Abstract
Many intracellular bacterial and protozoan pathogens reside within host cell vacuoles customized by the microbial invaders to fit their needs. Within such pathogen-containing vacuoles (PVs) microbes procure nutrients and simultaneously hide from cytosolic host defense systems. Among the many PV-resident human pathogens are the bacterium Chlamydia trachomatis and the protozoan Toxoplasma gondii. Immune responses directed against their PVs are poorly characterized. We reported that activation of host cells with IFNγ triggers the attachment of polyubiquitin chains to Toxoplasma- and Chlamydia-containing vacuoles and thereby marks PVs for destruction. In murine cells PV ubiquitination is dependent on IFNγ-inducible Immunity Related GTPases (IRGs). Human cells also decorate PVs with ubiquitin upon IFNγ priming; however, the molecular machinery promoting PV ubiquitination in human cells remains unknown and is likely to be distinct from the IRG-dependent pathway we described in murine cells. Thus, IFNγ-inducible PV ubiquitination constitutes a critical event in cell-autonomous immunity to C. trachomatis and T. gondii in mice and humans, but the molecular machinery underlying PV ubiquitination is expected to be multifaceted and possibly host species-specific.
Keywords: autophagy, Chlamydia, guanylate binding proteins, immunity related GTPases, interferon, pathogen-containing vacuoles, Toxoplasma, ubiquitin, vacuolar lysis
C. trachomatis and T. gondii are among the most prevalent human pathogen; C. trachomatis is the causative agent of the most common sexually transmitted bacterial infection in the Western world and the leading cause of preventable blindness worldwide.1 T. gondii infection is also exceptionally common. Seroprevalence of anti-T. gondii immunoglobulins varies substantially across the world but is typically in the range of 30 – 80% for a given human population.2 While most T. gondii infections remain asymptomatic, the parasite can induce serious illness in immunocompromised individuals and is able to cross the placenta causing spontaneous abortions.3
Both microbes are obligate intracellular pathogens highly adapted to a life inside tailor-made vacuoles known as C. trachomatis inclusions or T. gondii parasitophorous vacuoles, respectively.1,3 Both pathogens share a similar intracellular lifestyle and are susceptible to the same IFNγ-induced cell-autonomous immune responses.4-6 In IFNγ-primed murine cells members of the Immunity Related GTPase (IRG) protein family translocate to PV membranes surrounding C. trachomatis or T. gondii and subsequently induce the vesiculation and ultimate rupture of IRG-decorated PV membranes.7-11
The mechanism by which IRGs promote PV destruction is poorly characterized. In a recent publication we demonstrated that IFNγ priming of mouse fibroblasts or mouse macrophages prompts IRG-dependent ubiquitination of C. trachomatis and T. gondii PVs, a process that appears to precede PV disintegration.12 Ubiquitin is a small protein of 76 amino acids that can be covalently attached to protein substrates as a monomer or as lysine-linked polymers.13 We showed that K48- and K63-linked polyubiquitin chains are associated with C. trachomatis and T. gondii PVs in IFNγ-primed murine cells. We identified the ubiquitin E3 ligase TRAF6 as one mediator of PV ubiquitination. However, PV ubiquitination is only partly defective in TRAF6-deficient cells suggesting the involvement of additional E3 ligases. In support of this hypothesis we found that not only TRAF6 but also the E3 ligase Trim21 is recruited to PVs.12 The identification of the entire repertoire of PV-associated E3 ligases in future studies will be critical in order to understand how the host cell labels PVs with a variable ubiquitin code triggering potentially cell type- or pathogen-specific immune responses.
Ubiquitination of intracellular microbes has emerged as a focal point of cell-autonomous immunity to a variety of intracellular pathogens across many different host species.14,15 Accordingly, it comes as no surprise that IFNγ-primed human cells also tag T. gondii PVs with ubiquitin (see Fig. 1 and also Selleck et al.16). Although both murine and human cells apply ubiquitin-centered mechanisms to battle T. gondii infections, it is currently unknown whether any components of the machinery involved in T. gondii PV ubiquitination are conserved between mice and humans (Fig. 2). Some fundamental differences in the underlying molecular mechanisms of PV ubiquitination appear likely considering that human cells lack a subset of the IRG proteins that we have shown to be critical for PV ubiquitination in mice.12,17
Figure 1.
