Abstract
Objective
Co-infection with hepatitis C (HCV) is a major cause of morbidity and mortality among individuals with human immunodeficiency virus (HIV). Our objective was to assess the prognostic performance of non-invasive measures of liver fibrosis in predicting all cause mortality in women with HIV/HCV coinfection
Design
We studied HCV/HIV coinfected women enrolled in the prospective, multicenter Women’s Interagency HIV Study. APRI and FIB-4 were used to identify women without fibrosis at all visits and women who progressed to severe fibrosis.
Methods
Enhanced Liver Fibrosis (ELF), which utilizes direct measures of fibrosis, Hyaluronic Acid, Procollagen III aminoterminal peptide and Tissue Inhibitor of Matrix metalloproteinase was performed.
Results
Included were 381 women with 2296 ELF measurements, with mean follow up 8.3 ± 3.3y. There were 134 deaths (60% with severe liver fibrosis). Receiver operator characteristic curves at fixed time windows prior to death or at end of follow-up showed that ELF was best at predicting mortality when tested within a year of death (Area under the curve for ELF 0.85 vs. APRI 0.69, p<0.0001 and vs FIB-4 0.75, p=0.0036); and 1–3 years prior (ELF 0.71 vs. APRI 0.61, p=0.005 and vs FIB-4 0.65, p=0.06). Use of all three measures did not improve on ELF alone. In multivariate logistic regression models controlling for CD4 count, HIV viral load, antiretroviral use and age, ELF continued to perform better than APRI and FIB-4.
Conclusions
ELF predicted all cause mortality and was superior to APRI and FIB-4 in HIV/HCV co-infected women.
Keywords: liver fibrosis, non invasive markers, Hyaluronic Acid, Procollagen III aminoterminal peptide, Tissue Inhibitor of Matrix metalloproteinase
Co-infection with HCV leads to significant morbidity and mortality in HIV infected individuals(1). Assessment of liver disease severity, including the degree of hepatic fibrosis, is important in counselling patients with HCV and determining the need for treatment. Efforts have focused on the identification of non-invasive methods of assessing fibrosis that avoid the limitations of liver biopsy, which is costly, invasive and does not always stage fibrosis accurately(2, 3). Non invasive methods include serum markers and transient elastography. Transient elastography has been shown to predict mortality in HIV/HCV coinfected patients but cannot be studied retrospectively(4, 5). We have previously found that both APRI and FIB-4 are independently associated with all-cause mortality in HIV/HCV co-infected women in Women’s Interagency HIV Study (WIHS)(6). Although both tests are readily available, they are less sensitive and not specific as they lack biological plausibility as they measure markers of inflammation (alanine aminotransferase (ALT), aspartate aminotransferase (AST)) and platelets rather than so called “direct markers” of fibrosis, components of liver matrix and mediators of matrix remodelling. These latter components are incorporated in the Enhanced Liver Fibrosis test, which uses Hyaluronic Acid (HA), Procollagen III aminoterminal peptide (PIIINP) and Tissue Inhibitor of Matrix metalloproteinase (TIMP-1)(7, 8). The aim of this study was to assess the performance of ELF relative to other non-invasive markers in predicting all cause mortality in women with HIV and HCV co-infection. We chose ELF as it has been validated in HCV monoinfection and other chronic liver diseases; it utilizes direct measures of extra cellular matrix components as noted above; and it has not yet been studied in HIV infected individuals (7, 9). Accurate serum markers of fibrosis in HIV and HCV coinfection would allow for more frequent measurements without requiring invasive and costly procedures. We chose all cause mortality as prior work noted that death certificates have significant limitations and liver disease may have been the underlying or contributing cause of septic death, renal death or multisystem organ failure.(6)
Patients and Methods
Human Subjects
