Abstract
The upper jaw in vertebrates forms from several prominences that arise around the stomodeum or primitive mouth. These prominences undergo coordinated growth and morphogenesis to fuse and form the face. Undirected, regionalized cell proliferation is thought to be the driving force behind the morphogenesis of the facial prominences. However, recent findings suggest that directed cell behaviors in the mesenchyme (e.g., directed cell division, directed cell movement, convergent extension) might be required for successful face formation. Here we discuss the evidence for this view and how directed behaviors may interact with the basement membrane to regulate morphogenesis of the facial region. We believe that future research in these largely unexplored areas could significantly impact our understanding of facial morphogenesis.
Keywords: basement membrane, budding outgrowth, directed cell behaviors, epithelial evaginations
INTRODUCTION
The amniote face exhibits a remarkable range of morphological variation. Yet, during its initial formation, the morphology of the face is constrained by the need to coordinate the outgrowth of the facial prominences in order to successfully meet and fuse (Young et al. 2014). Initially, the facial region increases in width along the mediolateral axis as the forebrain expands (Patterson and Minkoff 1985; McGonnell et al. 1998; Marcucio et al. 2011). Subsequently, this mediolateral extension slows and facial growth is directed along the craniocaudal axis. At a tissue level, the face appears to undergo a convergent-extension pattern of growth (Patterson and Minkoff 1985; Geetha-Loganathan et al. 2014; Hu et al. 2015) (Fig. 1). After fusion of the facial primordia to form the early primary palate, the embryonic face grows mainly outwards, acquiring a profile that resembles the adult face (for a review see Jiang et al. 2006).
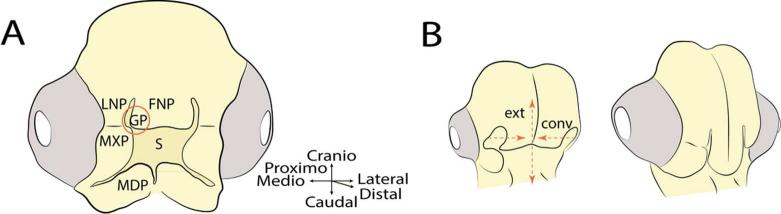
Fig. 1.
Morphology of the face of the chicken embryo. (A) A schematic representation of a chicken embryo at approximately HH24-26. At this time, the facial anlagen are apparent surrounding the stomodeum (S). The upper jaw forms from the paired Maxillary Prominences (MXP), the paired Lateral Nasal Prominences (LNP) and the median Frontonasal Prominence (FNP). The lower jaw forms from the paired Mandibular Prominences (MDP). The globular processes (GP) form the lateral edges of the FNP and will fuse to the MXP and LNP to form the early primary palate (red circle). (B) After fusion of the facial primordia, the facial tissues converge toward the midline (conv) and extend along the craniocaudal axis (ext). These morphogenetic changes make the face appear to undergo a convergent-extension-like pattern of growth (drawn from Hu et al. (2015).
During these stages, the secondary palate also forms. The secondary palate develops from two embryonic primordia, the palatal shelves, which evaginate from the lateral sides of the mouth. In mammals, the palatal shelves first grow vertically toward the tongue, and at later stages, they elevate and grow medially, meet at the midline, and fuse to form a continuous palate that separates the nasal and oral cavities (e.g., Ferguson 1988; Bush and Jiang 2012). In birds and most reptiles, the palatal shelves grow horizontally toward the midline but they do not fuse, giving rise to a natural cleft (Ferguson 1988). However, there are exceptions to these generalizations. For example, a complete secondary palate forms in crocodiles, which resembles the mammalian condition, and in some turtles the palatal anlagen do not form during development, leading to the absence of secondary palate in the adult (Abramyan et al. 2014).
Unravelling the mechanisms underlying facial morphogenesis is important because disruptions to these processes can lead to significant malformations such as cleft lip and/or cleft palate (CL/P). A relevant question is: how do the facial buds extend to form the face? Morphogenetic models of embryonic outgrowths such as limbs or facial prominences have been mainly focused on regionalized cell proliferation. In this model, cells divide at a higher rate at the tip of the bud and increase in density. This physically displaces adjacent cells and matrix to extend the anlage in a particular direction. Importantly, this model does not require that cell division is directed because isotropic growth would be enough to elongate the bud. Recently, this model was tested as an explanation for limb bud elongation using both computer simulation and live imaging (Boehm et al. 2010; Gros et al. 2010). The computer simulations demonstrated that local rates of cell proliferation alone cannot explain the shape changes that the limb bud undergoes during morphogenesis (Boehm et al. 2010). Further, the time-lapse imaging of living limb buds containing GFP-labeled mesenchymal cells revealed that cells move and divide directionally along preferred directions. These videos showed that the directed cell behaviors of the mesenchyme contribute to shaping the limb bud (Gros et al. 2010, see also Sato et al. 2010; Wyngaarden et al. 2010; Hopyan et al. 2011).
