Abstract
Rab proteins are small GTPases essential for controlling and coordinating intracellular traffic. The small GTPase Rab7b regulates the retrograde transport from late endosomes toward the Trans-Golgi Network (TGN), and is important for the proper trafficking of several receptors such as Toll-like receptors (TLRs) and sorting receptors. We recently identified the actin motor protein myosin II as a new interaction partner for Rab7b, and found that Rab7b transport is dependent on myosin II. Interestingly, we also discovered that Rab7b influences the phosphorylation state of myosin II by controlling the activation status of the small GTPase RhoA. Consequently, Rab7b is important for the remodeling of actin filaments in processes such as stress fiber formation, cell adhesion, polarization and cell migration. Our finding that Rab7b can control actomyosin reorganization reveals yet another important role for Rab proteins, in addition to their already established role as master regulators of intracellular transport. Here we discuss our findings and speculate how they can explain the importance of Rab7b in dendritic cells (DCs).
Keywords: actomyosin, cell migration, dendritic cells, Rab proteins, Rab7b
Rab GTPases are key regulators of all steps of membrane trafficking, from the sorting of cargo, budding and vesicle transport, to the tethering and fusion of vesicles to their target membrane.1 There are over 60 human Rab proteins identified, but only a fraction of these have been functionally characterized.2
The small GTPase Rab7b was initially thought to be an isoform of Rab7a, which regulates the transport from early to late endosomes/lysosomes,3 and thus named Rab7b because of its similarity to Rab7a and its localization to late endosomes and lysosomes.4 However Rab7b, in contrast to Rab7a, also associates to the Trans-Golgi Network (TGN) and the Golgi. Indeed, Rab7b regulates a different trafficking route, as it controls the transport from late endosomes toward the TGN.5 In particular, Rab7b is involved in the retrograde transport of different sorting receptors such as cation-independent mannose-6-phosphate receptor (CI-MPR) and sortilin.5,6 Furthermore, Rab7b is also important for the correct formation of carriers at the TGN.6 Interestingly, Rab7b is highly expressed in immune cells such as monocytes and monocyte-derived dendritic cells (MDDCs),4 and has been shown to be involved in regulation of Toll-like receptor (TLR) signaling in macrophages by negatively modulating both TLR4- and TLR9-mediated inflammatory responses.7,8
Recently, we identified the actin motor protein myosin II as an effector of Rab7b by a yeast 2-hybrid screen. The interaction was verified biochemically and proven to be direct. We further showed that Rab7b dynamics are altered when myosin II is chemically inhibited or depleted by siRNA, indicating that Rab7b-mediated transport is dependent on myosin II,9 (Fig. 1). It is generally recognized that members of the myosin family of motor proteins work together with Rab GTPases to regulate organelle transport.10 For example, Rab8, Rab10 and Rab11 interact with myosin V to regulate vesicle recycling,11 and Rab6 regulates fission of Rab6-positive vesicles from the Golgi by interacting with myosin II.12 However, myosins perform several additional cellular functions.
Figure 1.
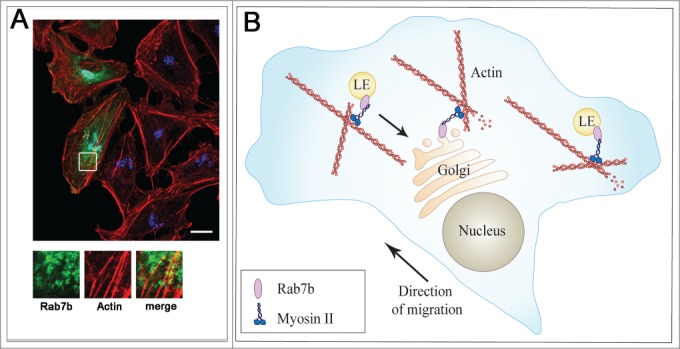
Rab7b in migrating cells. (A) A confluent monolayer of HeLa cells that had been transfected with GFP-Rab7b was scratched with a pipette tip and fixed after 2 hours. Cells were immunostained with an antibody against giantin followed by Alexa-647 (blue). Actin was labeled with Rhodamine-conjugated phalloidin (red). Scale bar 10 μm. Magnifications of the boxed area are shown in the insets. Rab7b-positive endosomes are visible along actin filaments. (B) Model illustrating Rab7b's intracellular roles. Rab7b localizes to late endosomes (LE) and TGN/Golgi and regulates the transport between these compartments. Rab7b interacts directly with the actin motor myosin II. This interaction is important for Rab7b dynamics, but also for actin cytoskeleton remodelling and thereby for cell migration.
