In distinction to anatomical and physiological imaging, molecular imaging aims to illuminate biological processes in vivo via sophisticated imaging agents targeted to key molecules and cells. As most disease processes have a biological basis, molecular imaging offers the following abilities: 1) to study precisely the role of important molecules and cells in a disease process in vivo, in serial fashion; 2) to evaluate the biological effects of molecular and cellular therapeutics; and 3) to refine risk prediction and risk stratification by incorporating biological information into traditional clinical assessments. Given the inability to routinely obtain tissue for biological analyses in many cardiovascular disease (CVD) conditions, molecular imaging approaches are anticipated to play an important role in CVD assessment.
In this inaugural review of the year in molecular imaging, we present important contributions chronologically, focusing on 4 areas: 1) atherosclerosis, 2) myocardial infarction, 3) stem cell therapy, and 4) multimodality imaging and theranostics. We close with an update on the clinical translation of this technology to CVD patients.
Novel Imaging Probes and Biological Applications
In the past several years, advances in molecular imaging agent and hardware technology have enabled rapid growth of experimental CVD investigations. An array of imaging probes are available for cardinal biological processes, including inflammation, angiogenesis, apoptosis, oxidative stress, calcification, cellular trafficking, and reporter gene expression. In addition, imaging agents now provide readouts for many clinical CVD imaging modalities including cardiac magnetic resonance (CMR), positron emission tomography (PET), single-photon emission computed tomography (SPECT), optical, ultrasound, and computed tomography (CT) imaging. The following sections highlight novel and substantive advances in the past year of preclinical molecular imaging studies of CVD. A number of promising agents/ approaches are expected to translate into the clinical arena.
Atherosclerosis
Atherosclerosis is a chronic inflammatory and lipid deposition disorder (1). Early atheromata develop in areas of activated endothelium that attract and bind circulating monocytes. Resident monocytes then differentiate into plaque macrophages that scavenge oxidized phospholipids and evolve into foam cells. Macrophages secrete proinflammatory mediators that may weaken the fibrous cap. Over time, apoptosis of macrophages promotes formation of a necrotic core and subsequent plaque expansion. Ultimately, this environment may promote fibrous cap disruption on consequent thrombosis leading to myocardial infarction, stroke, and ischemic limbs.
Molecular imaging can visualize the critical biological aspects that are otherwise undetected by conventional imaging methods. In addition to unraveling the molecular and cellular biology of atherogenesis in vivo, this approach may better identify high-risk plaques and patients and could offer refined risk stratification. In addition, as most atherosclerosis therapeutics possess a biological mechanism of action, molecular imaging offers a new approach to assess biological efficacy of atherosclerosis therapies.
Monocyte chemotactic protein (MCP)-1 detection with noninvasive radionuclide imaging
The macrophage MCP-1 peptide is up-regulated in atheromata and mediates monocyte recruitment to the vessel wall via its cognate chemokine (C-C motif) receptor 2 (CCR2). Hartung et al. (2) employed technetium Tc 99m–labeled recombinant MCP-1 (99mTc-MCP-1) to noninvasively detect CCR2-expressing monocyte/macrophages in atherosclerotic plaques of rabbits (2). The 99mTc-MCP-1 signal localized to atheromata on in vivo SPECT imaging and resected vessels demonstrated 4-fold greater radioactivity (0.06% injected dose per gram tissue [%ID/g]) than injected control rabbits on a normal chow diet. Immunoreactive macrophages correlated with mean 99mTc-MCP-1 uptake in aortic lesions (R2 = 0.76).
COMMENT
Radiolabeled 99mTc-MCP-1 expands the imaging armamentarium for noninvasive visualization of plaque macrophages. Additional gains are anticipated with integrated nuclear/CT imaging and analogous PET tracers offering higher spatial resolution. Mechanistic studies may clarify the relative binding of radiolabeled MCP-1 to trafficking monocytes versus resident monocytes/macrophages in atheroma.
Multichannel NIRF imaging of atheroma inflammation and osteogenic activity
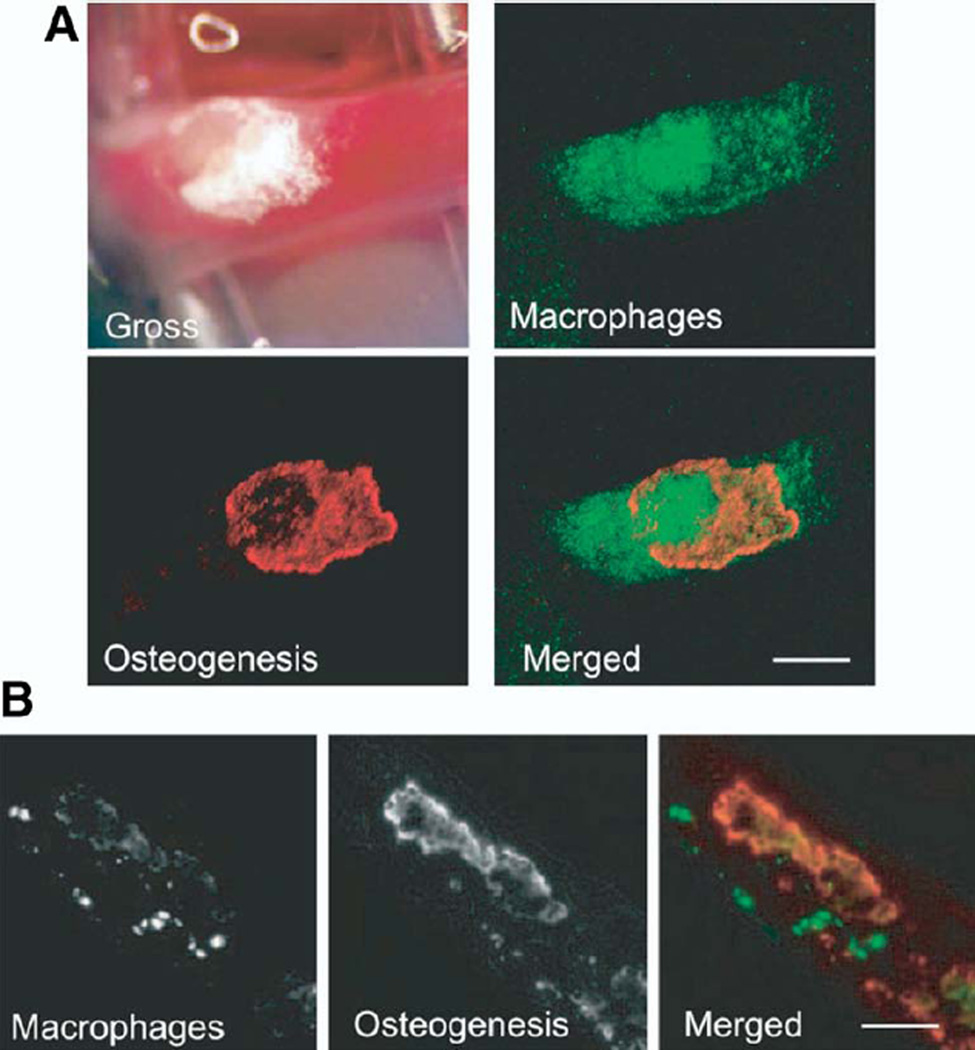
To explore the temporospatial relationship between in vivo osteogenesis (bone formation, plaque calcification) and plaque inflammation, Aikawa et al. (3) performed serial intravital confocal fluorescence microscopy of murine carotid plaques following coinjection of an osteogenic sensitive probe (near-infrared fluorochrome conjugated to a bisphosphonate) and a macrophage-avid near-infrared fluorescence (NIRF) iron oxide nanoparticle. Serial molecular imaging provided the first in vivo evidence that macrophage infiltration and plaque inflammation preceded osteogenesis (Fig. 1). Intriguingly, NIRF osteogenic activity was visualized in plaques without histological or CT evidence of calcification. Statin treatment decreased macrophage content and osteoblast-like activity, extending the association between inflammation and osteogenesis. Ex vivo fluorescence imaging of human carotid endarterectomy specimens demonstrated avid uptake of the NIRF nanoparticle and bisphosphonate agents that colocalized with immunoreactive macrophages and osteopontin, respectively.
Figure 1. In Vivo Multichannel NIRF Imaging of Plaque Macrophages and Osteogenesis in Aged ApoE −/− Mice.
(A) Calcified carotid artery atherosclerotic plaque gross morphology correlated with intravital fluorescence microscopy images of macrophages (green) and calcification (red). Merged intravital microscopy images reveal no colocalization between macrophages and calcification (bar = 500 µm). (B) Cross-sectional fluorescence microscopy with image overlay revealed a similar spatially distinct relationship (bar = 200 µm). Reprinted, with permission, from Aikawa et al. (3). ApoE−/−= apolipoprotein deficient; NIRF = near-infrared fluorescence.
COMMENT
Atherosclerotic calcification predicts future cardiovascular events, and superficial calcified nodules delineate a high-risk plaque subtype (4). Imaging of inflammation and osteogenesis via translatable NIRF imaging systems (e.g., intravascular catheters or tomographic systems) could help elucidate the role of lesional calcification in plaque rupture.
SPECT imaging of caspase inhibitor effects on plaque apoptosis
Dying cells may hasten plaque rupture by weakening of the protective fibrous cap and propagation of the underlying necrotic core (4). To image the effects of caspase inhibitors on plaque apoptosis in vivo, Sarai et al. (5) used 99mTc-labeled human recombinant annexin A5, a native protein that binds avidly to phosphatidylserine residues exposed on apoptotic cells. Rabbits with aortic atherosclerosis received a nonselective caspase inhibitor; or a specific inhibitor of caspase-1, −3, −8, or −9; or no inhibitor. Six hours after drug administration, 99mTc-labeled human recombinant or mutant annexin A5 was infused followed by micro-SPECT/CT imaging at 1 h. Compared with normal rabbits, radiolabeled annexin A5 signal was 10-fold greater in aortas of rabbits with atherosclerosis (0.05% ID/g). Annexin A5 apoptosis SPECT signal was markedly and modestly decreased by broad and specific caspase inhibition, respectively. Histology revealed reduced macrophage apoptotic figures (terminal deoxyribonucleotide transferase TdT-mediated nick-end labeling positive) after broad caspase, caspase-1, and caspase-3 inhibitor treatment.
COMMENT
Broad and specific caspase inhibitors suppressed atherosclerotic plaque apoptosis in vivo. Given the clinical potential of annexin A5 in apoptosis plaque imaging (6), this approach may offer a new method to assess antiapoptotic therapies.