We studied participants of the WIHS, the largest NIH-funded, prospective, multicenter cohort of women at risk for, or currently diagnosed with HIV(10). This study was approved by the WIHS Executive Committee, and the Institutional Review Boards at the six participating WIHS study sites, including the University of California San Francisco Institutional Review Board. Enrolment of women in this study took place during 1994–1995 and again in 2001–2002. WIHS participants are seen twice yearly and undergo detailed histories, physical exams, structured interviews, laboratory testing and storage of serum samples. Study eligibility included women with HIV/HCV co-infection at WIHS study entry as defined by detectable serum HCV RNA, positive HCV antibody, and positive HIV by Western blot and at least one visit with available serum measures for AST, ALT and platelets. Biomarkers of fibrosis from all eligible women who died and from eligible women who were alive at last follow-up visit and exhibited either severe or no/minimal fibrosis by APRI and FIB-4 were compared. Availability of specimen for ELF measurement was also required for inclusion. HCV infection was documented by testing for antibody to HCV by second or third-generation EIA (Ortho-Diagnostic Systems, Rochester, NY) and testing for the presence of HCV RNA by HCV branched DNA (Quantiplex 2.0 branched chain DNA-enhanced label amplification assay; Chiron, Emeryville, CA) and by RT-PCR (COBAS Amplicor HCV Detection Kit; Roche Diagnostic Systems, Pleasanton, CA). HIV RNA was measured using the isothermal nucleic acid sequence based amplification method (NASBA/Nuclisens, bioMerieaux, San Diego CA) with a detection limit of 80 copies/ml. Hepatitis B virus status was assessed via testing for the surface antigen (HBsAg) within the first year of entry into WIHS. The definition of highly active antiretroviral therapy (HAART) was based on the U.S. Department of Health and Human Services treatment guidelines (www.aidsinfo.gov) as previously described(6).
Serum markers of Fibrosis
ELF (Siemens Healthcare Diagnostics, Tarrytown, New York) utilizes direct serum measures of extracellular matrix components: HA, PIIINP and TIMP-1 were assayed on an automated IMMUNO 1 immunoanalyzer (Siemens Medical Solutions Diagnostics, Tarrytown, NY, USA). The assays are magnetic particle separation immunoassays and were identical to those used for the 2004 European Liver Fibrosis study(8). The TIMP-1 and PIIINP assays each use two monoclonal antibodies (MAbs) that bind to independent binding sites on their respective antigens. The HA assay uses HA binding protein, which is isolated from bovine nasal septum, in the place of MAbs. The ELF markers were analysed individually, and the results continually refereed to a set of quality standards to ensure accurate analysis. Tests were performed according to the manufacturer’s instructions at iQur Limited, London, UK. We also used two indirect markers of liver fibrosis: APRI(11) and FIB-4(12) with upper limit of normal for AST and ALT designated as 40 U/L. These measures can be calculated using readily available patient and laboratory data [AST, ALT, platelet count (109/L) and age] as described(11, 12). We defined women who had progressed to severe fibrosis by APRI >1.5 and FIB-4 >3.25 as previously described(11, 12); those women with minimal or no liver fibrosis at all WIHS visits were defined as having APRI <0.5 and FIB-4 <1.5. Moderate fibrosis was defined as APRI >0.5 but <1.5 and FIB-4 >1.5 but <3.25 as previously described(11, 12). ELF testing was performed on stored serum samples collected every 2 years during WIHS.
Statistical Analysis
The performance of each marker was assessed by receiver operator characteristic (ROC) curves showing sensitivity and specificity for predicting death. We evaluated all fibrosis markers as predictors of all cause mortality to compare their performance using the area under the curve (AUC). AUCs for each fibrosis marker were compared as predictors of mortality within one year, 1–3y, 3–5y, or 5–7y before our last observation for each woman. We also constructed linear logistic regression models using all three fibrosis markers (ELF, FIB-4 and APRI) to determine whether a linear combination of markers performed better than any one marker. We tested for statistically significant differences between the curves using the methods implemented in PROC LOGISTIC of SAS 9.2 (SAS Institute, Cary, NC)(13). Because differences in predictive power might arise from a serum marker’s sensitivity to processes unrelated to liver disease but related to mortality, multivariate logistic regression models were developed, adjusting for CD4, HIV viral load, antiretroviral (HAART) use and age. We believed that these were the four available variables that could plausibly influence both fibrosis markers and risk of death. Results from logistic regression employed fibrosis markers that were dichotomized as severe or non-severe fibrosis using standard cutoff values of 10.43 for ELF, 3.25 for FIB-4 and 1.5 for APRI as described(11, 12, 14).
