Abstract
Background:
Many studies showed a better recovery of cognitive function after administration of exogenous lactate during moderate-severe traumatic brain injury. However, the study evaluating lactate effect on mild traumatic brain injury is still limited.
Aims:
To evaluate the effect of exogenous lactate on cognitive function in mild traumatic brain injury patients.
Settings and Design:
Prospective, single blind, randomized controlled study on 60 mild traumatic brain injury patients who were undergoing neurosurgery.
Materials and Methods:
Subjects were randomly assigned into hyperosmolar sodium lactate (HSL) group or hyperosmolar sodium chloride (HSS) group. Patients in each group received either intravenous infusion of HSL or NaCl 3% at 1.5 ml/KgBW within 15 min before neurosurgery. During the surgery, patients in both groups received maintenance infusion of NaCl 0.9% at 1.5 ml/KgBW/hour.
Statistical Analysis:
Cognitive function, as assessed by Mini-Mental State Examination (MMSE) score at 24 h, 30 and 90 days post-surgery, was analyzed by Anova repeated measures test.
Results:
The MMSE score improvement was significantly better in HSL group than HSS group (P < 0.001). In HSL group the MMSE score improved from 16.00 (13.75-18.00) at baseline to 21.00 (18.75-22.00); 25.00 (23.75-26.00); 28.00 (27.00-29.00) at 24 h, 30, 90 days post-surgery, respectively. In contrast, in HSS group the MMSE score almost unchanged at 24 h and only slightly increased at 30 and 90 days post-surgery.
Conclusions:
Hyperosmolar sodium lactate infusion during mild traumatic brain injury improved cognitive function better than sodium chloride 3%.
Keywords: Cognitive function, mild brain injury, MMSE Score, sodium lactate
Introduction
Microstructural damage, and sequenced biomolecular impairment after mild traumatic brain injury (MTBI) could lead to cognitive impairment,[1,2] which affected most patients at 1 month post injury.[3,4,5,6] However, cognitive impairment could prolong to 3 months until 3 years post injury.[7] The deficits were most evident on tests of reasoning, information processing, verbal learning, inefficient organization, poor attention to detail, concentration, memory or judgment, and faulty error recognition.[8,9,10,11,12,13]
Lactate has been proven to be a preferred substrate for neuron and support the early recovery of synaptic function from ATP depletion after hypoxia.[14,15,16,17,18,19,20,21,22,23,24,25] L-lactate infusion in brain injured animal models significantly improves cognitive recovery[26,27,28] However, the studies in human are still limited. Therefore, this study aimed to evaluate the effect of hyperosmolar sodium lactate (HSL) infusion compared with 3% sodium chloride on cognitive function, assessed by Mini Mental State Examination (MMSE) score,[29,30] in MTBI patients
Materials and Methods
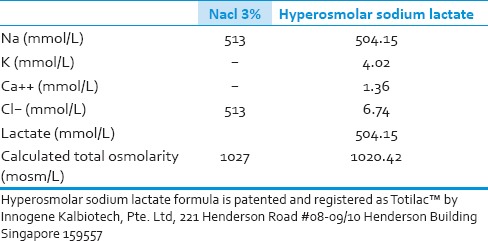
This was a prospective, randomized, single blind study aiming to evaluate the efficacy and safety of a scientifically formulated and patented hyperosmolar sodium-lactate infusion (Totilac®, manufactured by PT Finusolprima Farma International-Indonesia) in improving neurocognitive function post MTBI compared to commercially available hyperosmolar sodium chloride (HSS) (NaCl 3%, manufactured by PT. Otsuka – Indonesia). The composition of each solution is depicted in Table 1. Patients were asked to complete the MMSE questionnaire to assess the evolution of cognitive function.
Table 1.
Composition of sodium chloride 3% and hyperosmolar sodium lactate
Patients
This trial was conducted in 60 patients with MTBI who were undergoing emergency neurosurgical procedures (craniectomy debridement or craniotomy evacuation of hematoma) at Hasan Sadikin Hospital, Bandung, Indonesia. Craniectomy debridement means for open depressed skull fractures only and craniotomy evacuation means evacuating a subdural or epidural hemorrhage. Prior to the study, a written approval for the protocol was obtained from the Health Research Ethics Committee, Faculty of Medicine Universitas Padjadjaran – Hasan Sadikin Hospital, Bandung, Indonesia. Patients who met eligibility criteria were randomly assigned by permuted block into two groups immediately before surgery: the HSL (hyperosmolar sodium lactate) or the HSS (hyperosmolar sodium chloride 3% solution) group. Patients were selected according to the following inclusion criteria: male or female, age group of 14-60 years patients with MTBI with Glasgow Coma Scale (GCS) of 14-15 and required emergency neurosurgical procedures, physical status ASA I-II, onset of trauma at admission in the hospital was less than 9 h, patients had to be literate and also had written informed consent signed by patient's next of kin. Exclusion criteria were patients with multiple injury, pregnant or breastfeeding patients, patients with history of alcohol, barbiturate or opiate consumption prior to the injury. Patients were excluded from the analysis if they cannot be contacted or loss to follow up, died before the data collection completed, or experienced massive bleeding during neurosurgery procedures.
