Abstract
Microbial electrochemical systems exploit the metabolism of microorganisms to generate electrical energy or a useful product. In the past couple of decades, the application of microbial electrochemical systems has increased from the use of wastewaters to produce electricity to a versatile technology that can use numerous sources for the extraction of electrons on the one hand, while on the other hand these electrons can be used to serve an ever increasing number of functions. Extremophilic microorganisms grow in environments that are hostile to most forms of life and their utilization in microbial electrochemical systems has opened new possibilities to oxidize substrates in the anode and produce novel products in the cathode. For example, extremophiles can be used to oxidize sulfur compounds in acidic pH to remediate wastewaters, generate electrical energy from marine sediment microbial fuel cells at low temperatures, desalinate wastewaters and act as biosensors of low amounts of organic carbon. In this review, we will discuss the recent advances that have been made in using microbial catalysts under extreme conditions and show possible new routes that extremophilic microorganisms open for microbial electrochemical systems.
Keywords: anode-respiring bacteria, bioanode, bioelectrochemical systems, electricity generation, extremophiles, microbial electrolysis cells, microbial fuel cells, MEC, MFC
This review highlights the use of microbial electrochemical systems to catalyze environmental processes coupled to the production of energy or valuable resources and how utilizing extremophilic microorganisms opens up new possibilities such as bioremediation of environmentally hazardous wastes.
INTRODUCTION
Microbial electrochemical systems (MESs) describe all electrochemical devices that make use of microbial catalysts to drive or accelerate electrochemical reactions at the anode, the cathode or both electrodes (Rabaey and Rozendal 2010; Logan and Rabaey 2012). An MES in which energy is recovered is a microbial fuel cell (MFC), while a system which requires the input of electrical energy to drive a reduction reaction is a microbial electrolysis cell (MEC) (Logan et al.2006, 2008).
The discovery that an electrical current can be derived directly from organic electron donors (e.g. acetate) led to the testing of a nearly endless number of substrates for microbial-assisted production of electrons (Pant et al.2010). At first, this production was mainly used to (i) extract the energy available from wastewaters (Logan et al.2006), (ii) remove recalcitrant organic and inorganic compounds (Kim et al.2008; Catal, Bermek and Liu 2009; Mu et al.2009; Ter Heijne et al.2010; Virdis et al.2010), (iii) extract energy from plant rhizodeposits (Strik et al.2011) or (iv) power small-scale off-grid devices and sensors (Shantaram et al.2005). These applications have evolved into chemical or microbial catalyzed generation of products with a higher added value from the produced electrons, such as hydrogen (Logan et al.2008), methane (Cheng et al.2009), hydrogen peroxide (Rozendal et al.2009), and short- and medium-chain fatty acids (Steinbusch et al.2011; van Eerten-Jansen et al.2013). The more recent discovery that these electrons can be used to drive microbial metabolism opens up a whole new world of possible products of which we cannot yet see the full impact (Rabaey and Rozendal 2010; Nevin et al.2011). Nowadays, even the ionic current generated with the production of the electrical current is used to drive separation processes such as the recovery of ammonia (Kuntke et al.2012), the production of alkalinity (Sleutels, Hamelers and Buisman 2010; Modin et al.2011) or to provide additional energy to drive microbial activity (Logan and Elimelech 2012).
GENERAL PRINCIPLES OF MESs
Oxidation of a substrate can be spontaneous as well as chemically or microbially catalyzed reaction to form one or multiple products and in an electrochemical reaction the electrons are released (Fig. 1a). The derived electrons are transferred to the solid anode either directly or indirectly via mediators wherefrom they flow through an external electrical circuit to the cathode. At the cathode the electrons are combined with an electron acceptor to form a product. Again, similar to the anodic reaction, this can be catalyzed by microbes or metals (Fig. 1b).
Figure 1.

Schematic of a bioelectrochemical system. The system typically consists of anode (where substrate is oxidized (a)) and cathode (where the product is formed (b)) compartments separated by a membrane. Energy is applied by means of a power supply in MECs (c) or harvested from the system in MFCs (d). The anode and cathode chambers can be separated by various types of membranes (e–g) or be a single chamber without an ion exchange membrane (h).
Reactions at the anode
Microorganisms that predominantly act as catalysts in MESs and pass electrons to the anode are termed ‘electricigens’ (the term used in this review), ‘electrogens’, ‘anodophiles’ or ‘anode respiring bacteria’. Electricigens have been identified from a range of phyla but those commonly identified in high abundance are from the Proteobacteria and Firmicutes. The two most studied species are Geobacter sulfurreducens (Caccavo et al.1994) and Shewanella oneidensis (Venkateswaran et al.1999). However, many other species have been identified depending on the conditions in the anodic chamber. In this review, we will discuss alternative electricigens that have been reported to live under extreme conditions.
In ideal conditions, the anode compartment lacks any alternative external electron acceptors such that the cells pass their electrons to the anode via either the ‘direct’ or ‘indirect’ mechanisms (Fig. 1a). The direct mechanism involves anaerobic electron transport down the electron transport chain before transfer to the insoluble electron acceptor (the anode). This transfer is mediated by c-type cytochromes and iron-sulfur proteins localized to the cell surface (Liu et al.2014). An additional proposed method of direct electron transfer (at a distance) is via nanowires (Reguera et al.2005) that are produced by many electricigens. The mechanism by which electricigens transfer electrons to the anode via cytochromes and nanowires is under intense scrutiny (Lovley and Malvankar 2015; Malvankar et al.2015). Indirect transfer of electrons to the anode involves soluble redox shuttles, termed ‘mediators’. Mediators can be either organic (e.g. humic acids) or inorganic (e.g. the S0/H2S shuttle) and in a mixed microbial culture, electron transfer to the anode by mediators is not necessarily limited to the species that produces the shuttle. Microbial populations within the anode chamber can exist as planktonic cells that utilize mediators, as a biofilm formed on the surface of the anode that facilitates direct electron transfer, or a combination thereof. These populations can be single species or mixed populations that can act in a synergistic manner to break down complex organic compounds to simple compounds that can be utilized by the electricigens. However, competing microbial metabolisms that divert electrons from being transferred to the anode, such as methanogenesis, can also occur.
Reactions at the cathode
Electrons from the anode flow to the cathode where the reduction reaction takes place (Fig. 1b). The final electron acceptor varies depending on the type of system and its application. In an MFC for electricity production, oxygen is commonly used as the electron acceptor as it is abundant and sustainable due to its reduction product being water (Logan et al.2006; Clauwaert et al.2007; Virdis et al.2008). On the other hand, MECs rely on the input of additional external energy supplied by a power supply and can be used for production of valuable resources, such as hydrogen at the cathode (Logan et al.2008). Major advantages of hydrogen-producing MECs is that the energy consumption is substantially less than that required for conventional electrolysis of water (Liu, Grot and Logan 2005; Rozendal et al.2006) and a much higher yield of hydrogen can be reached (Logan et al.2008).
Separators
In many cases the electrodes are separated by an ion exchange membrane that allows the anolyte and catholyte to be of different compositions. The ion exchange membrane also prevents product and substrate crossover, makes production of a pure product in both anode and cathode possible, and enables transport of specific ionic charge and the rejection of the opposite ionic charge. An anion exchange membrane allows negatively charged ions to be transported and positive ions are rejected (Fig. 1e), while a cation exchange membrane transports positive ions and rejects anions (Fig. 1f). With the expanding field of MES applications, more severe conditions are being employed in operating these cells. For example, a bipolar membrane can be used to maintain a large pH gradient between the anode and cathode (Fig. 1g) (Ter Heijne et al.2006; Harnisch, Schröder and Scholz 2008; Harnisch and Schröder 2009). In some cases, this charge separation is not required and the ion exchange membrane can be left out or a non-selective barrier is used (Fig. 1h).
Thermodynamics
Depending on the value of the Gibbs free energy change (ΔGr) of both the oxidation reaction at the anode and the reduction reaction at the cathode, energy needs to be applied by means of a power supply (ΔGr > 0; Fig. 1c) or energy can be harvested from the system (ΔGr < 0; Fig. 1d). For the reaction vAA + vBB → vCC + vDD, the Gibbs free energy change can be calculated using equation 1:
 |
(1) |
where ΔGr0 is the Gibbs free energy change under standard conditions (298 K, 1 bar and 1 M concentration for all species), R is the universal gas constant (8.31 J mol−1 K−1), T is the temperature in degrees Kelvin, [i] is the concentration of a specific reactant (mol L−1) and vi is the reaction coefficient. In MESs, it is more convenient to express the Gibbs free energy change (kJ mol−1) of the overall reaction as the potential difference between the oxidation (anode) and reduction (cathode) reaction (ΔE; V) which is related to the Gibbs free energy of the overall reaction according to equation 2:
 |
(2) |
where n is the amount of electrons involved in the reaction. These anode (Ean) and cathode (Ecat) potentials can be calculated in a similar way as the energy change of the total reaction in an MFC but then related to a reference potential. From these anode and cathode potentials, the cell voltage can be calculated using equation 3:
 |
(3) |
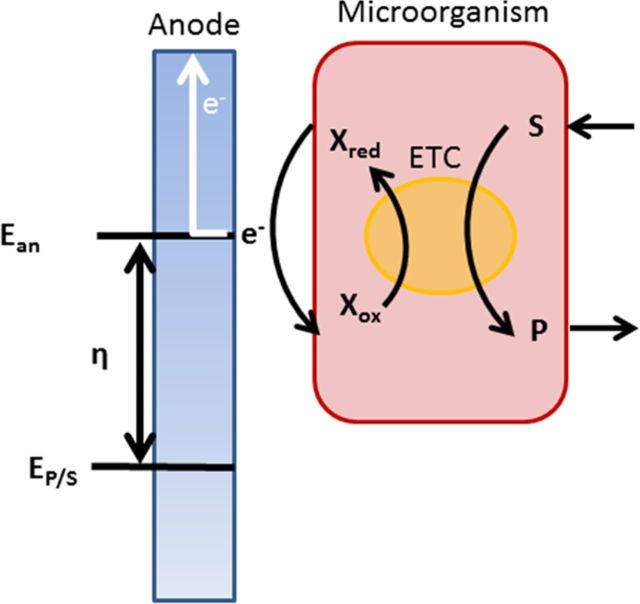
From a microbial perspective, it is interesting to see the amount of energy that is available to generate ATP. A schematic representation of the electron flow from microorganism to the electrode is given in Fig. 2. The microorganisms oxidize a substrate to a product from which electrons are released into the electron transfer chain. The energy level of these electrons is determined by the Gibbs free energy change of this oxidation reaction. Finally, the electrons are transferred to the electrode surface by a redox active enzyme or cytochrome. The energy at which the electrons are released is determined by the final step in the electron transfer chain and the enzyme that catalyzes this final step.
Figure 2.
Schematic representation of the electron flow from microorganism to the electrode. The microorganisms oxidize a substrate (S) to a product (P) from which electrons are released into the electron transfer chain. The energy level of these electrons is determined by the Gibbs free energy change of this oxidation reaction (EP/S). Finally, the electrons are transferred to the electrode surface by a redox active enzyme. The energy at which the electrons are released is determined by the final step in the electron transfer chain and the enzyme that catalyzes this final step (Ean). Figure adapted from Hamelers et al. (2011).
For the anode, the maximum amount of work that can be performed by the microorganisms is determined by the energy available from the substrate (Ep/s), the energy level at which these electrons are donated to electrode (Ean; anode potential) and the rate (I; current density) at which they are converted, which is in turn related to the anode potential. In more general terms, this potential difference between the thermodynamic potential and the actual potential is known as the electrode overpotential (η) and includes an activation and concentration term. The concentration term is determined by the concentration of the reacting species (both substrates and products) and the mass transfer in the system, which in most cases is limited by diffusion. The activation overpotential is mostly determined by the catalyst, in this case the microorganism, and the energy it requires to transfer the electrons.
EXTREME CONDITIONS AND EXTREMOPHILES
Extreme conditions are those that are harmful for most life on earth and encompass both physical (e.g. high and low temperatures) and geochemical (e.g. high metal concentrations or under extreme nutritional limitations) conditions (Fig. 3). Extreme milieu are defined taxonomically (rather than anthropocentrically) as environments with low species diversity where whole taxonomic groups are missing (Bott and Brock 1969). These environments can be natural in origin such as Mono Lake, California, that is both alkaline and hypersaline (Antony et al.2013); polar sites that have low temperatures and in some cases are arid (Cowan et al.2014); and hydrothermal vents that are high temperature (Dick et al.2013). In addition, extreme environments can be anthropogenic in origin such as metal laden, acidic mine waters (Dopson and Holmes 2014) or nuclear waste sites high in radioactivity (Morozkina et al.2010). Extremophilic microorganisms that inhabit these environments have been identified from all three domains of life (Pikuta, Hoover and Tang 2007). These organisms are adapted to live and thrive in these challenging conditions and in most cases are incapable of growth under non-extreme conditions. One example is the extreme acidophile Picrophilus oshimae that has an optimum growth pH of 0.7 and the cells burst above pH 4 (van de Vossenberg et al.1998). At least 15 classes of extremophiles have been defined such as cryptoendoliths that live in spaces between rocks and xerophiles that are able to survive in very dry conditions. The extremophile groups that have been utilized in MESs and are described in this review include acidophiles and alkaliphiles, psychrophiles and thermophiles, halophiles and oligotrophs.
