy na nervnuiu deiatel'nost' planarii. Dokl Akad Nauk SSSR 1966; 171:996-8; PMID:5994947 [PubMed] [Google Scholar]
- 92.Rilling M. The mystery of the vanished citations: James McConnell's forgotten 1960s quest for planarian learning, a biochemical engram, and celebrity. American Psychologist 1996; 51:589-98; http://dx.doi.org/ 10.1037/0003-066X.51.6.589 [DOI] [Google Scholar]
- 93.Ginsburg S, Jablonka E. Epigenetic learning in non-neural organisms. J Biosci 2009; 34:633-46; PMID:19920348; http://dx.doi.org/ 10.1007/s12038-009-0081-8 [DOI] [PubMed] [Google Scholar]
- 94.Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci 2005; 6:108-18; PMID:15654323; http://dx.doi.org/ 10.1038/nrn1604 [DOI] [PubMed] [Google Scholar]
- 95.Day JJ, Sweatt JD. DNA methylation and memory formation. Nat Neurosci 2010; 13:1319-23; PMID:20975755; http://dx.doi.org/ 10.1038/nn.2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arshavsky YI. “The seven sins” of the Hebbian synapse: can the hypothesis of synaptic plasticity explain long-term memory consolidation? Prog Neurobiol 2006; 80:99-113; PMID:17074430; http://dx.doi.org/ 10.1016/j.pneurobio.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 97.Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci 2014; 17:89-96; PMID:24292232; http://dx.doi.org/ 10.1038/nn.3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fehér O, Wang H, Saar S, Mitra PP, Tchernichovski O. De novo establishment of wild-type song culture in the zebra finch. Nature 2009; 459:564-8; http://dx.doi.org/ 10.1038/nature07994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rechavi O, Minevich G, Hobert O. Transgenerational inheritance of an acquired small RNA-based antiviral response in C. elegans. Cell 2011; 147:1248-56; PMID:22119442; http://dx.doi.org/ 10.1016/j.cell.2011.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mercer TR, Dinger ME, Mariani J, Kosik KS, Mehler MF, Mattick JS. Noncoding RNAs in Long-Term Memory Formation. Neuroscientist 2008; 14:434-45; PMID:18997122; http://dx.doi.org/ 10.1177/1073858408319187 [DOI] [PubMed] [Google Scholar]
- 101.Harper LV. Epigenetic inheritance and the intergenerational transfer of experience. Psychological bulletin 2005; 131:340-60; PMID:15869332; http://dx.doi.org/ 10.1037/0033-2909.131.3.340 [DOI] [PubMed] [Google Scholar]
- 102.Ungar G. Is there a chemical memory trace. Israel J Chem 1975; 14:169-76; http://dx.doi.org/ 10.1002/ijch.197500057 [DOI] [Google Scholar]
- 103.Ungar G. Molecular coding of memory. Life Sciences 1974; 14:595-604; PMID:4595997; http://dx.doi.org/ 10.1016/0024-3205(74)90394-4 [DOI] [PubMed] [Google Scholar]
- 104.Ungar G. Peptides and memory. Biochem Pharmacol 1974; 23:1553-8; PMID:4603211; http://dx.doi.org/ 10.1016/0006-2952(74)90366-9 [DOI] [PubMed] [Google Scholar]
- 105.Ungar G. Molecular code in memory. Recherche 1972; 3:19-& [Google Scholar]
- 106.Ungar G. Molecular coding of information in nervous-system. Naturwissenschaften 1972; 59:85-&; PMID:5019284; http://dx.doi.org/ 10.1007/BF00591779 [DOI] [PubMed] [Google Scholar]
- 107.McConnell JV, Shelby JM. Memory transfer experiments in invertebrates In: Ungar G, ed. Molecular mechanisms in memory and learning. New York: Plenum Press, 1970:71-101. [Google Scholar]
- 108.Ungar G. Molecular mechanisms in learning. Perspect Biol Med 1968; 11:217-&; PMID:4296038; http://dx.doi.org/ 10.1353/pbm.1968.0062 [DOI] [PubMed] [Google Scholar]
- 109.Ungar G. Chemical transfer of learning - its stimulus specificity. Federation Proceedings 1966; 25:207-& [Google Scholar]
- 110.Elliott SA, Sánchez Alvarado A. The history and enduring contributions of planarians to the study of animal regeneration. Wiley Interdiscip Rev Dev Biol 2013; 2:301-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Newmark P, Sánchez Alvarado A. Not your father's planarian: a classic model enters the era of functional genomics. Nat Rev Genet 2002; 3:210-9; PMID:11972158; http://dx.doi.org/ 10.1038/nrg759 [DOI] [PubMed] [Google Scholar]