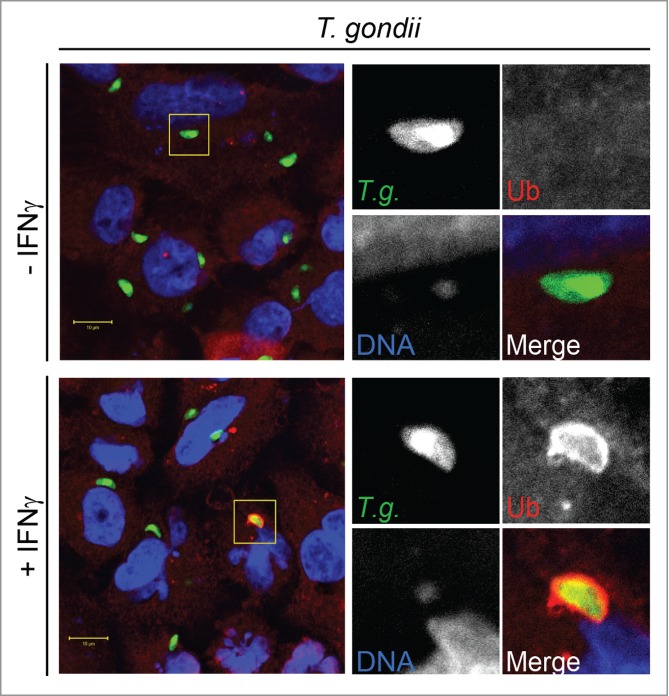
IFNγ-primed human cells decorate T. gondii PVs with ubiquitin. Human alveolar epithelial A549 cells were primed with IFNγ (200 U/mL) overnight or left untreated and subsequently infected with the avirulent GFP-expressing type II T. gondii strain Pru A7 (T.g.). At 1 hour post-infection cells were fixed and stained for DNA with Hoechst and for ubiquitin (Ub).
Figure 2.
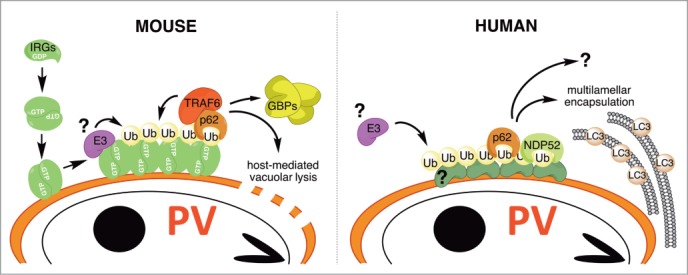
IFNγ-induced PV ubiquitination in mice and humans. In mice a subset of IFNγ-inducible IRGs (so-called GKS proteins) detect PV membranes through a missing-self principle.8,27 PV-bound IRGs facilitate the translocation of TRAF6 and other ubiquitin E3 ligases to PVs resulting in the decoration of PVs with ubiquitin. GKS proteins themselves are likely among the ubiquitinated PV-resident proteins, as GKS proteins in the GTP-bound active state are known to acquire K63-linked polyubiquitin chains.28 PV ubiquitination triggers PV lysis and the recruitment of IFNγ-inducible GBPs. In human cells IFNγ priming also leads to the decoration of T. gondii PVs but the underlying mechanism and the ubiquitinated substrates are unknown.16 Parasites inside ubiquitin-associated T. gondii PVs become encased within multilamellar autophagsome-like structures and cease replication.16
Our studies demonstrated that PV ubiquitination can lead to the destabilization of PVs.12 Specifically, we found that the adaptor protein p62 binds to ubiquitinated C. trachomatis inclusions and together with TRAF6 promotes the destruction of these PVs and their bacterial occupants. We further demonstrated that p62 escorts members of the Guanylate Binding Protein (GBP) family to ubiquitinated PVs.12 GBPs are host resistance proteins functionally linked to a plethora of innate immune responses that include inflammasome activation, antimicrobial autophagy (xenophagy) and host-mediated PV lysis.18-25 Because of the reported functional link between GBPs and PV destruction,21 it seems feasible that TRAF6 and p62 promote PV lysis through GBP recruitment. However, we have so far failed to confirm a direct role for GBPs in PV lysis.20 Therefore, the precise mechanism by which ubiquitination triggers vacuolar lysis requires further examination.