Figure 3.
Classes of extremophilic microorganisms that have been utilized in MESs, their defining characteristic and an example of an environment where they grow. Photographs are by the authors except of the chloride and soda lakes (courtesy Dimitry Sorokin) and the Antarctic Sea (courtesy Daniel Bengtsson).
Acidophiles are defined as having an optimum growth pH < 5 (extreme acidophiles have an optimum pH < 3) and include Bacteria, Archaea and Eukaryota. Species range from low temperature adapted acidophiles to thermoacidophiles. Various species can oxidize ferrous iron (Bonnefoy and Holmes 2012), sulfur and inorganic sulfur compounds (Dopson and Johnson 2012), and organic carbon for energy (Sato and Atomi 2011) and in some cases fix CO2 for organic carbon (Dopson 2012). Acidophiles utilize several mechanisms to cope with low pH including membranes that are resistant to the influx of protons, an internal negative membrane potential that inhibits the influx of protons, as well as primary and secondary proton transporters (reviewed in Slonczewski et al.2009). As a result of catalyzing metal sulfide dissolution, acidophiles are also often required to be tolerant to very high metal loads and the underlying mechanisms are a combination of both biotic and abiotic systems (reviewed in Dopson and Holmes 2014; Dopson et al.2014).
Alkaliphiles grow in a pH range of 8.5–11 and also include examples from all three domains of life. They are found in environments that include soda lakes, alkaline soils and the Red Sea and are often also halophilic, termed ‘haloalkaliphiles’. Alkaliphiles maintain their internal pH ≥ 2 pH units below that of the external milieu in part by exploiting a sodium motive force (sodium cannot be replaced by potassium) to conserve energy (Slonczewski et al.2009; Hicks et al.2010). Other alkaliphile pH homeostatic mechanisms include the Mrp Na+/H+ antiporter, additional transport systems and a voltage-gated sodium channel, NaVBP (reviewed in Slonczewski et al.2009). In contrast, some alkaliphiles (but not halophiles) grow best in the absence of sodium (Tiago et al.2006). Alkaliphiles have been extensively used in other biotechnologies, primarily enzymes for detergents (Sarethy et al.2011).
Psychrophiles (also known as cryophiles) are defined as having an optimum growth temperature <15°C and being able to replicate at <0°C while psychrotolerant species grow at low temperatures but their optimal temperature is >15°C. The absolute lowest temperature for life is unclear but cellular activity occurs as low as −20°C and cells can reproduce at −12°C (Rivkina et al.2000). Natural low-temperature environments include the deep oceans, Antarctica and the Arctic, mountain areas and glacial ice, while man-made fridges and freezers are also permanently cold. Due to these diverse areas, low-temperature environments cover approximately 80% of the globe resulting in psychrophiles being the most common and diverse of the known extremophiles. Much research has been directed at their low-temperature adaptations that include areas of the genome with high GC; proteins with increased flexibility, decreased thermostability and higher activity; expression of genes associated with the cold shock response; and physiological adaptations in the membrane, cryoprotectants, anti-freeze proteins and production of extracellular polysaccharide (reviewed in De Maayer et al.2014).
Thermophiles are predominantly archaea that grow at temperatures above 45°C and up to the present temperature maximum for growth, 122°C (Takai et al.2008). They are often found in geologically active areas such as Yellowstone National Park from where the first thermophile was discovered (Brock 1967). Thermophilic adaptations to life at high temperature include increased salt bridges and a hydrophobic core to increase the heat stability of proteins, a higher percentage of membrane saturated fatty acids and adaptations in the genome such as high GC content and positive supercoiling of the DNA (reviewed in Lewin, Wentzel and Valla 2013).
Halophiles grow in high salt concentrations and are mostly found within the Archaeal domain. They are classified as slight (0.3–0.8 M sodium chloride), moderate (0.8–3.4 M NaCl) and extreme (>3.4 M NaCl) halophiles (Cavicchioli and Thomas 2000). These microorganisms are adapted to a high salt environment by increasing their cytoplasmic osmolarity, such as via importing potassium ions (Oren 2013). The average salinity of seawater is about 3.5% (wt/vol; 599 mM NaCl) resulting in marine microorganisms being classified as slight halophiles.
Oligotrophs grow in extremely nutrient poor environments (e.g. the deep biosphere and sandplains of southern Western Australia) and are typically characterized as having low growth rates. For instance, carbon turnover rates in the deep biosphere estimate generation times to be in the 100s to 1000s of years and these species are hypothesized to have low energy maintenance systems (Hoehler and Jorgensen 2013). Further adaptations to oligotrophy include small cell size and a streamlined genome (Luef et al.2015).
EXTREMOPHILES IN MESs
Several different classes of extremophilic microorganisms have been utilized in MESs and this section (see categories below) will describe their use as well as comparing the different electrochemical efficiencies achieved.
The energy that is available for microbial activity is highly dependent on the electrode potential, the type of microorganism, the electron transfer chain it possess and its ability to deal with environmental conditions. The Gibbs free energy change is dependent on temperature, pressure, concentrations/activity and pH of the electrolyte that is altered when calculating the actual Gibbs free energy under extreme conditions compared to normal conditions. In non-dilute systems, the activity of a substance has to be used instead of the concentration in dilute systems. The correct value for the pH and the pressure of a certain gas can be directly used in the equation. As an example, the Gibbs free energy change for the oxidation of acetate was calculated at different pH values, assuming that the microorganisms are able to convert acetate at this entire pH range. This Gibbs free energy change not only alters because of the different conditions caused by the pH but also because the reaction equation changes due to the presence of different species (equations 4–6):
 |
(4) |
 |
(5) |
 |
(6) |
At standard conditions the Gibbs free energy change for the oxidation of acetate is 0.187 V (vs NHE) (Logan et al.2006), while at practical conditions (pH 7 and 0.005 M for reactive species) the value changes to −0.291 V (Fig. 4). At acidic conditions (pH 2), the anode potential is −0.022 V while the anode potential alkaliphiles experience at pH 10 is −0.609 V (Fig. 4). When the anode reaction is coupled to a cathode reaction and the cell voltage is calculated (equation 3), an increase in cell voltage is seen at increasing anolyte pH due to the additional pH gradient also representing a source of energy (chemical potential). The practical implication of an elevated cell voltage is that more energy can be gained from these systems at higher pH values. When more of this energy is available to the microorganisms, they can expend more maintenance energy to withstand the extreme conditions in which they live or produce more current.
Figure 4.

Example of the change in anode potential according to changes in the pH based on thermodynamics. Potentials at standard conditions are calculated with a concentration of 1 M, while the practical conditions are calculated with a concentration of 0.005 M. All potentials are reported vs NHE.
To date, few studies have been performed that systematically compare the performance of MESs (maximum power densities, Coulombic efficiencies, anode overpotentials, current densities and cell voltages) at extreme conditions. In addition, the reactor cell architecture and choice of electrolyte have a significant impact on performance. Therefore, it is difficult to draw hard conclusions from the reported effects of different extremes on the system performance.
Acidophiles
In this review, MES studies that exploit moderate and extreme acidophiles in the anode (e.g. Acidiphilium spp.; Borole et al.2008) as well as biocatalysts in the cathode (e.g. Acidithiobacillus ferrooxidans; Ter Heijne, Hamelers and Buisman 2007) are discussed (Table 1). An advantage of a low pH MES is that the protons will not cause diffusion limitations in the cathode compartment for reduction of oxygen and therefore, will not limit the current production (Erable, Etcheverry and Bergel 2009). However, a consequence of growth at low pH is that the cells have to maintain a near neutral cytoplasm (reviewed in Slonczewski et al.2009). This consumes a portion of the energy derived from electron transport for other processes such as proton export, increasing the anode overpotential (reducing whole cell voltage outputs) and lowering power generation. Despite a higher chemical oxygen demand removal rate at pH 4, lower voltage outputs and power generation are observed for neutrophilic microbe catalyzed MFCs operated at pH 7. This low Coulombic efficiency is attributed to a poor quality biofilm on the anode (Zhang et al.2011a). This indicates that current generation at these acidic conditions from acetate is possible but many electrons from the carbon source are directed to alternative electron acceptors than the electrode.
Table 1.
Comparison of the performance of selected bioelectrochemical systems under acidic conditions.
| BES | Condition | Inoculum | Electron donor | Voltage | Power density | Current density | CE | Anode potential (vs SHE) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| MFC | pH 2.0 | Acidithiobacillus ferrooxidans* | Electrode | – | – | 5.0 A m−2 | – | – | Carbajosa et al. (2010) |
| MFC | pH < 2.5 | Acidithiobacillus ferrooxidans* | FeSO4 | – | 1.2 W m−2 | 4.4 A m−2 | – | – | Ter Heijne, Hamelers and Buisman(2007) |
| MFC | pH 2.5 | Acidiphilium spp. | Glucose | – | – | 3 A m−2 | – | + 0.15 V (controlled) | Malki et al. (2008) |
| MFC | pH 2.5 | Mining wastewater | Tetrathionate | 0.18 V | 18 mW m−2 | 433 mA m−2 | <5% | 0.248 V | Sulonen et al. (2015) |
| MFC | pH 3.0 | Sediment | Glucose/glycerol | – | 300 mW m−2 | 3.5 A m−2 | – | – | Garcia-Munoz et al. (2011) |
| MFC | pH 4.0 | Anaerobic sludge | Acetate | – | – | – | 6.45% | – | Zhang et al. (2011a) |
| MFC | pH 4.0 | Acidiphilium cryptum | Glucose | 0.30 V (OCV) | 12.7 mW m−2 | – | – | – | Borole et al. (2008) |
| MFC | pH 4.0 | Anaerobic sludge | Synthetic food waste | 0.14 V (OCV) | 425 mW m−3 | – | 18% | – | Li, Cheng and Wong (2013) |
| MFC | pH 4.4 | Distillery wastewater | Distillery wastewater | 0.88 V | 12.9 W m−3 | 29 mA m−2 | – | – | Kim et al. (2014) |
| MES | pH 4.5 | Synthetic waste water | Organic carbon compounds | – | – | – | – | +0.003 V | Zheng et al. (2014) |
| MFC | pH 4.5 | Anaerobic sludge | Ethanol | – | – | – | 27% | – | Liang et al. (2013) |
| MFC | pH 4.7 | Wastewater/sludge | Wastewater | – | 454 mW m−3 | – | – | – | Li et al. (2013) |
Acidithiobacillus ferrooxidans was inoculated in the cathode for regeneration of Fe2+ to Fe3+. The anode contained a mixed culture.
The first acidophile demonstrated to generate electricity was Acidiphilium cryptum that oxidizes glucose coupled to the reduction of ferric iron at pH ≤ 4 (Borole et al.2008). However, accumulation of the electron acceptor ferric iron (potentially as Fe(OH)3) restricted current output. In order to eliminate this effect, the alternative electron mediators nitrilotriacetic acid and phenosafranin were added to the system that resulted in an increased power output of 12.7 mW m−2. Thus, the current generation in this study was based on electron transfer by the artificial mediators rather than direct electron transfer from the microorganisms to the electrodes (Borole et al.2008). In contrast, Acidiphilium sp. strain 3.2 sup 5 isolated from Rio Tinto, Spain, mediates direct electron transfer from glucose metabolism to the anode at pH 2.5 at a rate of 3 A m−2 even in the presence of air (Malki et al.2008). The ability of Acidiphilium sp. strain 3.2 sup 5 to donate electrons directly to the anode in the presence of air facilitates the MES design as a membrane halting oxygen diffusion from the cathode to anode compartments is unnecessary. This strain grows on carbon cloth and graphite anode materials, produces extracellular polymeric substances, forms a biofilm between the carbon microfibers and in pores on the graphite rod surface (Tapia et al.2009), and preferentially attaches to graphitic flakes as opposed to glass (Malki et al.2013). Acidiphilium strain 3.2 sup 5 forms a closely packed multilayered biofilm that contains iron potentially in c-type cytochromes that may mediate electron transport to the anode (Malki et al.2013). Although Acidiphilium strain 3.2 sup 5 reduces soluble ferric iron in the presence of air, thick biofilms of acidophilic microorganisms become anaerobic (Baker-Austin et al.2010). Therefore, electron transfer to the anode may occur in anaerobic conditions as oxygen cannot penetrate that deeply into the biofilm. The electricigen Acidiphilium strain PM genome has been sequenced but no genes are ascribed to mediate its ability to reduce ferric iron in this species (San Martin-Uriz et al.2011) or other acidophiles (Osorio et al.2013). A microcosm sediment–water interface MFC demonstrated electricity generation in a natural acidic system at pH 3 with Acidiphilium spp. predominantly colonizing the anode while A. ferrooxidans and Leptospirillum spp. colonize the cathode (Garcia-Munoz et al.2011). Due to the available protons and high ionic strength of the medium, the current density and maximum power generation are between 2- and 20-fold higher than similar sediment–water interface MFCs operated at neutral pH, respectively (Garcia-Munoz et al.2011).
