Abstract
Background
Breast cancer associated (BRCA) genes are critical for DNA repair. Mutations in BRCA1 and BRCA2 (BRCAm) result in loss of these repair mechanisms and potential carcinogenesis. Germline BRCAm are common in ovarian carcinomas, particularly in platinum-sensitive disease. The increased prevalence of BRCAm in platinum-sensitive disease is likely due to enhanced responsiveness to platinum chemotherapy from homologous recombination repair deficiency. The purpose of this study was to explore BRCA testing, treatment patterns and survival in platinum-sensitive recurrent (PSR) ovarian cancer.
Methods
This was an observational cohort analysis of PSR ovarian cancer treated at the Huntsman Cancer Institute from 1995 to 2012. Germline BRCA status was ascertained through chart review and categorized as BRCAm (BRCA1/2 positive), BRCAwt (BRCA wild type or variant of uncertain significance), and untested. Treatment patterns and survival were assessed from recurrence until death or last follow-up. The Kaplan-Meier method was used to evaluate survival from recurrence by BRCA status. Logistic regression and COX proportional hazard model was used to estimate predictors of BRCA testing and survival, respectively.
Results
Of the 168 PSR patients, 15 (9 %) were BRCAm, 25 (15 %) were BRCAwt, and 128 (76 %) were untested. Median age at PSR was 56 years for BRCAm and BRCAwt (p = 0.90) and 63 years for those untested (p = 0.033 vs BRCAm). Overall survival was similar between BRCAm and BRCAwt (median 50.4 vs 67.5 months, p = 0.86) and was 24.9 months in untested patients. Significant predictors for the likelihood of BRCA testing were age (OR = 0.93, 95 % CI 0.89, 0.97, p = 0.002), family history of breast or ovarian cancer (OR = 8.33, 95 % CI: 3.08, 22.59, p < 0.001), and cancer diagnosis year (OR = 10.02, 95 % CI: 3.22, 31.21, p < 0.001). BRCA-tested patients had a lower risk of death versus untested (HR 0.35, 95 % CI 0.17, 0.68, p = 0.001).
Conclusions
BRCAwt patients had similar outcomes to BRCAm patients, potentially owing to similar age at diagnosis, representing a BRCA testing channeling bias. Younger patients, those with a family history of breast or ovarian cancer, and those diagnosed more recently were more likely to be BRCA tested. BRCA tested patients had a lower risk of death.
Electronic supplementary material
The online version of this article (doi:10.1186/s13048-016-0227-x) contains supplementary material, which is available to authorized users.
Keywords: Platinum-sensitive ovarian cancer, BRCA testing, Survival, Systemic treatment
Background
It is estimated that in 2015, there will be 21,290 new cases of ovarian cancer diagnosed in the United States [1]. While this type of cancer is rare compared with other types, the percentage of patients surviving 5 years after diagnosis is only 45.6 % [1].
The current standard of care for late-stage ovarian cancer is cytoreductive surgery followed by 6–8 cycles of combination chemotherapy with a platinum-containing agent such as carboplatin [2]. Patients who respond to platinum-based therapy and experience a relapse of ovarian cancer greater than 6 months after treatment completion are considered to have platinum-sensitive ovarian cancer [3]. Those who experience a relapse during treatment or within 6 months after treatment are considered to have platinum-resistant ovarian cancer [4] and are unlikely to respond to additional platinum-based treatments. However, the majority of patients at first recurrence of ovarian cancer have platinum-sensitive disease [3] and standard therapy in these patients consists of retreatment with a platinum-containing regimen [2]. It has also been found that deleterious mutations in BRCA (breast cancer associated) genes are more prevalent in patients with platinum-sensitive ovarian cancer compared with platinum-resistant disease [5]; this finding has implications for improving the treatment of recurrent ovarian cancer.
BRCA1 and BRCA2 are genes that are critical for DNA repair through homologous recombination [6]. Germline and somatic mutations in either BRCA1 or BRCA2 result in a significant increase in genomic instability and errors leading to carcinogenesis due to homologous recombination repair deficiency [7]. Loss of function in both genes results in cell death, whereas loss of function in one gene allows for survival of cells with faulty DNA repair mechanisms [8]. Therefore, patients with germline BRCA1/2 mutations (BRCAm) have enhanced susceptibility to agents that target DNA, such as platinum-containing agents, because of induction of synthetic lethality [5, 9–11].
Owing to the enhanced susceptibility of platinum-containing agents in BRCAm platinum-sensitive recurrent ovarian cancer and the prevalence of BRCAm in recurrent disease, it is important to evaluate BRCA testing, treatment patterns and survival in patients who may benefit most from platinum therapy. The purpose of this study was to assess BRCA testing patterns, treatment patterns, and survival in patients with platinum-sensitive recurrent ovarian cancer.