Anesthesia and surgical procedures
Eligible patients were assessed for vital sign, GCS, and cognitive function with MMSE and the data were recorded as baseline data. Required demographic information and baseline laboratory data were also recorded. Anesthesia was administered identically in both groups as per standard protocol in Hasan Sadikin hospital. Anesthesia was induced with slow administration of 3 µg/KgBW fentanyl within 1 min and then continued with 2.5 mg/KgBW propofol and 0.1 mg/KgBW vecuronium. Immediately after the eyelash reflex disappeared, the patient received ventilation with 100% oxygen and sevoflurane. Blood pressure was closely monitored every minute. A dose of 1.5 mg/kgBW lidocaine and 1.5 mg/kgBW propofol was administered intravenously at 3 min and 1 min, respectively, before intubations. Intubation was performed as per standard procedure. Breathing volume and respiratory rate were closely controlled to 6 ml/KgBW and 12 times/minute, respectively. Anesthesia was maintained with the continuous infusion of 25-100 µg/Kg BW/min propofol and 1% sevoflurane.
Study drugs administration
Study drugs (HSL or HSS) were administered intravenously through a peripheral vein. After induction, the patients in each group received either HSLor HSS 3% accordingly at the same dose of 1.5 ml/kgBW within 15 min. During surgery, patients in both groups received maintenance infusion of NaCl 0.9% at a dose of 1.5 ml/kgBW/hour.
Patients monitoring
Throughout the surgery procedures, blood pressure, mean arterial pressure, respiratory rate, and oxygen saturation were evaluated and recorded every 5 min. Blood osmolality and sodium level were evaluated and recorded at baseline, 15, 30 min, and 6 h post-surgery. Serum osmolality was calculated using standard formula: serum osmolality = (2 × serum sodium level) + (blood glucosa/18) + (BUN/2.8). GCS was assessed and recorded at 24 h post-surgery. If the GCS of the patients was 14-15, then cognitive function at 24 h post-surgery was evaluated using MMSE and the data were recorded. During follow up period, the MMSE score was evaluated again at 30 and 90 days post-surgery. Any adverse event and serious adverse event during 24 h post-surgery were recorded.
Cognitive assessment
Cognitive function was assessed by using the MMSE questionnaire[30] at 24 h, 30, 90 days post-surgery.
Dose used for hyperosmolar sodium lactate infusion
The amount of exogenous lactate administration was extrapolated from previous preclinical data of the effect of exogenous lactate administration on neurocognitive function.[26]
Statistical analysis
Baseline data were assessed to show comparability; differences in categorical data were assessed by Chi-square test and differences in continuous data by independent Student's t-test. The t-test was run after equality of variance assumption was tested, and subsequently the appropriate test result was reported. Our statistical analysis revealed that the normality of the dependent variable mean of MMSE scores was not violated, except the fact that sphericty assumption (correlations among repeated measures) was found to be unequal. The skewness values were closer to 0 [standard observation for skewness value for normality: −1 to +1] for MMSE score parameters (data not shown). In addition, Anova with repeated measures is robust against two violations including multivariate normality and homogeneity of covariance matrices.[31,32] The unequal correlation was corrected by Greenhouse-Geisser correction factor, the Greenhouse-Geisser F-ratio is reported, as performed in our present analysis, which adjust the degrees of freedom in Anova repeated measures test. Therefore, MMSE score analysis in this study does not require a non-parametric Friedman test in this instance. The mean ± SD was reported. Statistical finding was reported as significant if P < 0.05. All statistical analysis was done using SPSS 15.0 for Windows.
Results
Patient and treatment characteristics
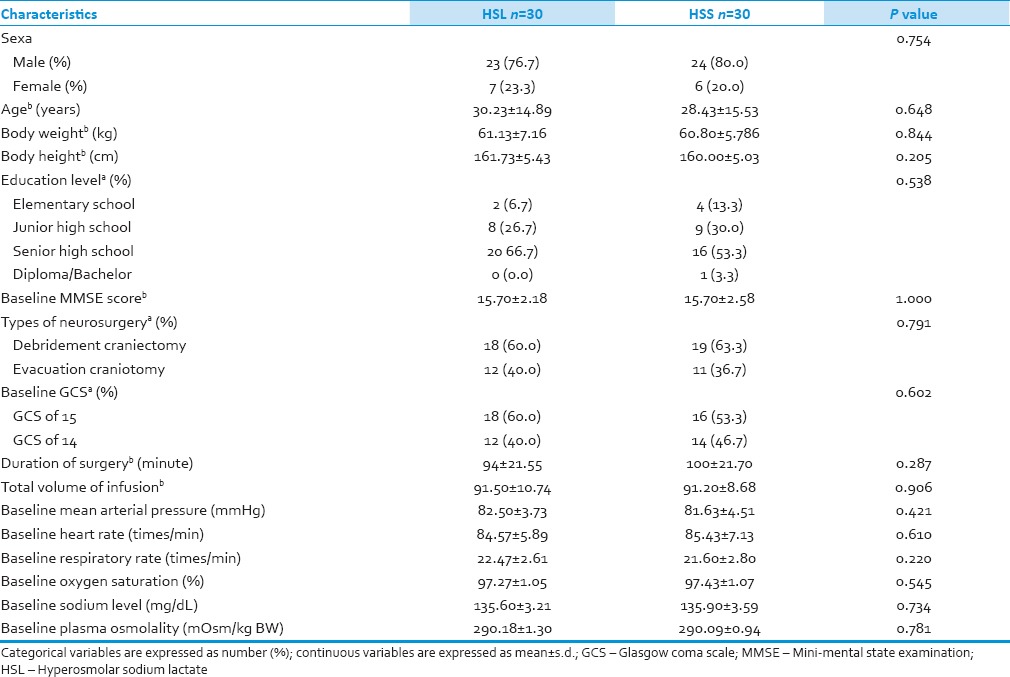
Sixty eligible patients were selected and randomized into HSL or HSS group; 30 patients in HSL group and 30 patients in HSS group. Randomization produced similar demographic and baseline data between patients in HSL and HSS group [Table 2]. Neurosurgical procedures (craniectomy debridement and craniotomy evacuation) and other managements were also standardized to be identical in both groups. All enrolled patients (60 patients) were analyzed for efficacy and safety data. No patient who loss to follow up, died before the data collection completed, or experienced massive bleeding during neurosurgery procedures.
