Abstract
Background
The biologically active phospholipids Platelet-activating Factor (PAF) and oxidatively truncated phospholipids from chemical oxidation are increased in the circulation of rats subject to the oxidant stress of chronic ethanol ingestion. Potentially, circulating inflammatory and apoptotic phospholipids correlate to physiologic oxidative stress.
Results
PAF and the common oxidatively truncated and biologically active phospholipid azelaoyl phosphatidylcholine (Az-PC) were significantly increased in the plasma of older mice, and in male mice. PAF and Az-PC are very rapidly cleared from the circulation, which was unaffected by age or sex. Platelets exposed to Az-PC display phosphatidylserine on their surface, and occlusive platelet carotid arterial thrombosis is enhanced by aging.
Conclusion
Biologically active phospholipids vary in the circulation, with the highest levels being found in older, male mice. Turnover of PAF and the biologically active Az-PC are rapid and are invariant with age and sex, so increased production accounts for the increased concentration and flux of both lipids. Platelets are exposed to plasma Az-PC that depolarizes their mitochondria to increase pro-thrombotic phosphatidylserine expression, and occlusive platelet thrombosis is enhanced in aged mice.
Significance
Oxidatively modified phospholipids are increased in the circulation during common, mild oxidant stresses of aging, or in male compared to female animals. Turnover of these biologically active phospholipids by rapid transport into liver and kidney is unchanged, so circulating levels reflect continuously increased production.
Abbreviations: Az-PC, azelaoyl phosphatidylcholine; PAF, Platelet-activating Factor; TMEM30a, transmembrane 30 kDa protein a
Keywords: Inflammation, Platelet-activating Factor, Oxidized phospholipid, Pharmacokinetics
Graphical abstract
Highlights
► Oxidatively truncated phospholipids accumulate in the circulation of aged and male animals. ► Clearance of oxidatively truncated phospholipids is both rapid, and constant. ► Circulating oxidized phospholipids report continual oxidative stress in aged, male mice. ► Aging promotes oxidant-induced occlusive platelet thrombosis in large vessels.
Introduction
Polyunsaturated fatty acids esterified in phospholipids are susceptible to free radical oxidation that fragments these sn-2 residues to small, and sometimes reactive, fragments from the distal end of the residue, while leaving the proximal fragment esterified in the parent phospholipid. These truncated phospholipids are not products of normal cellular metabolism, and are both excellent markers of oxidation even in complex in vivo responses to oxidative injury [1] and are unregulated mediators of inflammation and cell death.
Fragmentation of esterified phospholipid (hydro)peroxides generates phospholipids retaining the proximal fatty acyl fragment, and because oxidizable polyunsaturated fatty acyl residues are commonly incorporated into the sn-2 position, phospholipid oxidation produces a large number of oxidized and truncated phospholipids with chemically modified sn-2 residues. All of these are membrane disruptive, but many are biologically active because they stimulate the G protein coupled receptor for the phospholipid mediator Platelet-activating Factor (PAF), or enter cells to drive PPARγ-controlled gene transcription, or more commonly to depolarize mitochondria to initiate cell death through the intrinsic apoptotic cascade [2].
PAF, and the oxidatively truncated phospholipids, are specifically hydrolyzed to their component lysophospholipid and fatty acid fragment by either circulating PAF acetylhydrolase (lipoprotein-associated phospholipase A2; PLA2G7) [3], [4], or by intracellular PAF acetylhydrolase (PAFAH2) [5]. While a great deal of attention has focused on PLA2G2 inhibition [6], [7], [8], it turns out to be irrelevant to inactivation of circulating PAF or oxidatively truncated phospholipids. Inhibition of blood-borne PLA2G7 does not increase the levels of its substrates in the circulation [9] because the half life of PAF and Az-PC in blood is too transient for hydrolysis by this circulating enzyme. Rather, both phospholipids are removed from circulation with a half life of tens of seconds, so truncated phospholipids and PAF in plasma reflect only a small portion of what must be a large flux through the circulation.
The level of circulating oxidized phospholipids is increased in animals exposed to the oxidative stress of chronic ethanol catabolism, but whether this actually results from increased formation of oxidative phospholipid modification or instead reflects decreased removal from the circulation is unknown. The risk of lipid oxidation and atherosclerosis is increased in human males and in male animals for incompletely understood reasons, but increased oxidative stress is a potential effector of this.