Methods
Study design and data source
This was an observational cohort study of women with platinum-sensitive recurrent (PSR) ovarian cancer treated at the Huntsman Cancer Institute (HCI) in Salt Lake City, Utah. The Huntsman Cancer Institute tumor registry (HCI-TR) was used to identify patients with site and histology codes for epithelial ovarian cancer between January 1, 1995 and December 31, 2012. Identified patients were linked to electronic health record data from the University of Utah Enterprise Data Warehouse (EDW), which provided patient demographic and clinical information, including laboratory test results, medications, procedures, health status and physician notes.
Study population
Included patients were 18 years and older, with a diagnosis of epithelial ovarian cancer (ICD-10/ICD-O C56.9), fallopian tube cancer (C57.0), or primary peritoneal cancer (C48.1–48.3) in the HCI-TR, and had at least two health care visits separated by ≥30 days with ICD-9 codes for ovarian (183.X), fallopian tube (183.2) or primary peritoneal cancer (158.x) at HCI. Patients who received a platinum-based regimen (carboplatin or cisplatin) for initial systemic treatment of ovarian, fallopian tube, or primary peritoneal cancer and had a platinum-free interval (PFI) of at least 6 months before detection of recurrence were identified in the EDW or via chart review, classified as PSR ovarian cancer patients and included in the final analysis. Patients were excluded if they had a diagnosis of in situ ovarian cancer, had no documented use of platinum-containing agents, were platinum refractory, had a PFI of <6 months, or had no evidence of recurrence. The date of the first recurrence in the EDW was considered the index date for time to event data. The HCI-TR provided the date, stage, pathology, histological grade, and primary tumor site at ovarian cancer diagnosis. Clinical characteristics such as family history of hereditary breast or ovarian cancer and personal history of breast cancer were assessed at recurrence using chart review in the EDW. The University of Utah’s Institutional Review Board and the HCI Clinical Cancer Investigations Committee approved this study.
Classification of platinum-sensitive recurrent ovarian cancer
All patients who received platinum-based first-line treatment were identified in the EDW or via chart review and assessed for response and the PFI. PFI was defined as the number of months from the last platinum dose to recurrence. Those without evidence of recurrence after first-line treatment were excluded. Patients relapsing during first-line treatment or within 6 months of the last platinum treatment were categorized as platinum refractory and also excluded. Platinum sensitivity was defined as a response to first-line platinum treatment and a PFI of ≥6 months or physician-documented platinum sensitivity.
BRCA testing and status
BRCA status was ascertained through chart review. All medical records with mention of BRCA were reviewed by a semi-automated keyword search of the electronic notes with a custom text search tool that searched keywords or patterns of words using Boolean constructs. Patients were classified as BRCAm (BRCA1/2 positive) if a deleterious BRCA1 or BRCA2 germline mutation was documented in the medical record. Patients were classified as BRCAwt (BRCA wild type or variant of uncertain significance) if BRCA testing was conducted and documentation of the wild-type BRCA gene (no deleterious mutation detected) or a variant of uncertain significance was recorded. Lastly, patients were classified as untested if BRCA testing was not performed or if BRCA status was not recorded in the medical record.
Treatment patterns
Primary treatment modalities at the time of ovarian cancer diagnosis were categorized as systemic chemotherapy, radiation, or cytoreductive surgery; these treatments were also evaluated from recurrence until death or last follow-up. Systemic chemotherapy was further categorized as first-line, second-line, and third-line treatment based on the treatments received from recurrence as assessed by chart review. Systemic treatment lines after the initial treatment at recurrence were defined as a change in systemic treatment (addition and/or deletion of drug) due to disease progression, adverse events, or tolerability. Retreatment with the same systemic treatment after a delay in treatment as a result of an adverse event or the inability to tolerate the regimen was not considered a new treatment line. In patients who had a complete response to treatment and in whom systemic treatment was subsequently discontinued, an additional treatment line was considered if the same or alternative systemic treatment was restarted at subsequent disease recurrence/progression.
Statistical analysis
Descriptive statistical analyses, including mean and standard deviation for continuous variables and count and percentage for categorical variables, were performed. Student t-test and Wilcoxon rank-sum test were used for continuous variables and Fisher’s exact test was used for categorical variables. A significance level of 5 % was utilized for this study.
Logistic regression was conducted to estimate the likelihood of patients receiving BRCA testing (BRCAm or BRCAwt) versus untested patients. The covariates in the model included age at ovarian cancer diagnosis, ethnicity, family history of breast or ovarian cancer, personal history of breast cancer, ovarian cancer diagnosis stage, pathology, primary tumor site, and year of ovarian cancer diagnosis.
Kaplan-Meier methodology was used to evaluate survival from index date (date of PSR) until death or last follow-up. Survival was stratified by BRCA status and compared by log-rank test. A Cox proportional hazard model was constructed from recurrence to death or time of last follow-up, whichever occurred earlier, by including patient demographics, clinical characteristics, family history or personal history of breast or ovarian cancer, year of ovarian cancer diagnosis, and BRCA testing status (tested vs. untested) as covariates in a single model run.