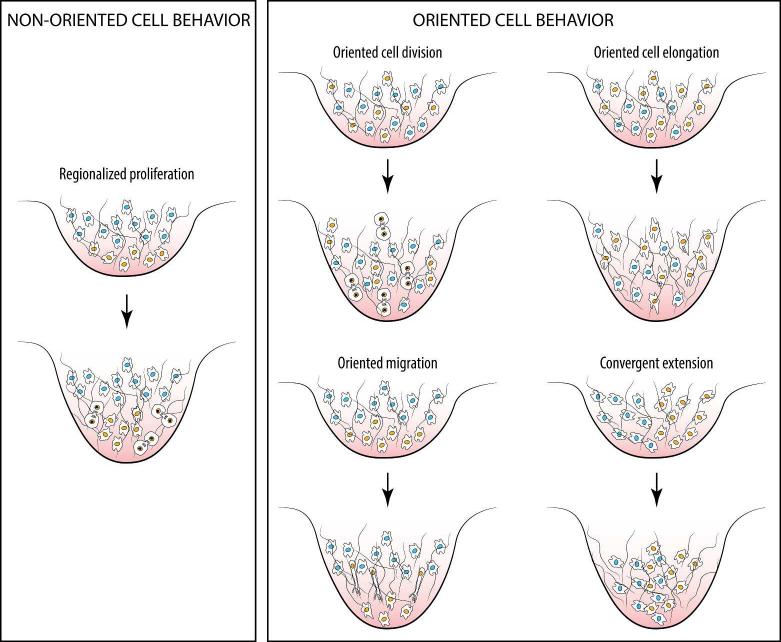
To our knowledge the undirected, regionalized cell proliferation model has not been explicitly tested in studies of facial outgrowth. This is despite the fact that there are indications that oriented cell behaviors, rather than cell proliferation alone, may play a significant role (Fig. 2). Here, we discuss available models for outgrowth of the facial primordia prior to the formation of the early primary palate. We suggest that directed cell behaviors are likely to play a role in this process as they do in the developing limb. We also discuss evidence that suggests a role for the basement membrane in facial budding outgrowth. Here we focus on early stages of primary palate morphogenesis in the avian embryo, but the outlined ideas could be useful for understanding facial morphogenesis in other organisms.
Fig. 2.
Examples of non-directed and directed behaviors of mesenchymal cells that can extend an embryonic evagination. Cells undergoing behavioral changes are depicted with an orange nucleus, except in convergent extension, where cells with blue nuclei are also actively intercalating at the midline. Hair-like lines represent the extracellular matrix.
CELLULAR MECHANISMS DURING EARLY PRIMARY PALATE MORPHOGENESIS
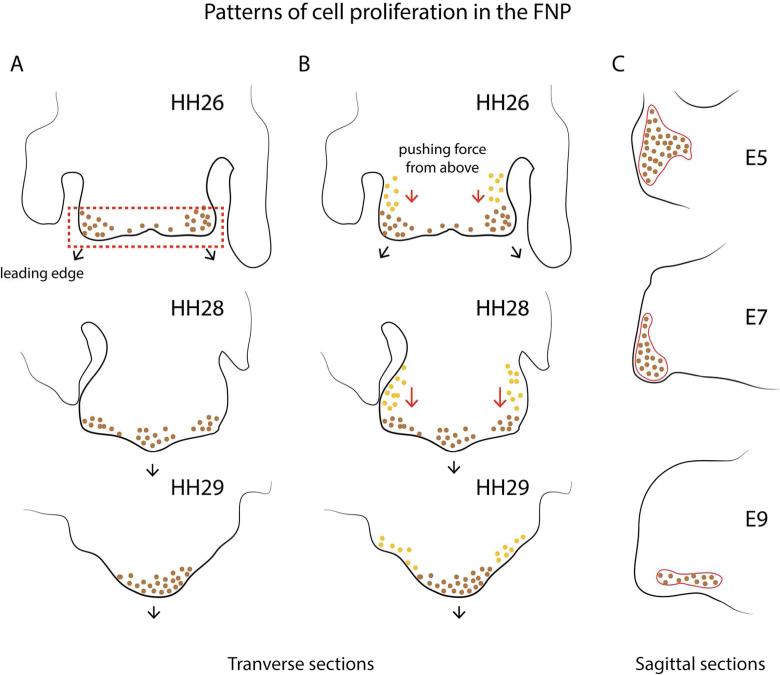
Minkoff and Kuntz (1977; 1978) showed the existence of regional differences in cell proliferation throughout the facial prominences. As in the limb bud, they reported more proliferation at the distal tips. However, this gradient appeared to result not from an increase in proliferation at the tips but rather a decrease at the base of each prominence. Subsequent to this work, models for facial bud morphogenesis have tended to posit regionalized cell proliferation as the driving force behind this process. However, it has not been clear which growth pattern would direct this morphogenetic event. Wu et al. (2006) found a higher proliferation rate within the paired globular processes of the FNP at stage 26 (Hamburger and Hamilton 1951), when the facial region increases mainly in width. Later in development, these two growth centers on either side of the face progressively converge towards the midline to form a single center of growth around stage 31 (Fig. 3A). The authors also showed that this growth pattern is regulated in part, by bone morphogenetic protein 4 (BMP4).