Table 2.
Demographic and baseline characteristics of patients
Cognitive function
In this study, we only included mild TBI patients with GCS score of 14-15 with consideration that patients who had GCS lower than 14 will not be capable in doing the MMSE test. As a consequence, MMSE score will be less accurate for describing the cognitive impairment in this group of patients.
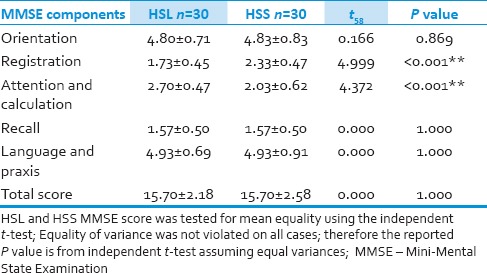
Analysis of the difference in each component of baseline MMSE score between the two groups showed that Registration and Attention scores and Calculation score were different before surgery, however the total baseline MMSE score was comparable between the two groups [Table 3].
Table 3.
Mean±SD of baseline MMSE components
HSL increased total MMSE score from 16.00 (13.75-18.00) at baseline to 21.00 (18.75-22.00); 25.00 (23.75-26.00); 28.00 (27.00-29.00) at 24 h, 30, 90 days post-surgery, respectively, while HSS increased total MMSE score from 16.00 (13.75-18.00) at baseline to 16.00 (14.00-18.00); 17.50 (15.75-20.00); 18.50 (16.00-21.00) at 24 h, 30, 90 days post-surgery, respectively, and the different between them was significant (P < 0.001). Table 3 and Figure 1 show the MMSE score evolution for every component over time. It was shown that HSL increased MMSE score in every component over time more rapidly than HSS (P < 0.001) except for the registration score (P = 0.820) [Table 3 and Figure 1].
Figure 1.
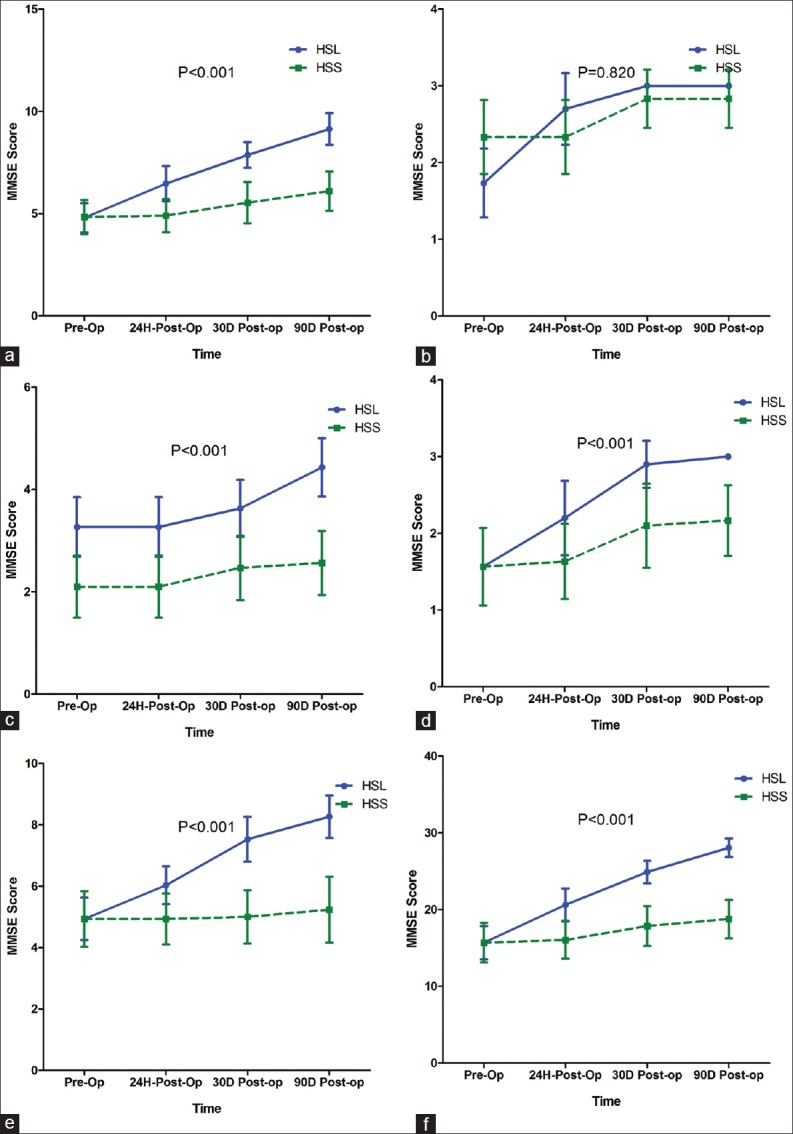
Straight line: HSL, dashed line: HSS. Panel (a) MMSE Orientation; (b) Registration; (c) Attention; (d) Recall; (e) Language; (f) Total score. Evaluation was done before surgery, 24 h, 30, and 90 days post-surgery. Comparisons by repeated measure ANOVA
Hemodynamic and respiratory parameters
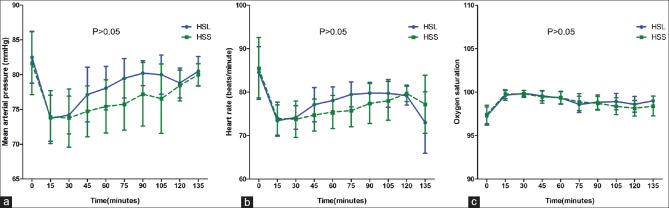
The changes of mean arterial pressure, heart rate, and oxygen saturation throughout the study period were comparable between HSL and HSS group (P > 0.05) [Figure 2].