Results
Patient characteristics
There were 732 unique adult patients identified in the HCI-TR with an ovarian cancer diagnosis between January 1, 1995 and December 31, 2012 with at least two visits for ovarian cancer in the EDW (Fig. 1). Of those diagnosed with ovarian cancer, 509 patients received a platinum agent, and of those, 168 (33 %) had documented PSR disease (final study cohort), 94 (18 %) had relapsed, platinum-refractory disease, and 247 (49 %) had no evidence of disease progression.
Fig. 1.
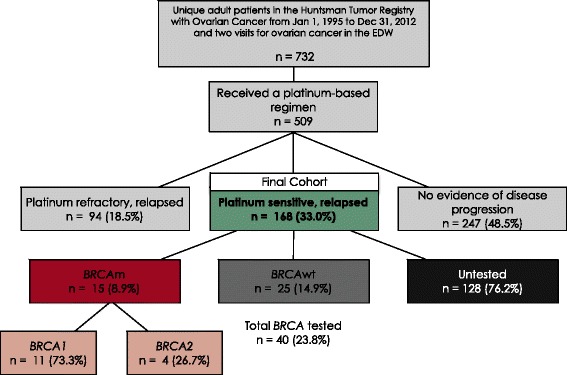
Patient Selection and BRCA Status
BRCA testing and mutational status
Of the 168 PSR patients, 15 (9 %) had BRCAm, 25 (15 %) had BRCAwt, and 128 (76 %) were classified as untested. Of the 15 BRCAm patients, 11 (73 %) had mutations in BRCA1 and four had mutations in BRCA2. BRCA testing was predominantly performed from 2006 to 2013, and only two patients received testing prior to 2006 (Fig. 2).
Fig. 2.
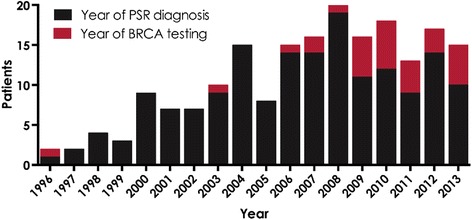
Number of Patients by Year of PSR Diagnosis and Year of BRCA Testing
Demographic and clinical characteristics
Median age at recurrence was 58 years (interquartile range [IQR]: 48–64) for BRCAm, 57 years (IQR: 50–63) for BRCAwt and 63 years (IQR: 54–71) for untested patients, Table 1. More than 50 % of the study patients were white (Table 1). BRCA tested patients had a higher percentage of those with a family history of breast or ovarian cancer compared to untested patients (65 % vs 20 %, p < 0.001). BRCA tested patients also had significantly higher rate of a personal history of breast cancer than untested patients (20 % vs. 5 %, p = 0.009) (Table 1), however a statistical difference was not observed between BRCAm and BRCAwt (33 % vs. 12 %, p = 0.13). There were no significant differences (p < 0.05) between the comparison groups for other clinical variables.
Table 1.
Demographics and Clinical Characteristics of Study Patients (N = 168)
|
BRCAm n = 15 |
BRCAwt n = 25 |
p-Value* | Untested n = 128 |
p-Value** | |
|---|---|---|---|---|---|
| Year of Ovarian Cancer Diagnosis | |||||
| Median (IQR) | 2007 (2004–2009) | 2008 (2005–2010) | 0.305 | 2004 (2000–2007) | 0.027 |
| Year of PSR Diagnosis | |||||
| Median (IQR) | 2008 (2006–2011) | 2010 (2008–2012) | 0.201 | 2006 (2003–2009) | 0.043 |
| Age at Recurrence, Years | |||||
| Median age (IQR) | 58 (48–64) | 57 (50–63) | 0.900 | 63 (54–71) | 0.052 |
| Mean age ± SD | 56 ± 11.0 | 56 ± 9.7 | 0.903 | 63 ± 12.7 | 0.033 |
| Age group | |||||
| <60 years | 8 (53.3 %) | 15 (60.0 %) | 0.231 | 50 (39.1 %) | 0.039 |
| 60–74 years | 5 (33.3 %) | 10 (40.0 %) | 55 (43.0 %) | ||
| ≥75 years | 2 (13.3 %) | 0 | 23 (18.0 %) | ||
| Ethnicity | |||||
| White | 8 (53 %) | 18 (72 %) | 0.138 | 84 (66 %) | 0.372 |
| Hispanic | 2 (13 %) | 0 (0 %) | 7 (5 %) | ||
| Other | 0 (0 %) | 2 (8 %) | 6 (5 %) | ||
| Unknown | 5 (33 %) | 5 (20 %) | 31 (24 %) | ||