Results
Cohort characteristics
We included 381 HIV/HCV coinfected women with a mean follow up of 8.3 ± 3.3 years. Table 1 shows the characteristics of all HIV/HCV co-infected women at last available visit. One hundred and thirty four of these women died during the study. Mean duration of follow up was shorter for women who died (7.9 ± 3.3y) than for women who were alive at last visit (8.6 ± 3.3y). Overall, the majority (63.0%) of women were African American. 44.4% of women had hypertension, 6.6% reported injecting drugs and 1.3% were HBsAg positive. These HIV/HCV coinfected women had modest elevations of AST and ALT. AST was higher among women who died. Those who died had poorer HIV control, as shown by higher last visit (p<0.001) and peak (p=0.013) HIV viral loads, lower CD4 count at last visit (p<0.001) and nadir (p<0.001), and less use of ART (p=0.0025). They also had lower BMI (p<0.0001), worse renal disease (as measured by both estimated estimated Glomerular Filtration Rate (eGFR, p=0.014) and the proportion of women with eGFR <60 ml/minute: p<0.0001). The primary cause of death reported on the death certificate was AIDS in 37 women; infection in 24 (16 lung, 1 neurologic, 7 unspecified); liver related in 18; cancer in 13; alcohol and drug abuse in 11; other [cardiac (6), renal (6), multiorgan (2), gastrointestinal (2) and one each pulmonary hypertension, neurologic, accident and psychiatric]; and HIV positive indeterminate cause in 11. No data were available about secondary causes of death. Only 6.2% of women had ever received HCV therapy at the time of this analysis.
Table 1.
Demographics at Last Visit of 381 HCV/HIV Coinfected Women of whom 134 Died
| Variable | ALL (n=381) |
Alive (n=247) |
Died (n=134) |
P value | |
|---|---|---|---|---|---|
| Age y (mean ± SD) | 49.4 ± 6.1 | 49.8 ± 6.0 | 48.8 ± 6.2 | 0.15 | |
| Race self described, (N) | 0.81 | ||||
| White | 58 | 39 | 19 | ||
| Hispanic | 77 | 48 | 29 | ||
| African American | 240 | 157 | 83 | ||
| Log10 HCV RNA IU/mL (Mean ± SD) | 6.2 ± 0.9 | 6.1 ± 0.8 | 6.3 ± 1.0 | 0.042 | |