Microorganisms from pH neutral environments have been inoculated into moderately acidic MESs (anode pH 4 to ∼5.5) to treat organic wastewaters such as from distilleries (Kim et al.2014) and food waste (Li et al.2013). Other MESs have been designed to remove sulfate in acidic wastewaters (pH 2.5–4.5) using organic carbon-fed neutrophilic sulfate-reducing microbes to produce sulfide that is chemically oxidized to elemental sulfur on the anode surface (Liang, Xiao and Zhao 2013; Zheng et al.2014). However, many wastewaters also contain inorganic carbon and energy sources such as inorganic sulfur compounds in mining wastewaters. To be able to treat mining wastewaters through oxidation and electron release, it is necessary to develop MESs containing acidophilic electricigens in the anodic chamber able to oxidize inorganic sulfur compounds. An MFC utilizing tetrathionate as electron donor has a mixed culture of A. ferrooxidans and Ferroplasma spp. coupled to the reduction of ferric iron (Sulonen et al.2015). However, the low Coulombic efficiency (<5%) suggests that many electrons were not passed to the anode. Acidithiobacillus ferrooxidans has also been utilized to catalyze oxygen reduction at the cathode at pH 2 to overcome kinetic limitations associated with the consumption of four protons per oxygen molecule reduced (Carbajosa et al.2010). Under practical conditions, the reduction of ferric iron on the cathode has a higher cathode potential than for the reduction of oxygen and the use of A. ferrooxidans to regenerate the ferric iron resulted in a 38% increase in power output compared to an MFC without the cathode biocatalyst (Ter Heijne, Hamelers and Buisman 2007). Of course, this increased power output cannot entirely be attributed to the presence of A. ferrooxidans as the elevated concentration of Fe3+ might help reduce the overpotential.
Looking at the general bioelectrochemical performance of systems employing acidophiles, it can be seen that the oxidation rate of substrate to produce current for these microorganisms is high and therefore, the removal rate is also high (e.g. 5 A m−2 for FeSO4 and 3 A m−2 for glucose). Unfortunately, overpotentials for the oxidation reaction are reported in very few studies. These overpotentials could give more insight into the energetic efficiency of the oxidation reaction and more solid conclusions about the process could be drawn.
Alkaliphiles
An overview of the studies reporting alkaliphiles in MESs is given in Table 2. In addition, alkaliphiles also capable of growth in high salt concentrations (making them haloalkaliphiles) that have been utilized for MESs are discussed in the multiple extremes section below. It is important to note that many MESs make use of alkaline medium as feed. However, since the anodic oxidation reaction is strongly acidifying, many of these systems actually operate under near pH neutral conditions. For example, urine is a well-known alkaline feed for MESs. While the urine itself has a pH of around 9, the effluent pH is around 7. Alkalinization of the medium in this case is caused by hydroxyl transport from the cathode and decreased current production drastically (Kuntke et al.2014).
Table 2.
Comparison of the performance of selected bioelectrochemical systems under alkaline conditions.
| BES | Condition | Inoculum | Electron donor | Voltage | Power density | Current density | CE | Anode potential (vs SHE) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| MFC | pH 9.0–11.0 | Bacillus spp. | Glucose | 0.90 V | – | – | – | – | Akiba et al. (1987) |
| MFC | pH 10.0 | Artificial wastewater | Organics | – | – | – | – | – | Wu et al. (2011) |
| MFC | pH 10.0 | Bacillus pseudofirmus | Organics | – | – | – | – | – | Ma et al. (2012) |
| MFC | pH 10.0 | Existing MFC | Brewery wastewater | 1.04 V (OCV) | 31 W m−3 | 135 A m−3 | 90% | – | Zhuang et al. (2010) |
| MFC | pH 9.5 | Pseudomonas alcaliphila | Citrate | 0.53 V (OCV) | – | 70 mA m−2 | – | −0.094 V | Zhang et al. (2011b) |
| MFC | pH 9.5 | Parental MFC | Urban sewage | 0.65 V (OCV) | 1.8 W m−3 | 13.7 A m−3 | 77% | – | Puig et al. (2010) |
| MFC | pH 9.5 | Corynebacterium sp. | Glucose | 0.45 V | 7.3 mW m−2 | 33 mA m−2 | 5.9% | – | Liu et al.2010 |
| MEC | pH 9.3 | Geoalkalibacter ferrihydriticus | Acetate | – | – | 8.3 mA m−2 | 84–95% | – | Badalamenti, Krajmalnik-Brown and Torres (2013) |
| MEC | pH 9.1 | Operational MFC | Urine | – | – | 23.1 A m−2 | 97% | – | Kuntke et al. (2014) |
| MFC | pH 9.0 | Waste water | Acetate | – | 2770 mW m−2 | 99 mA m−2 | – | – | Fan, Hu and Liu (2007) |
| MFC | pH 9.0 | Activated sludge | Acetate | – | 1170 mW m−2 | 0.18 mA m−2 | – | −0.393 V (OCP) | Yuan et al. (2011) |
| MFC | pH 9.0 | Anaerobic sludge | Synthetic waste | 0.60 V (OCV) | 5591 mW m−3 | – | 63% | – | Li, Cheng and Wong (2013) |
| MFC | pH 9.0 | Compost | Acetate | – | 6 W m−3 | 7 A m−2 | 23% | −0,041 V | Pocaznoi et al. (2012) |
| MFC | pH 9.0 | Compost | Acetate | – | – | 16 A m−2 | 33% | −0,041 V | Blanchet et al. (2014) |
| MFC | pH 9.0 | Paper mill effluent | Acetate | – | – | 7.5 A m−2 | – | −0.156 | Ketep et al. (2013) |
Operating the bioanode of MFCs at alkaline conditions has several advantages for electricity production. These include diminished competition for substrate by methanogenesis (Gutierrez et al.2009) and at high pH, the lower anode potential results in an increased cell voltage (Erable, Etcheverry and Bergel 2009). During MFC operation, the anode becomes acidified while the cathode becomes more alkaline leading to a reduced cell voltage and power output. An acetate-fed, high current density bioanode inoculated with a garden compost inoculum also resulted in a pH increase to 9.0 that was attributed to the spontaneous evolution of compost leachate (Pocaznoi et al.2012). To address this issue, an MFC with a pH 10 anode compartment and pH 2 cathode compartment was operated that had higher open circuit voltage, power density and the high anodic pH resulted in an increased efficient Coulombic efficiency than a MFC run at neutral pH (Zhuang et al.2010). Of course it is not possible to sustain these pH levels in practice as addition of chemicals would be too costly and their addition is an unsustainable solution. Further studies showing enhanced MFC performance with alkaliphiles include an MFC treating urban wastewater that has improved power generation at pH 9.5 compared to neutral pH (Puig et al.2010), a single-chamber air-cathode MFC operated at pH 9 that has 39% greater power density than that observed at pH 7 that is partly attributed to a reduction of charge-transfer and diffusion resistance when a high pH carbonate buffer is used compared to a lower pH phosphate buffer (Fan, Hu and Liu 2007) and a pH 9 MFC that has 29% and 89% higher power density compared to operation at pH 7 and 5, respectively (Yuan et al.2011). Again, the increase in power can be attributed to the addition of chemicals, in this case buffer species that decreased the diffusion resistance for protons. As in the previous case, the addition of chemicals is costly and unsustainable. The molecular mechanism underlying increased electricity production at alkaline pH in S. oneidensis MR-1 has been investigated (Yong et al.2013). Shewanella oneidensis MR-1 extracellular electron transfer to the anode is mediated by riboflavin and the amount synthesized increases between pH 6 and 9 and is directly proportional to electricity output (Yong et al.2013). Reversible bioelectrodes that alternate between substrate addition and aeration have been designed to exploit proton accumulation to increase the efficiency of the biocathode. A reversible acetate and oxygen bioelectrode with a garden compost inoculum reached a maximum pH of 9.9 (the pH increase was attributed to compost leachate) and maintained its anodic efficiency despite the aeration periods (Blanchet et al.2014). Analysis of the microbial population identified 49 ± 1% Chloroflexi, making this the first time this bacterial class has been identified as dominating bioanodes.
The first alkaliphile to be utilized in an MFC was a Bacillus sp. at pH 9.5–11 that requires the presence of a redox mediator (Akiba et al.1987). Since then further alkaliphiles have been utilized in pure culture MFCs including the Gram-positive Corynebacterium strain MFC03 that degrades glucose and donates electrons to the anode in the absence of exogenous mediators (Liu et al.2010). However, cyclic voltammetry suggested the involvement of exogenous mediators produced by the Corynebacterium sp. and the addition of anthroquinone-2,6-disulfonate increased the maximum power density (Liu et al.2010). A second pure culture of an anode-respiring alkaliphile utilized in MFCs is Geoalkalibacter ferrihydriticus that generates a high current density by oxidation of acetate and yeast extract at pH 9.3 (Badalamenti, Krajmalnik-Brown and Torres 2013).
Further strains isolated as pure cultures from MFCs operating at high pH include Corynebacterium humireducens MFC-5 (Wu et al.2011) and Bacillus pseudofirmus MC02 (Ma et al.2012) both isolated from humic acid/humus-degrading MFCs. Biological humic acid reduction has environmental benefits and humic substances, once reduced, can shuttle electrons to insoluble electron acceptors. The Wu et al. (2011) study revealed the possibility of concurrent biological humic acid reduction and biochemical electricity generation within an MFC system oxidizing several organic carbon electron donors including lactate, ethanol and sucrose. Another species capable of electricity production at high pH, Pseudomonas alcaliphila, excreted phenazine-1-carboxylic acid that acts as an electron shuttle during oxidation of citrate (Zhang et al.2011b). An MFC has also been developed to treat food wastes that comprise 30–55% (by weight) of all refuse in urban societies. The waste was first treated by anaerobic digestion (considered the most environmentally friendly treatment method) and the resulting food waste leachate was amended with 100 mM NaCl and electricity generated in a pH 9 MFC that had a maximum of 63% Coulombic efficiency (Li, Cheng and Wong 2013). Finally, MFCs have been used to treat alkaline paper mill effluents that reached an anode pH > 9 that was shown to contain Desulfuromonas acetexigens as the single dominant species in the tertiary microbial anodes (Ketep et al.2013).
Psychrophiles
In this section, studies of both psychrophilic and psychrotolerant microorganisms are reported (Table 3). In general terms, temperature has a great impact on the performance of MESs. It impacts the functioning of microorganisms, the electrochemical reactions and the Gibbs free energy change of the reactions. The microbial enzyme machinery has an optimum temperature for performance while the electrochemical reaction rate increases at increasing temperature. Lowering the operating temperature has an adverse effect on MFC performance in terms of power and current (Larrosa-Guerrero et al.2010), start-up time (Cheng, Xing and Logan 2011; Michie et al.2011) and substrate oxidation rate (Larrosa-Guerrero et al.2010; Michie et al.2011). This negatively impacts MESs for processes such as wastewater treatment since these streams are generally at low temperature. However, an advantage of low temperature MFCs is that they typically produce higher Coulombic efficiencies (Jadhav and Ghangrekar 2009; Catal et al.2011; Michie et al.2011; Zhang et al.2014).
Table 3.
Comparison of the performance of selected bioelectrochemical systems under low-temperature conditions.
| BES | Condition | Inoculum | Electron donor | Voltage | Power density | Current density | CE | Anode potential (vs SHE) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| MEC | 0°C | Activated sludge | Acetate | – | – | – | 46% | – | Xu et al. (2014) |
| MFC | 4°C | Marine sediment | Wastewater | – | 98.1 mW m−2 | – | 25% | – | Larrosa-Guerrero et al. (2010) |
| MFC | 4°C | Wastewater | Acetate | – | 425 mW m−2 | – | 31% | – | Cheng, Xing and Logan (2011) |
| MFC | 4°C | Seafloor sediment | Seafloor sediment | 0.62 V | 34 mW m−2 | 85 mA m−2 | – | −0.223 V | Reimers et al. (2006) |
| MEC | 4°C | Operational MFC | Glucose | – | – | – | 82% | – | Lu et al. (2012) |
| MEC | 4° & 9°C | Operational MFC/wastewater plant | Acetate | – | – | – | 94% | – | Lu et al. (2011) |
| MFC | 4–14°C | Domestic wastewater | Organics | 0.44 V | 602 mW m−2 | 41 mA m−2 | 38% | – | Catal et al. (2011) |
| MFC | 5–10°C | Marine sediment | – | 0.43 V (1000 Ω) | 160 mW m−2 | – | 24% | – | Zhang et al. (2014) |
| MFC | 8–22°C | Existing MFC | Synthetic wastewater | 0.075 | 15 mW m−2 | 1.5 mA | 5% | Jadhav and Ghangrekar (2009) | |
| MFC | 10°C | Anaerobic sludge | Wastewater | – | Michie et al. (2011) | ||||
| MEC | 9°C | Domestic wastewater | Molasses | 0.60 V applied | – | 77 A m−3 | – | – | Wang et al. (2014) |
| MES | 10°C | Activated sludge | Glucose | – | – | – | – | – | Kong et al. (2014) |
| MFC | 15°C | Marine sediment | Organics | – | – | ∼30 mA m−2 | – | – | Holmes et al. (2004a) |
| MFC | 15°C | Salt marsh sediment | Organics | – | – | ∼29 mA m−2 | – | – | Holmes et al. (2004a) |
| MFC | 15°C | Marine sediment | Organics | – | – | ∼21 mA m−2 | – | – | Holmes et al. (2004a) |
| MFC | 15°C | Anaerobic sludge | Acetate | 362 mV | 591 mW m−2 | 2.8 A m−2 | Liu et al. (2012) | ||
| 791 mV (OCV) |
The microbial community enriched from anaerobic sludge on the anode of an acetate-fed MFC operated at 15°C has been analyzed. The selected strains have 16S rRNA gene sequences most similar to the psychrophiles Simplicispira psychrophila LMG 5408 and G. psychrophilus P35 (Liu et al.2012). A second study of a 5°C–10°C MFC enriched for a community most similar to low-temperature microorganisms from the genera Arcobacter, Pseudomonas and Geobacter (Zhang et al.2014).