Myosin II interacts with actin to crosslink and contracts the actin filaments, thereby regulating cytokinesis, migration, intracellular transport, cell shape and polarity.13,14 Intriguingly, Rab7b, through the association with myosin II, regulates the remodeling of actin filaments and therefore stress fiber organization, as depletion of Rab7b causes a significant reduction in number of stress fibers in the cell. In addition, depletion of Rab7b reduces cell spreading and adhesion on fibronectin, and also strongly delays cell migration and polarization in response to a wound.9 In sum, Rab7b influences the actomyosin reorganization in the cell, and thus is important for functions such as polarization, adhesion and migration (Fig. 1).
The ability of myosin II to regulate actin cytoskeleton remodeling depends on the phosphorylation state of its light chains. One of the most important kinases responsible for the phosphorylation of the myosin light chains (MLC) is ROCK (Rho kinase), which is a direct target of the small GTPase RhoA.15 When RhoA is in its active GTP-bound form, it associates with and augments the kinase activity of ROCK,16 further leading to phosphorylation of the MLC, which causes myosin II to interact with the actin cytoskeleton.17 Interestingly, the depletion of Rab7b strongly decreases the amount of active RhoA, and correspondingly also decreases MLC phosphorylation.9 Therefore, Rab7b, by interfering with RhoA activation, can control MLC phosphorylation and thereby the actin reorganization in the cell.
While Rab proteins are most known for their role in intracellular trafficking, our data show that these small GTPases can also have a functional role in the remodeling of the cytoskeleton. This is a new and unexpected role for Rab proteins, and opens up new possibilities for the Rab GTPases that have earlier been linked to cell migration or other actomyosin-dependent functions only in regards to their role in intracellular transport. Indeed, Rab5 has been shown to coordinate endocytosis and cell migration,18 and a few other Rabs, like Rab11, Rab25 or Rab35, that interact with actin-binding proteins or members of the myosin family, also interfere with cell migration by controlling either integrin or cadherin transport. 19-22
As Rab7b is highly expressed in dendritic cells (DCs),4 we verified that the interaction between Rab7b and myosin II occurs also in these primary cells both by co-immunoprecipitation 9 and the proximity ligation assay (PLA) Duo-Link, a technique which allows visualization of protein-protein interaction in fixed cells.23 A strong positive signal was visible in DCs after amplification of Rab7b- and Myosin II- antibody labeling with PCR-based Duo-Link indicating that the 2 proteins are located at a distance inferior to 40 nm (Fig. 2).
Figure 2.