SPECT/CT imaging of monocyte trafficking to atheroma at baseline and following short-term statin therapy
Kircher et al. (7) developed a noninvasive 3-dimensional imaging, tracking, and quantification strategy to assess in vivo monocyte recruitment, a critical process in atherogenesis. Adoptively transferred monocytes labeled with the FDA-approved nuclear agent indium In 111 (111In) oxyquinolone were injected into apolipoprotein E deficient (ApoE−/−) mice with atherosclerosis. Mice next underwent serial integrated micro-SPECT/CT imaging from 30 min to 10 days. The SPECT/CT images revealed tracer localization to ascending aortic plaques by 5 days that was confirmed by ex vivo autoradiography and histology. Intriguingly, short-term statin therapy reduced plaque monocyte content by 5-fold, without altering plasma cholesterol.
COMMENT
This study offers a new in vivo approach to quantitatively study the role of monocytes in atherogenesis, and potentially in patients. In addition, the short-term statin results demonstrate how molecular imaging can connect biological insights and relevant clinical studies such as the MIRACL (Effects of Atorvastatin on Early Recurrent Ischemic Events in Acute Coronary Syndromes) trial (8).
Dual-targeted microparticle CMR of activated endothelial adhesion molecules
To image activated endothelium, a identifier of high-risk plaques (4), McAteer et al. (9) conjugated CMR-detectable 4.5-µm diameter microparticles of iron oxide (MPIO) to vascular cell adhesion molecule (VCAM)-1 and to P-selectin antibodies. After confirmation of VCAM-1-MPIO binding to mouse endothelium in vitro, mice received an intravenous or left ventricular injection of either MPIO conjugated to VCAM-1 or P-selectin alone, or in combination (dual-targeted MPIO). Compared with single-labeled particles, dual-labeling markedly increased MPIO binding to endothelium at sites overlying atheroma formation on ex vivo CMR, but not at areas without detectable atherosclerosis.
COMMENT
The dual-targeted MPIO extends the CMR library of activated endothelium targeted agents (10,11). Further studies will determine the ability of MPIO to provide in vivo MR images of activated endothelium of atheromata.
In vivo CMR evaluation of p38 MAPK inhibition on plaque inflammation
Magnetic resonance images enhanced with macrophage-targeted magnetic nanoparticle of ultrasmall particle of superparamagnetic iron oxide (USPIO) were employed to evaluate anti-inflammatory properties of SB-239063, a novel p38 mitogen-activated protein kinase (MAPK) inhibitor, on murine atherosclerosis (12). Following 21-day infusions of angiotensin II to activate p38 MAPK and alternate inflammatory pathways that promote leukocyte recruitment, T2-weighted 9.4-T CMR demonstrated high plaque signal loss indicating USPIO magnetic nanoparticle (MNP) deposition into plaques. In contrast, minimal plaque signal loss was evident in angiotensin II-treated ApoE−/− mice receiving the p38 MAPK inhibitor. Intriguingly, ex vivo CMR and histopathological analysis revealed minimal differences in plaque volume and macrophage content between SB-239063 and control animals, suggesting that p38 MAPK inhibition decreased macrophage uptake of MNP.
COMMENT
This study demonstrates the capability of molecular CMR to evaluate anti-inflammatory effects of novel atherosclerosis therapeutics and suggests that p38 MAPK inhibition may attenuate macrophage phagocytic activity.
Molecular CMR of MMP expression
Inflammatory proteases may degrade extracellular matrix protein and promote plaque rupture (1). Lancelot et al. (13) developed a new molecular imaging agent (P947) for CMR detection of matrix metalloproteinase (MMP) expression in vivo. The P947 agent is a gadolinium chelate attached to a MMP peptide inhibitor that blocks MMP-1, −2, −3, −8, −9, and −13 activity in vitro. In a detailed human carotid endarterectomy substudy, P947 enhanced MMP-rich complex internal carotid atheromata 2-fold more than MMP-poor external and common carotid segments. In vivo, ApoE−/− mice receiving P947 (100 µmol Gd/kg) showed significantly greater and more durable CMR signal enhancement than the control gadolinium-tetraazacy-clododecanetetraacetic acid (DOTA) group. The P947 enhancement concentrated at the fibrous cap and plaque shoulders and was notably absent from lipid cores.
COMMENT
The P947 agent offers a high-resolution noninvasive approach to detect plaque multi-MMP expression by CMR and complements the ability to noninvasively image MMP activity in vivo using NIRF imaging (14).
Plaque inflammation and thrombosis detection by combined 18FDG-PET/CT imaging
Computed tomographic angiography and fluorodeoxygenase F 18 (18FDG)-PET were employed to identify arterial thrombi overlying inflamed aortic atherosclerotic plaques in rabbits (15). Before imaging, thrombi were triggered by administration of intraperitoneal Russell’s viper venom and intravenous histamine. Computed tomographic angiography detected thrombi with 92% sensitivity and 89% specificity. Aziz et al. (15) found that rabbits subjected to thrombosis triggering had greater 18FDG accumulation than did untriggered animals, although changes were not significant among different triggered animals with and without thrombosis. On histology, macrophage density was highest in 18FDG-avid atheroma that developed thrombosis.
COMMENT
High 18FDG signal may identify thrombus-containing plaques. It is plausible that recruited leukocytes after thrombus triggering also contribute to the overall 18FDG signal.
Visualizing oxidized phospholipids via noninvasive CMR
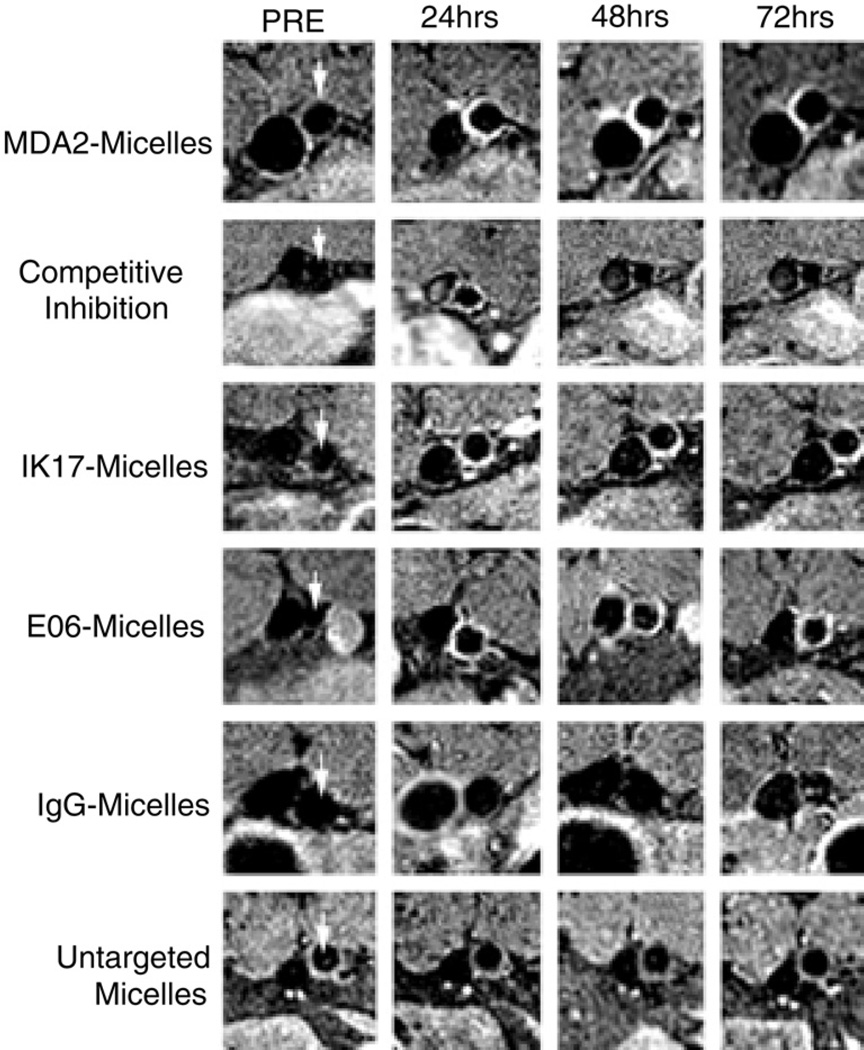
Oxidized phospholipids promote macrophage MMP release and apoptosis and consequent plaque instability (1). Briley-Saebo et al. (16) probed oxidized phospholipid constituents in atheroma of ApoE−/− mice using gadolinium-infused micelles (~20 nm) covalently bound to monoclonal antibodies (MDA2, IK17, or E06) that recognize oxidation-specific epitopes of lipoproteins. In vivo 9.4-T CMR showed maximal aortic wall uptake of targeted micelles (125% to 231% over control micelles) between 72 and 96 h (Fig. 2). Competition experiments pre-injecting unlabeled MDA2 antibody diminished the observed CMR signal enhancement, attesting to the in vivo specificity of the MDA2 imaging agent. Ex vivo experiments and confocal fluorescence microscopy revealed a close association of oxidation-specific antibody micelle probes with tissue macrophages in atheromata.
Figure 2. CMR of Oxidized Phospholipids in Atherosclerosis.
Serial MR molecular imaging of a representative abdominal aorta (arrows) in ApoE−/− mice. After injection of 0.075 mmol Gd/kg micelles targeted to oxidized phospholipids (MDA2, IK17, EO6), greater and more durable aortic plaque signal enhancement was seen when compared with control (untargeted, IgG) micelles. Competition experiments with unlabeled targeted antibodies limited the CMR signal increase. The inferior vena cava can be identified to the left of the aorta. Reproduced, with permission, from Briley-Saebo et al. (16). CMR = cardiac magnetic resonance; IgG = immunoglobulin G; other abbreviations as in Figure 1.
COMMENT
Noninvasive CMR of oxidized lipids using targeted micelles offers a new opportunity to study oxidative stress in vivo and to identify high-risk plaque subtypes.