Fig. 3.
Three models of morphogenesis of early primary palate in the chicken embryo based on undirected, regionalized cell proliferation. (A) In the model from Wu et al (2006) morphogenetic movements are directed by differential proliferation at the tip of the facial primordium. At HH26 regionalized proliferative zones (brown dots) in the globular processes of the FNP lead to outgrowth. Subsequently, at HH28-29, these proliferative zones merge to form a single growth center in the FNP (based on Wu et al. 2006) (B) In the model from Szabo-Rogers et al. (2008), in addition to the differential proliferation in the globular processes (brown dots), proliferating cells located more cranially (orange dots) exert a “pushing” force that directs outgrowth during this period. (C) In the model by Fritz et al. (2014) a single growth center is proposed to direct outgrowth. The shape changes of this growth zone determines the contour of the beak (adapted from Fritz et al. 2014).
Szabo-Rogers et al. (2008) demonstrated that high proliferation rates at the globular processes alone are not enough to extend the FNP. In their experiment, the implantation of a bead soaked in SU5402 close to the nasal pits induced a cleft lip. This observation indicates that the FNP extends not only at its caudal edge, but also elongates from above. According to Szabo-Roger et al. (2008), the caudal border of the FNP would not constitute a leading edge, as proposed by Wu et al (2006), but the bud also requires a pushing force exerted by the cranial mesenchyme to extend (Fig. 3B).
In a third model based on Darwin's finches, Fritz et al (2014) suggested that a single growth center in the midline of the FNP drives budding outgrowth. In their model, the shape of this growth center determines the shape of the beak in the adult (Fig. 3C). Accordingly, the FNP would extend along the proximodistal axis at early stages of primary palate formation, but this outgrowth has not been observed in other avian organisms such as chicken and duck where the FNP previously extends along the mediolateral axis and increases in width (McGonnell et al. 1998).
Regionalized growth may also be important in the maxillary prominences. In chicken embryos, proliferation rate is homogeneous throughout the mesenchyme of the maxillary buds at early stages of development. However, as they enlarge, a gradient of cell proliferation is established along the craniocaudal axis (Abramyan et al. 2014). As had earlier been shown by Minkoff and Kuntz (1977; 1978), this gradient forms by an uneven decline of cell proliferation throughout the bud, rather than by an increase at the tips. Overexpression of WNT11 in the maxillary mesenchyme has been shown to disrupt this proliferation gradient, leading to the formation of smaller and foreshortened maxillary buds.
CLUES IMPLICATING DIRECTED CELL BEHAVIORS IN EARLY PRIMARY PALATE MORPHOGENESIS
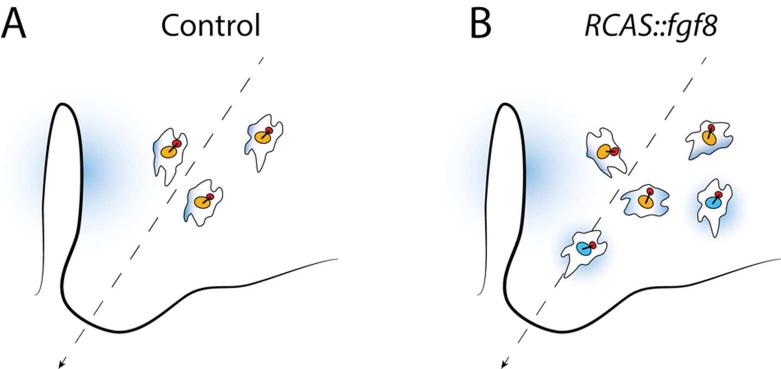
Cell proliferation certainly contributes significantly to morphogenesis of the facial primordia. However, this does not mean that undirected, regionalized cell proliferation is sufficient to explain morphogenesis of the facial prominences. Under such an isotropic growth framework, cells divide in a random, non-directional way, and therefore, do not exhibit signs of polarization. However, recent studies have shown that mesenchymal cells within the facial prominences are, in fact, polarized (Li et al. 2013; Geetha-Loganathan et al. 2014). In the FNP, measurements of the angle formed by the line connecting both the center of the nucleus and the Golgi and a reference line, have shown that mesenchymal cells located in the lateral regions of the FNP are oriented toward the globular processes (Li et al. 2013). Fgf8 is expressed by the epithelium surrounding the nasal pits during normal development, and ectopic expression of Fgf8 in the mesenchyme disrupts this cell polarity, and in combination with reduced rates of proliferation, leads to facial defects (Li et al. 2013) (Fig. 4).