We probed for the presence of the abundant oxidized phospholipid Az-PC as a marker for increased oxidative stress as a function of age and sex because Az-PC is both an abundant phospholipid oxidation marker, it is also highly effective in depolarizing cellular mitochondria to disrupt cellular function. We also determined whether the structurally related lipid mediator PAF was increased in parallel with Az-PC. PAF is both a remarkably potent and pleiotropic activator of numerous cells of the innate immune system [10], but both are cleared by the same endothelial cell TMEM30a phospholipid importer [11]. We find both biologically active phospholipids are increased in vivo as part of normal physiology, and that formation, not clearance, accounts for their presence.
Materials and methods
Cells
Human blood was isolated in a protocol approved by the Cleveland Clinic IRB. Human blood was drawn into acid-citrate-dextrose and centrifuged (200g, 20 min) without braking to obtain platelet-rich plasma. Platelets were stained with Annexin V-Alex 488 (1/100 dilution) to measure surface phosphatidylserine in binding buffer [140 mM NaCl, 10 mM Hepes (pH 7.4) and 2.5 mM CaCl2] for 15 min at room temperature. At the end of this incubation the samples were diluted four-fold with binding buffer and fluorescence was assessed by flow cytometry through the Cleveland Clinic Flow core.
Mice (C57/Bl6) were used in a protocol approved by the Cleveland Clinic's IACUC to induce occlusive thrombosis as described [12]. C57B/6 mice were anesthetized with ketamine (90 mg/kg)/xylazine (15 mg/kg) and the right jugular vein and the left carotid artery were exposed via a middle cervical incision. Platelets were labeled in vivo by injecting 100 μL of rhodamine 6G (0.5 mg/ml) in saline into the right jugular vein 15 min before FeCl3 injury. The left carotid artery was stripped of adventitia and a piece of black plastic was placed under the vessel to reduce background fluorescence. A 1×2 mm2 piece of filter paper saturated with 7.5% FeCl3 solution was applied to the carotid artery for 1 min, the filter paper was removed, and the vessel rinsed with saline. Fluorescent thrombus formation was observed in real-time under a water immersion objective at 10× magnification. Time to occlusive thrombosis was determined offline using real time video image capture with a QImaging Retigo Exi 12-bit mono digital camera (Surrey, Canada) and Streampix version 3.17.2 software (Norpix, Montreal, Canada). The end points were set as either cessation of blood flow for >30 s or no occlusion after 30 min (three times longer than the average occlusion time), in which case the time was recorded as 30 min for statistical comparison.
Supplementary material related to this article can be found online at doi:10.1016/j.redox.2012.11.011.
The following is the Supplementary material related to this article Video 1.
Mass spectrometry
Lipids were extracted [13] using [2H]PAF as an internal standard, and then purified over an aminopropyl column [14] before being quantified by liquid chromatography/electospray ionization/ tandem mass spectrometry (LC/MS/MS) [15]. These analyses were performed using a Quattro Ultima triple-quadrupole mass spectrometer (Micromass, Wythenshawe, UK) configured with the capillary voltage at 5 kV, the cone voltage at 60 V, the source temperature at 120 °C, and a desolvation temperature at 250 °C. N2 and desolvation gas flow were 90 and 811 liters/h. Collision induced dissociation used argon gas in a positive ion mode with multiple reaction monitoring. The phosphocholine m/z 184 product ion is the dominant ion of both PAF and Az-PC, and the precursor to product ion transition was m/z 667→184 for Az-PC. The identity of Az-PC was confirmed in comparison with synthetic standards in the negative mode.
In vivo phospholipid metabolism
[3H-acetyl]PAF in 0.5% albumin in PBS was injected into the retro-orbital plexus. 100 μL Of blood was collected by cardiac puncture, and intact [3H-acetyl]PAF was recovered by organic extraction for quantitation by liquid scintillation counting.
Materials
Annexin V and rhodamine 6G was from Invitrogen (Carlsbad, CA). [3H-acetyl]PAF was supplied by Perkin-Elmer (Boston, MA), and [2H-acetyl]PAF and Az-PC from Cayman Chemical (Ann Arbor, MI). Other reagents were from Sigma.
Expression of data and statistics
Experiments were performed at least three times, with assays performed in triplicate. The standard errors of the mean from all experiments are presented as error bars. Graphing of figures and statistical analyses were generated with Prism4 (GraphPad Software). A value of p<0.05 was considered statistically significant.