CMR detection of activated platelet glycoprotein IIb/IIIa for thrombosis detection and monitoring of fibrinolysis
Magnetic resonance imaging was used to noninvasively monitor thrombus formation and thrombolysis in mice (17). Nonocclusive carotid thrombi were formed with 6% ferric chloride (15% to 40% occlusive). Activated platelet targeted MPIO (sized 1 µm) were linked to a single-chain antibody specific for ligand-induced binding sites (LIBS) on conformationally active glycoprotein IIb/IIIa. Continuous CMR scans revealed progressive thrombus signal loss in mice injected with LIBS-MPIO but not in control MPIO mice. Successful thrombolysis of subocclusive thrombi with human or mouse urokinase diminished the LIBS-MPIO CMR signal loss and significantly reduced thrombus extent on histological examination. Histology confirmed the incorporation of LIBS-MPIO at platelet-rich areas at the thrombus surface. The total MPIO content correlated with overall thrombus size and with the magnitude of CMR signal void. In addition, ex vivo CMR studies of carotid endarterectomy specimens from 2 patients with symptomatic carotid disease also showed LIBS-MPIO–specific binding at the specimen surface that was confirmed on histological examination.
COMMENT
The LIBS-MPIO–enhanced CMR offers a new strategy to diagnose nonoccuslive thrombi with activated platelets and to monitor thrombolysis in vivo. The utility of this approach for fully occlusive thrombi remains to be determined. With the potential development of biodegradable MPIOs, clinical CMR of microthrombi on carotid atheromata may enable improved risk stratification.
Positive-contrast CMR to detect plaque macrophages
Detection of plaque-localized MNP signal loss may be challenging due to limited spatial resolution, low baseline signal-to-noise ratio, and susceptibility artifacts from surrounding tissues (e.g., lungs). To enable positive-contrast MNP/ USPIO detection, a new CMR sequence termed inversion recovery with ON-resonant water suppression (IRON) was evaluated in atherosclerotic rabbits (18). The IRON sequence uses a spectrally selective pre-pulse saturation signal to suppress on-resonant protons without saturating off-resonant protons induced by MNP-derived local field gradients. In 7 rabbits with atherosclerosis and 4 controls, MNP (MION-47 clinical-type USPIO, 500 µmol/kg) was administered followed by serial 3-T IRON-CMR at 1, 3, and 6 days. In atherosclerotic rabbits only, progressive aortic wall enhancement occurred at confirmed sites of atheroma. The IRON-CMR provided superior MNP contrast enhancement and detection when compared with traditional black blood approaches. Positive CMR contrast correlated with plaque macrophages and provided excellent operating characteristics (area under the curve: 0.92, sensitivity: 91%, specificity: 89%) for detecting macrophage-rich plaques.
COMMENT
The development of a positive-contrast clinical MNP imaging method is an important step toward improving the sensitivity to detect macrophage infiltration, which is a marker of high-risk atherosclerotic plaques. Alternative promising positive-contrast CMR reporters include a new gadolinium-containing immunomicelle targeting the macrophage scavenger receptors (19).
Myocardial Infarction
The sequelae of myocardial infarction (MI) are numerous and devastating. In the acute phase, myocardial ischemia compromises cardiac function and local inflammation at the infarct site may potentiate ventricular rupture. As the acute inflammatory phase resolves, infarcted myocardium is replaced by fibrotic scarring that predisposes to fatal arrhythmias and initiates a cascade of maladaptive neurohormonal and structural changes that promotes the development of congestive heart failure. Molecular imaging is positioned to shed light on many of these delicately balanced and highly coordinated biological processes. In particular, open questions remain about whether the inflammatory and angiogenic repair processes can be perturbed to favorably influence myocardial healing. It is envisioned that relevant molecular imaging readouts also might provide a biological basis for selecting specific combinations of therapeutic agents following MI, such as angiotensin-converting enzyme inhibitors, beta-blockers, angiotensin II receptor blockers, aldosterone receptor antagonists, and novel therapeutics.
SPECT/CT imaging of factor XIII activity and modulating therapies following acute MI
Factor XIII (FXIII) is a thrombin-activated transglutaminase that promotes infarct healing. Nahrendorf et al. (20) studied the role of FXIII in post-MI human subjects and then in vivo using SPECT molecular imaging of FXIII in mouse infarcts. In 9 patients that died of post-MI cardiac rupture, diminished myocardial FXIII protein was found compared with the amount of myocardial FXIII in 9 control hearts. In vivo, mice were subjected to infarction followed by SPECT/CT imaging with 111In-DOTA-FXIII, a radiolabeled activated FXIII peptide substrate. High SPECT signal was noted in the infarct zone. Computed tomographic delayed-enhancement CMR further colocalized the focal SPECT signal to the anterolateral infarct and increased FXIII activity correlated with leukocytes, collagen deposition, and angiogenesis. Following dalteparin (a low molecular weight heparin antithrombin agent) therapy, SPECT images revealed diminished FXIII infarct activity that was associated with increased mortality (64% by day 7 after MI vs. 10% in control animals) secondary to myocardial rupture. Remarkably, administration of supplemental FXIII increased FXIII SPECT signal, eliminated mortality (0% at 7 days), and improved the left ventricular (LV) ejection fraction on day 21 by 20%.
COMMENT
Noninvasive molecular imaging of FXIII may provide valuable insights into the integrity of the healing infarct and propensity to rupture. Supplementing with FXIII appears beneficial, and FXIII suppression by the antithrombin dalteparin appears detrimental, although potential clinical prothrombotic effects of these therapies remain unknown.
CMR of MPO activity and statin effects after MI
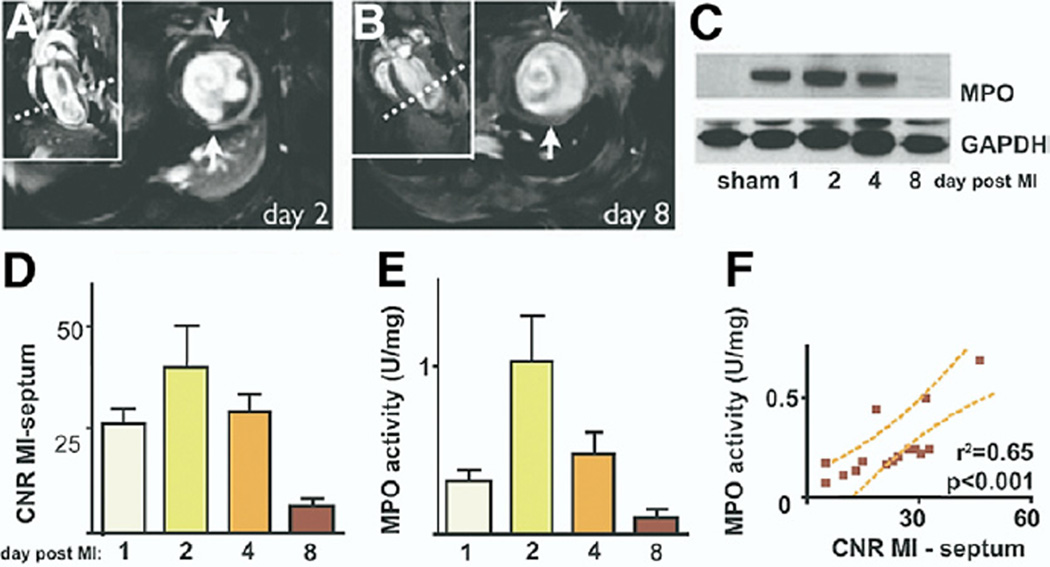
Myeloperoxidase (MPO) is a proinflammatory reactive oxygen species that may adversely effect infarct healing. Myeloperoxidase activity was evaluated in a murine MI model using an activatable gadolinium reporter that oligomerizes and enhances CMR signal in peroxidase-rich environments (21). In mice 2 days after surgical ligation of the left coronary artery, MPO-gadolinium (0.3 mmol/kg, 7-T CMR) preferentially and more durably localized to the infarct zone compared with unlabeled gadolinium. Repeat MPO-gadolinium contrast administration on day 5 showed reduced infarct MPO activity (Fig. 3). In vivo imaging findings correlated well with infarct MPO activity. In an ischemia-reperfusion injury model, statin pre-treatment significantly decreased MPO activity in vivo at 24 h.
Figure 3. Temporal Molecular CMR of MPO Activity in a Mouse MI Model.
Representative MR short-axis slices on days (A) 2 and (B) 8 after coronary artery ligation. Dotted lines identify the short-axis view locations. The significant MPO-gadolinium enhancement detected on day 2 after MI in the akinetic infarcted left ventricular free wall (arrows) diminished greatly by day 8. (C) In vivo MPO infarct activity peaked by day 2 after MI. (D) Guaiacol MPO assay of infarcts from the same animals demonstrated a similar MPO tissue activity time course. (E) Western blots confirmed significant infarct MPO expression on days 1 to 4 after MI that lessened by day 8. The MPO protein was minimal in hearts of sham-operated mice. (F) Infarct MPO activity correlated with guaiacol-derived MPO tissue activity. Reproduced, with permission, from Nahrendorf et al. (21). CNR = contrast-to-noise ratio; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; MI = myocardial infarction; MPO = myeloperoxidase; other abbreviations as in Figure 2.
COMMENT
Magnetic resonance imaging of MPO activity is a new approach to image oxidative stress in vivo and permits an integrated noninvasive molecular and functional assessment of MI.
PET/CT imaging of VEGF receptor expression after MI
Vascular endothelial growth factor (VEGF) receptor expression on neoangiogenic vessels is up-regulated after MI. To noninvasively image VEGF receptors, Rodriguez-Porcel et al. (22) employed the PET tracer copper Cu 64 (64Cu)-DOTA-VEGF121. Following left coronary artery ligation in rats, PET/CT imaging revealed abundant 64Cu-DOTA-VEGF121 uptake in infarct zones that peaked at day 3 after MI (0.97% ID/g) and was significantly greater than baseline myocardial uptake, sham controls, or infarcted animals receiving the control agent 64Cu-DOTA-VEGFmutant. The 64Cu-DOTA-VEGF121 signal progressively declined over 2 weeks to baseline. Autoradiography and immunofluorescence VEGF receptor studies confirmed the in vivo imaging findings.
COMMENT
Noninvasive imaging of myocardial VEGF receptor expression after MI may shed further insight into neoangiogenic changes that occur after myocardial damage. Shorter half-life PET tracers (e.g., fluorine F 18 or gallium Ga 68) could facilitate clinical imaging of VEGF receptors.