| Serum AST U/L (Mean ± SD) | 61.3 ± 48.3 | 56.2 ± 42.6 | 70.6 ± 56.3 | 0.017 | |
| Serum ALT U/L(Mean ± SD) | 44.5 ± 35.3 | 46.1 ± 36.9 | 41.6 ± 32.2 | 0.26 | |
| HCV therapy (any, ever) | 6.2% | 7.1% | 4.2% | 0.33 | |
| Hepatitis B infection | 1.3% | 1.2% | 1.5% | 0.82 | |
| Log10 HIV VL (mean ± SD) copies/mL | 3.0 ± 1.2 | 2.7 ± 1.1 | 3.5 ± 1.3 | <0.001 | |
| Peak log10 HIV VL (mean ± SD) copies/mL | 4.8 ± 0.8 | 4.7 ± 0.8 | 4.9 ± 0.9 | 0.013 | |
| CD4 cells/mm3(mean ± SD) | 410.5 ± 305.6 | 474.7 ± 298.2 | 292.1 ± 284.0 | <0.0001 | |
| CD4 nadir cells/mm3(mean±SD) | 190.5 ± 152.9 | 209.9 ± 152.2 | 154.9 ± 148.1 | <0.0001 | |
| On HAART | 59.6% | 65.2% | 49.2% | 0.0025 | |
| BMI (Mean ± SD) | 26.0 ± 6.7 | 26.9 ± 6.5 | 24.4 ± 7.0 | <0.0001 | |
| Last eGFR (ml/min Mean ± SD) | 83.5 ± 29.5 | 87.2 ± 25.0 | 76.5 ± 35.5 | 0.014 | |
| Chronic Renal Disease (EFGR2<60 ml/min) | 22.0% | 15.4% | 32.8% | <0.0001 | |
| Diabetes (%) | 12.1% | 9.7% | 16.4% | 0.055 | |
HAART= highly active antiretroviral therapy; eGFR= estimated glomerular filtration rate
Fibrosis assessment
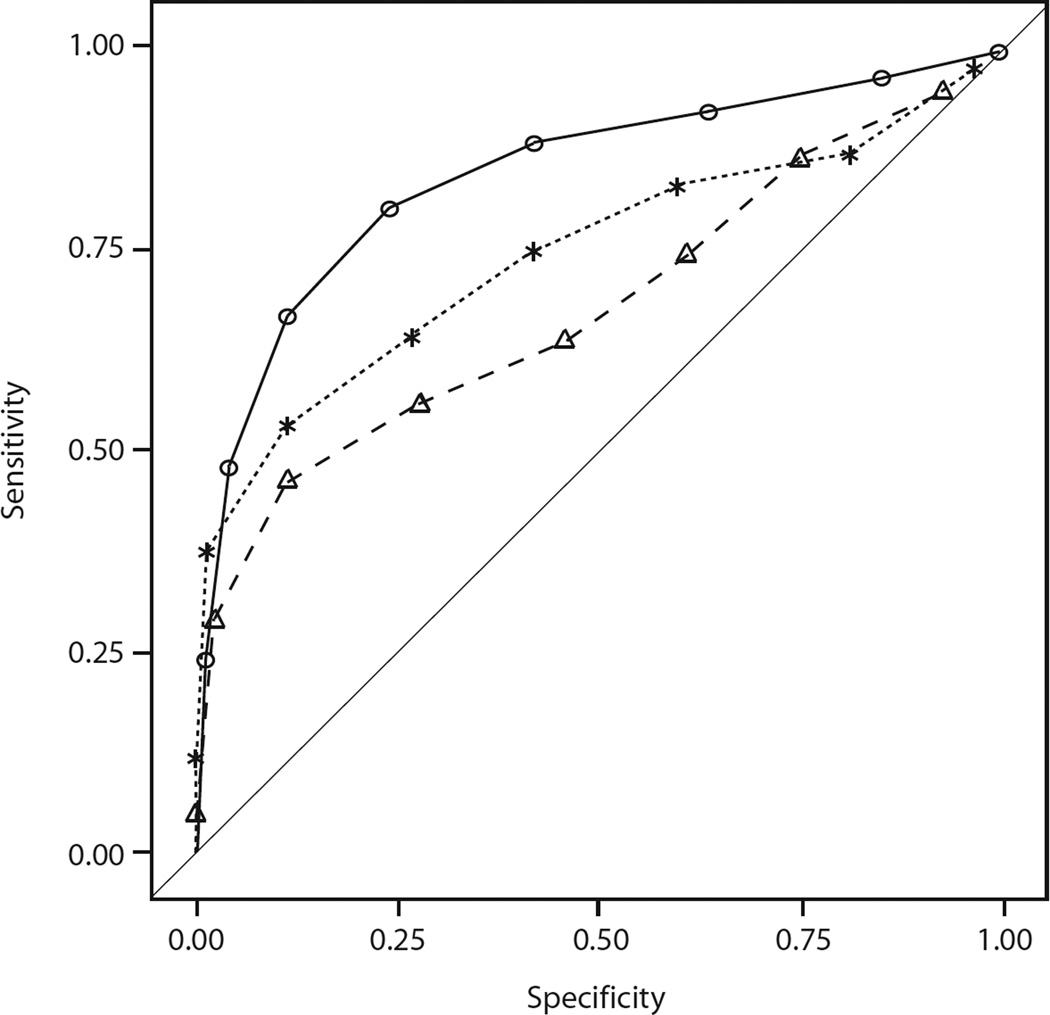
There were 2296 ELF measurements among 381 women: 270 women had no/ mild fibrosis; 33 had intermediate fibrosis and 78 had severe fibrosis. Nearly all (96.3%) women had 3 or more ELF measurements, and 60% had 6 or more ELF measurements. Figure 1 shows the ROC curves at year of death or last visit. ELF was best at predicting all cause mortality with AUC of 0.85, compared to AUC for FIB-4 of 0.75 (p=0.004 ELF vs FIB-4) and AUC for APRI of 0.69 (p<0.001 ELF vs APRI). The AUC for the model, which utilized all three markers, was 0.84, no better than ELF alone. At 2 years prior to death, ELF (AUC 0.71) continued to predict all cause mortality better than FIB-4 (AUC 0.65; p=0.06) or APRI (AUC 0.61; p=0.005). However, its accuracy waned at 4 years (ELF AUC 0.67) and 6 years (ELF AUC 0.61) and it was not substantially better than FIB-4 or APRI at these times points. These data are shown in Supplemental Table 1.