| Family History of Hereditary Breast or Ovarian Cancer | |||||
| No family history | 0 (0 %) | 14 (56 %) | <0.001 | 103 (80 %) | <0.001 |
| Familial risk | 15 (100 %) | 11 (44 %) | 25 (20 %) | ||
| Personal History of Breast Cancer | |||||
| No history | 10 (67 %) | 22 (88 %) | 0.126 | 121 (95 %) | 0.003 |
| History of breast cancer | 5 (33 %) | 3 (12 %) | 7 (5 %) | ||
| Stage at Ovarian Cancer Diagnosis | |||||
| ≤2 | 2 (13.3 %) | 5 (20 %) | 0.900 | 21 (16.4 %) | 0.917 |
| ≥3 | 10 (66.7 %) | 15 (60 %) | 87 (68.0 %) | ||
| Unknown | 3 (20 %) | 5 (20 %) | 20 (15.6 %) | ||
| Pathology | |||||
| Serous | 10 (66.7 %) | 18 (72 %) | 0.910 | 77 (60.2 %) | 0.759 |
| Adenocarcinoma | 1 (6.7 %) | 1 (4 %) | 14 (10.9 %) | ||
| Endometrioid | 0 | 1 (4 %) | 11 (8.6 %) | ||
| Other | 4 (26.7 %) | 5 (20 %) | 26 (20.3 %) | ||
| Tumor Histologic Grade at Ovarian Cancer Diagnosis | |||||
| 1 and 2 | 2 (13.3 %) | 3 (12 %) | 0.847 | 15 (11.7 %) | 0.855 |
| 3 | 7 (46.7 %) | 8 (32 %) | 68 (53.1 %) | ||
| 4 | 2 (13.3 %) | 5 (20 %) | 12 (9.4 %) | ||
| Unknown | 4 (26.7 %) | 9 (36 %) | 33 (25.8 %) | ||
| Primary Tumor Site | |||||
| Ovary | 12 (80 %) | 20 (80 %) | 0.574 | 113 (88.3 %) | 0.363 |
| Peritoneum | 3 (20 %) | 3 (12 %) | 11 (8.6 %) | ||
| Fallopian tube | 0 (0 %) | 2 (8 %) | 4 (3.1 %) | ||
| Ca-125 at Recurrence (Highest Value ±30 days), U/mL | |||||
| Median Ca-125 (IQR) | 195 (82–274) | 49.5 (36.5–178) | 0.218 | 90 (34–343) | 0.549 |
| ECOG score | |||||
| Median ECOG (IQR) | 1 (0–1) | 0 (0–1) | 0.457 | 1 (0–1) | 0.634 |
| ECOG group | |||||
| ≤2 | 6 (40 %) | 14 (56 %) | 0.514 | 34 (26.6 %) | 0.490 |
| ≥3 | 0 | 0 | 2 (1.6 %) | ||
| Unknown | 9 (60 %) | 11 (44 %) | 92 (71.9 %) | ||
| Initial Treatment at Ovarian Cancer Diagnosis | |||||
| Chemotherapy | 7 (46.7 %) | 12 (48 %) | 0.547 | 50 (39.1 %) | 0.504 |
| Neoadjuvant chemotherapy | 4 (26.7 %) | 3 (12 %) | 11 (8.6 %) | ||
| Radiation therapy | 0 | 0 | 2 (1.6 %) | ||
| Primary debulking or cytoreductive surgery | 9 (60 %) | 23 (92 %) | 110 (86 %) | ||
| Missing | 0 | 0 | 1 (0.8 %) | ||
| Duration of Follow-up, Days | |||||
| Median (IQR) | 1064 (656–2231) | 981.5 (434–1546) | 0.419 | 490 (219–1015) | 0.005 |
*BRCAm vs. BRCAwt Fisher’s exact test for categorical variables, Wilcoxon rank-sum or t-test for continuous variables; **BRCAm vs. Untested Fisher’s exact test for categorical variables, Wilcoxon rank-sum or t-test for continuous variables
The median time from ovarian cancer diagnosis to PSR disease was 20.1 months (BRCAm), 22.4 months (BRCAwt), and 19.0 months (untested) and was not significantly different between the comparison groups (BRCAm vs. BRCAwt, p = 0.850) (Additional file 1: Table S1). Similarly, the median PFI was 12.1 months (BRCAm), 14.5 months (BRCAwt), and 13.1 months (untested) and was not significantly different between the comparison groups (BRCAm vs. BRCAwt, p = 0.960) (Additional file 1: Table S1).