Fluorescence and SPECT imaging of angiotensin receptors following MI
Angiotensin (AT)-II receptor expression was assessed in a mouse model of ischemic heart failure using in vivo epifluorescence microscopy and noninvasive SPECT/CT imaging (23). Two AT-targeted agents were developed: a fluorescein-derivatized angiotensin peptide analog (picomolar affinity for AT type I and II receptors) for optical imaging, and 99mTc-labeled losartan, a clinical AT type I receptor blocker, for SPECT imaging. Following left coronary artery ligation, AT-targeted optical or SPECT tracers were injected at various time points from immediately after MI up to 12 weeks after MI. On real-time intravital epifluorescence microscopy of open-chested beating hearts, angiotensin peptide analog localized selectively to the infarcted region with minimal uptake in remote regions. Infarct fluorescence progressively increased from 1 to 3 weeks and then declined from 6 to 12 weeks, but angiotensin peptide analog colocalized primarily with myofibroblasts rather than cardiomyocytes or endothelial cells. On noninvasive SPECT/CT imaging, at 3 weeks, 99mTc-losartan enhanced infarcted regions with a 2.4-fold greater uptake compared to noninfarcted regions of control hearts.
COMMENT
Ventricular remodeling after MI is in part mediated by up-regulation of the renin-angiotensin system and myocardial AT-II receptor expression. Molecular imaging of myocardial AT-II receptor expression may predict the evolution of heart failure and help guide AT-II receptor blocker therapy, duration, and intensity.
Ultrasound imaging of inflammation during post-ischemic angiogenesis
Molecular imaging of inflammation in vascular development was assessed using contrast-enhanced ultrasound with 3-µm diameter microbubbles targeted to complement receptors on activated neutrophils, the alpha5-integrin of the fibronectin receptor on monocytes, or VCAM-1 on endothelium (24). Unlabeled microbubbles were employed for tissue perfusion imaging. Two in vivo models were employed: 1) subcutaneous Matrigel (BD Biosciences, San Jose, California) implants to facilitate de novo vasculogenesis; and 2) hind-limb ischemic arteriogenesis following ligation of the distal common iliac artery. In the middle of Matrigel implants, increased alpha5-integrin and VCAM-1 ultrasound signals temporally preceded increased perfusion signals, suggesting that inflammation occurred before the development of functional angiogenic vasculature. In hind-limb ischemia, targeted molecular imaging revealed that neutrophils, monocytes, and VCAM-1 expression preceded increased perfusion to the limb. Mice lacking the monocyte chemoattractant protein MCP-1 exhibited diminished monocyte signals when compared with wild-type mice as expected, however perfusion was not significantly reduced, presumably due to the early day 4 imaging time point.
COMMENT
Behm et al. (24) demonstrate the ability to monitor inflammatory biomarkers noninvasively during vascular development and remodeling in response to tissue ischemia. Detection of impaired inflammatory responses may guide earlier initiation of pro-angiogenic therapies engineered to salvage ischemic tissue.
CMR of collagen deposition in post-MI scarring
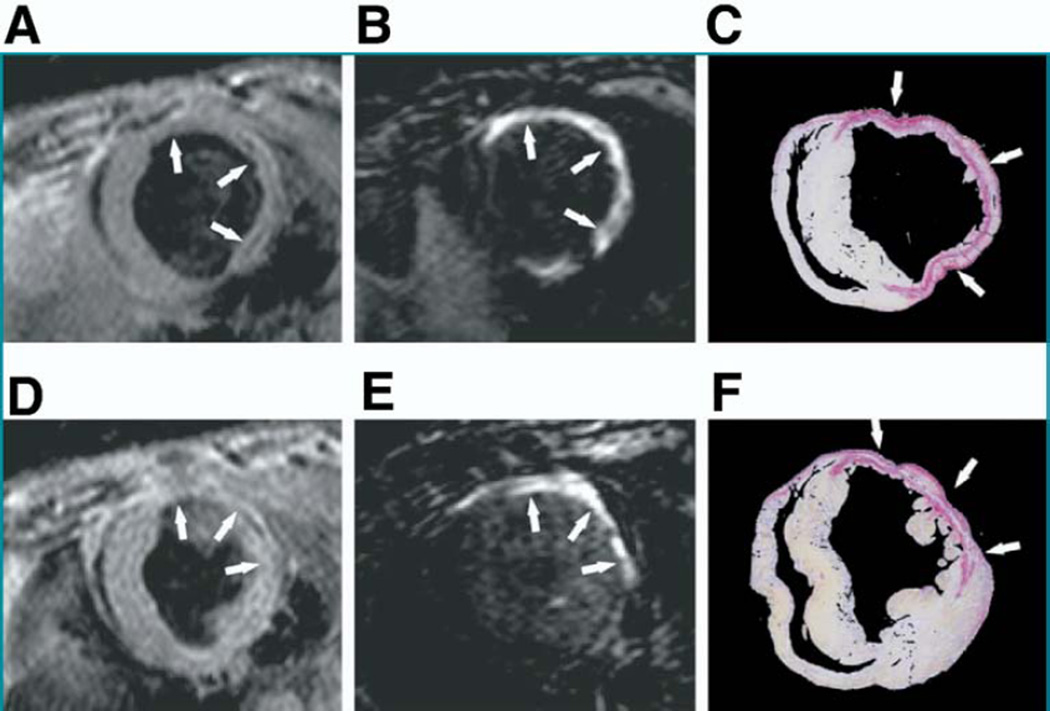
Helm et al. (25) investigated a new multivalent gadolinium-based collagen probe (EP-3533, 3 gadolinium ions per type I collagen binding peptide) in cardiac ischemia-reperfusion injury. The EP-3533 probe binds collagen reversibly with micromolar affinity. Multivalency enables very high R1 relaxivity for EP-3533, >5-fold that of untargeted gadolinium. Serial 4.7-T CMR scans at day 1 and day 42 post-MI after EP-3533 or gadolinium injection were performed. Compared with gadolinium, EP-3533 provided greater and more durable (195 vs. 25 min for gadolinium) infarct enhancement. The circumferential extent of fibrosis detected by EP-3533 enhancement correlated well with picrosirius red-detected collagen (R2 = 0.97) (Fig. 4). Inductively coupled plasma mass spectrometry confirmed higher gadolinium concentration in scarred versus normal myocardium.
Figure 4. Molecular CMR of Collagen Presence in MI.
(A, B, D, E) Mid-ventricular short-axis MR images at 2 levels and (C, F) tissue sections of corresponding locations in the left ventricular stained with picrosirius red were compared following mouse coronary artery ischemia-reperfusion. (A, D) Anatomic images obtained using a double inversion recovery gradient echo sequence of ischemic regions showed (B, E) areas of enhancement 40 min after EP-3533 administration that matched closely with (C, F) picrosirius red staining patterns on histology (9× magnification). Arrows identify locations of myocardial scarring. Reproduced, with permission, from Helm et al. (25). Abbreviations as in Figures 1 and 2.
COMMENT
Collagen molecular CMR detects post-infarction scarring with improved conspicuity than conventional delayed gadolinium enhancement does and may have broad applications for targeted fibrosis imaging.
Monitoring inflammation after ischemia with perfluorocarbon-enhanced fluorine CMR
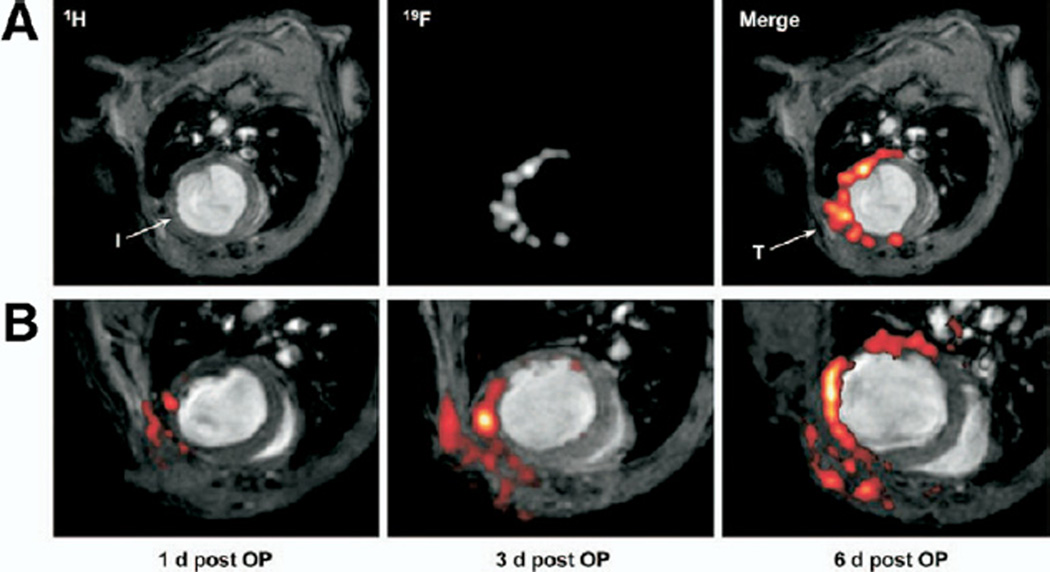
Nanoemulsions of biochemically inert perfluorocarbons (PFCs, specifically perfluoro-15-crown-5-ether) were investigated for detecting cellular inflammation in mouse models of acute cardiac (left anterior descending artery [LAD] ligation) and cerebral (photothrombosis) ischemia (26). Following PFC injection, fluorine F 19 (19F) CMR of density centrifugated blood samples revealed that mononuclear cell uptake of PFCs peaked by day 2 but was absent on day 3. Therefore, PFCs were injected 2 h after the initial MI and then reinjected at day 4. Multinuclear (proton and fluorine) CMR was performed serially at days 1, 3, and 6 after MI. Focal fluorine signal was progressively detected in the infarct only, as confirmed by anatomical CMR (Fig. 5). Fluorescent PFCs localized to the infarction border zone, but not noninfarcted nor necrotic areas. Flow cytometry revealed that >80% of PFCs colocalized with mononuclear cells. The CMR sensitivity detection threshold of this approach was estimated to be as few as 400 PFC-labeled cells.
Figure 5. In Vivo Molecular Imaging of Macrophage Infiltration Into Infarcted Hearts Using PFC-Enhanced 19F CMR.
(A) Corresponding 1H and 19F MR images 4 days after mouse left anterior descending ligation reveals 19F signal uptake within the infarct zone (I) and near the surgical thoracotomy site (T). Perfluorocarbons were initially injected 2 h after infarction. (B) Fusion 1H (grayscale) and 19F (pseudocolor) images show that PFC-labeled macrophages accumulate over time in the infarct and surgical injury zones. Of note, an additional PFC bolus was delivered on day 4 to compensate for PFC particle clearance by 3 days after injection. Reproduced, with permission, from Flogel et al. (26). OP = operative procedure; PFC = perfluorocarbon; 1H = hydrogen H 1; 19F = fluorine F 19; other abbreviations as in Figure 2.