Fig. 4.
Cell polarization in the FNP. (A) Measurements of the angle formed by a line connecting both the center of the Golgi (red) and the nucleus (orange) relative to a reference line (dashed arrow) have shown that mesenchymal cells in the FNP are oriented toward the direction of growth (dashed line) (Li et al. 2013). (B) Ectopic expression of fgf8 in the FNP (cells with blue nuclei), induced by injection of RCAS::Fgf8, disrupts this polarity, suggesting that a morphogenetic gradient of FGF8 (blue shading) emanating from the epithelium surrounding the nasal pits is involved in polarization of the mesenchyme in the FNP (Li et al. 2013).
In the maxillary buds, measurements of the angle formed by the major cell axis and a reference line showed that cells pointed preferentially towards the lateral nasal buds. Also in the maxillary buds, it has been shown that cells move directionally toward an ectopic source of WNT11, which suggests that this molecule can polarize cells and direct movement when spatially restricted to regions of the facial buds (Geetha-Loganathan et al. 2014).
Further suggestive evidence for cellular mechanisms other than undirected, regionalized cell proliferation comes from gain-of-function experiments that increase proliferation. Under the isotropic growth model, such experiments should lead to a premature lip fusion. However, promoting cell proliferation by placing beads soaked in FGF or SHH in the mesenchyme of the FNP does not lead to premature fusion of the facial primordia, but to clefting (Szabo-Rogers et al. 2008; Hu et al. 2015). With increased proliferation, facial prominences increased in volume, but this did not extend the prominences in a direction compatible for formation of an intact upper jaw. These results suggest that a directional cue is likely required to ensure that growth of the facial prominences proceeds toward a shape that allows closure of the prospective maxillary arch and does not lead to cleft lip and/or palate (Szabo-Rogers et al. 2008; Hu et al. 2015). In support of this hypothesis, knock out of planar cell polarity pathway components (Wnt5a, Ror2 and Vangl2), which are involved in the regulation of directed cell behaviors in several organs, led to mice embryos with markedly shorter faces (Gao et al. 2011). It would be also possible, however, that FGF signaling pathway leads to clefting by altering the components of the basement membrane (e.g., Li et al. 2001; Rebustini et al. 2007) (see below).
THE POTENTIAL ROLE OF THE BASEMENT MEMBRANE
In a previous study, Xu et al. (1990) showed that the thickness of the basement membrane varies across the embryonic face prior to fusion of the facial primordia. They showed that the basement membrane is thinner at regions that undergo extensive shape changes. This pattern suggested that the basement membrane might be directly involved in the regulation of facial morphogenesis, by allowing bud formation at regions where it is thinner, and stabilizing the facial contour at regions where it is thicker.
Previously observed in branching organs (e.g., Bernfield 1977), differences in basement membrane thickness suggested to some researchers that the mechanical compliance of the basement membrane could play an important role in epithelial budding (Ingber and Jamieson 1985). In a simple model of branching morphogenesis, a rounded bud formed by an epithelial layer surrounded by mesenchyme bifurcates at the tip to give rise to two sprouts. These two sprouts again bifurcate at their tips generating new sprouts which will continue dividing to form the characteristic treelike appearance of branching organs (e.g., lungs, lacrimal gland, submandibular gland, kidneys) (for a review see Iber and Menshykau 2013; Varner and Nelson 2014). How are the new sprouts generated? Regionalized cell proliferation was also proposed to explain branching morphogenesis. That is, epithelial cells dividing at a higher rate would accumulate and physically push each other and the basement membrane to form a sprout. Subsequently, it was shown that new sprouts emerge before the appearance of differential cell proliferation, which called this model into question (Nogawa et al. 1998).