The association of intracellular microbes with ubiquitin plays an important role in the capture of pathogens inside autophagosomes or autophagosome-like vacuoles (ALVs).14 Selleck et al reported that ubiquitinated T. gondii PVs in human Hela cells become encapsulated inside LC3-decorated multilamellar vacuoles.16 To determine whether the capture of T. gondii inside multilamellar ALVs is the predominant or potentially only fate of ubiquitinated T. gondii PVs in human cells, additional cell types will need to be examined. Similarly, future studies should address whether ubiquitination-dependent PV lysis also takes place in human cells and whether loss of vacuolar integrity is linked to the capture of PVs inside multilamellar ALVs.
Three independent studies demonstrated very recently that the attachment of ubiquitin to T. gondii or C. trachomatis PVs is advantageous to the host.12,16,26 Accordingly, virulent strains of T. gondii have evolved strategies to interfere with IFNγ-inducible PV ubiquitination pathways in both murine and human hosts.12,16,26 Although the mechanisms for bacterial evasion of this host defense pathway remain unexplored, we can expect several bacterial pathogens to deploy either distinct or convergent strategies to block IFNγ-inducible PV ubiquitination pathways. Defining these pathways on a molecular level and identifying the microbial evasion mechanisms may reveal novel microbial targets for the development of new drugs to treat bacterial and protozoan infections.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Belland R, Ojcius DM, Byrne GI. Chlamydia. Nat Rev Microbiol (2004); 2:530-531; PMID:15248311; http://dx.doi.org/ 10.1038/nrmicro931 [DOI] [PubMed] [Google Scholar]
- 2.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol (2009); 39:1385-1394; PMID:19433092; http://dx.doi.org/ 10.1016/j.ijpara.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 3.Boothroyd JC. Toxoplasma gondii: 25 years and 25 major advances for the field. Int J Parasitol (2009); 39:935-946; PMID:19630140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coers J, Starnbach MN, Howard JC. Modeling infectious disease in mice: co-adaptation and the role of host-specific IFNgamma responses. PLoS Pathog (2009); 5:e1000333; PMID:19478881; http://dx.doi.org/ 10.1371/journal.ppat.1000333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunn JP, Feng CG, Sher A, Howard JC. The immunity-related GTPases in mammals: a fast-evolving cell-autonomous resistance system against intracellular pathogens. Mamm Genome (2011); 22:43-54; PMID:21052678; http://dx.doi.org/ 10.1007/s00335-010-9293-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daubener W, MacKenzie CR. IFN-gamma activated indoleamine 2,3-dioxygenase activity in human cells is an antiparasitic and an antibacterial effector mechanism. Adv Exp Med Biol (1999); 467:517-524; PMID:10721095; http://dx.doi.org/ 10.1007/978-1-4615-4709-9_64 [DOI] [PubMed] [Google Scholar]
- 7.Coers J, Bernstein-Hanley I, Grotsky D, Parvanova I, Howard JC, Taylor GA, Dietrich WF, Starnbach MN. Chlamydia muridarum evades growth restriction by the IFN-gamma-inducible host resistance factor Irgb10. J Immunol (2008); 180:6237-6245; PMID:18424746; http://dx.doi.org/ 10.4049/jimmunol.180.9.6237 [DOI] [PubMed] [Google Scholar]
- 8.Haldar AK, Saka HA, Piro AS, Dunn JD, Henry SC, Taylor GA, Frickel EM, Valdivia RH, Coers J. IRG and GBP host resistance factors target aberrant, “non-self” vacuoles characterized by the missing of “self” IRGM proteins. PLoS Pathog (2013); 9:e1003414; PMID:23785284; http://dx.doi.org/ 10.1371/journal.ppat.1003414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, Yap GS. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med (2006); 203:2063-2071; PMID:16940170; http://dx.doi.org/ 10.1084/jem.20061318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog (2005); 1:e24; PMID:16304607; http://dx.doi.org/ 10.1371/journal.ppat.0010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao YO, Khaminets A, Hunn JP, Howard JC. Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog (2009); 5:e1000288; PMID:19197351; http://dx.doi.org/ 10.1371/journal.ppat.1000288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haldar AK, Foltz C, Finethy R, Piro AS, Feeley EM, Pilla-Moffett DM, Komatsu M, Frickel EM, Coers J. Ubiquitin systems mark pathogen-containing vacuoles as targets for host defense by guanylate binding proteins. Proc Natl Acad Sci U S A (2015); 112(41):E5628-37; PMID:26417105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komander D, Rape M. The ubiquitin code. Annual review of biochemistry (2012); 81:203-229; PMID:22524316; http://dx.doi.org/ 10.1146/annurev-biochem-060310-170328 [DOI] [PubMed] [Google Scholar]