Figure 2.
Straight line: HSL, dashed line: HSS. Panel (a) Mean Arterial Pressure (MAP); (b) Heart Rate; (c) Oxygen Saturation, (%). Evaluations were done every 15 min during surgery. Time variable was normalized to deal with the different length of surgery. Comparisons by repeated measure ANOVA; MAP: P =0.311; HR: P =0.9; OS: P =0.116
Biological parameters
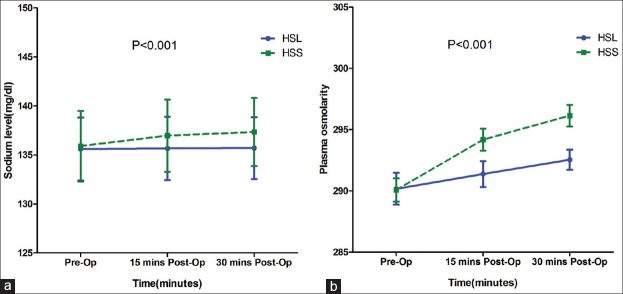
The evolution of blood sodium and osmolality was different between HSL and HSS group. Blood sodium and osmolality level increased in HSS group, whereas these parameters were almost unchanged in HSL group (P < 0.001 for both parameters) [Figure 3].
Figure 3.
Straight line: HSL, dashed line: HSS. Panel (a) Sodium; (b) Plasma Osmolality, mOsm/kg BW. Evaluations were done before surgery, 15 min, and 6 h post-surgery. Comparisons by repeated measure ANOVA
During the study and 24 h post-surgery, no adverse event or serious adverse event was noticed.
Discussion
This prospective randomized single blind study was aimed to evaluate the effect of exogenous lactate infusion contained in HSL solution, in comparison to 3% HSS solution on cognitive function as assessed by MMSE score in MTBI patients undergoing neurosurgical procedures (craniectomy debridement or craniotomy evacuation).
The 3% HSS was used as the reference solution because its total osmolarity is similar with 0.5 M HSL solution (1027 mOsmol/kg vs. 1020.42 mOsmol/kg, respectively). However, even though the total osmolarity of 0.5 M HSL and 3% HSS was equivalent; they have different inorganic ion composition. The 3% HSS has inorganic cation and anion of sodium and chloride, respectively, and both cannot cross plasma membrane so they will create hypertonicity toward intracellular space. HSL solution contains inorganic cation (Na+, K+, and Ca2+) and organic anion of lactate. After HSL infusion, lactate can cross cellular plasma membrane to be immediately metabolized and hence only sodium creates tonicity. Therefore, equiosmolar (1 osmole/L) of sodium chloride and sodium lactate do not develop the same tonicity toward intracellular space. Following the metabolism of lactate anions in the HSL group, the remaining inorganic cations create an electroneutrality imbalance (excess of extracellular cation). The excess of extracellular cation (Na+) is probably compensated by the net efflux of intracellular chloride because chloride is the principal intracellular anion and is responsible for intracellular tonicity. Therefore, net changes in cell volume after HSL infusion result from the combination of moderate changes in osmotic imbalance and electroneutrality imbalance while the changes after hypersomolar sodium chloride are only due to osmotic imbalance.[33,34]
Many studies showed that patients with traumatic brain injury, including MTBI commonly experienced late onset and long-term cognitive impairment. In clinical setting, MMSE can be used as a practical method in grading cognitive state post-TBI[35] Normal cognitive function is defined by MMSE score of 24-30; mild cognitive impairment by score of 18-23; and severe cognitive impairment by score of 0-17.[30] In this study, HSL group revealed an earlier and better improvement in cognitive function compared to HSS group. In HSL group, the obvious improvement of cognitive function from severe cognitive impairment to mild cognitive impairment was noticed since 24 h post-surgery. Moreover, the cognitive function continuously improved at 30 days post-surgery and reached normal function at 90-days post-surgery. Despite of similar molarity between HSL and HSS group, different evolution of cognitive function was noted between the two groups. In HSS group the cognitive function was almost unchanged at 24 h, 30 and 90 days post-surgery.