- 112.Blackiston D, Shomrat T, Nicolas CL, Granata C, Levin M. A second-generation device for automated training and quantitative behavior analyses of molecularly-tractable model organisms. PLoS One 2010; 5:e14370; PMID:21179424; http://dx.doi.org/ 10.1371/journal.pone.0014370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shomrat T, Levin M. An automated training paradigm reveals long-term memory in planarians and its persistence through head regeneration. J Exp Biol 2013; 216:3799-810; PMID:23821717; http://dx.doi.org/ 10.1242/jeb.087809 [DOI] [PubMed] [Google Scholar]
- 114.Baguñà J. The planarian neoblast: the rambling history of its origin and some current black boxes. Int J Dev Biol 2012; 56:19-37; http://dx.doi.org/ 10.1387/ijdb.113463jb [DOI] [PubMed] [Google Scholar]
- 115.Day JJ, Sweatt JD. Cognitive neuroepigenetics: a role for epigenetic mechanisms in learning and memory. Neurobiol Learn Mem 2011; 96:2-12; PMID:21195202; http://dx.doi.org/ 10.1016/j.nlm.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron 2011; 70:813-29; PMID:21658577; http://dx.doi.org/ 10.1016/j.neuron.2011.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harper LV. Epigenetic inheritance and the intergenerational transfer of experience. Psychol Bull 2005; 131:340-60; PMID:15869332; http://dx.doi.org/ 10.1037/0033-2909.131.3.340 [DOI] [PubMed] [Google Scholar]
- 118.Zovkic IB, Guzman-Karlsson MC, Sweatt JD. Epigenetic regulation of memory formation and maintenance. Learn Mem 2013; 20:61-74; PMID:23322554; http://dx.doi.org/ 10.1101/lm.026575.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature 2013; 501:373-9; PMID:23995685; http://dx.doi.org/ 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao J, Prough DS, McAdoo DJ, Grady JJ, Parsley MO, Ma L, Tarensenko YI, Wu P. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Experimental neurology 2006; 201:281-92; PMID:16904107; http://dx.doi.org/ 10.1016/j.expneurol.2006.04.039 [DOI] [PubMed] [Google Scholar]
- 121.Hoane MR, Becerra GD, Shank JE, Tatko L, Pak ES, Smith M, Murashov AK. Transplantation of neuronal and glial precursors dramatically improves sensorimotor function but not cognitive function in the traumatically injured brain. J Neurotrauma 2004; 21:163-74; PMID:15000757; http://dx.doi.org/ 10.1089/089771504322778622 [DOI] [PubMed] [Google Scholar]
- 122.Darsalia V, Allison SJ, Cusulin C, Monni E, Kuzdas D, Kallur T, Lindvall O, Kokaia Z. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J Cereb Blood Flow Metab 2011; 31:235-42; PMID:20531461; http://dx.doi.org/ 10.1038/jcbfm.2010.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lorber J. Is your brain really necessary? Nurs Mirror 1981; 152:29-30; PMID:6909910 [PubMed] [Google Scholar]
- 124.Lorber J. Is your brain really necessary. Arch Dis Child 1978; 53:834- [Google Scholar]
- 125.Heller HC. Hibernation: neural aspects. Annu Rev Physiol 1979; 41:305-21; PMID:373593; http://dx.doi.org/ 10.1146/annurev.ph.41.030179.001513 [DOI] [PubMed] [Google Scholar]
- 126.Hut RA, Barnes BM, Daan S. Body temperature patterns before, during, and after semi-natural hibernation in the European ground squirrel. J Comp Physiol B 2002; 172:47-58; PMID:11824403; http://dx.doi.org/ 10.1007/s003600100226 [DOI] [PubMed] [Google Scholar]
- 127.MacDonald JA, Storey KB. Regulation of ground squirrel Na+K+-ATPase activity by reversible phosphorylation during hibernation. Biochem Biophys Res Commun 1999; 254:424-9; PMID:9918854; http://dx.doi.org/ 10.1006/bbrc.1998.9960 [DOI] [PubMed] [Google Scholar]