COMMENT
This novel study by Flogel et al. (26) demonstrates a positive-contrast CMR approach to detect cellular inflammation after MI. Two advantages of this approach include: 1) the lack of 19F background in the body, enhancing signal-to-noise ratio, and 2) multinuclear proton imaging capability for concomitant cardiac anatomy, function, and scar imaging. Clinical fluorine CMR with PFCs such as perflubron, an artificial blood substitute, appears feasible.
Angiogenesis detection in hibernating myocardium
A swine model of hibernating myocardium was created using an Ameroid constrictor (Research Instruments NW, Lebanon, Oregon) placed around the left circumflex artery. After 2 to 3 weeks, swine underwent electroanatomic mapping-guided intramyocardial injection of either VEGF to stimulate therapeutic angiogenesis or saline control. Two weeks later, animals received an injection of Iodine I 123 (123I)-Gluco-Arginine-Glycine-Aspartate peptide motif (RGD), a glycosylated cyclic peptide SPECT imaging agent that binds integrin alphaV-beta3, a transmembrane glycoprotein up-regulated on the endothelial cells of neoangiogenic vessels (27). The 123I-Gluco-RGD SPECT imaging at 6 weeks in VEGF-injected swine revealed enhancement in the Ameroid constrictor territory that correlated with 15% greater perfusion and slight improvement in global and regional LV function. Comparatively, controls showed minimal 123I-Gluco-RGD enhancement, diminished myocardial blood flow, and progressive LV dysfunction. Injection of VEGF was associated with reduced fibrosis and higher capillary density on histology. Lectin staining correlated well (R2 = 0.8) with 123I-Gluco-RGD uptake.
COMMENT
Iodine I 123-Gluco-RGD SPECT imaging offers a noninvasive method to assess angiogenic-modulating therapies in ischemic issues. Clinical infarct angiogenesis imaging has been recently performed using the PET sister agent 18F-Galakto-RGD described in the Clinical Investigations section (28).
Stem Cell Therapy
Stem cell therapy holds great promise for the treatment of cardiovascular disease. Of particular interest is stem cell therapy in MI, which has the potential to reduce or even improve myocardial function not only through myocardial regeneration but also enhancement of neovascularization within the infarct zone to restore myocardial perfusion. Though clinical studies of stem cell therapy remain preliminarily encouraging, future trials incorporating molecular imaging may offer much greater insight into the critical processes of stem cell engraftment, survival, differentiation, and migration. In practice, current methods require labeling an ex vivo population of stem cells with an imaging agent or using stem cells with genetically encoded imaging reporters. Future screening efforts may identify injectable agents with specific targeting capabilities to particular stem cells.
Serial optical tracking of embryonic stem cell-derived endothelial cell therapy after MI
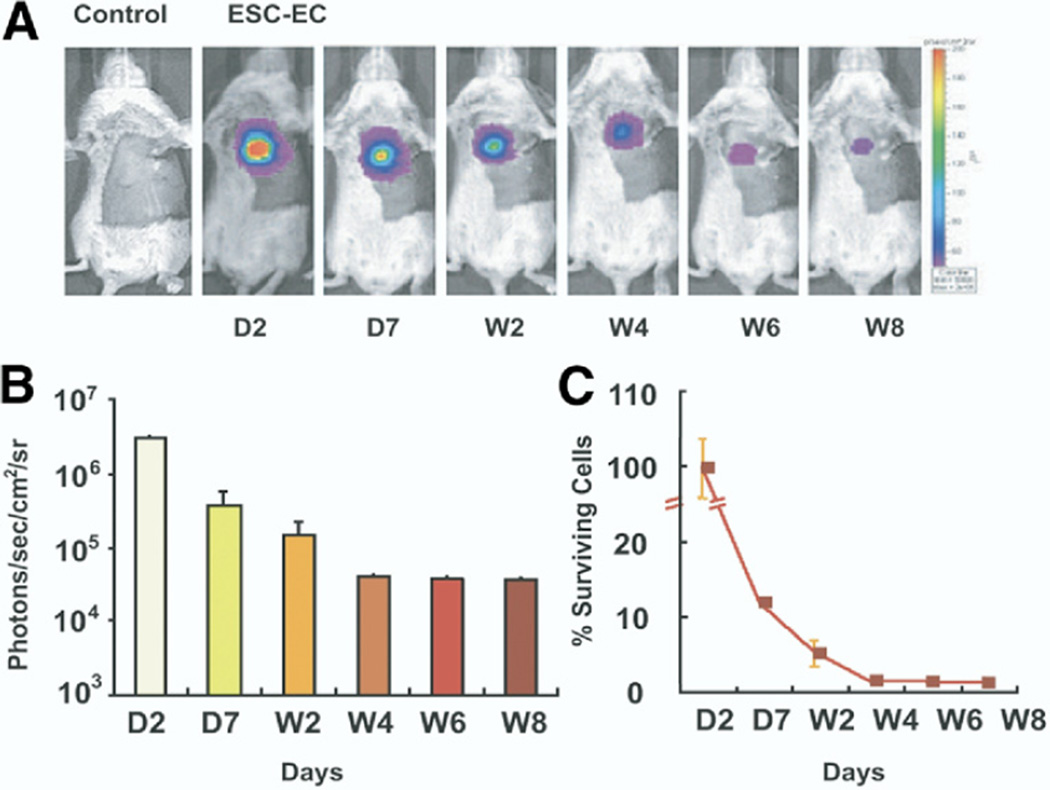
Regenerative stem cell therapy may offer the ability to restore viable myocytes that limit or reverse LV dysfunction. To quantitatively track the in vivo survival and effects of embryonic stem cell-derived endothelial cells (ESC-EC), Li et al. (29) implanted ESC-EC into murine hearts following experimentally induced MI. Murine ES cells were rendered optically detectable following viral transfection with ubiquitin promoter driving firefly luciferase and monomeric red fluorescence protein for bioluminescence and fluorescence detection, respectively. Transfected ESC were differentiated into EC. Ten minutes after left coronary ligation, ESC-EC were injected directly into the heart at the peri-infact zone and noninvasively imaged sequentially over 8 weeks. After day 1, there was significant progressive diminution of signal, corresponding to decreased cell survival from 12% to 1% over weeks 1 to 8 (Fig. 6). Compared with control phosphate-buffered saline injection, ESC-EC injection improved LV function at 8 weeks, and histological analysis demonstrated greater periinfarct neoangiogenesis. Interestingly, greater neovascularization occurred despite the poor overall survival of ESC-EC. No evidence of myocardial regeneration was evident. Compared with differentiated ES cells, undifferentiated ES cell injection induced teratoma formation associated with decreased survival.
Figure 6. Serial Bioluminescence Monitoring of ESC-EC Survival in MI.
(A) Representative images reveal significant bioluminescence activity detectable by day 2 in the post-MI mouse heart after injection of 5 × 105 ESC-ECs. The robust signal intensity gradually diminishes over the course of 8 weeks. (B) Temporal analysis of the ESC-EC bioluminescence signal from all animals. (C) Survival of donor cells over time from day 2 diminished markedly by week 8 (determined by percentage signal activity from baseline). Reproduced, with permission, from Li et al. (29). ESC-EC = embryonic stem cell-derived endothelial cell; other abbreviations as in Figure 3.
COMMENT
Molecular imaging strategies can track the fate and function of cardiac stem cell transplants in vivo. Clinical translation of this approach for assessing human stem cell transplants may be feasible via PET gene expression reporters such as herpes simplex virus type 1 thymidine kinase (30).
Tracking intracoronary progenitor cell delivery after MI with PET/CT
The temporal dynamics and efficiency of intracoronary-injected 18FDG-labeled circulating progenitor cell (CPC) myocardial engraftment was assessed in vivo by combined PET/CT imaging in a porcine MI model (31). Infarction was induced by catheter-based balloon occlusion of the mid left circumflex coronary artery for 90 min. Infarct size and cardiac function were evaluated by CMR at 48 h post-infarction, after which freshly harvested human and porcine CPC radiolabeled ex vivo with 18FDG (>98% viability) were administered via an intracoronary catheter within the left circumflex artery according to 2 delivery strategies: 1) 3 cycles of balloon occlusion followed by an equal duration of reperfusion (1 × 107 cells per injection); or 2) a high-concentration single-bolus injection (3 × 107 cells) without balloon occlusion. In the low-dose balloon occlusion group, dynamic 3-dimensional PET/CT revealed a repetitive cyclical rise in 18FDG-CPC myocardial signal during each balloon occlusion, followed by subsequent washout (average of 80% signal loss per cycle) after balloon deflation. Comparatively, high-dose single-bolus administration resulted in a larger initial peak 18FDG-CPC myocardial signal followed by a slower, gradual decline. At 1 h, the high-concentration single-bolus group demonstrated twice the PET signal of the low-concentration balloon occlusion group (p = 0.08).
COMMENT
This study identifies the time course of CPC delivery in a large-animal MI model and demonstrates in vivo noninvasive assessment of various cell-based myocardial regeneration therapies. Although overall engraftment is low, a novel high-dose single-bolus delivery strategy without balloon occlusion may be superior to a more labor-intensive occlusion technique that might induce downstream myocardial dysfunction.
PET/CT molecular imaging of gene expression
A minimally invasive clinical-type strategy was used to locally administer reporter gene products and then noninvasively evaluate their expression over time by hybrid PET/CT imaging (30). Genes (1010 plaque-forming units) were delivered using a percutaneous endomyocardial fluoroscopy-guided delivery system equipped with a helical needle injector that allowed high-precision steering of the catheter tip within the LV. Two days after local gene delivery to swine hearts, 18F-labeled 9-[4-fluoro-3-(hydroxymethyl)butyl] guanine (18F-FHBG), a reporter probe for imaging the expression of transfected genes, was intravenously injected (9.37 mCi per animal). The PET/CT images revealed signal enhancement at the site of initial gene product injection in the anteroseptal territory. Compared with saline-injected controls, PET signal was significantly greater in animals receiving myocardial reporter genes.
COMMENT
Noninvasive imaging of gene expression could play an important role in monitoring cardiac gene therapy in clinical trials.
Multimodality Imaging and Theranostics
The ability of a single imaging agent to provide readouts across multiple imaging modalities is intuitively attractive. In particular, derivatization of imaging agents with additional fluorescence reporters greatly augments the ability to rigorously validate the specificity of an imaging agent via fluorescence microscopy and flow cytometry. In addition, combining imaging platforms with complementary strengths (e.g., CMR for noninvasive, high-resolution detection, and PET or fluorescence for high-sensitivity detection) can augment the utility of an imaging agent. An important consideration for multimodality probes is the increased complexity encountered during agent synthesis.