In this study, we found that MAP evolution was comparable between HSL and HSS group. The MAP was within normal range throughout the study period in both groups. Adequate MAP is required to guarantee adequate brain perfusion. According to the guidelines from Brain trauma Foundation the cerebral perfusion pressure (CPP) in patient with traumatic brain injury ranges from 50 to 70 mmHg and CPP of 60 mmHg is generally accepted as the best CPP level. Cerebral perfusion pressure can be calculated by substracting MAP from ICP. The ICP of mild brain traumatic patients is commonly less than 20 mmHg,[36] therefore based on our finding that the MAP range was normal (72-78 mmHg) during this study, we assumed that the CPP of the patients were adequate throughout the study.
Significant improvement of cognitive function as indicated by the improvement of MMSE score was only found in the HSL group not in HSS group. There were several possible mechanisms responsible for the improvement of cognitive status after HSL infusion such as energetic substrate effect of lactate, the prevention of hyperchloremia, and the decrease of the brain cell edema.
Recent studies showed that lactate increase during hypoxia or ischemic condition is merely an adaptive response of our body to produce readily used energy substrate. During hypoxia or ischemic condition which frequently occurred after traumatic brain injury, the cells including brain cells are unable to use glucose as energy substrate because in hypoxic or ischemic condition the ATP is depleted while glucose metabolism requires ATP investment. The first few steps in the aerobic conversion of glucose to pyruvate require the investment of 2 moles of ATP for phosphorylation of 1 mole of glucose. The cellular ATP levels are practically very low after a long hypoxic period therefore glucose oxidation is inappropriate in supporting the recovery of organ function upon reoxygenation. In contrast, lactate conversion to pyruvate does not require investment of ATP. Therefore, lactate is a preferred energy substrate in hypoxia or ischemic recovery condition in many cells including brain cells.[14,15,16,17,18,19,20,21,22,23,24,25] This finding is supported by other studies which found that during traumatic brain injury, the extracellular lactate level increased and glucose level decreased. The increase of lactate level is suggested due to the need of lactate as an energy substrate for hypoxic brain cells to restore impaired brain homeostasis and synapse function after brain injury.[16,19,24,33,37,38] It was found in vitro that lactate can support synaptic function as the sole energy substrate and exogenously supplied lactate can support earlier recovery of synaptic function after hypoxia.[14,15] Further study showed that exogenous lactate administration significantly improved neurologic outcome in traumatic brain injury animal model.[26,27,28] Recent human study also showed that blood lactate is oxidized by the brain cells and is an important energy source in human brain.[39] Even during traumatic brain injury, it was obviously demonstrated that lactate is utilized as an energy substrate and protects the brain during injury.[40,41] Therefore, we strongly suggest that lactate contribute for the improvement of cognitive function in HSL group.
In this study, we found that the osmolality and sodium level in NaCl group was higher compared to HSL group; hence if the osmotic effect would be considered as a major beneficial effect we should have the opposite result. In other words, this indicates that in our study hyperosmolarity is not the major mechanism responsible for MMSE improvement. Indeed the electrolyte and osmolality should be evaluated more frequent and closer to the fluid infusion. In our study, we did not measure sodium level and osmolality immediately after study fluid infusion and we acknowledged this as one of study limitation. We evaluated these parameters 15 min post-operative and 6 h after surgery. The sodium level and osmolality at 15 min post-operative may represent sodium level and osmolality at 2 h after infusion (The average duration of the surgery was 90-100 min). Indeed we cannot expect the changes of sodium level and osmolality after more than 2 h after fluid infusion. However, we presented here the sodium level and osmolality at 6 h after Totilac infusion because Totilac is a novel solution; therefore, we need to evaluate the safety of the fluid infusion longer than 2 h.
As described previously, HSL infusion might decrease cell volume resulting from the exchange between the influx of metabolizable lactate into the cell and an efflux of chloride from the cell which must be accompanied by water.[33,34,42] This also may be responsible for possible explanation for the improvement cognitive function after HSL infusion in this study, but we cannot demonstrate it because we did not measure intracranial pressure.
Some studies supported the beneficial effect of balanced solution in many organ functions. But it remains controversial and we cannot separate the effect of acidosis with its root cause (hyperlactatemia vs. hyperchloremia). Moreover, experimental studies support that for same level of acidosis hyperchloremia has pro-inflammatory effect whereas hyperlactatemia did not.[43] Thus, we could suppose that by preventing hyperchloremia, sodium lactate infusion could improve neurological evolution of patients.
The composition of inorganic ions contained in HSL solution and NaCl 3% solution is also different. In addition to sodium, HSL solution contains potassium, calcium in physiologic concentration, and very low concentration of chloride. Intracellular calcium activation has been shown to be involved in the brain cell damage. However, there is no association between plasma calcium concentration and intracellular calcium activation.[44] Therefore, we assumed that by giving physiological concentration of calcium in the solution could not modify the activation of calcium in the cell. Finally, it is impossible to demonstrate that because it is impossible to measure intracellular calcium activation in clinical practice. However, we cannot totally exclude a possible effect of calcium in this as a component of HSL. The excess in extracellular glutamate availability during traumatic brain injury affects neurons and astrocytes and results in over-stimulation of ionotropic and metabotropic glutamate receptors with consecutive Ca2+, Na+, and K+fluxes.[45,46,47] However, extracellular potassium has never been described to be responsible for brain cell damage in addition to the only physiological concentration of potassium in the solution.