One promising application of low-temperature MFCs, especially for low power consuming applications like sensors that are intended to last for a long period of time, is energy production from marine sediments that function by placing the anode in the anaerobic sediment and the cathode in the aerobic seawater above (Bond et al.2002). This technology represents an extremely large energy reserve, although long-term power generation can be limited by the flow of substrates to the anode (Reimers et al.2006). Quantitative PCR of the 16S rRNA gene suggests marine, salt marsh and freshwater sediment energy-harvesting anode populations (incubated in the laboratory at 15°C) are 53–70% enriched in δ-Proteobacteria (Holmes et al.2004a). Of the identified δ-Proteobacteria, ∼65 to 75% of the 16S rRNA gene sequences are further classified as aligning with the Geobacteraceae family (Holmes et al.2004a). A psychrotolerant (grows between 4°C and 30°C and optimum at 22°C) microbial isolate grown from a marine sediment anode represented a novel lineage in the Geobacteraceae and was named Geopsychrobacter electrodiphilus (Holmes et al.2004b). Geopsychrobacter electrodiphilus grows utilizing the anode as sole electron acceptor and has a high Coulombic efficiency of approximately 90% while donating 90% of the available electrons from its substrate oxidation to the anode (Holmes et al.2004b). An alternative electron donor for marine sediment MFCs is sulfide and methane-rich fluids from cold seeps (Reimers et al.2006). The anode biofilm community in this environment altered according to the sediment depth that mimicked the geochemical environment. The shallow community (20–29 cm below the sediment surface) is dominated by strains similar to D. acetoxidans that changed with increasing depth (46–76 cm below the sediment) to comprise a more complex community of δ-Proteobacteria (including from the genus Desulfocapsa and Syntrophus) along with ε-Proteobacteria (Reimers et al.2006). However, a drawback of this system is decreasing current over time that was partially attributed to sulfide oxidation products (i.e. non-conductive solid sulfur) passivating the anode surface.
Another example of low-temperature MESs is hydrogen production in an MEC with glucose (Lu et al.2012), acetate (Lu et al.2011), waste-activated sludge and acetate (Xu et al.2014), or molasses wastewater (Wang et al.2014) as the electron donors. The amount of hydrogen produced from glucose at 4°C is similar to mesophilic MECs and has a microbial community carrying out negligible competing methanogenic and homoacetogic reactions (Lu et al.2012). This is because microorganisms mediating these pathways are generally inactive at low temperature. The dominating genus (37% of the total 16S rRNA gene reads) in the anode community at 4°C is Dysgonomonas that ferments glucose to acid (but not hydrogen gas) that exists syntrophically with other community members including known electricigens (Lu et al.2012). A second low-temperature study with acetate as the electron donor also has decreased methanogenesis as methanogenic archaea are suppressed at 4°C and 9°C while growth of G. psychrophilus was promoted (Lu et al.2011). Trehalose is a compatible solute that, among other functions, protects microorganisms against low temperatures (Kawahara 2008). Addition of trehalose to a waste-activated sludge and acetate-fed hydrogen-producing MEC operating at 0°C increases the energy recovery and Coulombic efficiencies (Xu et al.2014). Finally, biodegradation of the antibiotic chloramphenicol in a biocathode MES has been demonstrated at 10°C (Kong et al.2014). The study shows antibiotic removal in an MES after a temperature switch from 25°C to 10°C, although the chloramphenicol reduction rate was decreased.
Thermophiles
An overview of MESs operated under thermophilic conditions can be found in Table 4. The benefit of operating biochemical systems at thermophilic conditions could include a higher microbial activity, better substrate solubility, high mass transfer rate and lowered risk of contamination. An example of an improved current generation at high temperature (60°C) is a marine sediment MFC that generated 209–254 mA m−2 compared to 10–22 mA m−2 at 22°C (Mathis et al.2008). However, a drawback is higher rates of evaporation from MESs designed for applications at low or mesophilic temperatures. Two answers to this problem are possible, either to run the MES in continuous mode allowing replacement of the anolyte and catholyte or to utilize an MES that precludes evaporation, as designed by Carver, Vuoriranta and Tuovinen (2011).
Table 4.
Comparison of the performance of selected bioelectrochemical systems under high-temperature conditions.
| BES | Condition | Inoculum | Electron donor | Voltage | Power density | Current density | CE | Anode potential (vs SHE) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| MFC | 60°C | Marine marsh sediment | Acetate | – | 43 mW m−2 | 254 mA m−2 | 36% | – | Mathis et al. (2008) |
| MEC | 60°C | Thermincola ferriacetica | Acetate | – | – | 7.0–8.0 A m−2 | 93% | – | Parameswaran et al. (2013) |
| MFC | 60°C | Thermincola potens | Acetate | – | – | – | – | – | Wrighton et al. (2011) |
| MFC | 60°C | Thermincola ferriacetica | Acetate | – | 146 mW m−2 | 400 mA m−2 | 97% | – | Marshall and May (2009) |
| MFC | 57°C | Thermophilic compost | Glucose | 0.39 V (100 Ω) | 375 mW m−2 | – | – | – | Carver, Vuoriranta and Tuovinen (2011) |
| MFC | 55°C | Anaerobic digester | Acetate | 0.64 V | 436 mW m−2 | 8.3 mA | – | – | Fu et al. (2013b) |
| MFC | 55°C | Anaerobic digester | Acetate | – | 823 mW m−2 | 3.5 mA | – | – | Fu et al. (2013a) |
| MFC | 55°C | Calditerrivibrio nitroreducens | Acetate | – | 272 mW m−2 | 2.5 mA | – | – | Fu et al. (2013a) |
| MFC | 55°C | Anaerobic digester | Acetate | – | 1030 mW m−2 | – | 80% | – | Jong et al. (2006) |
| MFC | 55°C | Methanogenic digester | Acetate | – | 37 mW m−2 | – | 89% | – | Wrighton et al. (2008) |
| MFC | 55°C | Anaerobic digester | Waste water | – | 1000 W m−2 | 2300 mA m−2 | 89% | – | Ha et al. (2012) |
| MEC | 55°C | Thermophilic MFC | Acetate | – | – | 1280 mA m−2 | – | – | Fu et al. (2013c) |
| MFC | 50°C | Bacillus licheniformis/B. thermoglucosidansius | Glucose/lactose | 0.70 V (OCV) | – | – | 41% | – | Choi et al. (2004) |
| MFC | 50°C | Enrichment culture | Synthetic syngas | 0.68 V (OCV) | 34 mW L−1 | 200 mA L−1 | 34% | – | Hussain et al. (2012) |
An acetate-fed MFC inoculated with a mixed culture from a methanogenic anaerobic digester (55°C) was stable for 100 days with a Coulombic efficiency of 89% (Wrighton et al.2008). 16S rRNA clone library characterization of the mixed community showed 80% of the population responsible for electricity production to be from the Firmicutes. Isolation of pure cultures from the MFC identified Thermincola strain JR, which is the first reported strain from this genus to directly transfer electrons to the anode (Wrighton et al.2008). A pure culture of the ferric iron-reducing Gram-positive thermophile, Thermincola ferriacetica, is also able to donate electrons from acetate oxidation to the anode at 60°C and maintain a stable current (400 mA m−2) for over 3 months with 97% Coulombic efficiency (Marshall and May 2009). Cyclic voltammetry suggests electron transport from acetate to the anode and cell-free medium scans did not have any significant peaks. This suggests that direct electron transfer occurs from the T. ferriacetica biofilm to the anode without soluble mediators. Thermincolaferriacetica biofilms grow to a thickness of ∼38 μm and contain extracellular appendages similar to Geobacter sp., further supporting that electron transfer is via a solid conductive matrix (Parameswaran et al.2013). A second species from the Thermincola genus, T. potens was isolated from the anode surface of a MFC at 60°C (Wrighton et al.2011). Characterization of the T. potens anode biofilm supports direct transfer of electrons from acetate to the anode mediated by several multiheme c-type cytochromes (Carlson et al.2012), and a total of 32 multiheme cytochromes suggested to be located in the periplasm or on the cell surface are present on the T. potens genome (Byrne-Bailey et al.2010). Further MFCs inoculated with pure cultures of thermophilic electricigens at 50°C include B. thermoglucosidasius and B. licheniformis fed with various carbon sources including glucose and lactose, respectively (Choi et al.2004).
Several further studies utilize high-temperature anaerobic sludge as sources of thermophilic inocula in MFCs (Jong et al.2006; Ha et al.2012; Hussain et al.2012; Fu et al.2013a,b). The anode community of two separate studies of acetate-fed MFCs operated at 55°C is dominated by strains related to the Gram-negative nitrate-reducing species Calditerrivibrio nitroreducens (Jong et al.2006; Fu et al.2013a). A pure culture of C. nitroreducens generated a maximum power density of 272 mW m−2 in the absence of mediators (Fu et al.2013a). The C. nitroreducens genome encodes 12 hypothetical proteins with c-type cytochrome domain(s) (Pitluck, Sikorski and Zeytun 2011). Of these, the gene ORF Calni_1470 is predicted to encode a multiheme c-type cytochrome that is a candidate to mediate electron transfer to the anode as it is similar to the T. potens cytochrome that mediates extracellular electron transfer. An acetate-fed MFC operated at 55°C selected for a mixed population of species from the Firmicutes, Synergistetes, Coprothermobacteria and Chloroflexi with the Firmicutes making up 87% of the clone library sequences (Fu et al.2013b). The dominant-identified phylotype is similar to T. ferriacetica, while the second most abundant phylotype is similar to the genus Caloramator. A pure culture of Caloramator australicus reached a maximum current of 0.16 mA that is slightly lower than that attained with the mixed culture MFC (Fu et al.2013b).
Potential applications of high-temperature MFCs include the treatment of alcohol distillery wastewater (Ha et al.2012). Distillery wastewater usually contains high levels of organic carbon and sulfate, with temperatures typically ranging from 70°C to 80°C. In one study, the MFC system is able to achieve higher power output and Coulombic efficiency than previous wastewater MFCs at different temperatures while simultaneously reducing sulfate (Ha et al.2012). 16S rRNA sequencing identified the anode mixed culture to be dominated by Bacteroidetes with a single operational taxonomical unit constituting almost 40% of the reads (Ha et al.2012). Finally, an MEC for H2 production at 55°C had a rate of 377 ± 73 mmol day−1 m−2 and a cathodic recovery of approximately 70% (Fu et al.2013c). 16S rRNA gene analysis of the biocathode population identified 21 phylotypes of which Firmicutes (77%) followed by Coprothermobacter (20%) dominate the community.
Halophiles
Reports of halophilic microorganisms in MESs are summarized in Table 5, although marine sediment MFCs have not been included in this section and those operating at low- and high temperatures are described in the psychrophile and thermophile sections, respectively. A high salt concentration generally has a positive effect on the performance of MESs. First of all high salt concentrations decrease the internal resistance of the system as the resistance of the electrolyte is the reciprocal of the electrolyte conductivity. Second, when salt is added in the form of buffering compounds, the speed at which for example protons are removed from the biofilm increases and thereby, the proton diffusion resistance decreases. However, addition of salt and or buffering compounds is an unsustainable and costly method to increase the performance of MESs. In contrast, solutions with naturally occurring elevated salt concentrations could be of great use for the practical application of these types of systems. When reporting on the performance of halophiles in these systems under high ionic strength, care should be taken that the performance increase is indeed due to the microbial activity and not due to a reduced internal resistance.
Table 5.