Figure 1.
shows the ROC curves of various fibrosis markers at year of death or last visit in WIHS women with HIV/HCV coinfection. The curves represent ELF (open circles), FIB-4 (asterisks) and APRI (open triangles).
The performance of the different predictors in univariate analyses and in multivariate analyses, adjusting for ART, CD4, HIV VL and age are shown in Table 2. ELF was the only marker whose prediction of death remained statistically significant at all time periods. These results remained consistent whether CD4 was used as a continuous variable (shown) or tricotomized with cutpoints of either <100, >100 −350 and >350, or <200, >200–500 and >500 (data not shown). For predicting death within one year, ELF was the only marker whose effect increased with the addition of the other factors, from an OR of 12.2 to 15.0. At 2 and 4 years prior to death, ELF performed best, but all markers' effects declined when adjusted for the other factors (Table 2 and Supplemental Table 1). At 6 years prior to death, ELF again was the only measure whose magnitude of effect increased on adjustment for other factors (Table 2). The percentage of women with severe fibrosis was greatest at year of death. HCV treatment did not have p<0.05 when added to any of the multivariate models in Table 2, and ELF continued to have larger odds ratios than APRI or FIB-4 in those models.
Table 2.
Analyses of Fibrosis Markers as Predictors of All Cause Mortality in HIV/HCV coinfected women
| Years before Final Observation# |
Fibrosis Marker |
% with severe fibrosis |
Univariate Analysis (OR, 95% CI) |
p value | Multivariate Analysis* (OR, 95% CI) |
p value |
|---|---|---|---|---|---|---|
| 0 | ELF | 42.9 | 12.23 (5.63–26.55) | <0.0001 | 15.01 (5.42– 41.61) | <0.0001 |
| 0 | FIB-4 | 31.8 | 8.89 (3.88– 20.37) | <0.0001 | 6.82 (2.58–18.03) | <0.0001 |
| 0 | APRI | 27.9 | 6.45 (2.81–14.79) | <0.0001 | 4.86 (1.77–13.34) | 0.0022 |
| 2 | ELF | 32.3 | 5.19 (3.04 –8.88) | <0.0001 | 4.66 (2.60–8.37) | <0.0001 |
| 2 | FIB-4 | 25.5 | 2.79 (1.61–4.83) | 0.0003 | 2.41 (1.30– 4.47) | 0.0051 |
| 2 | APRI | 17.0 | 2.09 (1.12–3.92) | 0.021 | 1.90 (0.95– 3.80) | 0.0712 |
| 4 | ELF | 22.1 | 3.10 (1.77– 5.44) | <0.0001 | 2.72 (1.46– 5.06) | 0.0016 |
| 4 | FIB-4 | 24.7 | 2.71 (1.58–4.66) | 0.0003 | 2.33 (1.26– 4.31) | 0.0068 |
| 4 | APRI | 13.7 | 2.34 (1.20–4.55) | 0.0126 | 1.92 (0.91– 4.09) | 0.0885 |
| 6 | ELF | 19.0 | 2.48 (1.37–4.49) | 0.0028 | 2.55 (1.36–4.76) | 0.0034 |
| 6 | FIB-4 | 19.3 | 1.99 (1.09–3.60) | 0.0239 | 1.62 (0.85– 3.10) | 0.1449 |
| 6 | APRI | 16.0 | 2.68 (1.42–5.04) | 0.0023 | 2.37 (1.22– 4.60) | 0.0107 |
Years before Final Observation: rows labeled as 0 refer to measurements within one year of the final observation; those labeled 2, 4 and 6 are 1–3 years, 3–5 years and 5–7 years respectively before final observations. At year of death 154 women contributed data; 282 women at 2 years; 299 women at 4 years; and 300 women at 6 years from death.