In this study, we found that despite of higher osmolarity in the control group, the cognitive function did not significantly improve in each assessment until 3 months post TBI. The effect of HSS infusion has been evaluated in several studies however the findings were not consistent. A study by Sell et al.,[48] showed that HSL infusion in traumatic brain injury model improved neuronal survival and cognitive performance after injury. In contrast other studies showed that HSS infusion neither improved cognitive function[49] nor restored cerebral oxygen delivery and electroencephalographic after traumatic brain injury.[50] Even bolus administrations of HSS on brain injury model was associated with greater loss of hippocampal and brain cortex. We suggest that the osmolarity was not the major mechanism involved in the improvement of cognitive after administration of hyperosmolar solution in this study. The absence of lactate and its consequences in NaCl solution given to the control group may contribute to the insignificant improvement of cognitive function in the control group. In addition, despite similar total osmolarity between HSL and 3% hypertonic sodium chloride, other properties of HSL such as very low chloride content might also contribute to the significant improvement of cognitive function in HSL group.
During the study, no adverse event related to study drug infusion was recorded. Despite of high sodium load after HSL or HSS infusion, no hypernatremia was found in this study. In this study, we administered non-physiological isotonic sodium chloride for maintenance fluid in both group which could induce hyperchloremic acidosis. Hyperchloremic acidosis after sodium chloride infusion will occur after rapid and longer infusion of high doses of sodium chloride (such as 30 mL/kgBW/hour 0.9% NaCl).[51,52] In our study, we only administered low dose of 0.9% sodium chloride for maintenance, i.e. continuous infusion of 1.5 cc/kgBW/hour 0.9% NaCl. The mean time of surgery in this study was around 90-100 min [Table 1], therefore such low doses of NaCl 0.9% infusion for a short period will not induce hyperchloremic acidosis.
There were several studies showing that pyruvate is metabolized in the brain just like a lactate[53,54,55,56,57] except with a very high concentration of pyruvate. Despite potential deleterious effect of high concentration of pyruvate, the clinical neurological improvement of our patients does not support this hypothesis. Lactate load was just calculated to be completely oxidized without increasing pyruvate concentration.
This study had some limitations. The major methodological bias is that the study was not conducted in a double blind manner. For practical reason, we conducted with the only blind MMSE assessment by a different physician with the staff giving the study fluid. Moreover due to the well-known usual metabolic effect of sodium lactate infusion (alkalinizing effect)[42] it will be very easy to know which fluid has been given for the protocol. We did not measure the ICP and lactatemia during the study, therefore we cannot evaluate precisely cerebral edema and for the lactatemia we cannot exclude totally the development of hyperlactatemia. In addition, we cannot establish the correlation between lactate level and cognitive improvement after mild TBI in this study, and further study is warranted. In this study we did not measure blood sodium and osmolality immediately after study fluid infusion; hence we cannot evaluate the acute effect of the studied solution on these parameters. In addition to this limitation, we calculated blood osmolality rather than measuring it, thus this may constitute a limitation for interpreting precisely this parameter. Therefore, a more comprehensive clinical study is warranted to address precisely the mechanism of cognitive improvement after HSL infusion.
Conclusion
Infusion of HSL is safe and resulted in significantly better improvement of the cognitive function at 24 h, 30 and 90 days post-mild TBI as indicated by the increase of MMSE score in comparison to HSS 3% infusion.
Acknowledgment
We acknowledge Professor Carole Ichai for her valued contribution to give review and give feedback about the content of this manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 2.Smits M, Houston GC, Dippel DW, Wielopolski PA, Vernooij MW, Koudstaal PJ, et al. Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology. 2011;53:553–63. doi: 10.1007/s00234-010-0774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leininger BE, Gramling SA, Farrel AD, Kreutzer JS, Peck EA., 3rd Neuropsychological deficits in symptomatic minor head injury patients after concussion and mild concussion. J Neurol Neurosurg Psychiatry. 1990;53:293–6. doi: 10.1136/jnnp.53.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dikmen S, McLean A, Temkin N. Neuropsychological and psychosocial consequences of minor head injury. J Neurol Neurosurg Psychiatry. 1986;49:1227–32. doi: 10.1136/jnnp.49.11.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazarian JJ, Wong T, Harris M, Leahey N, Mookerjee S, Dombovy M. Epidemiology and predictors of postconcussive syndrome after minor head injury in an emergency population. Brain Inj. 1999;13:173–89. doi: 10.1080/026990599121692. [DOI] [PubMed] [Google Scholar]