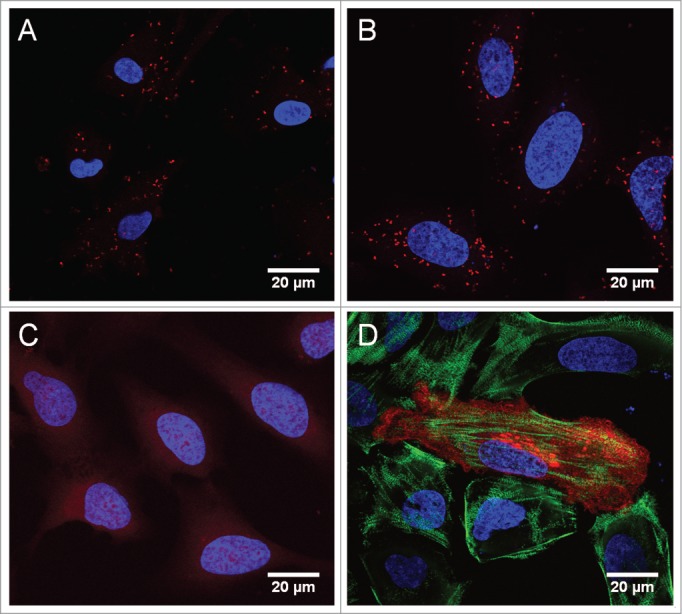
In situ PLA confirms the interaction between Rab7b and myosin II in DCs and U2OS cells. (A) Monocyte-derived dendritic cells were fixed and stained with antibodies against Rab7b and myosin II, combined with secondary PLA probes (Duolink, Sigma). The interaction events are visible as red dots. The nuclei are stained in blue (Hoechst). Scale bar 20 μm. (B) U2OS cells transiently transfected with HA-tagged Rab7b were fixed and stained with antibodies against Rab7b and myosin II, and further treated with secondary PLA probes (Duolink, Sigma). The interaction events are visible as red dots. Scale bar 20 μm. (C) U2OS cells transiently transfected with HA-tagged Rab7b were fixed and stained with antibodies against Rab7b and the early endosomal marker EEA1 as a negative control. No interaction events are visible after in situ PLA. Scale bar 20 μm. (D) U2OS cells transiently transfected with HA-tagged Rab7b were fixed and immunostained with primary antibodies against Rab7b and myosin II, followed by Alexa-555 (red) and Alexa-488 (green) conjugated secondary antibodies, respectively. Scale bar 20 μm.
DCs are cells of the immune system which patrol the body in order to find and take up antigens.24 Notably, cell migration is especially important for DCs that after antigen uptake in peripheral tissues move toward the lymph node to initiate a specific immune response. Initially after activation DCs decrease their intrinsic ability to migrate in order to better process antigens. However, DCs recover their migratory ability around 4 hours after pathogenic stimuli.25 Interestingly, also Rab7b is upregulated 4 hours after LPS stimulation, and then gradually down-regulated as the cells mature.26
In light of our recent results, where we show that Rab7b is important for cell migration by regulating actomyosin dynamics, we speculate that the sudden increase of Rab7b in activated DCs might be required for the actomyosin rearrangements needed for the proper migration of these cells. Importantly, myosin II activation through the RhoA-ROCK-pathway has been shown to be crucial for DC movement.24,27 Further studies are needed to elucidate the exact role of Rab7b in DCs, and investigate if Rab7b is important for the motility or for other actomyosin-dependent functions in activated DCs.
Recently, Rab7b was shown to associate with calpain-myosin in platelets, which are small non-nucleated cellular elements that after vessel damage are subjected to dramatic shape modification regulated by actin cytoskeleton rearrangements, confirming the role of this small GTPase for cytoskeleton organization.28
In conclusion, our data demonstrate that a Rab protein can control and coordinate cytoskeletal remodeling in addition to intracellular transport. It will be of great interest to further unravel the molecular mechanisms used by Rab7b to modulate RhoA activation and explore whether other Rab proteins can similarly influence cell migration by regulating actin cytoskeleton organization.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Work done in the author's laboratories is supported by the Norwegian Cancer Society [grants 5760850 to C.P. and 4604944 to O.B.], the Research Council of Norway [grants 239903 to C.P. and 230779 to O.B.] and through its Centers of Excellence funding scheme [project number 179573].