The observation that the basement membrane is thinner at regions of the bud where new sprouts are formed but thicker where the epithelium does not invaginate led to the formulation of a mechanical hypothesis of branching morphogenesis (Ingber and Jamieson 1985; Ingber 2006). Mesenchymal cells attach to the surrounding extracellular matrix (ECM) and “pull” on its fibers. The attachment of mesenchymal cells to the ECM is essential for their survival, which means that these cells are constantly pulling on the ECM. As a result of the force generated by pulling, the ECM could be considered a prestressed structure, or a structure that is permanently in tension (Ingber and Jamieson 1985). According to this mechanical hypothesis, the local increase in proliferation that makes the epithelium invaginate, would be triggered through the tension force the mesenchyme exerts on the epithelium at those regions where the basement membrane thins (Fig. 5A). That is, the thickness of the basement membrane would act as a “lock” that either allows or blocks the mechanical interactions between the epithelium and the surrounding mesenchyme. A thick basement membrane that is well-structured and comprised of a high density of fibers and/or crosslinks would resist the mechanical forces exerted by the mesenchyme and thus block epithelial budding. In contrast, a thin basement membrane with a low density of fibers and/or crosslinks would enable shape changes, because it would be more pliable to the forces placed on it by the mesenchymal cells. Recent studies have shown the existence of a variety of cellular mechanisms underlying branching morphogenesis in different organs, and some of these indeed imply mechanical forces in the regulation of budding morphogenesis (e.g. Iber and Menshykau 2013; Varner and Nelson 2014).
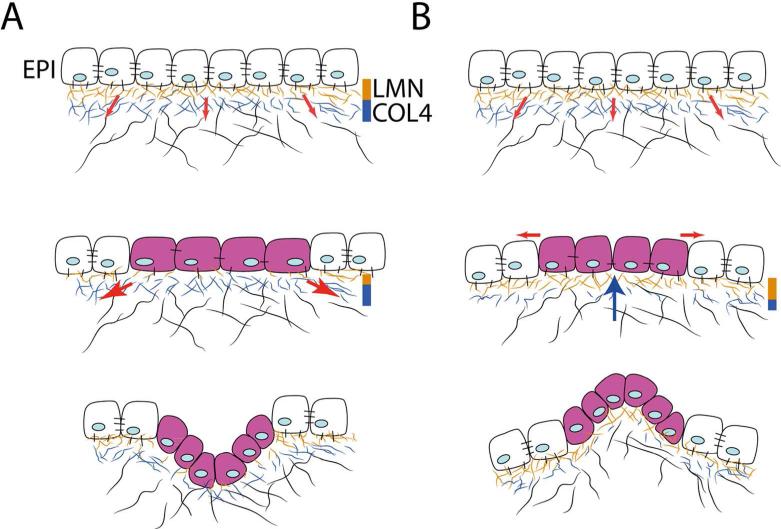
Fig. 5.
Mechanical model explaining epithelial invaginations and evaginations. (A) Top: Prior to budding, the basement membrane underlying the epithelium (EPI) is well-structured and contains abundant laminin (LMN) and collagen (COL4) fibers. Tension exerted (red arrows) on the extracellular matrix by the mesenchymal cells is resisted and the epithelium remains stabilized. Middle: At sites where laminin is reduced, epithelial cells are stretched (purple cells) due to tension exerted by mesenchymal cells (red arrows), which triggers proliferation. Bottom: At these sites the epithelium invaginates (adapted from Ingber 2006). (B) This diagram illustrates how the mechanical hypothesis described in A can be applied to explain evaginations. Top: The epithelium is stabilized by a well-formed basement membrane (see A Top). Middle: At sites where COL4 is reduced, tension exerted on the epithelium from the extracellular matrix is relaxed. At these sites, a “pushing” force (blue arrow) exerted by the growing mesenchyme may generate tension along the outer surface of the epithelium (red arrows), triggering proliferation (purple cells) or other cell behaviors (see Lao et at. 2015) Bottom: At these sites, the epithelium evaginates.
In contrast to branching organs where the epithelium invaginates to produce the sprouts, facial buds form as epithelial outgrowths or evaginations. How can the mechanical model of morphogenesis, in which epithelial invaginations are hypothesized to occur at sites where the basement membrane becomes thinner, be applied to understanding formation of epithelial evaginations? We hypothesize that different alterations of the basement membrane could determine where the epithelium forms an invagination or an evagination. The basement membrane forms between the epithelial cells and the interstitial matrix to which the mesenchymal cells attach. The epithelial cells anchor to a laminin-rich layer of the basement membrane, whereas the layer contacting the interstitial matrix is rich in collagen IV (COL4) (e.g., Paulsson 2008; Barber et al. 2014; Mouw et al. 2014). During morphogenesis, the basement membrane of branching buds and facial buds thin at regions where they are remodeled, but the thinning seems to occur differently in the two systems. In some branching organs, the basement membrane thins mainly by reduction of the laminin layer (e.g., Rebustini et al. 2007). In contrast, in the facial buds the basement membrane appears to become thinner by reduction of COL4, while laminin remains evenly distributed (Xu et al. 1990). This observation suggests that when the laminin layer, which helps stabilize the epithelium, becomes thinner, the tension exerted by the mesenchyme would pull the epithelium inward to produce an invagination. However, if the collagen layer that attaches the interstitial matrix to the basement membrane becomes thinner, then the tension on the matrix that is generated by the mesenchymal cells would not be transmitted to the epithelium and no invagination would occur. At these regions, a pushing force generated by the growing mesenchyme could help form an epithelial evagination (Fig. 5B).