Treatment patterns
Systemic treatment, including chemotherapy and hormonal agents, was administered in the majority of patients at recurrence (93.3 % BRCAm, 92.0 % BRCAwt, and 78.9 % untested, Table 2). Secondary cytoreductive surgery was performed in 46.7 % (n = 7) of BRCAm, 52 % (n = 13) of BRCAwt, and 40.6 % (n = 52) of untested patients. Radiation therapy was used more frequently in the BRCAm (n = 7, 46.7 %) and BRCAwt (n = 9, 36 %) patients compared with untested patients (n = 25, 19.5 %). All patients in the BRCAm and BRCAwt groups received at least one treatment (systemic, cytoreductive surgery or radiation therapy). However, in the untested group, 2.3 % of patients (n = 3) received no treatment and 3.9 % of patients (n = 5) had unknown/missing treatments (Table 2). The proportion of patients who received a platinum-containing regimen any time after recurrence was 87 % (n = 13) in the BRCAm, 76 % (n = 19) in the BRCAwt, and 67 % (n = 118) in untested group. No statistical difference in the number of treatment lines were observed between BRCAm and BRCAwt groups (median 4 BRCAm vs. 3 BRCAwt, p = 0.83). The median number of systemic treatment lines was two in the untested group (Fig. 3).
Table 2.
Systemic Treatment, Surgery, Radiation and Utilization Post-Recurrence
| Treatment Type | BRCAm | BRCAwt | Untested | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 15 | % or Range | p-Value | n = 25 | % or Range | p-Value* | n = 128 | % or Range | p-Value** | |
| Systemic Treatment | |||||||||
| Systemic treatment received, n | 14 | 93.3 % | ref | 23 | 92.0 % | 0.876 | 101 | 78.9 % | 0.138 |
| Missing/unknown, n | 1 | 6.7 % | - | 1 | 4.0 % | - | 16 | 12.5 % | - |
| Median number of treatment lines (IQR) | 4 (2–4) | 0–6 | ref | 3 (1–5) | 0–11 | 0.832 | 2 (1–3) | 0–6 | 0.001 |
| Platinum-containing regimen post-recurrence, n | 13 | 86.7 % | ref | 19 | 76.0 % | 0.404 | 86 | 67.2 % | 0.098 |
| Cytoreductive Surgery | |||||||||
| Secondary cytoreductive surgery, n | 7 | 46.7 % | ref | 13 | 52.0 % | 0.744 | 52 | 40.6 % | 0.655 |
| Missing/unknown, n | 0 | 0.0 % | - | 0 | 0.0 % | - | 5 | 3.9 % | - |
| Median months to cytoreductive surgery from recurrence (IQR) | 8.8 (1.5–38.8) | 0–93.5 | ref | 0.8 (−0.4–3.4) | −0.5–11.7 | 0.067 | 0.4 (−0.1–3.4) | −0.8–71.9 | 0.064 |
| Radiation Therapy | |||||||||
| Radiation therapy, n | 7 | 46.7 % | ref | 9 | 36.0 % | 0.506 | 25 | 19.5 % | 0.027 |
| Missing/unknown, n | 0 | 0.0 % | - | 0 | 0.0 % | - | 5 | 3.9 % | - |
| Median months to radiation therapy from recurrence (IQR) | 15.8 (7.2–21.9) | 1.7–29.4 | ref | 19.7 (17.0–26.5) | 11.7–34.5 | 0.333 | 3.5 (1.3–11.7) | 0.1–81.5 | 0.109 |
| No Treatment (Surgery, Radiation, Systemic), n | 0 | 0.0 % | - | 0 | 0.0 % | - | 3 | 2.3 % | - |
| Total Unknown/Missing (Surgery, Radiation, Systemic), n | 0 | 0.0 % | - | 0 | 0.0 % | - | 5 | 3.9 % | - |
*BRCAm vs. BRCAwt;**BRCAm vs. Untested
Fig. 3.
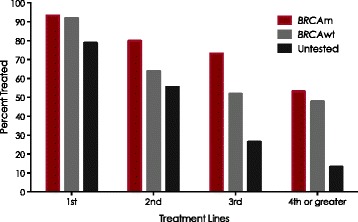
Systemic Treatment Lines Received for Platinum-Sensitive Recurrence Date
Survival analysis
The overall survival for patients with a BRCAm was similar to the BRCAwt group (log rank, p = 0.855), Fig. 4 and Additional file 1: Table S2. Median overall survival from recurrence was 50.4 months in BRCAm, 67.5 months in BRCAwt and 24.9 months in the untested group.
Fig. 4.
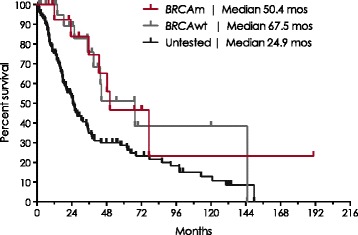
Kaplan-Meier Survival Estimates from Platinum-Sensitive Recurrence Date
Predictors of BRCA testing
The significant predictors for the likelihood of BRCA testing were age (decreased likelihood with a 1-year increase in age, odds ratio [OR] = 0.93, 95 % CI: 0.89, 0.97, p = 0.002), family history of breast or ovarian cancer (increased likelihood with positive history, OR = 8.33, 95 % CI: 3.08, 22.59, p < 0.001), and year of ovarian cancer diagnosis (increased likelihood after 2006, OR = 10.02, 95 % CI: 3.22, 31.21, p < 0.001) (Additional file 1: Table S3).