- 128.Hindle AG, Martin SL. Cytoskeletal regulation dominates temperature-sensitive proteomic changes of hibernation in forebrain of 13-lined ground squirrels. PLoS One 2013; 8:e71627; PMID:23951209; http://dx.doi.org/ 10.1371/journal.pone.0071627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.von der Ohe CG, Darian-Smith C, Garner CC, Heller HC. Ubiquitous and temperature-dependent neural plasticity in hibernators. J Neurosci 2006; 26:10590-8; PMID:17035545; http://dx.doi.org/ 10.1523/JNEUROSCI.2874-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Magarinos AM, McEwen BS, Saboureau M, Pevet P. Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters. Proc Natl Acad Sci U S A 2006; 103:18775-80; PMID:17121986; http://dx.doi.org/ 10.1073/pnas.0608785103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Popov VI, Bocharova LS, Bragin AG. Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation. Neuroscience 1992; 48:45-51; PMID:1584424; http://dx.doi.org/ 10.1016/0306-4522(92)90336-Z [DOI] [PubMed] [Google Scholar]
- 132.Popov VI, Medvedev NI, Patrushev IV, Ignat'ev DA, Morenkov ED, Stewart MG. Reversible reduction in dendritic spines in CA1 of rat and ground squirrel subjected to hypothermia-normothermia in vivo: A three-dimensional electron microscope study. Neuroscience 2007; 149:549-60; PMID:17919827; http://dx.doi.org/ 10.1016/j.neuroscience.2007.07.059 [DOI] [PubMed] [Google Scholar]
- 133.Mateo JM, Johnston RE. Retention of social recognition after hibernation in Belding's ground squirrels. Anim Behav 2000; 59:491-9; PMID:10715170; http://dx.doi.org/ 10.1006/anbe.1999.1363 [DOI] [PubMed] [Google Scholar]
- 134.Mateo JM. Self-referent phenotype matching and long-term maintenance of kin recognition. Animal Behav 2010; 80:929-35; http://dx.doi.org/ 10.1016/j.anbehav.2010.08.019 [DOI] [Google Scholar]
- 135.Millesi E, Prossinger H, Dittami JP, Fieder M. Hibernation effects on memory in European ground squirrels (Spermophilus citellus). J Biol Rhythms 2001; 16:264-71; PMID:11407786; http://dx.doi.org/ 10.1177/074873001129001971 [DOI] [PubMed] [Google Scholar]
- 136.Clemens LE, Heldmaier G, Exner C. Keep cool: Memory is retained during hibernation in Alpine marmots. Physiol Behav 2009; 98:78-84; PMID:19393672; http://dx.doi.org/ 10.1016/j.physbeh.2009.04.013 [DOI] [PubMed] [Google Scholar]
- 137.Popov VI, Kraev IV, Ignat'ev DA, Stewart MG. Suspension of mitotic activity in dentate gyrus of the hibernating ground squirrel. Neural Plast 2011; 2011:867525; PMID:21773054; http://dx.doi.org/ 10.1155/2011/867525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stieler JT, Bullmann T, Kohl F, Toien O, Bruckner MK, Hartig W, Barnes BM, Arendt T. The Physiological Link between Metabolic Rate Depression and Tau Phosphorylation in Mammalian Hibernation. PLoS One 2011; 6:e14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martin SJ, Grimwood PD, Morris RGM. Synaptic plasticity and memory: An evaluation of the hypothesis. Annual Review Of Neuroscience 2000; 23:649-711; PMID:10845078; http://dx.doi.org/ 10.1146/annurev.neuro.23.1.649 [DOI] [PubMed] [Google Scholar]
- 140.Bliss T, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 1973; 232:331-56; PMID:4727084; http://dx.doi.org/ 10.1113/jphysiol.1973.sp010273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Massey PV, Bashir ZI. Long-term depression: multiple forms and implications for brain function. Trends Neurosci 2007; 30:176-84; PMID:17335914; http://dx.doi.org/ 10.1016/j.tins.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 142.Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 2012; 484:381-5; PMID:22441246; http://dx.doi.org/ 10.1038/484410a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci 2010; 33:121-9; PMID:20138375; http://dx.doi.org/ 10.1016/j.tins.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 144.Cohen LD, Zuchman R, Sorokina O, Müller A, Dieterich DC, Armstrong JD, Ziv T, Ziv NE. Metabolic turnover of synaptic proteins: kinetics, interdependencies and implications for synaptic maintenance. PLoS One 2013; 8:e63191; PMID:23658807; http://dx.doi.org/ 10.1371/journal.pone.0063191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ziv NE, Ahissar E. Neuroscience: New tricks and old spines. Nature 2009; 462:859-61; PMID:20016588; http://dx.doi.org/ 10.1038/462859a [DOI] [PubMed] [Google Scholar]