Comparison of the performance of selected bioelectrochemical systems under high salt conditions.
| BES | Condition | Inoculum | Electron donor | Voltage | Power density | Current density | CE | Anode potential (vs SHE) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| MEC | 17 g L−1 NaCl | Geoalkalibacter subterraneus | Acetate/yeast extract | – | – | 3.3 Am−2 | 84–95% | – | Badalamenti, Krajmalnik-Brown and Torres (2013) |
| MFC | 151 g L−1 NaCl | Saline sediments | Lactate/acetate/H2 | – | 18 mW m−2 | – | – | – | Miller and Oremland (2008) |
| MFC | 158 g L−1 NaCl | Haloferax volcanii | Yeast extract | 0.57 V | 510 mW m−2 | 560 mA m−2 | – | – | Abrevaya et al. (2011) |
| MFC | 91 g L−1 NaC | Costal sediment | Lactate | – | 5.2 mW m−2 | – | – | – | Huang, Sun and Zhang (2010) |
| MES | 45 g L−1 NaCl | Salt marsh | Acetate | – | – | 85 A m−2 | 24% | – | Rousseau et al. (2013) |
| MFC | 18 g L−1 NaCl | Wastewater | Acetate | 0.42 V (OCV) | 22 W m−3 | 52 mA m−2 | 52% | – | Liu et al. (2008) |
| MFC | 35 g L−1 NaCl | Geoalkalibacter subterraneus | Acetate | – | – | 4.68 A m−2 | – | +0.441 V | Carmona-Martinez et al. (2013) |
| MFC | 35 g L−1 NaCl | Salt lake/salt factory | Butyrate/acetate | – | – | 8.5 A m−2 | 70% | −0.047 V (controlled) | Pierra et al. (2015) |
| MFC | 40 g L−1 NaCl | Domestic wastewater | Acetate | 0.42 (OCV) | 18 W m−3 | – | 22% | – | Lefebvre et al. (2012) |
| MEC | 20 g L−1 NaCl | Saline microbial mats | Acetate | – | – | 4.45 A m−2 | – | −0.103 V | Miceli et al. (2012) |
| MEC | 25 g L−1 NaCl | Existing MFC | Acetate | 1.2 V (applied) | – | 7.8 mA m−2 | 100% | −0.197 V | Kim and Logan (2013) |
Raising the ionic strength (as well as decreasing the distance between the electrodes) in an acetate-fed, wastewater-inoculated single chamber MFC lowers the internal resistance and increases power generation and output (Liu et al.2008). In a second study, electricity generation by S. marisflavi also increases due to lessened internal resistance in anolytes containing up to 1.5 M ionic strength (Huang, Sun and Zhang 2010), although the S. marisflavi type species is only a slight halophile (Yoon et al.2004). Finally, adding 20 g L−1 (342 mM) NaCl increases the power production by 30% in an acetate-fed MFC inoculated with domestic wastewater (Lefebvre et al.2012). These data demonstrate that halophiles are able to produce electrons, but the increase in performance is due to a reduction in internal resistance.
Other pure cultures of halophiles have been tested for electricity generation. These include testing the performance of halophilic Haloferax volcanii (anode ionic strength 2.68 M, pH 5.9) compared to the haloalkaliphilic Natrialba magadii (anode ionic strength 3.63 M, pH 10; discussed in the multiple extremes section) and the neutrophilic Escherichia coli (ionic strength 0.09 M, pH 7) in dual chamber MFCs (Abrevaya et al.2011). Of the three, H. volcanii has the highest maximum power and current (Abrevaya et al.2011). A second pure culture halophile tested in MFCs is Ge. subterraneus (Badalamenti, Krajmalnik-Brown and Torres 2013; Carmona-Martinez et al.2013). Geoalkalibacter subterraneus was selected from an MES inoculated with sediments from a salt plant and forms a 76 ± 7 μm thick biofilm on the anode where it is suggested to directly transfer electrons (Carmona-Martinez et al.2013). This pure culture produced a high current density of 4.7 A m−2 at a poised anode potential of +200 mV vs SCE. Finally, the arsenate-respiring halophiles B. selenitireducens and candidatus Halarsenatibacter silvermanii strain SLAS-1 isolated from moderately hypersaline Mono Lake and extremely hypersaline (salt saturated) Searles Lake, respectively, generated similar power levels (Miller and Oremland 2008). The cultures oxidize lactate and donate electrons to the anode in the absence of added mediators (although it was not discerned if the microbes excreted endogenous mediators). The Coulombic efficiencies of the MFCs are 0.3% and 6.7%, respectively suggesting alternative electron acceptors are used or a large part of the substrate is consumed for other processes (Miller and Oremland 2008).
Mixed cultures of anode-respiring microorganisms have been selected in MFCs with inocula from various sources: a saline microbial mat from Cabo Rojo, PR, USA (Miceli et al.2012); a Mediterranean Sea (Gruissan, France) salt marsh (Rousseau et al.2013); and a salt lake or salt factory wastewater lagoon (Pierra et al.2015). The anode community selected in the Cabo Rojo saline microbial mat enrichment contains a diverse community with approximately one third of each of Bacteroidia and Clostridia (Miceli et al.2012); the microbial community from the salt march was dominated by Desulfuromonas and Marinobacter spp. (Rousseau et al.2014); and the salt lake or salt factory wastewater population was dominated by one or both of Ge. subterraneus and D. acetoxidans (Pierra et al.2015).
An application of MESs is for the desalination of saline waters that are produced by many industries (Lefebvre and Moletta 2006) and these MESs can remove up to 95% of salt from sea water (e.g. Cao et al.2009; Luo et al.2012). A three-chamber microbial saline-wastewater electrolysis cell was designed that metabolized organic carbon in the anode compartment in the presence of 0.34 M NaCl (20 g L−1) while simultaneously desalinating wastewaters (Kim and Logan 2013). The majority of the substrate was removed in the anode compartment with a Coulombic efficiency of approximately 100%.
Oligotrophs
Traditionally MFCs were looked at as wastewater treatment devices that have the ability to recover energy. Therefore, microorganisms are expected to have high metabolic rates to achieve high power and current. In contrast, MFCs have been utilized with oligotrophic organisms (Table 6) for low biochemical oxygen demand sensors that are more efficient, stable and accurate compared to conventional biochemical oxygen demand monitoring techniques (reviewed in Abrevaya et al.2015). An MFC-based biosensor for wastewater BOD measurement was stable for over five years using an enrichment of microorganisms from a starch processing plant (Kim et al.2003). Oligotrophic extremophiles have been enriched from surface waters containing 6 mg L−1 BOD and artificial wastewater containing glucose and glutamate and used for an MFC-based sensor with good stability (Kang et al.2003). Based upon16S rRNA gene sequences, the microbial communities in the above biosensors contain distinct populations with 46% of the river water enriched sequences aligning with β-Proteobacteria while the artificial wastewater enriched for 64% α-Proteobacteria (Phung et al.2004). Finally, MFC biosensors with oligotrophic microorganisms have been further developed for continuous online monitoring of BOD (Moon et al.2005).
Table 6.
Comparison of the performance of selected bioelectrochemical systems under low substrate conditions.
| BES | Condition | Inoculum | Electron donor | Voltage | Power | Current | CE | Anode potential (vs SHE) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| density | density | ||||||||
| MFC | – | River sediment | Organics | – | – | – | – | – | Moon et al. (2005) |
| MFC | – | River sediment | Glucose/glutamate | – | – | – | 20% | – | Kang et al. (2003) |
| MFC/BOD sensor | – | Starch wastewater | Starch wastewater | – | – | – | – | – | Kim et al. (2003) |
Multiple extremes
Microorganisms with multiple extremophilic characteristics utilized in MESs are haloalkaliphiles that grow in high salt and basic pH (Table 7). A pure culture of haloalkaliphilic N. magadii (ionic strength 3.63 M, pH 10) generated an electrical current in the absence of mediators (Abrevaya et al.2011). A second study inoculated with a mixed culture from Soap Lake, Washington, enriched for a culture growing optimally at 7% salinity (1.2 M NaCl) and pH 11 that generated a current density of 12.5 mA m−2 (Paul et al.2014). 16S rRNA gene sequencing showed that the MFC was enriched with a single strain most similar (99%) to Halanaerobium hydrogeniformans. Finally, soil microorganisms from the high pH and saline Texaco Lake, Mexico, were enriched on a bioanode at pH 10.5–11 and 1.2 M NaCl (Sathish-Kumar et al.2012, 2013). Low currents have been detected, and cyclic voltammetry suggests that electron transfer is mediated by alkaliphilic cytochromes.
Table 7.
Comparison of the performance of selected bioelectrochemical systems under multiple extreme conditions.
| BES | Condition | Inoculum | Electron donor | Voltage | Power | Current | CE | Anode potential (vs SHE) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| density | density | ||||||||
| MFC | 210 g L−1 NaCl/pH 10 | Natrialba magadii | Yeast extract | 0.58 V | 54 mW m−2 | 560 mA m−2 | – | – | Abrevaya et al. (2011) |
| MFC | 75 g L−1 NaCl/pH 11 | Soap lake sediment | Yeast extract | 0.8 V (OCV) | 1.66 mW m−2 | 12.5 mA m−2 | – | – | Paul et al. (2014) |
| MFC | 60–80 g L−1 NaCl/pH 9 | Texaco lake | Acetate | – | 49 mW m−2 | – | – | 0.094 V | Sathish-Kumar et al. (2013) |
| MFC | 70 g L−1 NaCl/pH 11−1 | Texaco lake | Acetate | 0.294 V | 41 mW m−2 | 138 mA m−2 | – | −0.091 V | Sathish-Kumar et al. (2012) |
| 0.6 V (OCV) |
FUTURE OPPORTUNITIES FOR EXTREMOPHILES IN MESs
The application of extremophiles in MESs has only just begun and oxidation of particular substrates in combination with specific inocula may reveal new applications for the recovery of energy or the remediation of polluted water streams. Bioremediation of wastewaters that represent an extreme environment offers a major opportunity for further application of these microorganisms in MESs. In this case, the energy costs of the remediation of these polluted streams might be compensated by the energy production of the MES. A key research need is the discovery of extremophile electricigens to be able to treat the desired wastewaters and this will require sampling of diverse extreme environments likely to contain suitable microorganisms (e.g. those described in Fig. 3). However, further unanswered questions remain including which pollutants can be used as electrons donors are electron acceptors available to give sufficient energy gain when coupled to these donors to fulfill the energy need, and will both donor and acceptor be available at the same site.
To date, MESs for sulfate removal from acidic wastewaters have utilized inocula from neutral pH environments (Li et al.2013; Kim et al.2014). These studies identified sulfate-reducing species typically growing at pH 7 and it would be interesting to test truly acidophilic sulfate reducers such as Desulfosporosinus acidiphilus (Jameson et al.2010) in these systems. In addition, inocula from other anaerobic low pH environments could be tested in MESs to select for potential electricigens. For instance, acid mine drainage sludge possibly contains electricigens able to treat low pH, high metal concentration wastewaters. An example of this research is the European FP7 project ‘BioelectroMET’ where the oxidation of tetrathionate is coupled to the reduction of ferric iron (Sulonen et al.2015). High pH wastewaters may also be treated with MESs that has advantages for electricity production (described above). However, there are only a few reports of the use of alkaliphiles in MESs and very few pure and mixed culture inocula have been tested. The possibilities for discovering new electricigens are good and their enrichment from high pH environments such as soda lake sediments and slag dumps may prove successful. Examples of wastewaters that could be treated with alkaliphilic electricigens are from the textile (Choe et al.2005) and brewing industries (Rao et al.2007).
Both psychrophiles and thermophiles can be applied to treat waste streams at different temperatures providing an economic advantage as the waters would not need to be heated or cooled, respectively. However, little is known about the actual performance of these systems in real-life conditions, and systematic studies are required to see how MESs perform over a longer period of time. The start-up temperature of low-temperature MESs employing psychrotolerant microbes has been suggested to be important (Cheng, Xing and Logan 2011), and further characterization of this parameter would also be warranted. Benthic low-temperature MESs have successfully been used to oxidize sulfide but the resultant deposition of sulfide on the anode surface causes deactivation (Reimers et al.2006). Future research strategies to attempt to solve this issue include stripping the passivating layer on the anode surface and cycling periods of current harvesting and no harvesting (Reimers et al.2006). For the thermophiles, one study has addressed the issue of evaporation (Carver, Vuoriranta and Tuovinen 2011) but further development will likely result in more efficient reactor designs.
Using halophiles for the desalination of water (e.g. Cao et al. (2009)) has to date only been carried out in batch systems. Further development is needed as commercial use would require a continuous system. When it comes to the use of oligotrophs in biosensors, it is important to increase the signal-to-noise ratio, reproducibility, sensitivity, ability of the microbial consortia to degrade a wide range of substrates and to investigate the many novel species that were enriched (Abrevaya et al.2015).
The use of MESs also offers possibilities to treat streams with different extreme conditions, as the anode and cathode are generally separated by an ion exchange membrane. This is best illustrated by the pH gradient that in most cases develops over the membrane and is considered as one of the major disadvantages of MESs (Rozendal et al.2008a,b). The pH gradient develops since the oxidation (proton producing) and reduction (proton consuming) reactions are separated and do not take place in a single compartment (as in a bacterial cell). This charge difference is not compensated as the charge transport in the system is mainly by cations other than protons causing the pH to decrease in the anode and to increase in the cathode. The electrical potential loss associated with this pH gradient is 60 mV per pH unit and severely reduces the power output of MFCs or increases the required power input in MECs (Sleutels et al.2012, 2013). When a pH 3 stream is fed to the anode, the chance of the charge transport through the membrane to be in the form of protons is 10 000 times higher than when a stream of pH 7 is used (Rozendal, Hamelers and Buisman 2006). Another potential advantage of extremophiles in MESs is to use a low pH catholyte in combination with an alkaline anolyte to create a negative pH gradient which may help the energetics of the system. Of course the pH gradient in these types of systems remains a serious topic of research. The use of a bipolar membrane, which in theory could sustain the pH in both compartments, has not shown its full potential due to the high-energy demand of water splitting (Ter Heijne et al.2006; Harnisch and Schröder 2009). Further research on these types of membranes and especially how to make them more selective in the electrolyte conditions used in MESs is required to prove their purpose in MESs.