Predictors of survival from platinum-sensitive recurrence
Patients who received a BRCA test had a lower risk of death than untested patients (hazard ratio [HR] = 0.35, 95 % CI 0.17, 0.68, p = 0.001). Also, the risk of death increased by 2 % for each increasing year of age (HR = 1.020, 95 % CI 1.001, 1.040, p = 0.039) (Additional file 1: Table S4).
Discussion
This study provides a unique perspective into BRCA testing, clinical characteristics, treatment patterns, and survival outcomes in unselected, consecutive patients with PSR ovarian cancer. These data suggest that tested patients are younger at diagnosis, receive more treatment lines and have improved survival compared with untested patients.
This study demonstrates that in an academic oncology center with extensive genetic services support, approximately 24 % (n = 40) of patients with PSR ovarian cancer (n = 168) were tested for BRCAm. Of those who were tested, 37.5 % (n = 15) tested positive for a deleterious mutation in BRCA1 or BRCA2, indicating a high pre-test probability for BRCAm, since it is thought that only 12–15 % of invasive ovarian cancers are associated with BRCAm [12, 13]. The high probability for BRCAm was also supported by other factors such as family history of breast or ovarian cancer, which was observed in all BRCAm patients and is known to be positively associated with BRCA testing.
BRCA1/2 testing was made commercially available in 1996 by Myriad Laboratories, Inc. Utilization of BRCA testing increased during the study period, with more patients with PSR ovarian cancer being BRCA tested after 2006, which is substantiated by outside reports of increased utilization of BRCA testing [14]. Also, it was in 2006 that HCI instituted a Hereditary Risk Evaluation Program, which may have contributed to increased BRCA testing in subsequent years and may account for the variation in the number of tested patients before and after 2006.
These data affirm that patients with a family history of hereditary breast or ovarian cancer are more likely to have been BRCA tested, with all BRCAm patients having a history compared with 44 % of BRCAwt and 20 % of untested patients. Additionally, BRCAm patients (33 %, n = 5) were more likely to have a personal history of breast cancer, either prior to ovarian cancer diagnosis or concurrently, compared to untested (5 %, n = 7) and BRCAwt patients (20 %, n = 3).
BRCA-tested patients were also significantly younger than untested patients. The median age for BRCAm patients was similar to those in other reports [15]. The similar age of BRCAm and BRCAwt patients potentially represents a channeling bias as patients diagnosed at a younger age are more likely have a BRCAm and clinicians would be more likely to offer genetic testing to younger patients based on guideline recommendations [2].
Overall, younger age at diagnosis of ovarian cancer, family history of hereditary breast or ovarian cancer, and personal history of breast cancer were all significant predictors of BRCA testing in this population and suggests that testing was motivated by genetic risk assessment. Additionally, based on other population-based studies indicating a BRCAm rate of 12–15 % [12, 13] in invasive ovarian cancer, our cohort would be expected to contain 20–25 BRCAm carriers. Therefore, over the study period, genetic risk assessment and BRCA testing potentially discovered 60–75 % of the expected BRCAm carriers. However, the expected BRCAm rate may be higher in patients with PSR ovarian cancer. Universal BRCA testing is now recommended for all patients with high-grade serous ovarian cancer and would have likely identified additional BRCA carriers [2].
Our study identified 11 BRCA1 and 4 BRCA2 mutation carriers thus a BRCA1-to-BRCA2 ratio of ~3:1. There are geographic and ethnic differences in BRCA1 and BRCA2 mutations [16]. Common founder BRCA1/2 mutations with a significant role in the population occur in Ashkenazi Jews, as well as in Iceland, Russia, Germany, Hungary, Norway, Finland, Sweden, Denmark, France, the Netherlands, and the UK [16]. Overall, 8–40 % of all BRCA1 mutations have been identified in families from the UK, USA, France, Germany, Italy, and the Netherlands [16]. In 13 studies containing at least 60 families in which one or more cases of ovarian cancer were ascertained, the frequency of BRCA1 ranged from 24.2 to 76.2 % and the frequency of BRCA2 ranged from 1.0 to 16.7 % [16]. The overall ratio of BRCA1 to BRCA2 mutations ranged from 2:1 to 62:1 [16]. These 13 studies mostly took place in European countries (aside from Australia and the USA). Thus, the BRCA1: BRCA2 ratio of 3:1 in our study is consistent given that the majority of Utah inhabitants are of Northern European descent.