- 146.Nader K, Einarsson EO. Memory reconsolidation: an update. Ann N Y Acad Sci 2010; 1191:27-41; PMID:20392274; http://dx.doi.org/ 10.1111/j.1749-6632.2010.05443.x [DOI] [PubMed] [Google Scholar]
- 147.Kwapis JL, Helmstetter FJ. Does PKM(zeta) maintain memory? Brain Res Bull 2014; 105:36-45; PMID:24076105; http://dx.doi.org/ 10.1016/j.brainresbull.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci 2005; 6:119-30; PMID:15685217; http://dx.doi.org/ 10.1038/nrn1607 [DOI] [PubMed] [Google Scholar]
- 149.Muntz WRA, Sutherland NS, Young JZ. Simultaneous Shape Discrimination in Octopus after Removal of the Vertical Lobe. J Exp Biol 1962; 39:557-66; PMID:13936679 [DOI] [PubMed] [Google Scholar]
- 150.Muntz WRA. Intraocular transfer and the function of the optic lobes in Octopus. Q J Exp Psychol 1963; 15:116-24; http://dx.doi.org/ 10.1080/17470216308416562 [DOI] [Google Scholar]
- 151.Barbour B, Brunel N, Hakim V, Nadal JP. What can we learn from synaptic weight distributions? Trends Neurosci 2007; 30:622-9; PMID:17983670; http://dx.doi.org/ 10.1016/j.tins.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 152.Hepper PG, Waldman B. Embryonic olfactory learning in frogs. Q J Exp Psychol 1992; 44:179-97; PMID:1598418 [DOI] [PubMed] [Google Scholar]
- 153.Mathis A, Ferrari MC, Windel N, Messier F, Chivers DP. Learning by embryos and the ghost of predation future. Proc Biol Sci 2008; 275:2603-7; PMID:18682368; http://dx.doi.org/ 10.1098/rspb.2008.0754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Levin M, Pezzulo G. Re-Membering the Body: applications of computational neuroscience to the top-down control of regeneration of limbs and other complex organs. Ann Rev Biomed Engineering 2015; 17:(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alvarez L, Friedrich BM, Gompper G, Kaupp UB. The computational sperm cell. Trends Cell Biol 2013; 198-207; PMID:24342435 [DOI] [PubMed] [Google Scholar]
- 156.Zhu L, Aono M, Kim SJ, Hara M. Amoeba-based computing for traveling salesman problem: Long-term correlations between spatially separated individual cells of Physarum polycephalum. Bio Systems 2013; 112:1-10; PMID:23438635; http://dx.doi.org/ 10.1016/j.biosystems.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 157.Caudron F, Barral Y. A super-assembly of Whi3 encodes memory of deceptive encounters by single cells during yeast courtship. Cell 2013; 155:1244-57; PMID:24315096; http://dx.doi.org/ 10.1016/j.cell.2013.10.046 [DOI] [PubMed] [Google Scholar]
- 158.Gagliano M, Renton M, Depczynski M, Mancuso S. Experience teaches plants to learn faster and forget slower in environments where it matters. Oecologia 2014; 175:63-72; PMID:24390479; http://dx.doi.org/ 10.1007/s00442-013-2873-7 [DOI] [PubMed] [Google Scholar]
- 159.Gremiaux A, Yokawa K, Mancuso S, Baluska F. Plant anesthesia supports similarities between animals and plants: Claude Bernard's forgotten studies. Plant Signal Behav 2014; 9:e27886; PMID:24476640; http://dx.doi.org/ 10.4161/psb.27886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Scialdone A, Mugford ST, Feike D, Skeffington A, Borrill P, Graf A, Smith AM, Howard M. Arabidopsis plants perform arithmetic division to prevent starvation at night. Elife 2013; 2:e00669; PMID:23805380; http://dx.doi.org/ 10.7554/eLife.00669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Trewavas A. How plants learn. Proc Natl Acad Sci U S A 1999; 96:4216-8; PMID:10200239; http://dx.doi.org/ 10.1073/pnas.96.8.4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Trewavas A. Aspects of plant intelligence. Ann Bot (Lond) 2003; 92:1-20; http://dx.doi.org/ 10.1093/aob/mcg101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bose I, Karmakar R. Simple models of plant learning and memory. Physica Scripta 2003; T106:9-12; http://dx.doi.org/ 10.1238/Physica.Topical.106a00009 [DOI] [Google Scholar]