FUNDING
This work was supported by the TTIW-cooperation framework of Wetsus, European Centre of Excellence for Sustainable Water Technology (www.wetsus.nl). Wetsus is funded by the Dutch Ministry of Economic Affairs, the European Union Regional Development Fund, the Province of Fryslân, the City of Leeuwarden and the EZ/Kompas program of the ‘Samenwerkingsverband Noord-Nederland’. The authors also wish to acknowledge funding from the European Union Seventh Framework Programme (FP7/2012-2016) project ‘Bioelectrochemical systems for metal production, recycling, and remediation’ under grant agreement n° 282970.
Conflict of interest. None declared.
REFERENCES
- Abrevaya XC, Sacco N, Mauas PJD, et al. Archaea-based microbial fuel cell operating at high ionic strength conditions. Extremophiles. 2011;15:633–42. doi: 10.1007/s00792-011-0394-z. [DOI] [PubMed] [Google Scholar]
- Abrevaya XC, Sacco NJ, Bonetto MC, et al. Analytical applications of microbial fuel cells. Part I: biochemical oxygen demand. Biosens Bioelectron. 2015;63:580–90. doi: 10.1016/j.bios.2014.04.034. [DOI] [PubMed] [Google Scholar]
- Akiba T, Bennetto HP, Stirling JL, et al. Electricity production from alkalophilic organisms. Biotechnol Lett. 1987;9:611–6. [Google Scholar]
- Antony CP, Kumaresan D, Hunger S, et al. Microbiology of Lonar Lake and other soda lakes. ISME J. 2013;7:468–76. doi: 10.1038/ismej.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badalamenti JP, Krajmalnik-Brown R, Torres CI. Generation of high current densities by pure cultures of anode-respiring Geoalkalibacter spp. under alkaline and saline conditions in microbial electrochemical cells. mBio. 2013;4:1–8. doi: 10.1128/mBio.00144-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Austin C, Potrykus J, Wexler M, et al. Biofilm development in the extremely acidophilic archaeon ‘Ferroplasma acidarmanus’ Fer1. Extremophiles. 2010;14:485–91. doi: 10.1007/s00792-010-0328-1. [DOI] [PubMed] [Google Scholar]
- Blanchet E, Pécastaings S, Erable B, et al. Protons accumulation during anodic phase turned to advantage for oxygen reduction during cathodic phase in reversible bioelectrodes. Bioresource Technol. 2014;173:224–30. doi: 10.1016/j.biortech.2014.09.076. [DOI] [PubMed] [Google Scholar]
- Bond DR, Holmes DE, Tender LM, et al. Electrode-reducing microorganisms that harvest energy from marine sediments. Science. 2002;295:483–5. doi: 10.1126/science.1066771. [DOI] [PubMed] [Google Scholar]
- Bonnefoy V, Holmes DS. Genomic insights into microbial oxidation and iron homeostasis in extremely acidic environments. Environ Microbiol. 2012;14:1597–611. doi: 10.1111/j.1462-2920.2011.02626.x. [DOI] [PubMed] [Google Scholar]
- Borole AP, O'Neill H, Tsouris C, et al. A microbial fuel cell operating at low pH using the acidophile Acidiphilium cryptum. Biotechnol Lett. 2008;20:1367–72. doi: 10.1007/s10529-008-9700-y. [DOI] [PubMed] [Google Scholar]
- Bott TL, Brock TD. Bacterial growth rates above 90°C in Yellowstone hot springs. Science. 1969;164:1411–2. doi: 10.1126/science.164.3886.1411. [DOI] [PubMed] [Google Scholar]
- Brock TD. Life at high temperatures. Science. 1967;158:1012–9. doi: 10.1126/science.158.3804.1012. [DOI] [PubMed] [Google Scholar]
- Byrne-Bailey KG, Wrighton KC, Melnyk RA, et al. Complete genome sequence of the electricity-producing ‘Thermincola potens’ strain JR. J Bacteriol. 2010;192:4078–9. doi: 10.1128/JB.00044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccavo F, Lonergan DJ, Lovley DR, et al. Geobacter sulfurreducens sp. nov., a hydrogen-oxidizing and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microb. 1994;60:3752–9. doi: 10.1128/aem.60.10.3752-3759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Huang X, Liang P, et al. A new method for water desalination using microbial desalination cells. Environ Sci Technol. 2009;43:7148–52. doi: 10.1021/es901950j. [DOI] [PubMed] [Google Scholar]
- Carbajosa S, Malki M, Caillard R, et al. Electrochemical growth of Acidithiobacillus ferrooxidans on a graphite electrode for obtaining a biocathode for direct electrocatalytic reduction of oxygen. Biosens Bioelectron. 2010;26:877–80. doi: 10.1016/j.bios.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Carlson HK, Iavarone AT, Gorur A, et al. Surface multiheme c-type cytochromes from Thermincola potens and implications for respiratory metal reduction by Gram-positive bacteria. P Natl Acad Sci USA. 2012;109:1702–7. doi: 10.1073/pnas.1112905109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Martinez AA, Pierra M, Trably E, et al. High current density via direct electron transfer by the halophilic anode respiring bacterium Geoalkalibacter subterraneus. Phys Chem Chem Phys. 2013;15:19699–707. doi: 10.1039/c3cp54045f. [DOI] [PubMed] [Google Scholar]
- Carver SM, Vuoriranta P, Tuovinen OH. A thermophilic microbial fuel cell design. J Power Sources. 2011;196:3757–60. [Google Scholar]
- Catal T, Bermek H, Liu H. Removal of selenite from wastewater using microbial fuel cells. Biotechnol Lett. 2009;31:1211–6. doi: 10.1007/s10529-009-9990-8. [DOI] [PubMed] [Google Scholar]
- Catal T, Kavanagh P, O'Flaherty V, et al. Generation of electricity in microbial fuel cells at sub-ambient temperatures. J Power Sources. 2011;196:2676–81. [Google Scholar]
- Cavicchioli R, Thomas T. Extremophiles. In: Lederberg J, editor. Encyclopedia of Microbiology. Vol. 2. San Diego: Academic Press; 2000. pp. 317–37. [Google Scholar]
- Cheng S, Xing D, Call DF, et al. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ Sci Technol. 2009;43:3953–8. doi: 10.1021/es803531g. [DOI] [PubMed] [Google Scholar]
- Cheng SA, Xing DF, Logan BE. Electricity generation of single-chamber microbial fuel cells at low temperatures. Biosens Bioelectron. 2011;26:1913–7. doi: 10.1016/j.bios.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Choe EK, Son EJ, Lee BS, et al. NF process for the recovery of caustic soda and concentration of disodium terephthalate from alkaline wastewater from polyester fabrics. Desalination. 2005;186:29–37. [Google Scholar]
- Choi Y, Jung E, Park H, et al. Construction of microbial fuel cells using thermophilic microorganisms, Bacillus licheniformis and Bacillus thermoglucosidasius. B Korean Chem Soc. 2004;25:813–8. [Google Scholar]
- Clauwaert P, Rabaey K, Aelterman P, et al. Biological denitrification in microbial fuel cells. Environ Sci Technol. 2007;41:3354–60. doi: 10.1021/es062580r. [DOI] [PubMed] [Google Scholar]
- Cowan DA, Makhalanyane TP, Dennis PG, et al. Microbial ecology and biogeochemistry of continental Antarctic soils. Front Microbiol. 2014;5:154. doi: 10.3389/fmicb.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maayer P, Anderson D, Cary C, et al. Some like it cold: understanding the survival strategies of psychrophiles. EMBO Rep. 2014;15:508–17. doi: 10.1002/embr.201338170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GJ, Anantharaman K, Baker BJ, et al. The microbiology of deep-sea hydrothermal vent plumes: ecological and biogeographic linkages to seafloor and water column habitats. Front Microbiol. 2013;4:124. doi: 10.3389/fmicb.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopson M. Physiological adaptations and biotechnological applications of acidophiles. In: Anitori R, editor. Extremophiles: Microbiology and Biotechnology. Norwich: Horizon Scientific Press; 2012. pp. 265–94. [Google Scholar]
- Dopson M, Holmes DS. Metal resistance in acidophilic microorganisms and its significance for biotechnologies. Appl Microbiol Biot. 2014;98:8133–44. doi: 10.1007/s00253-014-5982-2. [DOI] [PubMed] [Google Scholar]
- Dopson M, Johnson DB. Biodiversity, metabolism and applications of acidophilic sulfur- metabolizing micro-organisms. Environ Microbiol. 2012;14:2620–31. doi: 10.1111/j.1462-2920.2012.02749.x. [DOI] [PubMed] [Google Scholar]
- Dopson M, Ossandon F, Lövgren L, et al. Metal resistance or tolerance? Acidophiles confront high metal loads via both abiotic and biotic mechanisms. Front Microbiol. 2014;5:157. doi: 10.3389/fmicb.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erable B, Etcheverry L, Bergel A. Increased power from a two-chamber microbial fuel cell with a low-pH air-cathode compartment. Electrochem Comm. 2009;11:619–22. [Google Scholar]
- Fan YZ, Hu HQ, Liu H. Sustainable power generation in microbial fuel cells using bicarbonate buffer and proton transfer mechanisms. Environ Sci Technol. 2007;41:8154–8. doi: 10.1021/es071739c. [DOI] [PubMed] [Google Scholar]
- Fu Q, Kobayashi H, Kawaguchi H, et al. A thermophilic Gram-negative nitrate-reducing bacterium, Calditerrivibrio nitroreducens, exhibiting electricity generation capability. Environ Sci Technol. 2013a;47:12583–90. doi: 10.1021/es402749f. [DOI] [PubMed] [Google Scholar]
- Fu Q, Kobayashi H, Kawaguchi H, et al. Electrochemical and phylogenetic analyses of current-generating microorganisms in a thermophilic microbial fuel cell. J Biosci Bioengin. 2013b;115:268–71. doi: 10.1016/j.jbiosc.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Fu Q, Kobayashi H, Kuramochi Y, et al. Bioelectrochemical analyses of a thermophilic biocathode catalyzing sustainable hydrogen production. Int J Hydrogen Energ. 2013c;38:15638–45. [Google Scholar]
- Garcia-Munoz J, Amils R, Fernandez VM, et al. Electricity generation by microorganisms in the sediment-water interface of an extreme acidic microcosm. Int Microbiol. 2011;14:73–81. doi: 10.2436/20.1501.01.137. [DOI] [PubMed] [Google Scholar]
- Gutierrez O, Park D, Sharma KR, et al. Effects of long-term pH elevation on the sulfate-reducing and methanogenic activities of anaerobic sewer biofilms. Water Res. 2009;43:2549–57. doi: 10.1016/j.watres.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Ha PT, Lee TK, Rittmann BE, et al. Treatment of alcohol distillery wastewater using a Bacteroidetes-dominant thermophilic microbial fuel cell. Environ Sci Technol. 2012;46:3022–30. doi: 10.1021/es203861v. [DOI] [PubMed] [Google Scholar]
- Hamelers HVM, ter Heijne A, Stein N, et al. Butler-Volmer-Monod model for describing bio-anode polarization curves. Bioresour Technol. 2011;102:381–7. doi: 10.1016/j.biortech.2010.06.156. [DOI] [PubMed] [Google Scholar]
- Harnisch F, Schröder U. Selectivity versus mobility: separation of anode and cathode in microbial bioelectrochemical systems. ChemSusChem. 2009;2:921–6. doi: 10.1002/cssc.200900111. [DOI] [PubMed] [Google Scholar]
- Harnisch F, Schröder U, Scholz F. The suitability of monopolar and bipolar ion exchange membranes as separators for biological fuel cells. Environ Sci Technol. 2008;42:1740–6. doi: 10.1021/es702224a. [DOI] [PubMed] [Google Scholar]
- Hicks DB, Liu J, Fujisawa M, et al. F1F0-ATP synthases of alkaliphilic bacteria: lessons from their adaptations. BBA - Bioenergetics. 2010;1797:1362–77. doi: 10.1016/j.bbabio.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehler TM, Jorgensen BB. Microbial life under extreme energy limitation. Nat Rev Microbiol. 2013;11:83–94. doi: 10.1038/nrmicro2939. [DOI] [PubMed] [Google Scholar]
- Holmes DE, Bond DR, O'Neil RA, et al. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb Ecol. 2004a;48:178–90. doi: 10.1007/s00248-003-0004-4. [DOI] [PubMed] [Google Scholar]
- Holmes DE, Nicoll JS, Bond DR, et al. Potential role of a novel psychrotolerant member of the family Geobacteraceae, Geopsychrobacter electrodiphilus gen. nov., sp. nov., in electricity production by a marine sediment fuel cell. Appl Environ Microb. 2004b;70:6023–30. doi: 10.1128/AEM.70.10.6023-6030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JX, Sun BL, Zhang XB. Electricity generation at high ionic strength in microbial fuel cell by a newly isolated Shewanella marisflavi EP1. Appl Microbiol Biot. 2010;85:1141–9. doi: 10.1007/s00253-009-2259-2. [DOI] [PubMed] [Google Scholar]