Improved ovarian cancer survival outcomes in BRCAm carriers have been reported [15, 17]. The largest and most recent meta-analysis demonstrated that BRCA1 and BRCA2 mutations have positive prognostic effects on ovarian cancer overall and progression-free survival [18]. The results of this meta-analysis revealed that BRCA1 mutation carriers were associated with better overall survival than non-carriers, with a pooled HR of 0.76 (95 % CI: 0.70, 0.83) [18]. However, BRCA2 mutation carriers were associated with even better survival outcomes compared with non-carriers, with a pooled HR of 0.58 (95 % CI: 0.50, 0.66) [18]. Platinum-free survival was also improved in BRCA1 mutation carriers (HR = 0.65; 95 % CI: 0.52, 0.81) and BRCA2 mutation carriers (HR = 0.61; 95 % CI: 0.47, 0.80) [18]. Though we did not evaluate survival in BRCA1 and BRCA2 patients because of sample size limitations, median survival from recurrence was not significantly different between BRCAm and BRCAwt patients in our study, whereas it was lower in untested patients.
These data indicated that tested patients had better OS than untested patients. However, this result should be interpreted with caution as the tested patients tended to be younger and their diagnoses were more recent and thus changes in treatment practice may be contributing. Furthermore, these data support the necessity for testing patients to ensure early diagnosis and optimization of the treatment options.
Potentially contributing to the improved survival, tested patients also received more systemic treatment lines after diagnosis of PSR ovarian cancer than untested patients. However there were no significant differences between BRCAm and BRCAwt patients, potentially reflecting the lack of available targeted therapies. The proportion of patients who received a platinum-containing regimen at any time after PSR diagnosis was 87 % (n = 13) in BRCAm, 76 % (n = 19) BRCAwt, and 67 % (n = 118) in untested patients. Utilization of secondary cytoreductive surgery and palliative radiation therapy was similar between BRCAm (46.7 %) and BRCAwt (36 %) patients; however, fewer untested patients received palliative radiation therapy (19.5 %). The increased use of palliative radiation therapy in BRCA tested patients may be partially explained by their increased duration of survival and therefore increased opportunity to receive radiation therapy.
There are several limitations of this research that should be considered when interpreting the results of this study. These results were from a single institution and may not be generalizable to a larger population or geographic area where clinical practices differ. Furthermore, the demographic composition of the study resulted in under-representation of some demographic categories and small sample sizes for some groups, such as races other than Caucasian/white, precluding meaningful comparisons across some categories of interest. The practice patterns observed at the HCI, a National Cancer Institute Designated Center and member of the National Comprehensive Cancer Network, may not be typical of those in other types of treatment facilities and institutions.
Data from medical charts and tumor registries can be subject to missing data and coding errors. Although most of the demographic and clinical characteristics and treatment patterns of interest for this study were reported as known, there were several variables in the overall population with non-negligible missing or unknown values such as race/ethnicity, stage at ovarian cancer diagnosis, and ECOG performance status, which may have an impact on survival.
Lastly, this study was limited by the small number of BRCA tested patients (n = 40) versus the larger cohort of untested patients (n = 128) and in particular, the resulting small sample size of BRCAm carriers (n = 15) which did not allow for meaningful characterization of survival and treatment patterns between BRCA1 and BRCA2 mutation carriers. An inherent problem of observational studies is the possibility of selection bias; as this was an observational study of real-world BRCA testing practices, the BRCA test selection bias (younger age and more recent year of diagnosis) made it difficult to compare differences between BRCAm and BRCAwt patients. Also, owing to the limitations of available observational data, the untested group was used as a comparison group; this group was assumed to contain predominantly non-BRCAm carriers.
Conclusion
This study demonstrates in an academic oncology center with extensive genetic services support that BRCA testing rates increased during the study period and that the likelihood of BRCA testing increased with lower age, positive family history, and presenting time. However, wider BRCA testing may have identified additional BRCA carriers. Patients tested for BRCAm had greater median overall survival rates versus untested patients and a corresponding greater number of systemic treatment lines. BRCAwt patients had similar outcomes to BRCAm patients. Overall, this study continues to demonstrate the distinct clinical behavior of BRCA-mutated ovarian cancer and underscores the importance of appropriate genetic risk assessment and BRCA testing.
Statement of ethics approval
The study was approved by the Institutional Review Board of the University of Utah via IRB Number IRB_00068779 and the Huntsman Cancer Institute’s Clinical Cancer Investigations Committee.
Consent to publish
This is not applicable here as all data included in this study that was extracted from the University of Utah Enterprise Data Warehouse (EDW) was de-identified without any personal health information. The data that was collected through patient chart reviews was also collected without any personal health information
Availability of data and materials
The data for this study is housed in a secure dedicated database server in the Pharmacotherapy Outcomes Research Center at the University of Utah in Salt Lake City, Utah.
Source of funding
This work was supported by a research grant from AstraZeneca.