- 164.Masi E, Ciszak M, Stefano G, Renna L, Azzarello E, Pandolfi C, Mugnai S, Baluska F, Arecchi FT, Mancuso S. Spatiotemporal dynamics of the electrical network activity in the root apex. Proc Natl Acad Sci U S A 2009; 106:4048-53; PMID:19234119; http://dx.doi.org/ 10.1073/pnas.0804640106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sahu S, Ghosh S, Hirata K, Fujita D, Bandyopadhyay A. Multi-level memory-switching properties of a single brain microtubule. Appl Phys Lett 2013; 102; http://dx.doi.org/ 10.1063/1.4793995 [DOI] [Google Scholar]
- 166.Volkov AG, Carrell H, Adesina T, Markin VS, Jovanov E. Plant electrical memory. Plant Signal Behav 2008; 3:490-2; PMID:19704496; http://dx.doi.org/ 10.4161/psb.3.7.5684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Inoue J. A simple Hopfield-like cellular network model of plant intelligence. Prog Brain Res 2008; 168:169-74; PMID:18166394; http://dx.doi.org/ 10.1016/S0079-6123(07)68014-5 [DOI] [PubMed] [Google Scholar]
- 168.Koch C, Segev I. The role of single neurons in information processing. Nat Neurosci 2000; 3 Suppl:1171-7; PMID:11127834; http://dx.doi.org/ 10.1038/81444 [DOI] [PubMed] [Google Scholar]
- 169.Cohen IR. The cognitive principle challenges clonal selection. Immunol Today 1992; 13:441-4; PMID:1476598; http://dx.doi.org/ 10.1016/0167-5699(92)90071-E [DOI] [PubMed] [Google Scholar]
- 170.Cohen IR. The cognitive paradigm and the immunological homunculus. Immunol Today 1992; 13:490-4; PMID:1463581; http://dx.doi.org/ 10.1016/0167-5699(92)90024-2 [DOI] [PubMed] [Google Scholar]
- 171.Turner CH, Robling AG, Duncan RL, Burr DB. Do bone cells behave like a neuronal network? Calcif Tissue Int 2002; 70:435-42; PMID:12149636; http://dx.doi.org/ 10.1007/s00223-001-1024-z [DOI] [PubMed] [Google Scholar]
- 172.Spencer GJ, Genever PG. Long-term potentiation in bone–a role for glutamate in strain-induced cellular memory? BMC Cell Biol 2003; 4:9; PMID:12892570; http://dx.doi.org/ 10.1186/1471-2121-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zoghi M. Cardiac memory: do the heart and the brain remember the same? J Interv Card Electrophysiol 2004; 11:177-82; PMID:15548883; http://dx.doi.org/ 10.1023/B:JICE.0000048567.18088.a2 [DOI] [PubMed] [Google Scholar]
- 174.Chakravarthy SV, Ghosh J. On Hebbian-like adaptation in heart muscle: a proposal for ‘cardiac memory’. Biol Cybern 1997; 76:207-15; PMID:9151418 [DOI] [PubMed] [Google Scholar]
- 175.Goel P, Mehta A. Learning theories reveal loss of pancreatic electrical connectivity in diabetes as an adaptive response. PLoS One 2013; 8:e70366; PMID:23936417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mcculloch WS. Why the Mind is in the Head? In: Jeffress LA, ed. Cerebral Mechanisms in Behavior: The Hixon Symposium, New York: Jon Wiley Publ; 1951. 42-57 p. [Google Scholar]
- 177.Mustard J, Levin M. Bioelectrical Mechanisms for Programming Growth and Form: Taming Physiological Networks for Soft Body Robotics. Soft Robotics 2014; 1:169-91 [Google Scholar]