The continued evolution of molecular imaging agents has also witnessed the development of theranostic (combined therapeutic and diagnostic) imaging strategies. Theranostic agents offer the ability to localize, track, and quantify a therapeutic molecular target in a diseased tissue of interest. Such information could be used to predict therapeutic efficacy and/or modulate the delivered dose (e.g., injecting an additional dose of agent in tissues with suboptimal uptake). These futuristic-sounding molecular approaches have gained increasing traction in recent years.
Multimodality PET-CMR-fluorescence imaging of plaque macrophages
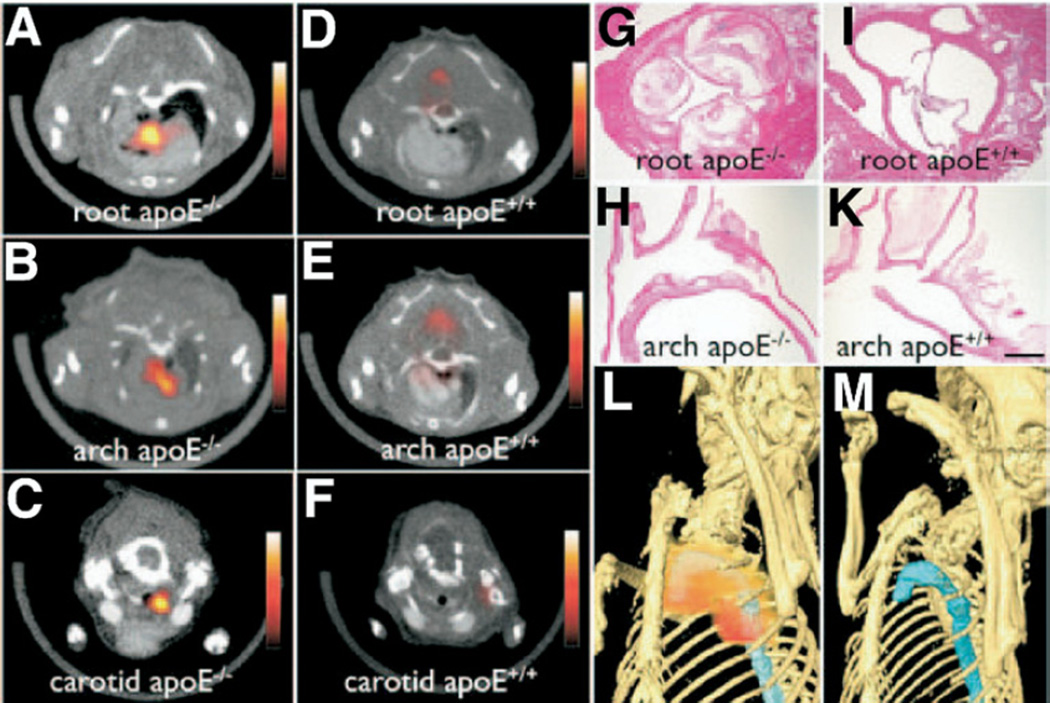
Nahrendorf et al. (32) developed and validated a trimodality nanoparticle (TNP) for PET, CMR, and NIRF imaging of lesional macrophages. Based on the backbone of an earlier NIR fluorescent MNP (33), TNP was derivatized with 64Cu (half-life: 12.7 h) to enable concomitant PET imaging. The PET-based detection of TNP was found to be 50-fold more sensitive than CMR in vitro. Then TNP was injected into ApoE−/− mice (dose: 303 µCi, 1.5 mg Fe/kg). After 24 h, PET, CMR, and NIRF imaging were performed. In vivo TNP localized to aortic and carotid plaques with a 260% to 392% signal increase over wild-type mice (Fig. 7). Flow cytometry and fluorescence microscopy confirmed macrophage targeting of TNP. Compared with 18FDG-PET imaging in the same mouse, TNP enabled higher in vivo plaque target-to-background ratios (TBRs).
Figure 7. Multimodality PET, CMR, and NIRF Imaging of Plaque Macrophages Using a 64Cu-Labeled TNP.
Strong 64Cu-TNP activity was observed in the (A) aortic root, (B) arch, and (C) carotid artery on fused PET/CT images at sites of atherosclerotic plaque burden in ApoE−/− mice. (D to F) Comparatively, scant 64Cu-TNP signal was identified in the same vascular beds of wild-type mice. Histology of respective vascular territories identified high plaque content in ApoE−/− mice but (I, K) not in wild-type controls (magnification: 400× for G and I, 200× for H and K; bar = 0.4 mm). Maximum intensity reconstruction of the 3-dimensional fused PET/CT data set demonstrates (L) focal 64Cu-TNP uptake (red) in the proximal thoracic aorta (blue) of ApoE−/− mice that is (M) absent in matched wild-type control animals. Reproduced, with permission, from Nahrendorf et al. (32). CT = computed tomography; PET = positron emission tomography; TNP = trimodality nanoparticle; 64Cu = copper Cu 64; other abbreviations as in Figures 1 and 2.
COMMENT
By combining the sensitivity of PET imaging, the excellent soft tissue contrast of CMR, and the fluorescence capabilities of reflectance imaging, flow cytometry, and fluorescence microscopy, the TNP agent is an important multimodal imaging advance in atherosclerosis molecular imaging.
Simultaneous PET-CMR molecular imaging
A significant technological advance, molecular imaging is now possible using combined 18FDG-PET and CMR. Judenhofer et al. (34) developed solid-state silicon photo sensors that were insensitive to magnetic field variations and insertable directly into CMR scanners. Benchmark testing comparing the PET insert alone or in combination with CMR revealed little signal degradation or inhomogeneities. Excellent coregistration of molecular, anatomical, and functional integrated CMR-PET images was demonstrated in vivo in mice brain and colon cancer studies.
COMMENT
Combining PET’s high-sensitivity (subpicomolar) detection of molecular targets with CMR’s anatomical, functional, and spectroscopic capabilities is a major technological advance that will strongly influence cardiovascular, neurological, cancer, and stem cell imaging. The simultaneous acquisition of PET and CMR signals is particularly important for functional imaging where physiological states can change dynamically. Clinical PET-CMR scanners are under active development.
Detection and treatment of plaque neovascularization
Plaque angiogenesis may facilitate intraplaque hemorrhage, demarcate high-risk plaques, and represent an intriguing therapeutic target for atherosclerosis. Winter et al. (35) developed a theranostic antiangiogenic CMR-based strategy for atherosclerosis. An angiogenesis-targeted gadolinium-containing nanoparticle, alphaV-beta3-MNP, was further derivatized by fumagillin, an antiangiogenic endothelial mycotoxin. Atherosclerotic rabbits received a single dose of fumagillin or control alphaV-beta3-MNP (1 ml/ kg) and underwent T1-weighted turbo spin echo CMR before and 3 h after injection. Magnetic resonance imaging was then performed weekly for 4 weeks after control alphaV-beta3-MNP injection. In a second study, rabbits received 1 or 2 doses of the fumagillin alphaV-beta3-MNP with or without statin therapy (atorvastatin 44 mg/kg), with serial alphaV-beta3-MNP molecular CMR over 8 weeks to assess angiogenesis. Neovessel MNP signal enhancement diminished by more than 50% at 1 week after fumagillin treatment and returned gradually to baseline levels by 4 weeks. Signal enhancement remained elevated in animals receiving control alphaV-beta3-MNP without fumagillin. Remarkably, atorvastatin alone did not reduce angiogenesis, however atorvastatin combined with serial fumagillin dosing sustained an antiangiogenic effect in atheromata. In vivo findings were confirmed with fluorescent MNP studies as well as with histology.
COMMENT
This is an elegant approach employing a single agent to diagnose, monitor, and treat angiogenesis in atherosclerosis. Serial monitoring of plaque angiogenesis also enabled optimization of the timing and dosing of antiangiogenic agents, as well as concomitant potentiators such as statins.
Clinical Investigations
Within the extensive area of imaging agent development, a major goal is to develop clinically useful molecular imaging agents—specifically agents that are nontoxic, sensitive, specific, efficient, and that possess favorable pharmacokinetic properties. Advances in clinical CVD molecular imaging over the past 3 years have already made a substantial impact in atherosclerosis imaging of patients (e.g., 18FDG for PET imaging, iron oxide MNP for CMR, 99mTc-annexin V for SPECT imaging). In the past year, clinical atherosclerosis molecular imaging studies provided new insights into the relationship between atherosclerosis and inflammation. In addition, new clinical molecular imaging approaches were also demonstrated for thrombosis and MI.
Prevalence of inflammation in carotid atherosclerosis determined by 18FDG PET/CT imaging
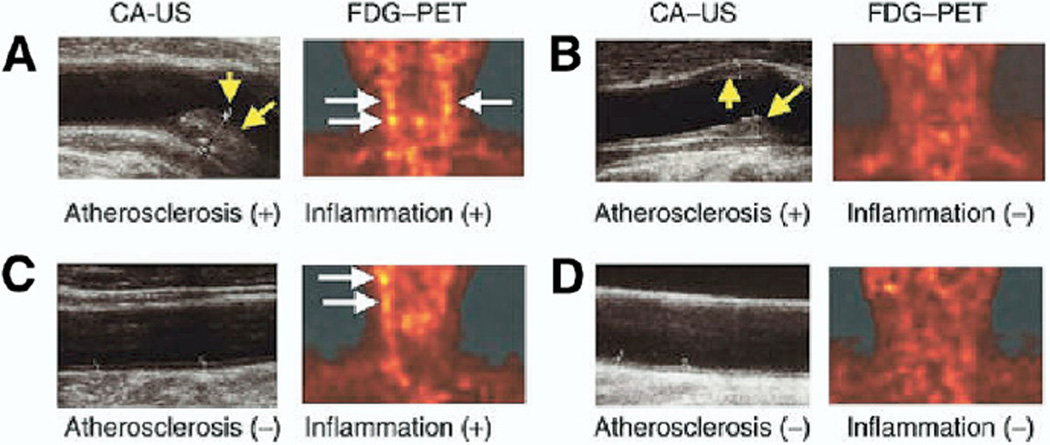
Uptake of 18FDG, a PET tracer that accumulates in metabolically active cells, correlates with plaque macrophages. To evaluate the prevalence of 18FDG-derived carotid plaque inflammation in asymptomatic individuals, Tahara et al. (36) performed carotid 18FDG-PET and CT imaging on 100 consecutive subjects undergoing screening carotid ultrasonography. Exclusion criteria included active inflammatory syndromes (aortitis, vasculitis, acute coronary syndromes) as well as the use of statins, previously shown to reduce 18FDG signal in human carotid plaques (37). On ultrasound, 41% of patients harbored carotid atherosclerosis. However, only 30% of those with carotid atherosclerosis demonstrated elevated 18FDG carotid plaque signal indicating inflammation (standardized uptake value: >1.60) (Fig. 8). Enhanced 18FDG signal was noted in 10% of patients without atherosclerosis, possibly identifying subjects at greater future risk. Inflammation derived from 18FDG correlated with elevated body mass index, waist circumference, the use of antihypertensive medications, and the number of atherosclerotic plaques.