Results
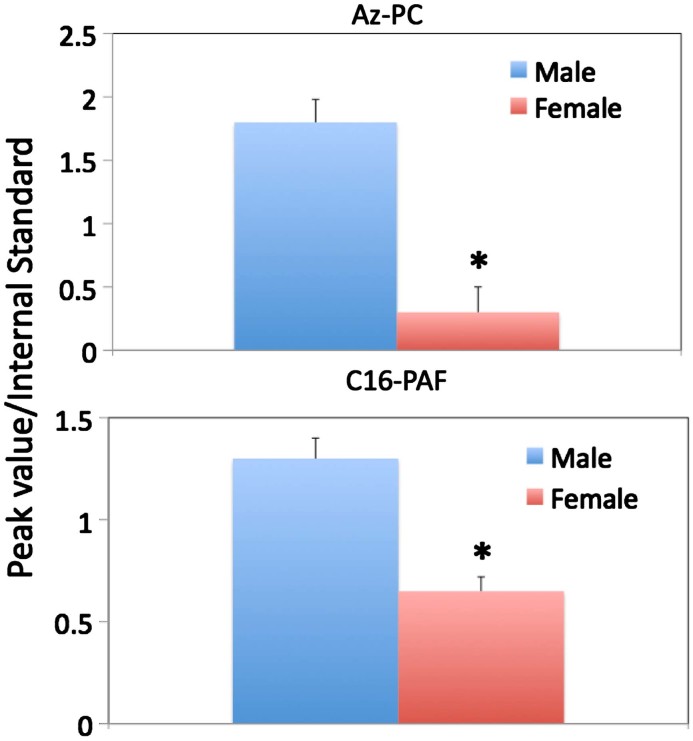
The oxidized phospholipid Az-PC is present in the circulation of male mice at just over six times the amount circulating in female C57/Bl6 mice (Fig. 1). There was little variation among animals, reflected in a small standard error, so sex is a key determinant of circulating Az-PC. This phospholipid is not a product of cellular metabolism and is a prominent product of phospholipid oxidation, so its presence signals prior phospholipid oxidation. The phospholipid PAF also was more prominent in the serum of male animals, although this difference was less than two fold. PAF is thought to primarily reflect cellular production either constitutively [16] or after stimulation of inflammatory cells [17], but also is a product of phospholipid oxidation [18].
Fig. 1.
Circulating phospholipids are increased in male mice. AzPC and PAF levels in mice at 3 months of age were measured by mass spectrometry by comparing the abundance of the m/z 184 phosphocholine transition peak from the molecular ion of the sample phospholipid to the m/z 184 phosphocholine transition peak from an [2H]PAF internal standard as described in Section 2. Bars indicate average (±SD) of 5–10 mice ⁎ Indicates p<0.05. Figures are a representative of three independent experiments.
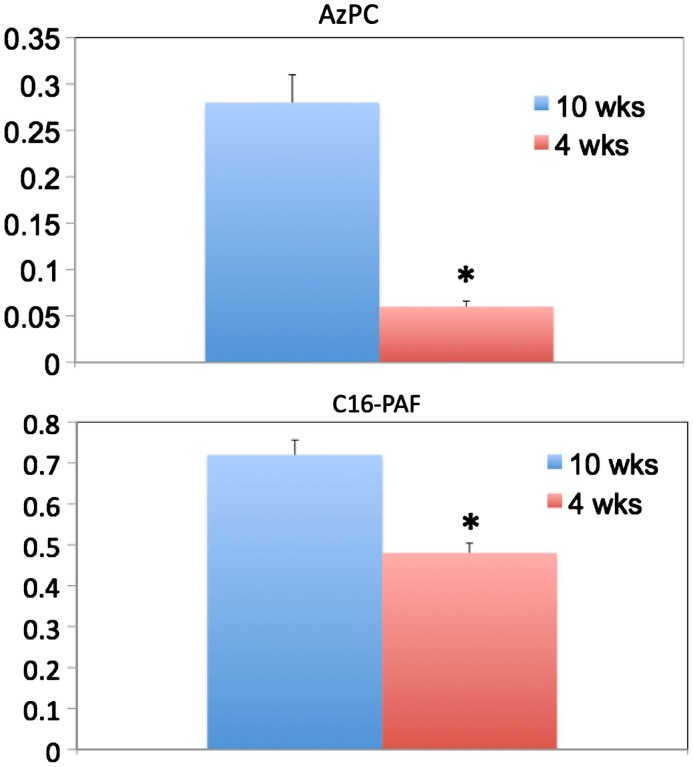
Circulating levels of Az-PC and PAF have been correlated with the oxidative stress of ethanol catabolism [19], but also varied with age. Young animals contained less of each phospholipid than mice 10 weeks of age (Fig. 2), and again the difference was greatest for Az-PC, 4-fold, relative to the 1.5 fold difference for PAF abundance over the six week period of time.