CONCLUSIONS
In this commentary, we have critically assessed the widely studied model of undirected, regionalized cell proliferation during early stages of upper jaw formation, focusing on avian models. We argue that it is unlikely that this model alone can account for morphogenesis of the face. The undirected, regionalized cell proliferation model is at odds with new data showing the relevance of cell polarity in facial budding outgrowth (Li et al. 2013; Geetha-Loganathan et al. 2014), and also with the observation that an increase in cell proliferation leads not to premature lip fusion but, instead to clefting (Szabo-Rogers et al. 2008; Hu et al. 2015). Directed cell behaviors have recently been shown to underlie limb bud elongation, for which undirected, regionalized cell growth was the predominant hypothesis for decades (for a review see Hopyan et al. 2011). Similarly, regionalized cell proliferation has also been discarded as the principal cellular mechanism underlying branching morphogenesis (Nogawa et al. 1998; Iber and Menshykau 2013; Varner and Nelson 2014). Undirected, regionalized cell proliferation certainly contributes to facial morphogenesis, but the extent to which it participates in directing budding outgrowth of the face would require further examination.
The observation of cell polarization in the facial buds hints at the involvement of directed cell behaviors during early primary palate morphogenesis. However, much work would need to be done to better understand how directed cell behaviors contribute to the morphogenesis of the face. Identifying which cell behaviors, such as cell division, movement, or cell shape are polarized is an important step in understanding the role of these processes in facial morphogenesis. If cells are moving directionally, it is also relevant to determine whether they are actively moving or just passively displaced by the dividing cells and growing tissues that surround them. Further, how these behaviors, combined with the effects of cell proliferation, may impact the distribution of mechanical forces in the bud is unknown and could have significant consequences on facial morphogenesis. As illustrated by secondary palate elongation, more than one cellular mechanism may be involved in morphogenesis of an organ (Economou et al. 2013). Quantifying the contributions of each process would be necessary to better understand how they shape the facial prominences and how perturbations to those processes results in structural birth defects of the face.
We have argued for a specific potential role of the basement membrane in facial budding outgrowth. We have suggested how the mechanical hypothesis proposed to explain epithelial invaginations can be extended to explain the formation of the evaginations that form the face. According to current models, early facial budding outgrowth is a mesenchymal-driven process, i.e., the epithelium would play a passive role, simply expanding at those regions where cell proliferation in the mesenchyme is higher. However, as recently proposed by Lau et al. (2015) in the limb bud, the epithelium is a mechanically responsive tissue capable to contract in response to forces exerted by the mesenchyme, and therefore, capable to impinge direction to the process of budding outgrowth. It would be relevant to better understand the role of the epithelium in facial morphogenesis.
Epithelial-mesenchymal transformation is thought to be the mechanism by which alterations of matrix components lead to clefting in humans (Bueno et al. 2011, see also Pratt and King 1972; Bosi et al. 1998; Singh et al. 1998; Letra et al. 2007; Gagliano et al. 2010). However, if the role of the basement membrane in facial budding outgrowth is confirmed, it would provide a novel etiological cause for the formation of orofacial clefts. Under this framework, alterations in the basement membrane during early palate morphogenesis could alter the shape of the facial buds. Consequently, these shape changes could prevent the facial buds to physically contact to one another, leading to the formation of clefts. Understanding the full range of mechanisms that could be perturbed to produce CL/P is foundational to the important goal of individualizing treatments for patients. Such understanding may even help lead to interventions that diagnose and prevent CL/P before it occurs.
ACKNOWLEDGEMENTS
This work was supported by NIDCR R01-DE018234 to R.M. and R01-DE019638 to R.M. and B.H. We would like to thank the anonymous reviewers for their very constructive comments.