Figure 8. Clinical PET Imaging of Atherosclerosis Inflammation.
Paired carotid 18FGD-PET and B-mode ultrasound images of human patients having atherosclerotic disease (A) with or (B) without 18FDG-derived evidence of plaque inflammation and patients without detectable atherosclerosis (C) with or (D) without inflammation. White arrows = 18FDG-PET signal uptake. Yellow arrows = carotid atherosclerotic plaque. Reproduced, with permission, from Tahara et al. (36). CA-US = carotid atherosclerosis ultrasound; 18FGD = fluorodeoxygenase F 18; other abbreviations as in Figure 7.
COMMENT
Roughly one-third of carotid plaques from asymptomatic patients are inflamed as determined by 18FDG-PET imaging. Prospective studies are needed to determine if the presence of 18FDG-defined plaque inflammation portends a greater risk of stroke or cardiovascular events.
Reproducibility of atherosclerosis inflammation measurements obtained by 18FDG-PET/CT imaging
The short-term reproducibility of 18FDG-PET imaging of inflammatory lesions in carotid, iliac, and femoral arteries was assessed in 19 high-risk patients (38). Patients were imaged twice 14 days apart with combined vascular 18FDG-PET/CT. Excellent interscan, interobserver, and intraob-server agreement was present for all vessel beds (interclass coefficients: >0.8). In addition to the standardized uptake value analysis, Rudd et al. (38) derived new TBR measures to quantify 18FDG plaque signal. The investigators suggested tracking the mean TBR to assess systemic atherosclerosis therapies and using the maximum plaque TBR within a diseased segment to monitor local therapies.
COMMENT
Clinical 18FDG-PET/CT imaging of carotid and peripheral arterial plaques is reproducible over 14 days and strengthens this approach to monitor anti-inflammatory atherosclerosis treatments. Ongoing 18FDG-PET phase II studies may help determine which novel pharmacotherapies will be supported for costly phase III outcome studies.
Correlation of carotid plaque inflammation with luminal stenosis employing iron oxide nanoparticle-enhanced CMR
Patients (n = 71) with asymptomatic carotid stenosis >40% were injected with a macrophage-targeted MNP for CMR, specifically dextran-coated USPIO, to evaluate the relationship between plaque inflammation and luminal stenosis (39). Spearman rank correlation coefficients demonstrated no significant relationship between MNP-derived plaque inflammation and stenosis (rho = 0.15, p > 0.05).
COMMENT
This hypothesis-generating cross-sectional study of asymptomatic subjects with moderate carotid stenoses sheds light on the incongruity of anatomic indices and inflammation. Elevated plaque inflammation is present over a wide range (40% to 80%) of stenosis. Clinical outcomes studies are needed to determine the risk of moderate and severe asymptomatic lesions based on the underlying degree of plaque inflammation.
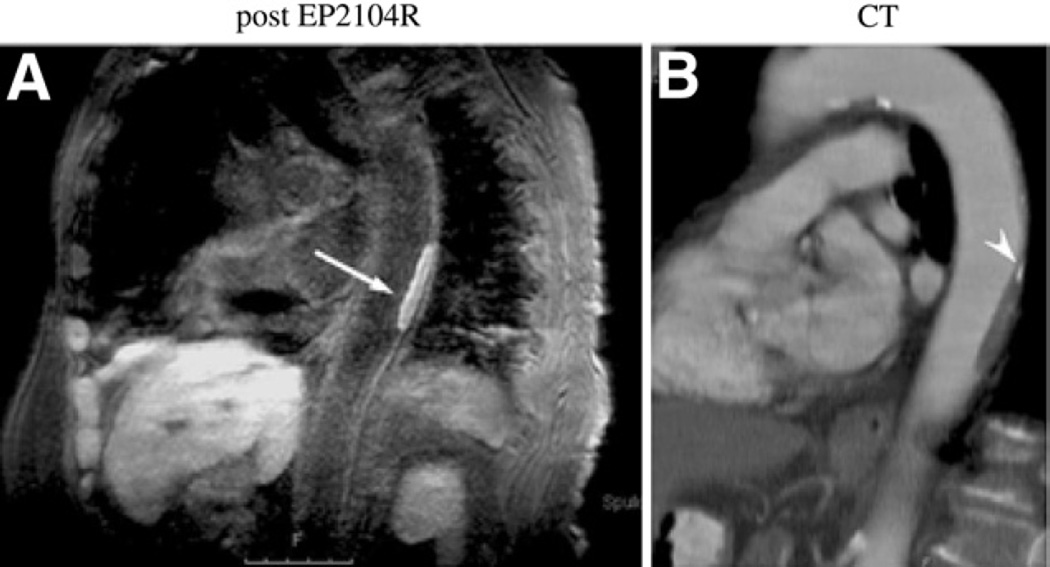
Imaging fibrin in cardiovascular thrombi using CMR
Spuentrup et al. (40) performed a multicenter phase II clinical trial of 11 patients with recent thrombosis (intracardiac, thoracic aorta, carotid artery). Patients received the new fibrin-targeted peptide contrast agent EP-2104R for CMR (40). The EP-2104R agent contains 4 gadolinium molecules per peptide, providing a multivalent signal amplification strategy. Black-blood CMR was performed 3.5 h after agent injection (16 µmol Gd/kg). The thrombus-to-blood and thrombus-to-adjacent soft tissue contrast-to-noise ratio increased by >500% after EP-2104R injection, predominantly on the surface of thrombi (Fig. 9).
Figure 9. Clinical CMR of Fibrin-Rich Thrombi.
(A) Magnetic resonance molecular imaging of a descending aortic thrombus with a fibrin-specific probe (EP-2104R) in an 82-year-old female patient. The thrombus is visualized as positive contrast (arrow) on the inversion recovery black-blood gradient-echo imaging sequence. (B) Reconstructed images from a corresponding contrast-enhanced CT confirm the thrombotic filling defect and note a small region of calcification (arrow-head). Reproduced, with permission, from Spuentrup et al. (40). Abbreviations as in Figures 2 and 7.
COMMENT
The EP-2104R agent is a novel clinical molecular CMR agent for thrombosis that appears safe and well tolerated. Although the relatively long administration-to-imaging time likely precludes widespread application of EP-2104R for acute arterial thrombosis syndromes, fibrin CMR could offer a noninvasive option to transesophageal echocardiography for left atrial thrombi. In addition, detection of subclinical thrombi on carotid atheroma could identify high-risk patients.
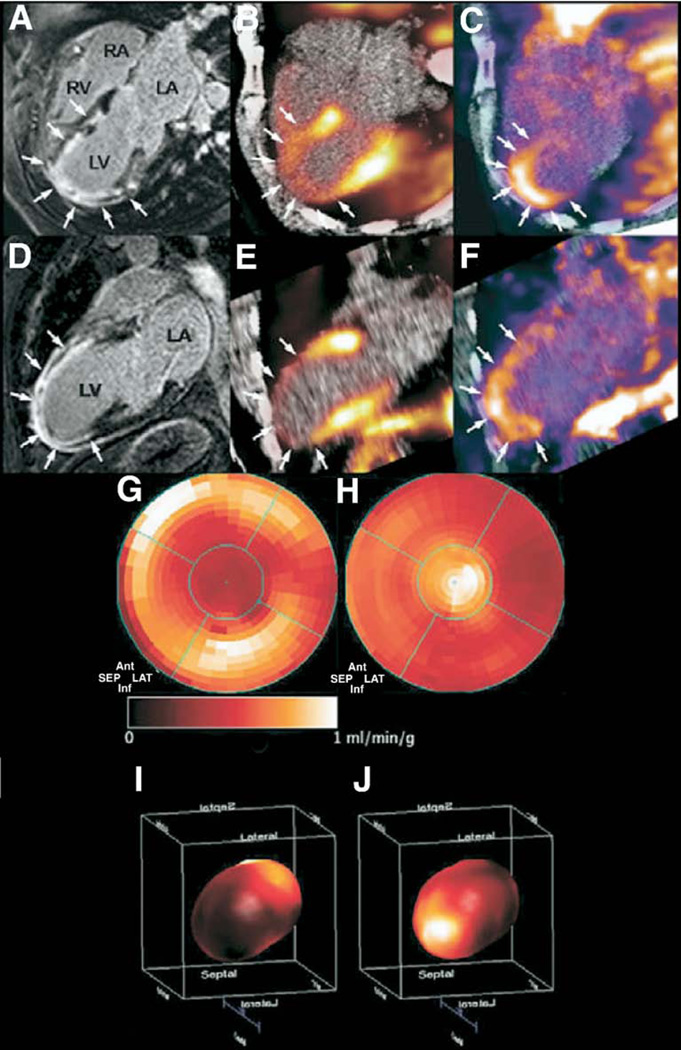
Detection of post-infarction angiogenesis by SPECT/CT imaging
Positron emission tomography imaging with the angiogenesis-targeted imaging agent 18F-Galakto-RGD was used to evaluate post-MI angiogenesis in a case report of a patient with a large anterolateral MI (28). The patient was emergently revascularized with proximal LAD stenting. Two weeks later, CMR revealed systolic dysfunction (LV ejection fraction: 33%) and transmural infarction as detected by delayed gadolinium enhancement. Severely reduced myocardial blood flow in the infarct zone was also demonstrated by Nitrogen N 13 ammonia PET imaging. The PET images following injection of 18F-Galakto-RGD, an agent that targets integrins up-regulated during post-MI neovascularization, showed focal uptake in the infarct suggesting possible myocardial healing (Fig. 10).
Figure 10. Clinical PET Imaging of Infarct Angiogenesis.