Abbreviations
- BRCA
breast cancer associated
- BRCAm
breast cancer associated mutation
- BRCAwt
breast cancer associated wild type or variant of uncertain significance
- PSR
platinum-sensitive recurrent
Additional file
Supplementary Tables and Figures. (DOCX 80 kb)
Footnotes
Competing interest
Sudhir K. Unni, Marisa B. Schauerhamer, Rishi Deka, Vanessa Stevens, Diana Brixner, and David Stenehjem were paid from a research grant provided for this study by AstraZeneca. Jerzy E. Tyczynski and Ancilla W. Fernandes are employees of AstraZeneca.
Authors’ contributions
SU helped in designing the study and conducting the statistical analysis. MS and RD performed the statistical analysis and helped to draft the manuscript. JT, AF, VS, and DB helped in conceptualizing and designing. DS was involved in conceptualizing, designing, analyzing, and interpreting the results. All authors read and approved the final manuscript.
Contributor Information
Sudhir K. Unni, Email: Sudhir.Unni@pharm.utah.edu
Marisa B. Schauerhamer, Email: Marisa.Schauerhamer@pharm.utah.edu
Rishi Deka, Email: r.deka@utah.edu.
Jerzy E. Tyczynski, Email: Jerzy.Tyczynski@astrazeneca.com
Ancilla W. Fernandes, Email: Ancilla.Fernandes@astrazeneca.com
Vanessa Stevens, Email: Vanessa.Stevens@pharm.utah.edu.
Diana I. Brixner, Email: Diana.Brixner@utah.edu
David D. Stenehjem, Phone: (801) 587-9715, Email: david.Stenehjem@hsc.utah.edu
References
- 1.Cancer of the Ovary - SEER Stat Fact Sheets [Internet]. SEER. [cited 2015 May 14]. Available from: http://seer.cancer.gov/statfacts/html/ovary.html
- 2.NCCN . Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer v3.2014. 2012. pp. 1–77. [Google Scholar]
- 3.Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24(29):4699–707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 4.Parmar MKB, Ledermann JA, Colombo N, Bois du A, Delaloye J-F, Kristensen GB, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361(9375):2099–106. doi: 10.1016/S0140-6736(03)13718-X. [DOI] [PubMed] [Google Scholar]
- 5.Dann RB, DeLoia JA, Timms KM, Zorn KK, Potter J, Flake DD, et al. BRCA1/2 mutations and expression: response to platinum chemotherapy in patients with advanced stage epithelial ovarian cancer. Gynecol Oncol. 2012;125(3):677–82. doi: 10.1016/j.ygyno.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Powell SN, Kachnic LA. Roles of BRCA1 and BRCA2 in homologous recombination, DNA replication fidelity and the cellular response to ionizing radiation. Oncogene. 2003;22(37):5784–91. doi: 10.1038/sj.onc.1206678. [DOI] [PubMed] [Google Scholar]
- 7.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108(2):171–82. doi: 10.1016/S0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004;95(11):866–71. doi: 10.1111/j.1349-7006.2004.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iglehart JD, Silver DP. Synthetic lethality―a New direction in cancer-drug development. N Engl J Med. 2009;361(2):189–91. doi: 10.1056/NEJMe0903044. [DOI] [PubMed] [Google Scholar]
- 10.Amir E, Seruga B, Serrano R, Ocana A. Targeting DNA repair in breast cancer: a clinical and translational update. Cancer Treat Rev. 2010;36(7):557–65. doi: 10.1016/j.ctrv.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Konstantinopoulos PAP, Spentzos DD, Karlan BYB, Taniguchi TT, Fountzilas EE, Francoeur NN, et al. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J Clin Oncol. 2010;28(22):3555–61. doi: 10.1200/JCO.2009.27.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104(12):2807–16. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 13.Risch HA, McLaughlin JR, Cole DEC, Rosen B, Bradley L, Kwan E, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68(3):700–10. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeStefano F, Whitehead N, Lux LJ, Lohr KN. Infrastructure To Monitor Utilization and Outcomes of Gene-Based Applications: An Assessment. Rockville: Agency for Healthcare Research and Quality; 2008. pp. 1–100. [Google Scholar]
- 15.Da Y, Khan S, Sun Y, Hess K, Shmulevich I, Sood AK, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306(14):1557–65. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramus SJ, Gayther SA. The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol Oncol. 2009;3(2):138–50. doi: 10.1016/j.molonc.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyman DM, Zhou Q, Iasonos A, Grisham RN, Arnold AG, Phillips MF, et al. Improved survival for BRCA2-associated serous ovarian cancer compared with both BRCA-negative and BRCA1-associated serous ovarian cancer. Cancer. 2012;118(15):3703–9. doi: 10.1002/cncr.26655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Q, Peng H-L, Zhao X, Zhang L, Hwang W-T. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin Cancer Res. 2014;21(1):211–20. doi: 10.1158/1078-0432.CCR-14-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this study is housed in a secure dedicated database server in the Pharmacotherapy Outcomes Research Center at the University of Utah in Salt Lake City, Utah.