- 6.Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, et al. Prognosis for mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;36(Suppl 43):84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 7.Ewing R, McCarthy D, Gronwall D, Wrightson P. Persisting effects of minor head injury observable during hypoxic stress. J Clin Neuropsychol. 1980;2:147–55. [Google Scholar]
- 8.Rimel RW, Giordani B, Barth JT, Boll TJ, Jane JA. Disability caused by minor head injury. Neurosurgery. 1981;9:221–8. [PubMed] [Google Scholar]
- 9.Barth, Macciocchi SN, Giordani B, Rimel R, Jane JA, Boll TJ. Neuropsychological sequelae of minor head injury. Neurosurgery. 1983;13:529–33. doi: 10.1227/00006123-198311000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Gentilini M, Nichelli P, Schoenhuber R, Bortolotti P, Tonelli L, Falasca A, et al. Neuropsychological evaluation of mild head injury. J Neurol Neurosurg Psychiatry. 1985;48:137–40. doi: 10.1136/jnnp.48.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hugenholtz H, Stuss DT, Stethem LL, Richard MT. How long does it take to recover from amild concussion? Neurosurgery. 1988;22:853–8. [PubMed] [Google Scholar]
- 12.Vanderploeg RD, Curtiss G, Belanger HG. Long-term neuropsychological outcomes following mild traumatic brain injury. J Int Neuropsychol Soc. 2005;11:228–36. doi: 10.1017/S1355617705050289. [DOI] [PubMed] [Google Scholar]
- 13.O’Jile JR, Ryan LM, Betz B, Parks-Levy J, Hilsabeck RC, Rhudy JL, et al. Information processing following mild head injury. Arch Clin Neuropyschol. 2006;21:293–6. doi: 10.1016/j.acn.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Schurr A, Payne RS, Miller JJ, Rigor BM. Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation, an in vitro study. Brain Res. 1997;744:105–11. doi: 10.1016/s0006-8993(96)01106-7. [DOI] [PubMed] [Google Scholar]
- 15.Schurr A, Payne RS, Miller JJ, Rigor BM. Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: Further in vitro validation. J Neuroschem. 1997;69:423–6. doi: 10.1046/j.1471-4159.1997.69010423.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen T, Qian YZ, Rice A, Zhu JP, Di X, Bullock R. Brain lactate uptake increases at the site of impact after traumatic brain injury. Brain Res. 2000;861:281–7. doi: 10.1016/s0006-8993(00)01992-2. [DOI] [PubMed] [Google Scholar]
- 17.Bliss TM, Sapolsky RM. Interactions among glucose, lactate and adenosine regulate energy substrate utilization in hippocampal cultures. Brain Res. 2001;899:134–41. doi: 10.1016/s0006-8993(01)02218-1. [DOI] [PubMed] [Google Scholar]
- 18.Schurr A. Lactate, glucose and energy metabolism in the ischemic brain (Review) Int J Mol Med. 2002;10:131–6. [PubMed] [Google Scholar]
- 19.Serres S, Bouyer JJ, Bezancon E, Canioni P, Merle M. Involvement of brain lactate in neuronal metabolism. NMR Biomed. 2003;16:430–9. doi: 10.1002/nbm.838. [DOI] [PubMed] [Google Scholar]
- 20.Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK, Amiel SA. Lactate: A preferred fuel for human brain metabolism in vivo. J Cerebr Blood Flow Metab. 2003;23:658–64. doi: 10.1097/01.WCB.0000063991.19746.11. [DOI] [PubMed] [Google Scholar]
- 21.Cater HL, Chandratheva A, Benham CD, Morrison B, 3rd, Sundstrom LE. Lactate and glucose as energy substrates during, and after, oxygen deprivation in rat hippocampal acute and cultured slices. J Neurochem. 2003;87:1381–90. doi: 10.1046/j.1471-4159.2003.02100.x. [DOI] [PubMed] [Google Scholar]
- 22.Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23:1298–306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- 23.Hertz L, Dienel GA. Lactate transport and transporters: General principles and functional roles in brain cells. J Neurosci Res. 2004;79:11–8. doi: 10.1002/jnr.20294. [DOI] [PubMed] [Google Scholar]
- 24.Gladden IB. Lactate metabolism: A new paradigm for the third millenium. J Physiol. 2004;558:5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schurr A. Lactate: The ultimate cerebral oxidative energy substrate? J Cereb Blood Flow Metab. 2006;26:142–52. doi: 10.1038/sj.jcbfm.9600174. [DOI] [PubMed] [Google Scholar]
- 26.Rice AC, Zsoldos R, Chen T, Wilson MS, Alessandri B, Hamm RJ, et al. Lactate administration attenuates cognitive deficits following traumatic brain injury. Brain Res. 2002;928:156–9. doi: 10.1016/s0006-8993(01)03299-1. [DOI] [PubMed] [Google Scholar]
- 27.Holloway R, Zhou1 Z, Harvey HB, Levasseur JE, Rice AC, Sun D, et al. Effect of lactate therapy upon cognitive deficits after traumatic brain injury in the rat. Acta Neurochir (Wien) 2007;149:919–27. doi: 10.1007/s00701-007-1241-y. [DOI] [PubMed] [Google Scholar]
- 28.Berthet C, Lei H, Thevenet J. Neuroprotective role of lactate after cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:1780–9. doi: 10.1038/jcbfm.2009.97. [DOI] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein S, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Folstein MF, Crum RM, Antony JC, Basset SS. Instruction for administration of mini mental state examination (MMSE) J Am Med Assoc. 1993;169:2386–91. [Google Scholar]
- 31.James SP. Applied Multivariate Statistics for the Social Sciences. In: Mahway NJ, editor. 3rd edn. New Jersey: Lawrence Erlbaum Associates, Inc; 1996. [Google Scholar]
- 32.SAS Library -Repeated Measures ANOVA Using SAS PROC GLM. [Last accessed on 2012 Sep 15]. Available from: http://www.ats.ucla.edu/stat/sas/library/repeated_ut.htm .