- 178.Levin M. Endogenous bioelectrical networks store non-genetic patterning information during development and regeneration. J Physiol 2014; 592:2295-305; PMID:24882814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tseng A, Levin M. Cracking the bioelectric code: Probing endogenous ionic controls of pattern formation. Commun Integ Biol 2013; 6:1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Levin M. Reprogramming cells and tissue patterning via bioelectrical pathways: molecular mechanisms and biomedical opportunities. Wiley Interdisciplinary Reviews: Systems Biology and Medicine 2013; 5:657-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Levin M. The wisdom of the body: future techniques and approaches to morphogenetic fields in regenerative medicine, developmental biology and cancer. Regen Med 2011; 6:667-73; PMID:22050517 [DOI] [PubMed] [Google Scholar]
- 182.Levin M. Morphogenetic fields in embryogenesis, regeneration, and cancer: non-local control of complex patterning. Bio Systems 2012; 109:243-61; PMID:22542702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Levin M, Buznikov GA, Lauder JM. Of minds and embryos: left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci 2006; 28:171-85; PMID:16679764 [DOI] [PubMed] [Google Scholar]
- 184.Buznikov G, Shmukler Y, Lauder J. From oocyte to neuron: do neurotransmitters function in the same way throughout development? Cell Molec Neurobiol 1996; 16:537-59; PMID:8956008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stewart S, Rojas-Munoz A, Izpisua Belmonte JC. Bioelectricity and epimorphic regeneration. Bioessays 2007; 29:1133-7; PMID:17935197 [DOI] [PubMed] [Google Scholar]
- 186.McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev 2005; 85:943-78; PMID:15987799 [DOI] [PubMed] [Google Scholar]
- 187.Levin M. Molecular bioelectricity in developmental biology: new tools and recent discoveries: control of cell behavior and pattern formation by transmembrane potential gradients. Bioessays 2012; 34:205-17; PMID:22237730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Levin M, Stevenson CG. Regulation of cell behavior and tissue patterning by bioelectrical signals: challenges and opportunities for biomedical engineering. Annu Rev Biomed Eng 2012; 14:295-323; PMID:22809139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Levin M. Molecular bioelectricity: how endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol Biol Cell 2014; 25:3835-50; PMID:25425556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lahoz-Beltra R, Hameroff SR, Dayhoff JE. Cytoskeletal logic: a model for molecular computation via Boolean operations in microtubules and microtubule-associated proteins. Biosystems 1993; 29:1-23; PMID:8318677 [DOI] [PubMed] [Google Scholar]
- 191.Rasmussen S, Karampurwala H, Vaidyanath R, Jensen KS, Hameroff S. Computational Connectionism within Neurons - a Model of Cytoskeletal Automata Subserving Neural Networks. Physica D 1990; 42:428-49; http://dx.doi.org/ 10.1016/0167-2789(90)90093-5 [DOI] [Google Scholar]
- 192.Ling H, Samarasinghe S, Kulasiri D. Novel recurrent neural network for modelling biological networks: oscillatory p53 interaction dynamics. Biosystems 2013; 114:191-205; PMID:24012741; http://dx.doi.org/ 10.1016/j.biosystems.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 193.Friston K, Levin M, Sengupta B, Pezzulo G. Knowing one's place: a free-energy approach to pattern regulation. J R Soc Interface 2015; 12:pii: 20141383; PMID:25788538; http://dx.doi.org/ 10.1098/rsif.2014.1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ray S. Survival of olfactory memory through metamorphosis in the fly Musca domestica. Neuroscience Letters 1999; 259:37-40; PMID:10027550; http://dx.doi.org/ 10.1016/S0304-3940(98)00892-1 [DOI] [PubMed] [Google Scholar]
- 195.Maldonado H, Tablante A. Behavioral transfer in praying mantis by injection of brain homogenate. Physiol Behav 1976; 16:617-21; PMID:987593; http://dx.doi.org/ 10.1016/0031-9384(76)90223-7 [DOI] [PubMed] [Google Scholar]
- 196.Martin U, Martin H, Lindauer M. Transplantation of a Time-Signal in Honeybees. J Comparative Physiol 1978; 124:193-201; http://dx.doi.org/ 10.1007/BF00657051 [DOI] [Google Scholar]