Multivariate analysis adjusting for log10 CD4 count (current and nadir CD4), and log10 HIV viral load, HAART use, and age as a continuous variable.
Results from logistic regression employed fibrosis markers that were dichotomized as severe or non-severe fibrosis using standard cutoff values of 10.43 for ELF, 3.25 for FIB-4 and 1.5 for APRI.
Discussion
This study showed that in a large cohort of women with HIV and HCV coinfection, ELF was better in predicting all cause mortality than APRI and FIB-4. Using all three measures did not improve on the predictive value of ELF alone. To determine whether this superior performance was a consequence of ELF’s sensitivity to non-liver related causes of comorbidity, we compared the predictors’ performance with and without adjusting for CD4 count, HIV viral load, HAART use and age in logistic regression models. In these multivariate models, ELF continued to have higher Odds Ratios than APRI and FIB-4 up to 6 years prior to death, but it was not superior after 3 years prior to death in univariate ROC curve analyses. We have previously shown that two indirect markers of hepatic fibrosis, APRI and FIB-4 scores, were independently predictive of mortality in 450 WIHS HIV/HCV co-infected women on HAART(6). Others have shown that APRI and FIB-4 are predictive of liver fibrosis in HIV HCV coinfected subjects but most accurate for diagnosis of cirrhosis(15). These tests are currently widely available in clinical practice as they are derived from algorithms that combine commonly measured serum analytes. Nevertheless, they were found to be inferior to ELF in this current study. We performed an analysis to address the possibility of selection bias with a model weighted by the sampling fractions in women with different degrees of fibrosis defined by APRI and FIB-4. This sensitivity analysis provided similar results, which is evidence against selection bias accounting for the apparent superiority of ELF over APRI and FIB-4.
Tissue injury leads to ongoing extracellular matrix remodelling with fibrogenesis and the production of proteins such as TIMP and fibrolysis with degradation by metalloproteinases(16). If fibrogenesis exceeds fibrolysis, then fibrosis is the net result(17). Mild fibrosis leads to mild functional compromise but with ongoing progression, cirrhosis develops with elevation of portal pressure, shunting of blood from the liver and impaired synthetic and metabolic activity. The assessment of the extent of fibrosis is much more difficult in the absence of the clinical findings of advanced disease. In the US, liver biopsy has been considered the gold standard for determining the degree of hepatic fibrosis. But we have previously estimated that only 37% of samples from patients with true stage 3 fibrosis were accurately classified by liver biopsy(3, 18). In addition, liver biopsy is an invasive and costly, which limits the frequency of measurements, further compromising its utility as a monitoring tool. Fewer measurements also make it difficult to average out measurement error. Even liver biopsy performs poorly in the differentiation of minor and moderate degrees of fibrosis(18). WIHS is an observational cohort and few women had liver biopsies. We assessed the three serum fibrosis markers against all cause mortality rather than liver biopsy because of these limitations. Since we studied stored serum samples, we could not assess transient elastography as another non-invasive assessment of liver fibrosis. The analytes measured by ELF are stable over protracted storage at −70 (Siemens IFU and data on file). An advantage of serum based tests over transient elastography is the ability to study stored serum samples, to replicate frequently and lack of failure to test that is reported with transient elastography. Transient elastography is increasingly being used as an alternative to liver biopsy in many centers and its association with mortality in HIV/HCV coinfected individuals has been demonstrated.(5, 19). Because we studied subjects retrospectively, we did not have such data on transient elastography. Transient elastography is subject to a failure rate of 5–15% in the best hands and is influenced by inflammation, obesity and inter-operator variability(20, 21). None of these are true for ELF, making it more amenable to routine use in both hospital and community based settings. In a number of published studies, equivalent performance has been reported for ELF and LSM (when successful), and in “intention to diagnose” analyses, ELF outperforms LSM(21–23).