- Hussain A, Mehta P, Raghavan V, et al. The performance of a thermophilic microbial fuel cell fed with synthesis gas. Enzyme Microb Tech. 2012;51:163–70. doi: 10.1016/j.enzmictec.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Jadhav GS, Ghangrekar MM. Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresource Technol. 2009;100:717–23. doi: 10.1016/j.biortech.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Jameson E, Rowe OF, Hallberg KB, et al. Sulfidogenesis and selective precipitation of metals at low pH mediated by Acidithiobacillus spp. and acidophilic sulfate-reducing bacteria. Hydrometallurgy. 2010;104:488–93. [Google Scholar]
- Jong BC, Kim BH, Chang IS, et al. Enrichment, performance, and microbial diversity of a thermophilic mediatorless microbial fuel cell. Environ Sci Technol. 2006;40:6449–54. doi: 10.1021/es0613512. [DOI] [PubMed] [Google Scholar]
- Kang KH, Jang JK, Pham TH, et al. A microbial fuel cell with improved cathode reaction as a low biochemical oxygen demand sensor. Biotechnol Lett. 2003;25:1357–61. doi: 10.1023/a:1024984521699. [DOI] [PubMed] [Google Scholar]
- Kawahara H. Cryoprotectants and ice-binding proteins. In: Margesin R, Schinner F, Marx JC, et al., editors. Psychrophiles: From Biodiversity to Biotechnology. Berlin: Springer; 2008. pp. 229–46. [Google Scholar]
- Ketep SF, Bergel A, Bertrand M, et al. Lowering the applied potential during successive scratching/re-inoculation improves the performance of microbial anodes for microbial fuel cells. Bioresource Technol. 2013;127:448–55. doi: 10.1016/j.biortech.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Kim BH, Chang IS, Gil GC, et al. Novel BOD (biological oxygen demand) sensor using mediator-less microbial fuel cell. Biotechnol Lett. 2003;25:541–5. doi: 10.1023/a:1022891231369. [DOI] [PubMed] [Google Scholar]
- Kim H, Kim B, Kim J, et al. Electricity generation and microbial community in microbial fuel cell using low-pH distillery wastewater at different external resistances. J Biotechnol. 2014;186:175–80. doi: 10.1016/j.jbiotec.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Kim JR, Dec J, Bruns MA, et al. Removal of odors from Swine wastewater by using microbial fuel cells. Appl Environ Microb. 2008;74:2540–3. doi: 10.1128/AEM.02268-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Logan BE. Simultaneous removal of organic matter and salt ions from saline wastewater in bioelectrochemical systems. Desalination. 2013;308:115–21. [Google Scholar]
- Kong D, Liang B, Lee DJ, et al. Effect of temperature switchover on the degradation of antibiotic chloramphenicol by biocathode bioelectrochemical system. J Environ Sci. 2014;26:1689–97. doi: 10.1016/j.jes.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Kuntke P, Sleutels T, Saakes M, et al. Hydrogen production and ammonium recovery from urine by a Microbial Electrolysis Cell. Int J Hydrogen Energ. 2014;39:4771–8. [Google Scholar]
- Kuntke P, Smiech KM, Bruning H, et al. Ammonium recovery and energy production from urine by a microbial fuel cell. Water Res. 2012;46:2627–36. doi: 10.1016/j.watres.2012.02.025. [DOI] [PubMed] [Google Scholar]
- Larrosa-Guerrero A, Scott K, Head IM, et al. Effect of temperature on the performance of microbial fuel cells. Fuel. 2010;89:3985–94. [Google Scholar]
- Lefebvre O, Moletta R. Treatment of organic pollution in industrial saline wastewater: a literature review. Water Res. 2006;40:3671–82. doi: 10.1016/j.watres.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Lefebvre O, Tan Z, Kharkwal S, et al. Effect of increasing anodic NaCl concentration on microbial fuel cell performance. Bioresource Technol. 2012;112:336–40. doi: 10.1016/j.biortech.2012.02.048. [DOI] [PubMed] [Google Scholar]
- Lewin A, Wentzel A, Valla S. Metagenomics of microbial life in extreme temperature environments. Curr Opin Biotechnol. 2013;24:516–25. doi: 10.1016/j.copbio.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Li XM, Cheng KY, Selvam A, et al. Bioelectricity production from acidic food waste leachate using microbial fuel cells: effect of microbial inocula. Process Biochem. 2013;48:283–8. doi: 10.1016/j.biortech.2013.09.037. [DOI] [PubMed] [Google Scholar]
- Li XM, Cheng KY, Wong JW. Bioelectricity production from food waste leachate using microbial fuel cells: effect of NaCl and pH. Bioresource Technol. 2013;149:452–8. doi: 10.1016/j.biortech.2013.09.037. [DOI] [PubMed] [Google Scholar]
- Liang F, Xiao Y, Zhao F. Effect of pH on sulfate removal from wastewater using a bioelectrochemical system. Chem Eng J. 2013;218:147–53. [Google Scholar]
- Liu H, Cheng S, Huang L, et al. Scale-up of membrane-free single-chamber microbial fuel cells. J Power Sources. 2008;179:274–9. [Google Scholar]
- Liu H, Grot S, Logan BE. Electrochemically assisted microbial production of hydrogen from acetate. Environ Sci Technol. 2005;39:4317–20. doi: 10.1021/es050244p. [DOI] [PubMed] [Google Scholar]
- Liu L, Tsyganova O, Lee D-J, et al. Anodic biofilm in single-chamber microbial fuel cells cultivated under different temperatures. Int J Hydrogen Energ. 2012;37:15792–800. [Google Scholar]
- Liu M, Yuan Y, Zhang LX, et al. Bioelectricity generation by a Gram-positive Corynebacterium sp strain MFC03 under alkaline condition in microbial fuel cells. Bioresource Technol. 2010;101:1807–11. doi: 10.1016/j.biortech.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Z, Liu J, et al. A trans-outer membrane porin-cytochrome protein complex for extracellular electron transfer by Geobacter sulfurreducens PCA. Environ Microbiol Rep. 2014;6:776–85. doi: 10.1111/1758-2229.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan BE, Call D, Cheng S, et al. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ Sci Technol. 2008;42:8630–40. doi: 10.1021/es801553z. [DOI] [PubMed] [Google Scholar]
- Logan BE, Elimelech M. Membrane-based processes for sustainable power generation using water. Nature. 2012;488:313–9. doi: 10.1038/nature11477. [DOI] [PubMed] [Google Scholar]
- Logan BE, Hamelers B, Rozendal R, et al. Microbial fuel cells: methodology and technology. Environ Sci Technol. 2006;40:5181–92. doi: 10.1021/es0605016. [DOI] [PubMed] [Google Scholar]
- Logan BE, Rabaey K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science. 2012;337:686–90. doi: 10.1126/science.1217412. [DOI] [PubMed] [Google Scholar]
- Lovley DR, Malvankar NS. Seeing is believing: novel imaging techniques help clarify microbial nanowire structure and function. Environ Microbiol. 2015;17:2209–15. doi: 10.1111/1462-2920.12708. [DOI] [PubMed] [Google Scholar]
- Lu L, Ren NQ, Zhao X, et al. Hydrogen production, methanogen inhibition and microbial community structures in psychrophilic single-chamber microbial electrolysis cells. Energy Environ Sci. 2011;4:1329–36. [Google Scholar]
- Lu L, Xing DF, Ren NQ, et al. Syntrophic interactions drive the hydrogen production from glucose at low temperature in microbial electrolysis cells. Bioresource Technol. 2012;124:68–76. doi: 10.1016/j.biortech.2012.08.040. [DOI] [PubMed] [Google Scholar]
- Luef B, Frischkorn KR, Wrighton KC, et al. Diverse uncultivated ultra-small bacterial cells in groundwater. Nat Commun. 2015;6:6372. doi: 10.1038/ncomms7372. [DOI] [PubMed] [Google Scholar]
- Luo H, Xu P, Roane TM, et al. Microbial desalination cells for improved performance in wastewater treatment, electricity production, and desalination. Bioresource Technol. 2012;105:60–6. doi: 10.1016/j.biortech.2011.11.098. [DOI] [PubMed] [Google Scholar]
- Ma C, Zhuang L, Zhou SG, et al. Alkaline extracellular reduction: isolation and characterization of an alkaliphilic and halotolerant bacterium, Bacillus pseudofirmus MC02. J Appl Microbiol. 2012;112:883–91. doi: 10.1111/j.1365-2672.2012.05276.x. [DOI] [PubMed] [Google Scholar]
- Malki M, Casado S, Lopez MF, et al. Physicochemical characterization of Acidiphilium sp. biofilms. ChemPhysChem. 2013;14:1237–44. doi: 10.1002/cphc.201201034. [DOI] [PubMed] [Google Scholar]
- Malki M, De Lacey AL, Rodriguez N, et al. Preferential use of an anode as an electron acceptor by an acidophilic bacterium in the presence of oxygen. Appl Environ Microb. 2008;74:4472–6. doi: 10.1128/AEM.00209-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvankar NS, Vargas M, Nevin K, et al. Structural basis for metallic-like conductivity in microbial nanowires. MBio. 2015;6:e00084. doi: 10.1128/mBio.00084-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CW, May HD. Electrochemical evidence of direct electrode reduction by a thermophilic Gram-positive bacterium, Thermincola ferriacetica. Energy Environ Sci. 2009;2:699–705. [Google Scholar]
- Mathis B, Marshall C, Milliken C, et al. Electricity generation by thermophilic microorganisms from marine sediment. Appl Microbiol Biot. 2008;78:147–55. doi: 10.1007/s00253-007-1266-4. [DOI] [PubMed] [Google Scholar]
- Miceli JF, Parameswaran P, Kang DW, et al. Enrichment and analysis of anode-respiring bacteria from diverse anaerobic inocula. Environ Sci Technol. 2012;46:10349–55. doi: 10.1021/es301902h. [DOI] [PubMed] [Google Scholar]
- Michie IS, Kim JR, Dinsdale RM, et al. The influence of psychrophilic and mesophilic start-up temperature on microbial fuel cell system performance. Energy Environ Sci. 2011;4:1011–9. [Google Scholar]
- Miller LG, Oremland RS. Electricity generation by anaerobic bacteria and anoxic sediments from hypersaline soda lakes. Extremophiles. 2008;12:837–48. doi: 10.1007/s00792-008-0191-5. [DOI] [PubMed] [Google Scholar]
- Modin O, Fukushi K, Rabaey K, et al. Redistribution of wastewater alkalinity with a microbial fuel cell to support nitrification of reject water. Water Res. 2011;45:2691–9. doi: 10.1016/j.watres.2011.02.031. [DOI] [PubMed] [Google Scholar]
- Moon H, Chang IS, Jang JK, et al. On-line monitoring of low biochemical oxygen demand through continuous operation of a mediator-less microbial fuel cell. J Microbiol Biotechn. 2005;15:192–6. [Google Scholar]
- Morozkina EV, Slutskaya ES, Fedorova TV, et al. Extremophilic microorganisms: biochemical adaptation and biotechnological application. Appl Biochem Micro. 2010;46:1–14. [PubMed] [Google Scholar]
- Mu Y, Rabaey K, Rozendal RA, et al. Decolorization of azo dyes in bioelectrochemical systems. Environ Sci Technol. 2009;43:5137–43. doi: 10.1021/es900057f. [DOI] [PubMed] [Google Scholar]
- Nevin KP, Hensley SA, Franks AE, et al. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl Environ Microb. 2011;77:2882–6. doi: 10.1128/AEM.02642-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A. Life at high salt concentrations, intracellular KCl concentrations, and acidic proteomes. Front Microbiol. 2013;4:315. doi: 10.3389/fmicb.2013.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio H, Mangold S, Denis Y, et al. Anaerobic sulfur metabolism coupled to dissimilatory iron reduction in the extremophile Acidithiobacillus ferrooxidans. Appl Environ Microb. 2013;79:2172–81. doi: 10.1128/AEM.03057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant D, Van Bogaert G, Diels L, et al. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresource Technol. 2010;101:1533–43. doi: 10.1016/j.biortech.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Parameswaran P, Bry T, Popat SC, et al. Kinetic, electrochemical, and microscopic characterization of the thermophilic, anode-respiring bacterium Thermincola ferriacetica. Environ Sci Technol. 2013;47:4934–40. doi: 10.1021/es400321c. [DOI] [PubMed] [Google Scholar]
- Paul VG, Minteer SD, Treu BL, et al. Ability of a haloalkaliphilic bacterium isolated from Soap Lake, Washington to generate electricity at pH 11.0 and 7% salinity. Environ Technol. 2014;35:1003–11. doi: 10.1080/09593330.2013.858186. [DOI] [PubMed] [Google Scholar]