Fig. 2.
Circulating oxidized phospholipid and PAF are increased in older animals. (A) AzPC and (B) C16-PAF levels in mice at age 4 or 10 weeks. C16-PAF was more abundant than PAF containing an sn-1 C18 fatty ether residue (not shown), as expected. Bars indicate average (±SD) of 5 mice per group. ⁎ Indicates p<0.05. Figures are a representative of two independent experiments.
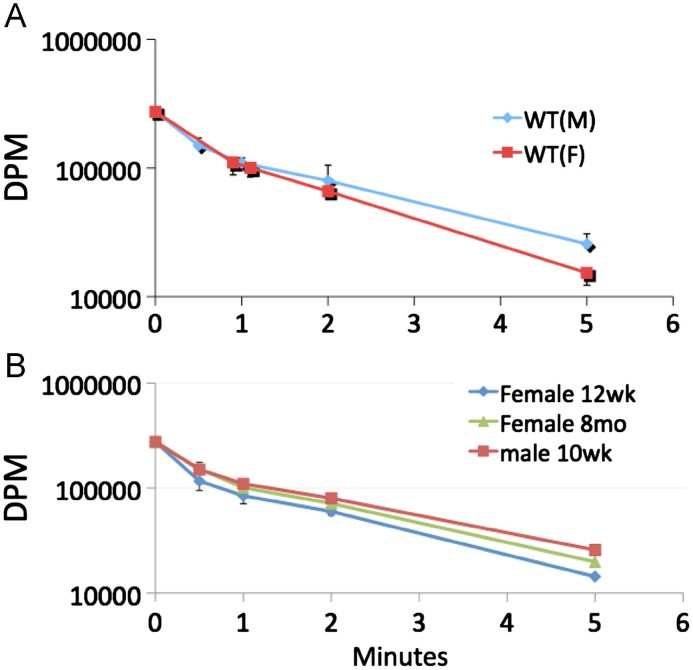
PAF and Az-PC are cleared from the circulation not by hydrolysis, but rather by remarkably facile uptake into liver and kidney so the half life of these two lipids in the circulation is far less than a minute [11]. This rapid clearance did not depend on the sex of the mice (Fig. 3A), nor did it depend on their age. The same rapid clearance obtained in juvenile animals, males at 10 weeks or females at 12 weeks, as it did in old female mice at 8 months of age (Fig. 3B).
Fig. 3.
Clearance of circulating PAF is rapid, and independent of age or sex. [3H-acetyl]PAF (10 μCi) in 100 μL of PBS containing 0.5% human serum albumin was injected into (A) 12-week-old wild-type male (n=5) or female (n=5) mice, or (B) wild-type male or female (8–12 weeks, n=5 each group) through the retro-orbital plexus. At the stated post-injection times, 100 μL of blood was collected by cardiac puncture, and intact [3H-acetyl]PAF was recovered by organic extraction for quantitation by liquid scintillation counting.
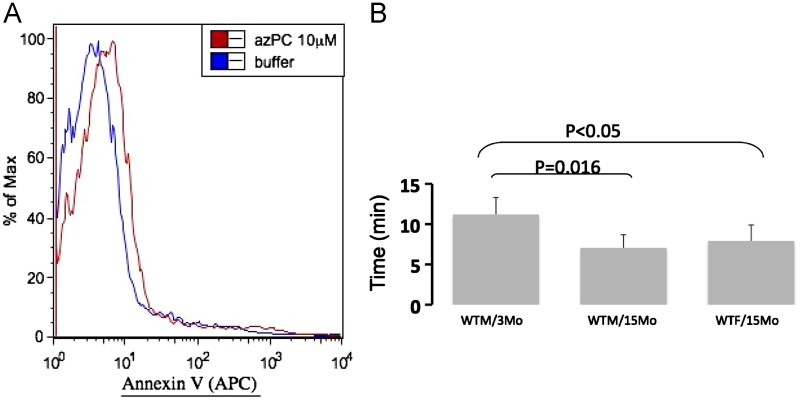
Platelets circulating in plasma will be exposed to Az-PC, and this phospholipid depolarizes these anucleate cells [20] as it does nucleated cells of the innate immune system [21], [22]. Maintenance of phospholipid asymmetry depends on the availability of cellular energy, and Az-PC promoted phosphatidylserine expression on the surface of platelets (Fig. 4A). Platelet reactivity can be assessed in vivo by determining the time it takes to form a thrombus and stop blood flow in a major vessel damaged by ectopic FeCl3 oxidative damage [12], [23]. Aging results in hypersensitive platelets with a higher propensity to undergo thrombosis where the time to occlusion fell from 11.2 min to 7.1 min as male animals aged from 3 to 15 months. There was a significant decrease in older female animals as well where occlusive thrombosis took an average of 7.9 min after oxidative damage. The slight difference between aged female and male animals itself was not significant.