As noted in previous work, death certificates have certain limitations and liver disease may have been the underlying cause of septic death, renal death or multisystem organ failure but we were not able to verify this.(6) As noted in Table 2, a higher proportion of women who died had severe fibrosis, suggesting that liver fibrosis may have played a role even in women reported to have died from HIV or non liver causes. For this reason, we assessed all cause mortality and showed that ELF was the best predictor of death in these HIV/HCV coinfected women.
Serum markers of fibrosis have been studied for some years and different tests measure a variety of proteins including acute phase reactants; proteins of fibrinolysis and fibrogenesis; as well as breakdown products of inflammatory response or collagen deposition(9, 11, 24, 25). The ideal measure of fibrosis in serum should be an accurate measure of the amount of liver fibrosis, and should to be able to distinguish between stages of fibrosis and predict the onset of complications of fibrosis. It should be reproducible, validated in large cohorts, and able to be monitored over time. ELF has been shown to be of prognostic value for both all-cause, and liver-related morbidity and mortality in a range of liver diseases including hepatitis C but HIV patients were not included(7). Our results are similar to those reported in HCV monoinfected individuals(12,5). ELF has also been shown to be useful in assessing fibrosis progression and clinical liver decompensation in different types of liver disease, including hepatitis C, primary biliary cirrhosis and nonalcoholic fatty liver disease(14, 26, 27). However many of these studies did not include women and this is the first study showing that ELF predicts death in HIV/HCV co-infected individuals. ELF could be useful in stratifying patients to identify those HCV patients at greatest and most immediate risk of liver decompensation and in need of new all oral HCV therapies.
Supplementary Material
Acknowledgments
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange).
Sources of support
This work is supported by the National Institute of Allergy and Infectious Diseases (NIAID: R21 AI08835 1to MGP and NIAID R01 AI087176 to PCT). The WIHS is funded by NIAID (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590;) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co- funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
WMR is an inventor of the ELF test, which is owned by Siemens. He is a consultant for Siemens but receives no income from ELF testing.
Footnotes
MGP, PB, JP and WR were involved in all aspects of the study: design, data and statistical analyses, and writing of the manuscript. RB was involved in statistical analyses and critical review of the results and manuscript. ALF, PCT, MWP, MJG, MA, ETG, RK were involved in data collection, and critical review of the results and manuscript.
Financial disclosure: Other authors have no conflicts of interest with regard to the work.