(A) Four- and (D) 2-chamber MR images after gadolinium administration reveal delayed enhancement (arrows) of the anterior wall and apical regions corresponding with (B, E) anatomically matched severely decreased myocardial perfusion (arrows) by 13N-ammonia radionuclide scanning. (C, F) Focal 18FDG-PET uptake colocalized (arrows) with the infarct zone, possibly representing regions of neovascularization. (G) Two- and (I) 3-dimensional polar maps of 13N-ammonia myocardial blood flow demonstrate a significant reduction in the distal left anterior descending artery territory. (H, J) The 18F-Galakto-RGD signal on PET-imaging coregistered with areas of severely diminished 13N-ammonia perfusion. Reproduced, with permission, from Makowski et al. (28). RGD = Arginine-Glycine-Aspartate peptide motif; 13N = nitrogen N 13; other abbreviations as in Figures 2, 7, and 8.
COMMENT
This case report demonstrates the potential to image post-MI angiogenesis using non-invasive PET imaging. Clinical studies are needed to determine whether angiogenesis imaging can predict long-term LV function after MI.
Conclusions
Molecular imaging of CVD has witnessed an exciting year. An array of reporter imaging strategies now provides the ability to sense fundamental biological processes across a range of CVD states and imaging platforms. In the clinical arena, rapid growth is anticipated in molecular imaging studies designed to evaluate new pharmaceuticals in phase II clinical trials (in particular, imaging of drug-induced effects on plaque inflammation). The next phase of clinical molecular imaging studies will aim to provide longitudinal biological information about the evolution of CVD in patients and will undoubtedly offer new opportunities to identify patients at risk for rapid disease progression and adverse outcomes.
Acknowledgments
Dr. Jaffer is a consultant for VisEn Medical and has received financial support from Howard Hughes Medical Institute Early Career Award, American Heart Association Scientist Development Grant, and the Donald W. Reynolds Foundation.
REFERENCES
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Hartung D, Petrov A, Haider N, et al. Radiolabeled monocyte chemotactic protein 1 for the detection of inflammation in experimental atherosclerosis. J Nucl Med. 2007;48:1816–1821. doi: 10.2967/jnumed.107.043463. [DOI] [PubMed] [Google Scholar]
- 3.Aikawa E, Nahrendorf M, Figueiredo JL, et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 4.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 5.Sarai M, Hartung D, Petrov A, et al. Broad and specific caspase inhibitor-induced acute repression of apoptosis in atherosclerotic lesions evaluated by radiolabeled annexin A5 imaging. J Am Coll Cardiol. 2007;50:2305–2312. doi: 10.1016/j.jacc.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 6.Kietselaer BL, Reutelingsperger CP, Heidendal GA, et al. Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis. N Engl J Med. 2004;350:1472–1473. doi: 10.1056/NEJM200404013501425. [DOI] [PubMed] [Google Scholar]
- 7.Kircher MF, Grimm J, Swirski FK, et al. Noninvasive in vivo imaging of monocyte trafficking to atherosclerotic lesions. Circulation. 2008;117:388–395. doi: 10.1161/CIRCULATIONAHA.107.719765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIR-ACL study: a randomized controlled trial. JAMA. 2001;285:1711–1718. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 9.McAteer MA, Schneider JE, Ali ZA, et al. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb Vasc Biol. 2008;28:77–83. doi: 10.1161/ATVBAHA.107.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res. 2005;96:327–336. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 11.Nahrendorf M, Jaffer FA, Kelly KA, et al. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 12.Morris JB, Olzinski AR, Bernard RE, et al. p38 MAPK inhibition reduces aortic ultrasmall superparamagnetic iron oxide uptake in a mouse model of atherosclerosis: MRI assessment. Arterioscler Thromb Vasc Biol. 2008;28:265–271. doi: 10.1161/ATVBAHA.107.151175. [DOI] [PubMed] [Google Scholar]
- 13.Lancelot E, Amirbekian V, Brigger I, et al. Evaluation of matrix metalloproteinases in atherosclerosis using a novel noninvasive imaging approach. Arterioscler Thromb Vasc Biol. 2008;28:425–432. doi: 10.1161/ATVBAHA.107.149666. [DOI] [PubMed] [Google Scholar]
- 14.Deguchi JO, Aikawa E, Libby P, et al. Matrix metalloproteinase-13/ collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation. 2005;112:2708–2715. doi: 10.1161/CIRCULATIONAHA.105.562041. [DOI] [PubMed] [Google Scholar]
- 15.Aziz K, Berger K, Claycombe K, Huang R, Patel R, Abela GS. Noninvasive detection and localization of vulnerable plaque and arterial thrombosis with computed tomography angiography/positron emission tomography. Circulation. 2008;117:2061–2070. doi: 10.1161/CIRCULATIONAHA.106.652313. [DOI] [PubMed] [Google Scholar]
- 16.Briley-Saebo KC, Shaw PX, Mulder WJ, et al. Targeted molecular probes for imaging atherosclerotic lesions with magnetic resonance using antibodies that recognize oxidation-specific epitopes. Circulation. 2008;117:3206–3215. doi: 10.1161/CIRCULATIONAHA.107.757120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von zur Muhlen C, von Elverfeldt D, Moeller JA, et al. Magnetic resonance imaging contrast agent targeted toward activated platelets allows in vivo detection of thrombosis and monitoring of thrombolysis. Circulation. 2008;118:258–267. doi: 10.1161/CIRCULATIONAHA.107.753657. [DOI] [PubMed] [Google Scholar]
- 18.Korosoglou G, Weiss RG, Kedziorek DA, et al. Noninvasive detection of macrophage-rich atherosclerotic plaque in hyperlipidemic rabbits using “positive contrast” magnetic resonance imaging. J Am Coll Cardiol. 2008;52:483–491. doi: 10.1016/j.jacc.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amirbekian V, Lipinski MJ, Briley-Saebo KC, et al. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc Natl Acad Sci U S A. 2007;104:961–966. doi: 10.1073/pnas.0606281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nahrendorf M, Aikawa E, Figueiredo JL, et al. Transglutaminase activity in acute infarcts predicts healing outcome and left ventricular remodelling: implications for FXIII therapy and antithrombin use in myocardial infarction. Eur Heart J. 2008;29:445–454. doi: 10.1093/eurheartj/ehm558. [DOI] [PubMed] [Google Scholar]
- 21.Nahrendorf M, Sosnovik D, Chen JW, et al. Activatable magnetic resonance imaging agent reports myeloperoxidase activity in healing infarcts and noninvasively detects the antiinflammatory effects of atorvastatin on ischemia-reperfusion injury. Circulation. 2008;117:1153–1160. doi: 10.1161/CIRCULATIONAHA.107.756510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Porcel M, Cai W, Gheysens O, et al. Imaging of VEGF receptor in a rat myocardial infarction model using PET. J Nucl Med. 2008;49:667–673. doi: 10.2967/jnumed.107.040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verjans JWH, Lovhaug D, Narula N, et al. Noninvasive imaging of angiotensin receptors after myocardial infarction. J Am Coll Cardiol Img. 2008;1:354–362. doi: 10.1016/j.jcmg.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behm CZ, Kaufmann BA, Carr C, et al. Molecular imaging of endothelial vascular cell adhesion molecule-1 expression and inflammatory cell recruitment during vasculogenesis and ischemia-mediated arteriogenesis. Circulation. 2008;117:2902–2911. doi: 10.1161/CIRCULATIONAHA.107.744037. [DOI] [PubMed] [Google Scholar]
- 25.Helm PA, Caravan P, French BA, et al. Postinfarction myocardial scarring in mice: molecular MR imaging with use of a collagen-targeting contrast agent. Radiology. 2008;247:788–796. doi: 10.1148/radiol.2473070975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flogel U, Ding Z, Hardung H, et al. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 2008;118:140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson LL, Schofield L, Donahay T, Bouchard M, Poppas A, Haubner R. Radiolabeled arginine-glycine-aspartic acid peptides to image angiogenesis in swine model of hibernating myocardium. J Am Coll Cardiol Img. 2008;1:500–510. doi: 10.1016/j.jcmg.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makowski MR, Ebersberger U, Nekolla S, Schwaiger M. In vivo molecular imaging of angiogenesis, targeting alphavbeta3 integrin expression, in a patient after acute myocardial infarction. Eur Heart J. 2008;29:2201. doi: 10.1093/eurheartj/ehn129. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Wu JC, Sheikh AY, et al. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116:I46–I54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Porcel M, Brinton TJ, Chen IY, et al. Reporter gene imaging following percutaneous delivery in swine moving toward clinical applications. J Am Coll Cardiol. 2008;51:595–597. doi: 10.1016/j.jacc.2007.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyle B, Kemp BJ, Chareonthaitawee P, et al. Dynamic tracking during intracoronary injection of 18F-FDG-labeled progenitor cell therapy for acute myocardial infarction. J Nucl Med. 2007;48:1708–1714. doi: 10.2967/jnumed.107.042838. [DOI] [PubMed] [Google Scholar]
- 32.Nahrendorf M, Zhang H, Hembrador S, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaffer FA, Nahrendorf M, Sosnovik D, Kelly KA, Aikawa E, Weissleder R. Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol Imaging. 2006;5:85–92. [PubMed] [Google Scholar]
- 34.Judenhofer MS, Wehrl HF, Newport DF, et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14:459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- 35.Winter PM, Caruthers SD, Zhang H, Williams TA, Wickline SA, Lanza GM. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. J Am Coll Cardiol Img. 2008;1:624–634. doi: 10.1016/j.jcmg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tahara N, Kai H, Nakaura H, et al. The prevalence of inflammation in carotid atherosclerosis: analysis with fluorodeoxyglucose-positron emission tomography. Eur Heart J. 2007;28:2243–2248. doi: 10.1093/eurheartj/ehm245. [DOI] [PubMed] [Google Scholar]
- 37.Tahara N, Kai H, Ishibashi M, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825–1831. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 38.Rudd JH, Myers KS, Bansilal S, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49:871–878. doi: 10.2967/jnumed.107.050294. [DOI] [PubMed] [Google Scholar]
- 39.Tang TY, Howarth SP, Miller SR, et al. Correlation of carotid atheromatous plaque inflammation using USPIO-enhanced MR imaging with degree of luminal stenosis. Stroke. 2008;39:2144–2147. doi: 10.1161/STROKEAHA.107.504753. [DOI] [PubMed] [Google Scholar]
- 40.Spuentrup E, Botnar RM, Wiethoff AJ, et al. MR imaging of thrombi using EP-2104R, a fibrin-specific contrast agent: initial results in patients. Eur Radiol. 2008;18:1995–2005. doi: 10.1007/s00330-008-0965-2. [DOI] [PubMed] [Google Scholar]