Fig. 4.
Platelets are depolarized by Az-PC, and occlusive platelet thrombosis is enhanced by aging. (A) Platelets exposed to Az-PC express phosphatidylserine on their surface. Human platelets were treated with 10 μM Az-PC, or not, for 4 h before staining with Alexa488-conjugated Annexin V before flow cytometry. (B) Time to thrombotic occlusion of carotid arteries from WT C57/Bl6 male mice 3 months of age (n=3) and WT male mice at 15 months (n=4) and WT female also 15 months old (n=5) was measured after topical application of 7.5% FeCl3 to exposed carotid arteries as described in Section 2.
Discussion
Polyunsaturated fatty acids are esterified in phospholipids of cellular membranes and circulating lipoproteins preferentially in the sn-2 position of the glycerol backbone of the various phospholipid classes. Polyunsaturated fatty acids are preferred oxidation targets because the vinyl carbon–hydrogen bond between two olefinic bonds is weak [24]. Free radical attack and hydrogen abstraction at these positions with bond rearrangement, if possible, result in oxygen adduction at the beginning and end of a run of double bonds. What this means in practice is that phospholipid peroxides tend to reside at the first or last carbon of the run of double bonds. The 4, 5 bond of arachidonoyl or the 9, 10 bond of esterified linoleoyl residues are therefore favored sites, and because the most abundant polyunsaturated fatty acid are esterified linoleoyl residues, the 9-(hydro)peroxide is a common oxidation product.
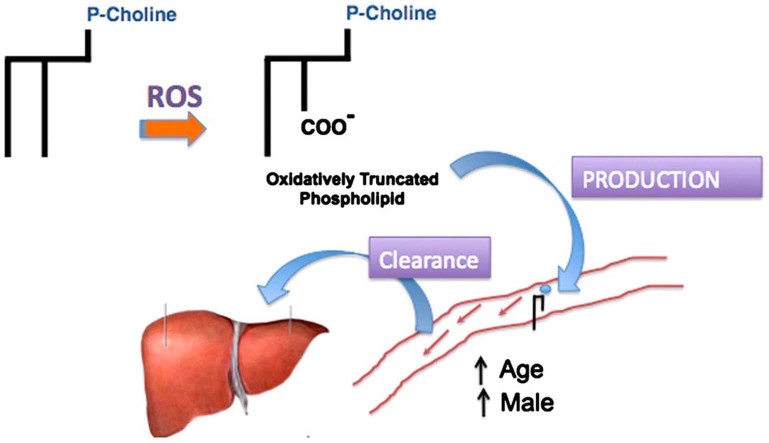
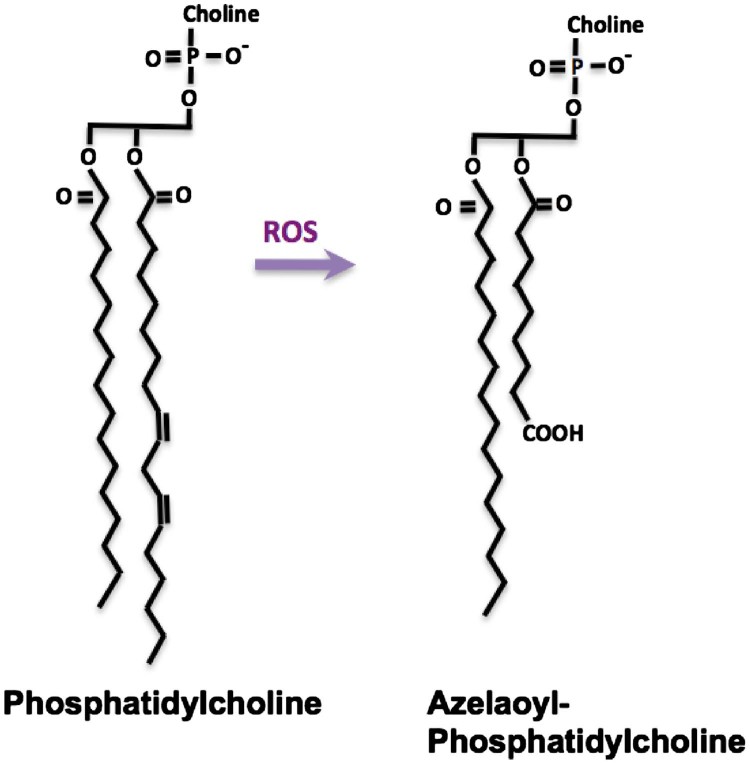
Lipid peroxides may fragment on either side of the oxygen function, which for the common 9-(hydro)peroxide produces either a 9-carbon fragment bearing the (hydro)peroxide at the ω-end of the shortened chain or, if fragmentation occurred on the proximal side of the peroxide, an 8-carbon long fragment that lacks the oxygen function. The trivial name for a 9-carbon dicarboxylic fatty acid is azelaic acid, so oxidation of the most common linoleoyl polyunsaturated fatty residue generates phospholipids with sn-2 azelaoyl residues (Fig. 5). Accordingly, oxidation of low density lipoprotein particles, which contain a shell of phosphatidylcholine around a core of neutral cholesterol and cholesterol esters, yields azelaoyl-phosphatidylcholine (Az-PC) [25]. Intermediate length dicarboxylic fatty acids like azelaic acid are not esterified into cellular phospholipids, so Az-PC is a marker of oxidative stress, not cellular metabolism.
Fig. 5.