- 14.Cemma M, Brumell JH. Interactions of pathogenic bacteria with autophagy systems. Current biology : CB (2012); 22, R540-545; PMID:22790007; http://dx.doi.org/ 10.1016/j.cub.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 15.Randow F, MacMicking JD, James LC. Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science (2013); 340:701-706; PMID:23661752; http://dx.doi.org/ 10.1126/science.1233028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selleck EM, Orchard RC, Lassen KG, Beatty WL, Xavier RJ, Levine B, Virgin HW, Sibley LD. A Noncanonical Autophagy Pathway Restricts Toxoplasma gondii Growth in a Strain-Specific Manner in IFN-gamma-Activated Human Cells. mBio (2015); 6:pii: e01157-15; PMID:26350966; http://dx.doi.org/ 10.1128/mBio.01157-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bekpen C, Hunn JP, Rohde C, Parvanova I, Guethlein L, Dunn DM, Glowalla E, Leptin M, Howard JC. The interferon-inducible p47 (IRG) GTPases in vertebrates: loss of the cell autonomous resistance mechanism in the human lineage. Genome Biol (2005); 6:R92; PMID:16277747; http://dx.doi.org/ 10.1186/gb-2005-6-11-r92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rupper AC, Cardelli JA. Induction of guanylate binding protein 5 by gamma interferon increases susceptibility to Salmonella enterica serovar Typhimurium-induced pyroptosis in RAW 264.7 cells. Infect Immun (2008); 76:2304-2315; PMID:18362138; http://dx.doi.org/ 10.1128/IAI.01437-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, MacMicking JD. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science (2012); 336:481-485; PMID:22461501; http://dx.doi.org/ 10.1126/science.1217141 [DOI] [PubMed] [Google Scholar]
- 20.Pilla DM, Hagar JA, Haldar AK, Mason AK, Degrandi D, Pfeffer K, Ernst RK, Yamamoto M, Miao EA, Coers J. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc Natl Acad Sci U S A (2014); 111:6046-6051; PMID:24715728; http://dx.doi.org/ 10.1073/pnas.1321700111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meunier E, Dick MS, Dreier RF, Schürmann N, Kenzelmann Broz D, Warming S, Roose-Girma M, Bumann D, Kayagaki N, Takeda K, et al.. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature (2014); 509:366-370; PMID:24739961; http://dx.doi.org/ 10.1038/nature13157 [DOI] [PubMed] [Google Scholar]
- 22.Kim BH, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD. A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science (2011); 332:717-721; PMID:21551061; http://dx.doi.org/ 10.1126/science.1201711 [DOI] [PubMed] [Google Scholar]
- 23.Meunier E, Wallet P, Dreier RF, Costanzo S, Anton L, Rühl S, Dussurgey S, Dick MS, Kistner A, Rigard M, et al.. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat Immunol (2015); 16(5):476-84; PMID:25774716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Man SM, Karki R, Malireddi RK, Neale G, Vogel P, Yamamoto M, Lamkanfi M, Kanneganti TD. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol (2015); 16(5):467-75; PMID: 25774715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finethy R, Jorgensen I, Haldar AK, de Zoete MR, Strowig T, Flavell RA, Yamamoto M, Nagarajan UM, Miao EA, Coers J. Guanylate Binding Proteins enable rapid activation of canonical and noncanonical inflammasomes in Chlamydia-infected macrophages. Infect Immun (2015); PMID:26416908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Y, Sasai M, Ma JS, Sakaguchi N, Ohshima J, Bando H, Saitoh T, Akira S, Yamamoto M. p62 Plays a specific role in interferon-gamma-induced presentation of a toxoplasma vacuolar Antigen. Cell reports (2015); 13(2):223-33; PMID: 26440898 [DOI] [PubMed] [Google Scholar]
- 27.Coers J. Self and Non-self Discrimination of Intracellular Membranes by the Innate Immune System. PLoS Pathog (2013); 9:e1003538; http://dx.doi.org/ 10.1371/journal.ppat.1003538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Traver MK, Henry SC, Cantillana V, Oliver T, Hunn JP, Howard JC, Beer S, Pfeffer K, Coers J, Taylor GA. Immunity-related gtpase M (IRGM) proteins influence the localization of guanylate-binding protein 2 (GBP2) by modulating macroautophagy. J Biol Chem (2011); 286(35):30471-80; PMID:21757726 [DOI] [PMC free article] [PubMed] [Google Scholar]