REFERENCES
- Abramyan J, Leung KJ, Richman JM. Divergent palate morphology in turtles and birds correlates with differences in proliferation and BMP2 expression during embryonic development. J Exp Zool Part B. 2014;322:73–85. doi: 10.1002/jez.b.22547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber T, Esteban-Pretel G, Marín MP, Timoneda J. Vitamin A deficiency and alterations in the extracellular matrix. Nutrients. 2014;6:4984–5017. doi: 10.3390/nu6114984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M. The basal lamina in epithelial-mesenchymal morphogenetic interactions. Upsala J Med Sci. 1977;82:111–112. doi: 10.3109/03009737709179091. [DOI] [PubMed] [Google Scholar]
- Boehm B, Westerberg H, Lesnicar-Pucko G, Raja S, Rautschka M, Cotterell J, Swoger J, Sharpe J. The role of spatially controlled cell proliferation in limb bud morphogenesis. PLoS Biol. 2010;8:e1000420. doi: 10.1371/journal.pbio.1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosi G, Evangelisti R, Valeno V, Carinci F, Pezzetti F, Calastrini C, Bodo M, Carinci P. Diphenylhydantoin affects glycosaminoglycans and collagen production by human fibroblasts from cleft palate patients. J Dent Res. 1998;77:1613–1621. doi: 10.1177/00220345980770080901. [DOI] [PubMed] [Google Scholar]
- Bush JO, Jiang R. Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development. 2012;139:231–243. doi: 10.1242/dev.067082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou AD, Brock LJ, Cobourne MT, Green JB. Whole population cell analysis of a landmark-rich mammalian epithelium reveals multiple elongation mechanisms. Development. 2013;140:4740–4750. doi: 10.1242/dev.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MW. Palate development. Development. 1988;103:41–60. doi: 10.1242/dev.103.Supplement.41. [DOI] [PubMed] [Google Scholar]
- Fritz JA, Brancale J, Tokita M, Burns KJ, Hawkins MB, Abzhanov A, Brenner MP. Shared developmental programme strongly constrains beak shape diversity in songbirds. Nat Commun. 2014;5:3700. doi: 10.1038/ncomms4700. [DOI] [PubMed] [Google Scholar]
- Gagliano N, Carinci F, Moscheni C, Torri C, Pezzetti F, Scapoli L, Martinelli M, Gioia M, Stabellini G. New insights in collagen turnover in orofacial cleft patients. Cleft Palate Craniofac J. 2010;47:393–399. doi: 10.1597/07-196.1. [DOI] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha-Loganathan P, Nimmagadda S, Fu K, Richman JM. Avian Facial Morphogenesis Is Regulated by c-Jun N-terminal Kinase/Planar Cell Polarity (JNK/PCP) Wingless-related (WNT) Signaling. J Biol Chem. 2014;289:24153–24167. doi: 10.1074/jbc.M113.522003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros J, Hu JK- H, Vinegoni C, Feruglio PF, Weissleder R, Tabin CJ. WNT5A/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. Curr Biol. 2010;20:1993–2002. doi: 10.1016/j.cub.2010.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H. Series of embryonic chicken growth. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hopyan S, Sharpe J, Yang Y. Budding behaviors: Growth of the limb as a model of morphogenesis. Dev Dyn. 2011;240:1054–1062. doi: 10.1002/dvdy.22601. [DOI] [PubMed] [Google Scholar]
- Hu D, Young NM, Li X, Xu Y, Hallgrímsson B, Marcucio RS. A dynamic Shh expression pattern, regulated by SHH and BMP signaling, coordinates fusion of primordia in the amniote face. Development. 2015;142:567–574. doi: 10.1242/dev.114835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber D, Menshykau D. The control of branching morphogenesis. Open Biol. 2013;3:130088. doi: 10.1098/rsob.130088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE, Jamieson JD. Cells as tensegrity structures: Architectural regulation of histodifferentiation by physical forces tranduced over basement membranes. In: Andersson L, Gahmberg C, Ekblom P, editors. Gene Expression During Normal and Malignent Differentiation. Academic; Orlando, FL: 1985. pp. 13–32. [Google Scholar]
- Ingber DE. Mechanical control of tissue morphogenesis during embryological development. Int J Dev Biol. 2006;50:255. doi: 10.1387/ijdb.052044di. [DOI] [PubMed] [Google Scholar]
- Jiang R, Bush JO, Lidral AC. Development of the upper lip: morphogenetic and molecular mechanisms. Dev Dyn. 2006;235:1152–1166. doi: 10.1002/dvdy.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K, Tao H, Liu H, Wen J, Sturgeon K, Sorfazlian N, Lazic S, Burrows JT, Wong MD, Li D, Deimling S, Scott I, Simmons C, Henkelman RM, Williams T, Hadjantonakis AK, Fernández-González R, Sun Y, Hopyan S. Anisotropic stress orients remodelling of mammalian limb bud ectoderm. Nat Cell Biol. 2015;17:569–579. doi: 10.1038/ncb3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letra A, Silva RA, Menezes R, Astolfi CM, Shinohara A, de Souza AP, Granjeiro JM. MMP gene polymorphisms as contributors for cleft lip/palate: association with MMP3 but not MMP1. Arch Oral Biol. 2007;52:954–960. doi: 10.1016/j.archoralbio.