Azelaoyl-phosphatidylcholine is generated from oxidative phospholipid truncation. Phosphatidylcholine is the most abundant phospholipid of cellular membranes, and is synthesized with two long chain fatty acyl residues esterified to the sn-1 and sn-2 positions of the glycerol backbone. The sn-2 residues tend to be both longer and with more unsaturation than the sn-1 residue. The most common polyunsaturated fatty acyl residue is the C18:2 linoleoyl residue with two olefinic bonds between carbons 9,10 and 12, 13. Oxidation to a fatty acyl peroxyl radical preferentially rearranges to the 9 or 13 carbon atom. This fatty acyl peroxyl radical can fragment on either side of the newly introduced oxygen, so a common oxidation product is a truncated phospholipid containing the 9-carbon azelaic acid residue. This phospholipid is a product of oxidation, not physiologic anabolism.
The phospholipids Az-PC and PAF slightly differ from one another at the sn-1 position, where the bond in PAF is a carbon–oxygen–carbon ether bond, compared to the ester bond of Az-PC. The largest difference is the acetyl sn-2 residue of PAF compared to the 9-carbon dicarboxy azelaoyl residue of Az-PC, but both are choline phospholipids and both are reasonably soluble. Both are also transport substrates for the phospholipid importer TMEM30a in mammalian cells [11] and its ROS3 (also identified as LEM3 [26]) homolog in Saccharomyces cerevisiae [27]. TMEM30a is expressed by endothelial cells and labeled PAF and Az-PC both are rapidly cleared from the circulation by transport as the intact molecule into liver and kidney [11].
The flux of polar choline phospholipids out of the circulation is one component of the balance of production and loss that defines plasma phospholipid levels, but the rate of efflux from the vascular compartment has not been examined to determine whether it is variable. PAF and Az-PC are primarily products of different systems, inflammation and oxidation, respectively, so the increase in both lipids in the circulation of male and older mice suggests their clearance mechanism would vary in common. Instead, we found that under conditions where both Az-PC and PAF were increased in the circulation that the rate of efflux was fast, but remained constant. This implies the rate of PAF and Az-PC production, that is oxidation and inflammation, are both enhanced in older, male animals. That is, the continued flux of Az-PC shows both the male sex and age promote on-going oxidative stress that is greater than in their counterparts.
Oxidatively truncated phospholipids like Az-PC traffic to mitochondria after internalization where they depolarize mitochondria in a concentration dependent process [22]. Maintenance of phospholipid asymmetry is energy dependent, so for platelets the appearance of phosphatidylserine on their surface after loss of mitochondrial function will promote tissue factor induced thrombin formation and thrombosis. This pro-thrombotic effect will be enhanced in male animals with increased numbers of circulating platelets.