References
- 1. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513-25; PMID:19603039; http://dx.doi.org/ 10.1038/nrm2728 [DOI] [PubMed] [Google Scholar]
- 2. Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 2011; 91:119-49; PMID:21248164; http://dx.doi.org/ 10.1152/physrev.00059.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vitelli R, Santillo M, Lattero D, Chiariello M, Bifulco M, Bruni CB, Bucci C. Role of the small GTPase Rab7 in the late endocytic pathway. J Biol Chem 1997; 272:4391-7; PMID:9020161; http://dx.doi.org/ 10.1074/jbc.272.7.4391 [DOI] [PubMed] [Google Scholar]
- 4. Yang M, Chen T, Han C, Li N, Wan T, Cao X. Rab7b, a novel lysosome-associated small GTPase, is involved in monocytic differentiation of human acute promyelocytic leukemia cells. Biochem Biophys Res Commun 2004; 318:792-9; PMID:15144907; http://dx.doi.org/ 10.1016/j.bbrc.2004.04.115 [DOI] [PubMed] [Google Scholar]
- 5. Progida C, Cogli L, Piro F, De Luca A, Bakke O, Bucci C. Rab7b controls trafficking from endosomes to the TGN. J Cell Sci 2010; 123:1480-91; PMID:20375062; http://dx.doi.org/ 10.1242/jcs.051474 [DOI] [PubMed] [Google Scholar]
- 6. Progida C, Nielsen MS, Koster G, Bucci C, Bakke O. Dynamics of rab7b-dependent transport of sorting receptors. Traffic 2012; 13:1273-85; PMID:22708738; http://dx.doi.org/ 10.1111/j.1600-0854.2012.01388.x [DOI] [PubMed] [Google Scholar]
- 7. Wang Y, Chen T, Han C, He D, Liu H, An H, Cai Z, Cao X. Lysosome-associated small Rab GTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood 2007; 110:962-71; PMID:17395780; http://dx.doi.org/ 10.1182/blood-2007-01-066027 [DOI] [PubMed] [Google Scholar]
- 8. Yao M, Liu X, Li D, Chen T, Cai Z, Cao X. Late endosome/lysosome-localized Rab7b suppresses TLR9-initiated proinflammatory cytokine and type I IFN production in macrophages. J Immunol 2009; 183:1751-8; PMID:19587007; http://dx.doi.org/ 10.4049/jimmunol.0900249 [DOI] [PubMed] [Google Scholar]
- 9. Borg M, Bakke O, Progida C. A novel interaction between Rab7b and actomyosin reveals a dual role in intracellular transport and cell migration. J Cell Sci 2014; 127:4927-39; PMID:25217632; http://dx.doi.org/ 10.1242/jcs.155861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seabra MC, Coudrier E. Rab GTPases and myosin motors in organelle motility. Traffic 2004; 5:393-9; PMID:15117313; http://dx.doi.org/ 10.1111/j.1398-9219.2004.00190.x [DOI] [PubMed] [Google Scholar]
- 11. Roland JT, Bryant DM, Datta A, Itzen A, Mostov KE, Goldenring JR. Rab GTPase-Myo5B complexes control membrane recycling and epithelial polarization. Proc Natl Acad Sci U S A 2011; 108:2789-94; PMID:21282656; http://dx.doi.org/ 10.1073/pnas.1010754108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miserey-Lenkei S, Chalancon G, Bardin S, Formstecher E, Goud B, Echard A. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat Cell Biol 2010; 12:645-54; PMID:20562865; http://dx.doi.org/ 10.1038/ncb2067 [DOI] [PubMed] [Google Scholar]
- 13. Conti MA, Kawamoto S, Adelstein RS. Non-muscle myosin II. Myosins 2008:223-487 [Google Scholar]
- 14. Heissler SM, Manstein DJ. Nonmuscle myosin-2: mix and match. Cell Mol Life Sci 2013; 70:1-21; PMID:22565821; http://dx.doi.org/ 10.1007/s00018-012-1002-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 1996; 271:20246-55; PMID:8702756; http://dx.doi.org/ 10.1074/jbc.271.34.20246 [DOI] [PubMed] [Google Scholar]