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Li X, Talts U, Talts JF, Arman E, Ekblom P, Lonai P. Akt/PKB regulates laminin and collagen IV isotypes of the basement membrane. PNAS. 2001;98:14416–14421. doi: 10.1073/pnas.251547198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Young NM, Tropp S, Hu D, Xu Y, Hallgrímsson B, Marcucio RS. Quantification of shape and cell polarity reveals a novel mechanism underlying malformations resulting from related FGF mutations during facial morphogenesis. Hum Mol Genet. 2013;22:5160–5172. doi: 10.1093/hmg/ddt369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucio RS, Young NM, Hu D, Hallgrimsson B. Mechanisms that underlie co-variation of the brain and face. Genesis. 2011;49:177–189. doi: 10.1002/dvg.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonnell IM, Clarke JDW, Tickle C. Fate map of the developing chick face: Analysis of expansion of facial primordia and establishment of the primary palate. Dev Dyn. 1998;212:102–118. doi: 10.1002/(SICI)1097-0177(199805)212:1<102::AID-AJA10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Minkoff R, Kuntz AJ. Cell proliferation during morphogenetic change; analysis of frontonasal morphogenesis in the chick embryo employing DNA labeling indices. J Embryol Exp Morp. 1977;40:101–113. [PubMed] [Google Scholar]
- Minkoff R, Kuntz AJ. Cell proliferation and cell density of mesenchyme in the maxillary process and adjacent regions during facial development in the chick embryo. J Embryol Exp Morp. 1978;46:65–74. [PubMed] [Google Scholar]
- Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014;15:771–785. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa H, Morita K, Cardoso WV. Bud formation precedes the appearance of differential cell proliferation during branching morphogenesis of mouse lung epithelium in vitro. Dev Dyn. 1998;213:228–235. doi: 10.1002/(SICI)1097-0177(199810)213:2<228::AID-AJA8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Patterson SB, Minkoff R. Morphometric and autoradiographic analysis of frontonasal development in the chick embryo. Anat Rec. 1985;212:90–99. doi: 10.1002/ar.1092120113. [DOI] [PubMed] [Google Scholar]
- Paulsson M. Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol. 2008;27:93–127. doi: 10.3109/10409239209082560. [DOI] [PubMed] [Google Scholar]
- Pratt RM, King CTG. Inhibition of collagen cross-linking associated with β-aminopropionitrile-induced cleft palate in the rat. Dev Biol. 1972;27:322–328. doi: 10.1016/0012-1606(72)90171-6. [DOI] [PubMed] [Google Scholar]
- Rebustini IT, Patel VN, Stewart JS, Layvey A, Georges-Labouesse E, Miner JH, Hoffman MP. Laminin α5 is necessary for submandibular gland epithelial morphogenesis and influences FGFR expression through α1 integrin signaling. Dev Biol. 2007;308:15–29. doi: 10.1016/j.ydbio.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Seki R, Noro M, Yokoyama H, Tamura K. Morphogenetic change of the limb bud in the hand plate formation. J Exp Zool Part B. 2010;314:539–551. doi: 10.1002/jez.b.21359. [DOI] [PubMed] [Google Scholar]
- Singh GD, Johnston J, Ma W, Lozanoff S. Cleft palate formation in fetal Br mice with midfacial retrusion: tenascin, fibronectin, laminin, and type IV collagen immunolocalization. Cleft Palate Craniofac J. 1998;35:65–76. doi: 10.1597/1545-1569_1998_035_0065_cpfifb_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Szabo-Rogers HL, Geetha-Loganathan P, Nimmagadda S, Fu KK, Richman JM. FGF signals from the nasal pit are necessary for normal facial morphogenesis. Dev Biol. 2008;318:289–302. doi: 10.1016/j.ydbio.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Varner VD, Nelson CM. Cellular and physical mechanisms of branching morphogenesis. Development. 2014;141:2750–2759. doi: 10.1242/dev.104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Shen JY, Widelitz RB, Chuong C- M. Morphoregulation of avian beaks: comparative mapping of growth zone activities and morphological evolution. Dev Dyn. 2006;235 doi: 10.1002/dvdy.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyngaarden LA, Vogeli KM, Ciruna BG, Wells M, Hadjantonakis AK, Hopyan S. Oriented cell motility and division underlie early limb bud morphogenesis. Development. 2010;137:2551–2558. doi: 10.1242/dev.046987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Parker SB, Minkoff R. Distribution of type IV collagen, laminin, and fibronectin during maxillary process formation in the chick embryo. Am J Anat. 1990;187:232–246. doi: 10.1002/aja.1001870303. [DOI] [PubMed] [Google Scholar]
- Young NM, Hu D, Lainoff AJ, Smith FJ, Diaz R, Tucker AS, Trainor PA, Schneider RA, Hallgrímsson B, Marcucio RS. Embryonic bauplans and the developmental origins of facial diversity and constraint. Development. 2014;141:1059–1063. doi: 10.1242/dev.099994. [DOI] [PMC free article] [PubMed] [Google Scholar]