Platelet function can be assessed in vivo by oxidatively damaging carotid arteries by briefly applying FeCl3 to the external aspect of the closed, intact vessel. The flow of platelets, previously labeled with fluorescent rhodamine dye can be assessed by video microscopy, their accretion at the site of damage can be imaged, and the time it takes before blood flow is abolished by formation of an occlusive platelet thrombus can be quantified. We found that aging greatly increased the rapidity of occlusive thrombus formation.
Overall, we found that oxidized phospholipid increases in the circulation during normal physiologic process of aging and is increased in male animals. We also discovered that the rate of clearance of these markers of chemical oxidation and for inflammatory PAF was invariant, so the relative abundance of polar choline phospholipids reflects their relative rates of generation and introduction to the circulation. Circulating Az-PC is thus a systemic marker of continual oxidative attack on cellular phospholipids. Its rapid clearance means it reports this oxidation in real time. We therefore document a continual and enhanced oxidative attack on the cellular phospholipids of older, male animals.
Contribution of authors
Drs. Liu and Li, and Ms. Chen performed the reported experiments and contributed to the manuscript production. Dr. McIntyre organized the research and the manuscript.
Funding
NIH HL092747, AA017748.
Conflict of interest
None.
References
- 1.Adachi J., Matsushita S., Yoshioka N., Funae R., Fujita T., Higuchi S. Plasma phosphatidylcholine hydroperoxide as a new marker of oxidative stress in alcoholic patients. Journal of Lipid Research. 2004;45(5):967–971. doi: 10.1194/jlr.M400008-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.McIntyre T.M. Bioactive oxidatively truncated phospholipids in inflammation and apoptosis: formation, targets, and inactivation. Biochimica et Biophysica Acta. 2012;1818(10):2456–2464. doi: 10.1016/j.bbamem.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stafforini D.M. Biology of platelet-activating factor acetylhydrolase (PAF-AH, lipoprotein associated phospholipase A2) Cardiovascular Drugs and Therapy. 2009;23(1):73–83. doi: 10.1007/s10557-008-6133-8. [DOI] [PubMed] [Google Scholar]
- 4.McIntyre T.M., Prescott S.M., Stafforini D.M. The emerging roles of PAF acetylhydrolase. Journal of Lipid Research. 2009;50(Suppl.):S255–S259. doi: 10.1194/jlr.R800024-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arai H., Koizumi H., Aoki J., Inoue K. Platelet-activating factor acetylhydrolase (PAF-AH) Journal of Biochemistry (Tokyo) 2002;131(5):635–640. doi: 10.1093/oxfordjournals.jbchem.a003145. [DOI] [PubMed] [Google Scholar]
- 6.MacPhee C.H., Moores K.E., Boyd H.F., Dhanak D., Ife R.J., Leach C.A. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochemical Journal. 1999;338(2):479–487. [PMC free article] [PubMed] [Google Scholar]
- 7.Zalewski A., Nelson J.J., Hegg L., Macphee C. Lp-PLA2: a new kid on the block. Clinical Chemistry. 2006;52(9):1645–1650. doi: 10.1373/clinchem.2006.070672. [DOI] [PubMed] [Google Scholar]
- 8.Macphee C.H., Nelson J., Zalewski A. Role of lipoprotein-associated phospholipase A2 in atherosclerosis and its potential as a therapeutic target. Current Opinion in Pharmacology. 2006;6(2):154–161. doi: 10.1016/j.coph.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Serruys P.W., Garcia-Garcia H.M., Buszman P., Erne P., Verheye S., Aschermann M. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118(11):1172–1182. doi: 10.1161/CIRCULATIONAHA.108.771899. [DOI] [PubMed] [Google Scholar]
- 10.Prescott S.M., McIntyre T.M., Zimmerman G.A. Platelet-activating Factor: a phospholipid mediator of inflammation. In: Gallin J.I., Snyderman R., editors. Inflammation: Basic Principles and Clinical Correlates. Lippincott Williams and Wilkins; Philadelphia: 1999. pp. 387–396. [Google Scholar]