References
- 1.Weber R, Sabin CA, Friis-Moller N, Reiss P, El Sadr WM, Kirk O, Dabis F, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 2.Bacchetti P, Boylan R, Astemborski J, Shen H, Mehta SH, Thomas DL, Terrault NA, et al. Progression of biopsy-measured liver fibrosis in untreated patients with hepatitis C infection: non-Markov multistate model analysis. PLoS ONE. 2011;6:e20104. doi: 10.1371/journal.pone.0020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am. J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 4.Macias J, Giron-Gonzalez JA, Gonzalez-Serrano M, Merino D, Cano P, Mira JA, Arizcorreta-Yarza A, et al. Prediction of liver fibrosis in human immunodeficiency virus/hepatitis C virus coinfected patients by simple non-invasive indexes. Gut. 2006;55:409–414. doi: 10.1136/gut.2005.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Montero JV, Barreiro P, Vispo E, Labarga P, Sanchez-Parra C, Soriano V. Liver stiffness predicts liver-related complications and mortality in HIV patients with chronic hepatitis C on antiretroviral therapy. Aids. 2013;27:1129–1134. doi: 10.1097/QAD.0b013e32835e063f. [DOI] [PubMed] [Google Scholar]
- 6.Bambha K, Pierce C, Cox C, French AL, Tien PC, Sharp GB, Augenbraun M, et al. Assessing mortality in women with hepatitis C virus and HIV using indirect markers of fibrosis. AIDS. 2012;26:599–607. doi: 10.1097/QAD.0b013e32834fa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkes J, Roderick P, Harris S, Day C, Mutimer D, Collier J, Lombard M, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010 doi: 10.1136/gut.2009.203166. [DOI] [PubMed] [Google Scholar]
- 8.Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol. 2006;44:462–474. doi: 10.1016/j.jhep.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 10.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 11.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 12.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, Sulkowski S, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 13.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 14.Parkes J, Guha IN, Roderick P, Harris S, Cross R, Manos MM, Irving W, et al. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat. 2010 doi: 10.1111/j.1365-2893.2009.01263.x. [DOI] [PubMed] [Google Scholar]
- 15.Resino S, Asensio C, Bellon JM, Carmona R, Miralles P, Lopez JC, Cosin J, et al. Diagnostic accuracy of the APRI, FIB-4, and the Forns index for predicting liver fibrosis in HIV/HCV-coinfected patients: a validation study. J Infect. 2011;63:402–405. doi: 10.1016/j.jinf.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 17.Hui AY, Friedman SL. Molecular basis of hepatic fibrosis. Expert. Rev. Mol. Med. 2003;2003:1–23. 1–23. doi: 10.1017/S1462399403005684. [DOI] [PubMed] [Google Scholar]
- 18.Bacchetti P, Boylan R. Estimating complex multi-state misclassification rates for biopsy-measured liver fibrosis in patients with hepatitis C. Int J Biostat. 2009;5 doi: 10.2202/1557-4679.1139. Article 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macias J, Camacho A, Von Wichmann MA, Lopez-Cortes LF, Ortega E, Tural C, Rios MJ, et al. Liver stiffness measurement versus liver biopsy to predict survival and decompensations of cirrhosis among HIV/hepatitis C virus-coinfected patients. Aids. 2013;27:2541–2549. doi: 10.1097/QAD.0b013e32836381f3. [DOI] [PubMed] [Google Scholar]
- 20.Castera L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828–835. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 21.Castera L, Winnock M, Pambrun E, Paradis V, Perez P, Loko MA, Asselineau J, et al. Comparison of transient elastography (FibroScan), FibroTest, APRI and two algorithms combining these non-invasive tests for liver fibrosis staging in HIV/HCV coinfected patients: ANRS CO13 HEPAVIH and FIBROSTIC collaboration. HIV Med. 2014;15:30–39. doi: 10.1111/hiv.12082. [DOI] [PubMed] [Google Scholar]
- 22.Deuffic-Burban S, Deltenre P, Buti M, Stroffolini T, Parkes J, Muhlberger N, Siebert U, et al. Predicted effects of treatment for HCV infection vary among European countries. Gastroenterology. 2012;143:974–985. e914. doi: 10.1053/j.gastro.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 23.Wahl K, Rosenberg W, Vaske B, Manns MP, Schulze-Osthoff K, Bahr MJ, Bantel H. Biopsy-controlled liver fibrosis staging using the enhanced liver fibrosis (ELF) score compared to transient elastography. PLoS One. 2012;7:e51906. doi: 10.1371/journal.pone.0051906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, Bruguera M, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 25.Kelleher TB, Mehta SH, Bhaskar R, Sulkowski M, Astemborski J, Thomas DL, Moore RE, et al. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J Hepatol. 2005;43:78–84. doi: 10.1016/j.jhep.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, Newman D, Vizzutti F, et al. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology. 2009;136:160–167. doi: 10.1053/j.gastro.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Mayo MJ, Parkes J, Adams-Huet B, Combes B, Mills AS, Markin RS, Rubin R, et al. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology. 2008;48:1549–1557. doi: 10.1002/hep.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.