- Phung NT, Lee J, Kang KH, et al. Analysis of microbial diversity in oligotrophic microbial fuel cells using 16S rDNA sequences. FEMS Microbiol Lett. 2004;233:77–82. doi: 10.1016/j.femsle.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Pierra M, Carmona-Martínez AA, Trably E, et al. Specific and efficient electrochemical selection of Geoalkalibacter subterraneus and Desulfuromonas acetoxidans in high current-producing biofilms. Bioelectrochemistry. 2015;106(Pt A):221–5. doi: 10.1016/j.bioelechem.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Pikuta EV, Hoover RB, Tang J. Microbial extremophiles at the limits of life. Crit Rev Microbiol. 2007;33:183–209. doi: 10.1080/10408410701451948. [DOI] [PubMed] [Google Scholar]
- Pitluck S, Sikorski J, Zeytun A, et al. Complete genome sequence of Calditerrivibrio nitroreducens type strain (Yu37-1T) Stand Genomic Sci. 2011;4:54–62. doi: 10.4056/sigs.1523807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocaznoi D, Erable B, Etcheverry L, et al. Towards an engineering-oriented strategy for building microbial anodes for microbial fuel cells. Phys Chem Chem Phys. 2012;14:13332–43. doi: 10.1039/c2cp42571h. [DOI] [PubMed] [Google Scholar]
- Puig S, Serra M, Coma M, et al. Effect of pH on nutrient dynamics and electricity production using microbial fuel cells. Bioresource Technol. 2010;101:9594–9. doi: 10.1016/j.biortech.2010.07.082. [DOI] [PubMed] [Google Scholar]
- Rabaey K, Rozendal RA. Microbial electrosynthesis - revisiting the electrical route for microbial production. Nat Rev Microbiol. 2010;8:706–16. doi: 10.1038/nrmicro2422. [DOI] [PubMed] [Google Scholar]
- Rao AG, Reddy TSK, Prakash SS, et al. pH regulation of alkaline wastewater with carbon dioxide: a case study of treatment of brewery wastewater in UASB reactor coupled with absorber. Bioresource Technol. 2007;98:2131–6. doi: 10.1016/j.biortech.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Reguera G, McCarthy KD, Mehta T, et al. Extracellular electron transfer via microbial nanowires. Nature. 2005;435:1098–101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- Reimers CE, Girguis P, Stecher HA, et al. Microbial fuel cell energy from an ocean cold seep. Geobiology. 2006;4:123–36. [Google Scholar]
- Rivkina EM, Friedmann EI, McKay CP, et al. Metabolic activity of permafrost bacteria below the freezing point. Appl Environ Microb. 2000;66:3230–3. doi: 10.1128/aem.66.8.3230-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau R, Dominguez-Benetton X, Délia M-L, et al. Microbial bioanodes with high salinity tolerance for microbial fuel cells and microbial electrolysis cells. Electrochem Comm. 2013;33:1–4. [Google Scholar]
- Rousseau R, Santaella C, Achouak W, et al. Correlation of the electrochemical kinetics of high-salinity-tolerant bioanodes with the structure and microbial composition of the biofilm. ChemElectroChem. 2014;1:1966–75. [Google Scholar]
- Rozendal RA, Hamelers HVM, Buisman CJN. Effects of membrane cation transport on pH and Microbial Fuel Cell performance. Environ Sci Technol. 2006;40:5206–11. doi: 10.1021/es060387r. [DOI] [PubMed] [Google Scholar]
- Rozendal RA, Hamelers HVM, Euverink GJW, et al. Principle and perspectives of hydrogen production through biocatalyzed electrolysis. Int J Hydrogen Energ. 2006;31:1632–40. [Google Scholar]
- Rozendal RA, Hamelers HVM, Rabaey K, et al. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008a;26:450–9. doi: 10.1016/j.tibtech.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Rozendal RA, Leone E, Keller J, et al. Efficient hydrogen peroxide generation from organic matter in a bioelectrochemical system. Electrochem Comm. 2009;11:1752–5. [Google Scholar]
- Rozendal RA, Sleutels TH, Hamelers HV, et al. Effect of the type of ion exchange membrane on performance, ion transport, and pH in biocatalyzed electrolysis of wastewater. Water Sci Technol. 2008b;57:1757–62. doi: 10.2166/wst.2008.043. [DOI] [PubMed] [Google Scholar]
- SanMartin-Uriz P, Gomez MJ, Arcas A, et al. Draft genome sequence of the electricigen Acidiphilium sp. strain PM (DSM 24941) J Bacteriol. 2011;193:5585–6. doi: 10.1128/JB.05386-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarethy IP, Saxena Y, Kapoor A, et al. Alkaliphilic bacteria: applications in industrial biotechnology. J Ind Microbiol Biot. 2011;38:769–90. doi: 10.1007/s10295-011-0968-x. [DOI] [PubMed] [Google Scholar]
- Sathish-Kumar K, Solorza-Feria O, Tapia-Ramirez J, et al. Electrochemical and chemical enrichment methods of a sodic-saline inoculum for microbial fuel cells. Int J Hydrogen Energ. 2013;38:12600–9. [Google Scholar]
- Sathish-Kumar K, Solorza-Feria O, Vazquez-Huerta G, et al. Electrical stress-directed evolution of biocatalysts community sampled from a sodic-saline soil for microbial fuel cells. J New Mat Electr Sys. 2012;15:181–6. [Google Scholar]
- Sato T, Atomi H. Novel metabolic pathways in Archaea. Curr Opin Microbiol. 2011;14:307–14. doi: 10.1016/j.mib.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Shantaram A, Beyenal H, Raajan R, et al. Wireless sensors powered by microbial fuel cells. Environ Sci Technol. 2005;39:5037–42. doi: 10.1021/es0480668. [DOI] [PubMed] [Google Scholar]
- Sleutels TH, Hamelers HV, Buisman CJ. Reduction of pH buffer requirement in bioelectrochemical systems. Environ Sci Technol. 2010;44:8259–63. doi: 10.1021/es101858f. [DOI] [PubMed] [Google Scholar]
- Sleutels THJA, Heijne AT, Buisman CJN, et al. Steady-state performance and chemical efficiency of Microbial Electrolysis Cells. Int J Hydrogen Energ. 2013;38:7201–8. [Google Scholar]
- Sleutels THJA, Ter Heijne A, Buisman CJN, et al. Bioelectrochemical systems: an outlook for practical applications. ChemSusChem. 2012;5:1012–9. doi: 10.1002/cssc.201100732. [DOI] [PubMed] [Google Scholar]
- Slonczewski JL, Fujisawa M, Dopson M, et al. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv Microb Physiol. 2009;55:1–79. doi: 10.1016/S0065-2911(09)05501-5. [DOI] [PubMed] [Google Scholar]
- Steinbusch KJJ, Hamelers HVM, Plugge CM, et al. Biological formation of caproate and caprylate from acetate: fuel and chemical production from low grade biomass. Energy Environ Sci. 2011;4:216–24. [Google Scholar]
- Strik DP, Timmers RA, Helder M, et al. Microbial solar cells: applying photosynthetic and electrochemically active organisms. Trends Biotechnol. 2011;29:41–9. doi: 10.1016/j.tibtech.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Sulonen ML, Kokko ME, Lakaniemi AM, et al. Electricity generation from tetrathionate in microbial fuel cells by acidophiles. J Hazard Mater. 2015;284:182–9. doi: 10.1016/j.jhazmat.2014.10.045. [DOI] [PubMed] [Google Scholar]
- Takai K, Nakamura K, Toki T, et al. Cell proliferation at 122°C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. P Nat Acad Sci USA. 2008;105:10949–54. doi: 10.1073/pnas.0712334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia JM, Munoz JA, Gonzalez F, et al. Interrelation between cells and extracellular polymeric substances (EPS) from Acidiphilium 3.2Sup(5) on carbon surfaces. Adv Mat Res. 2009;71–73:287–90. [Google Scholar]
- Ter Heijne A, Hamelers HV, Buisman CJ. Microbial fuel cell operation with continuous biological ferrous iron oxidation of the catholyte. Environ Sci Technol. 2007;41:4130–4. doi: 10.1021/es0702824. [DOI] [PubMed] [Google Scholar]
- Ter Heijne A, Hamelers HV, De Wilde V, et al. A bipolar membrane combined with ferric iron reduction as an efficient cathode system in microbial fuel cells. Environ Sci Technol. 2006;40:5200–5. doi: 10.1021/es0608545. [DOI] [PubMed] [Google Scholar]
- Ter Heijne A, Liu F, Weijden R, et al. Copper recovery combined with electricity production in a microbial fuel cell. Environ Sci Technol. 2010;44:4376–81. doi: 10.1021/es100526g. [DOI] [PubMed] [Google Scholar]
- Tiago I, Pires C, Mendes V, et al. Bacillus foraminis sp. nov., isolated from a non-saline alkaline groundwater. Int J Syst Evol Micr. 2006;56:2571–4. doi: 10.1099/ijs.0.64281-0. [DOI] [PubMed] [Google Scholar]
- van de Vossenberg JLCM, Driessen AJM, Zillig W, et al. Bioenergetics and cytoplasmic membrane stability of the extremely acidophilic, thermophilic archaeon Picrophilus oshimae. Extremophiles. 1998;2:67–74. doi: 10.1007/s007920050044. [DOI] [PubMed] [Google Scholar]
- van Eerten-Jansen MCAA, Ter Heijne A, Grootscholten TIM, et al. Bioelectrochemical production of caproate and caprylate from acetate by mixed cultures. SusChemEngin. 2013;1:513–8. [Google Scholar]
- Venkateswaran K, Moser DP, Dollhopf ME, et al. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int J Syst Bacteriol. 1999;49:705–24. doi: 10.1099/00207713-49-2-705. [DOI] [PubMed] [Google Scholar]
- Virdis B, Rabaey K, Rozendal RA, et al. Simultaneous nitrification, denitrification and carbon removal in microbial fuel cells. Water Res. 2010;44:2970–80. doi: 10.1016/j.watres.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Virdis B, Rabaey K, Yuan Z, et al. Microbial fuel cells for simultaneous carbon and nitrogen removal. Water Res. 2008;42:3013–24. doi: 10.1016/j.watres.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo WQ, Xing DF, et al. Hydrogen production using biocathode single-chamber microbial electrolysis cells fed by molasses wastewater at low temperature. Int J Hydrogen Energ. 2014;39:19369–75. [Google Scholar]
- Wrighton KC, Agbo P, Warnecke F, et al. A novel ecological role of the Firmicutes identified in thermophilic microbial fuel cells. ISME J. 2008;2:1146–56. doi: 10.1038/ismej.2008.48. [DOI] [PubMed] [Google Scholar]
- Wrighton KC, Thrash JC, Melnyk RA, et al. Evidence for direct electron transfer by a Gram-positive bacterium isolated from a microbial fuel cell. Appl Environ Microb. 2011;77:7633–9. doi: 10.1128/AEM.05365-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, Zhuang L, Zhou SG, et al. Corynebacterium humireducens sp. nov., an alkaliphilic, humic acid-reducing bacterium isolated from a microbial fuel cell. Int J Syst Evol Bacteriol. 2011;61:882–7. doi: 10.1099/ijs.0.020909-0. [DOI] [PubMed] [Google Scholar]
- Xu L, Liu W, Wu Y, et al. Trehalose enhancing microbial electrolysis cell for hydrogen generation in low temperature (0°C) Bioresource Technol. 2014;166:458–63. doi: 10.1016/j.biortech.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Yong YC, Cai Z, Yu YY, et al. Increase of riboflavin biosynthesis underlies enhancement of extracellular electron transfer of Shewanella in alkaline microbial fuel cells. Bioresource Technol. 2013;130:763–8. doi: 10.1016/j.biortech.2012.11.145. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Yeo SH, Kim IG, et al. Shewanella marisflavi sp. nov. and Shewanella aquimarina sp. nov., slightly halophilic organisms isolated from sea water of the Yellow Sea in Korea. Int J Syst Evol Mic. 2004;54:2347–52. doi: 10.1099/ijs.0.63198-0. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhao B, Zhou SG, et al. Electrocatalytic activity of anodic biofilm responses to pH changes in microbial fuel cells. Bioresource Technol. 2011;102:6887–91. doi: 10.1016/j.biortech.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li C, Ding L, et al. Influences of initial pH on performance and anodic microbes of fed-batch microbial fuel cells. J Chem Technol Biotechnol. 2011a;86:1226–32. [Google Scholar]
- Zhang L, Shen J, Wang L, et al. Stable operation of microbial fuel cells at low temperatures (5–10°C) with light exposure and its anodic microbial analysis. Bioproc Biosyst Eng. 2014;37:819–27. doi: 10.1007/s00449-013-1054-8. [DOI] [PubMed] [Google Scholar]
- Zhang TT, Zhang LX, Su WT, et al. The direct electrocatalysis of phenazine-1-carboxylic acid excreted by Pseudomonas alcaliphila under alkaline condition in microbial fuel cells. Bioresource Technol. 2011b;102:7099–102. doi: 10.1016/j.biortech.2011.04.093. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Xiao Y, Yang Z-H, et al. The bacterial communities of bioelectrochemical systems associated with the sulfate removal under different pHs. Process Biochem. 2014;49:1345–51. [Google Scholar]
- Zhuang L, Zhou SG, Li YT, et al. Enhanced performance of air-cathode two-chamber microbial fuel cells with high-pH anode and low-pH cathode. Bioresource Technol. 2010;101:3514–9. doi: 10.1016/j.biortech.2009.12.105. [DOI] [PubMed] [Google Scholar]