- 11.Chen R., Brady E., McIntyre T.M. Human TMEM30a promotes uptake of anti-tumor and bioactive choline phospholipids into mammalian cells. Journal of Immunology. 2011;186(5):3215–3225. doi: 10.4049/jimmunol.1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen K., Li W., Major J., Rahaman S.O., Febbraio M., Silverstein R.L. Vav guanine nucleotide exchange factors link hyperlipidemia and a prothrombotic state. Blood. 2011;117(21):5744–5750. doi: 10.1182/blood-2009-01-201970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 14.Marathe G.K., Davies S.S., Harrison K.A., Silva A.R., Murphy R.C., Castro-Faria-Neto H. Inflammatory platelet-activating factor-like phospholipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. Journal of Biological Chemistry. 1999;274(40):28395–28404. doi: 10.1074/jbc.274.40.28395. [DOI] [PubMed] [Google Scholar]
- 15.Latchoumycandane C., Marathe G.K., Zhang R., McIntyre T.M. Oxidatively truncated phospholipids are required agents of tumor necrosis factor alpha (TNFalpha)-induced apoptosis. Journal of Biological Chemistry. 2012;287(21):17693–17705. doi: 10.1074/jbc.M111.300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder F., Fitzgerald V., Blank M.L. Biosynthesis of platelet-activating factor and enzyme inhibitors. Advances in Experimental Medicine and Biology. 1996;416:5–10. doi: 10.1007/978-1-4899-0179-8_2. [DOI] [PubMed] [Google Scholar]
- 17.Prescott S.M., Zimmerman G.A., Stafforini D.M., McIntyre T.M. Platelet-activating factor and related lipid mediators. Annual Review of Biochemistry. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 18.Tsoukatos D.C., Arborati M., Liapikos T., Clay K.L., Murphy R.C., Chapman M.J. Copper-catalyzed oxidation mediates PAF formation in human LDL subspecies. Protective role of PAF:acetylhydrolase in dense LDL. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17(12):3505–3512. doi: 10.1161/01.atv.17.12.3505. [DOI] [PubMed] [Google Scholar]
- 19.Yang L., Latchoumycandane C., McMullen M.R., Pratt B.T., Zhang R., Papouchado B.G. Chronic alcohol exposure increases circulating bioactive oxidized phospholipids. Journal of Biological Chemistry. 2010;285:22211–22220. doi: 10.1074/jbc.M110.119982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen R., Chen X., Salomon R.G., McIntyre T.M. Platelet activation by low concentrations of intact oxidized LDL particles involves the PAF receptor. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29(3):363–371. doi: 10.1161/ATVBAHA.108.178731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen R., Feldstein A.E., McIntyre T.M. Suppression of mitochondrial function by oxidatively truncated phospholipids is reversible, aided by bid, and suppressed by Bcl-XL. Journal of Biological Chemistry. 2009;284(39):26297–26308. doi: 10.1074/jbc.M109.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen R., Yang L., McIntyre T.M. Cytotoxic phospholipid oxidation products. Cell death from mitochondrial damage and the intrinsic caspase cascade. Journal of Biological Chemistry. 2007;282(34):24842–24850. doi: 10.1074/jbc.M702865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W., Febbraio M., Reddy S.P., Yu D.Y., Yamamoto M., Silverstein R.L. CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. Journal of Clinical Investigation. 2010;120(11):3996–4006. doi: 10.1172/JCI42823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frankel E.N. Chemistry of free radical and singlet oxidation of lipids. Progress in Lipid Research. 1984;23:197–221. doi: 10.1016/0163-7827(84)90011-0. [DOI] [PubMed] [Google Scholar]
- 25.Davies S.S., Pontsler A.V., Marathe G.K., Harrison K.A., Murphy R.C., Hinshaw J.C. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor gamma ligands and agonists. Journal of Biological Chemistry. 2001;276(19):16015–16023. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- 26.Noji T., Yamamoto T., Saito K., Fujimura-Kamada K., Kondo S., Tanaka K. Mutational analysis of the Lem3p-Dnf1p putative phospholipid-translocating P-type ATPase reveals novel regulatory roles for Lem3p and a carboxyl-terminal region of Dnf1p independent of the phospholipid-translocating activity of Dnf1p in yeast. Biochemical and Biophysical Research Communications. 2006;344(1):323–331. doi: 10.1016/j.bbrc.2006.03.095. [DOI] [PubMed] [Google Scholar]
- 27.Kato U., Emoto K., Fredriksson C., Nakamura H., Ohta A., Kobayashi T. A novel membrane protein, Ros3p, is required for phospholipid translocation across the plasma membrane in Saccharomyces cerevisiae. Journal of Biological Chemistry. 2002;277(40):37855–37862. doi: 10.1074/jbc.M205564200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.