- 16. Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 1996; 15:2208-16; PMID:8641286 [PMC free article] [PubMed] [Google Scholar]
- 17. Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 2009; 10:778-90; PMID:19851336; http://dx.doi.org/ 10.1038/nrm2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell 2008; 134:135-47; PMID:18614017; http://dx.doi.org/ 10.1016/j.cell.2008.05.034 [DOI] [PubMed] [Google Scholar]
- 19. Kessler D, Gruen GC, Heider D, Morgner J, Reis H, Schmid KW, Jendrossek V. The action of small GTPases Rab11 and Rab25 in vesicle trafficking during cell migration. Cell Physiol Biochem 2012; 29:647-56; PMID:22613965; http://dx.doi.org/ 10.1159/000295249 [DOI] [PubMed] [Google Scholar]
- 20. Allaire PD, Seyed Sadr M, Chaineau M, Seyed Sadr E, Konefal S, Fotouhi M, Maret D, Ritter B, Del Maestro RF, McPherson PS. Interplay between Rab35 and Arf6 controls cargo recycling to coordinate cell adhesion and migration. J Cell Sci 2013; 126:722-31; PMID:23264734; http://dx.doi.org/ 10.1242/jcs.112375 [DOI] [PubMed] [Google Scholar]
- 21. Lindsay AJ, Jollivet F, Horgan CP, Khan AR, Raposo G, McCaffrey MW, Goud B. Identification and characterization of multiple novel Rab-myosin Va interactions. Mol Biol Cell 2013; 24:3420-34; PMID:24006491; http://dx.doi.org/ 10.1091/mbc.E13-05-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seabra MC, Coudrier E. Rab GTPases and Myosin Motors in Organelle Motility. Traffic 2004; 5:393-9; PMID:15117313; http://dx.doi.org/ 10.1111/j.1398-9219.2004.00190.x [DOI] [PubMed] [Google Scholar]
- 23. Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods 2006; 3:995-1000; PMID:17072308; http://dx.doi.org/ 10.1038/nmeth947 [DOI] [PubMed] [Google Scholar]
- 24. Heuze ML, Vargas P, Chabaud M, Le Berre M, Liu YJ, Collin O, Solanes P, Voituriez R, Piel M, Lennon-Dumenil AM. Migration of dendritic cells: physical principles, molecular mechanisms, and functional implications. Immunological reviews 2013; 256:240-54; PMID:24117825; http://dx.doi.org/ 10.1111/imr.12108 [DOI] [PubMed] [Google Scholar]
- 25. Granucci F, Ferrero E, Foti M, Aggujaro D, Vettoretto K, Ricciardi-Castagnoli P. Early events in dendritic cell maturation induced by LPS. Microbes Infect 1999; 1:1079-84; PMID:10572310; http://dx.doi.org/ 10.1016/S1286-4579(99)00209-9 [DOI] [PubMed] [Google Scholar]
- 26. Berg-Larsen A, Landsverk OJ, Progida C, Gregers TF, Bakke O. Differential regulation of Rab GTPase expression in monocyte-derived dendritic cells upon lipopolysaccharide activation: a correlation to maturation-dependent functional properties. PLoS One 2013; 8:e73538; PMID:24039975; http://dx.doi.org/ 10.1371/journal.pone.0073538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 2008; 453:51-5; PMID:18451854; http://dx.doi.org/ 10.1038/nature06887 [DOI] [PubMed] [Google Scholar]
- 28. Tsai JC, Lin YW, Huang CY, Lin CY, Tsai YT, Shih CM, Lee CY, Chen YH, Li CY, Chang NC, et al. The role of calpain-myosin 9-Rab7b pathway in mediating the expression of Toll-like receptor 4 in platelets: a novel mechanism involved in alpha-granules trafficking. PLoS One 2014; 9:e85833; PMID:24489676; http://dx.doi.org/ 10.1371/journal.pone.0085833 [DOI] [PMC free article] [PubMed] [Google Scholar]