- 33.O’Neill WC. Physiological significance of volume regulatory transporters. Am J Physiol. 1999;276:C995–1011. doi: 10.1152/ajpcell.1999.276.5.C995. [DOI] [PubMed] [Google Scholar]
- 34.Jiang G, Klein JD, O’Neill WC. Growth factors stimulate the Na-K-2Cl cotransporter NKCC1 through a novel Cl(-)-dependent mechanism. Am J Physiol Cell Physiol. 2001;281:C1948–53. doi: 10.1152/ajpcell.2001.281.6.C1948. [DOI] [PubMed] [Google Scholar]
- 35.Chih CP, Roberts EL. Energy substrates for neurons during neural activity: A critical review of the astrocyte-neuron lactate shuttle hypothesis. J Cereb Blood Flow Metab. 2003;23:1263–81. doi: 10.1097/01.WCB.0000081369.51727.6F. [DOI] [PubMed] [Google Scholar]
- 36.Smith M. Monitoring intracranial pressure in traumatic brain injury. Anesth Analg. 2008;106:240–8. doi: 10.1213/01.ane.0000297296.52006.8e. [DOI] [PubMed] [Google Scholar]
- 37.Magistretti PJ, Pellerin L, Martin JL. Brain energy metabolism. Neuropsychopharmacology. 2000;5:1–13. [Google Scholar]
- 38.Levassuer JE, Alessandri B, Reinert M, Clausen T, Zhou Z, Altememi N, et al. Lactate, not glucose, up-regulates mitochondrial oxygen consumption both in sham and lateral fluid percused at brain. Neurosurgery. 2006;59:1122–31. doi: 10.1227/01.NEU.0000245581.00908.AF. [DOI] [PubMed] [Google Scholar]
- 39.Hall G, Stromstad M, Rasmussen P, Jans O, Zaar M, Gam C, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29:1121–9. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- 40.Cureton EL, Kwan RO, Dozier KC, Sadjadi J, Pal JD, Victorino GP. A different view of lactate in trauma patients: Protecting the injured brain. J Surg Res. 2010;159:468–73. doi: 10.1016/j.jss.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 41.Gallagher CN, Carpenter KL, Grice P, Howe DJ, Mason A, Timofeev I, et al. The human brain utilizes lactate via the tricarboxylic acid cycle: A 13 C-labelled microdialysis and high resolution nuclear magnetic resonance study. Brain. 2009;132:2839–49. doi: 10.1093/brain/awp202. [DOI] [PubMed] [Google Scholar]
- 42.Ichai C, Armando G, Orban JC, Berthier F, Rami L, Samat-Long C, et al. Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intensive Care Med. 2009;35:471–9. doi: 10.1007/s00134-008-1283-5. [DOI] [PubMed] [Google Scholar]
- 43.Kellum JA, Song M, Almasri E. Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest. 2006;130:962–7. doi: 10.1378/chest.130.4.962. [DOI] [PubMed] [Google Scholar]
- 44.Berridge MJ, Bootman MD, Lipp P. Calcium: A life and death signal. Nature. 1998;395:645–8. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 45.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 46.Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D. Molecular mechanisms of ischemic cerebral edema. Role of electroneutral ion transport. Physiology (Bethesda) 2009;24:257–65. doi: 10.1152/physiol.00015.2009. [DOI] [PubMed] [Google Scholar]
- 47.Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 2010;112:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 48.Sell SL, Avila MA, Yu G, Vergara L, Prough DS, Grady JJ, et al. Hypertonic resuscitation improves neuronal and behavioral outcomes after traumatic brain injury plus hemorrhage. Anesthesiology. 2008;108:873–81. doi: 10.1097/ALN.0b013e31816c8a15. [DOI] [PubMed] [Google Scholar]
- 49.Quigley A, Tan Arlene A, Hoane MR. The effects of hypertonic saline and nicotinamide on sensorimotor and cognitive function following cortical contusion injury in the rat. Brain Res. 2009;1304:138–48. doi: 10.1016/j.brainres.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeWitt DS, Prough DS, Deal DD, Vines SM, Hoen H. Hypertonic saline does not improve cerebral oxygen delivery after head injury and mild hemorrhage in cats. Crit Care Med. 1996;24:109–17. doi: 10.1097/00003246-199601000-00019. [DOI] [PubMed] [Google Scholar]
- 51.Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produced hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265–70. doi: 10.1097/00000542-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Morino PL. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2007. The ICU Book; pp. 236–7. [Google Scholar]
- 53.Desagher S, Glowinski J, Premont J. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J Neurosci. 1997;17:9060–7. doi: 10.1523/JNEUROSCI.17-23-09060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang XF, Cynader MS. Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity. J Neurosci. 2001;21:3322–31. doi: 10.1523/JNEUROSCI.21-10-03322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.González-Falcón A, Candelario-Jalil E, García-Cabrera M, León OS. Effects of pyruvate administration on infarct volume and neurological deficits following permanent focal cerebral ischemia in rats. Brain Res. 2003;990:1–7. doi: 10.1016/s0006-8993(03)03378-x. [DOI] [PubMed] [Google Scholar]
- 56.Mazzio E, Soliman KF. Pyruvic acid cytoprotection against 1-methyl-4-phenylpyridinium, 6-hydroxydopamine and hydrogen peroxide toxicities in vitro. Neurosci Lett. 2003;337:77–80. doi: 10.1016/s0304-3940(02)01327-7. [DOI] [PubMed] [Google Scholar]
- 57.Mazzio EA, Soliman KF. Cytoprotection of pyruvic acid and reduced beta-nicotinamide adenine dinucleotide against hydrogen peroxide toxicity in neuroblastoma cells. Neurochem Res. 2003;28:733–41. doi: 10.1023/a:1022813817743. [DOI] [PubMed] [Google Scholar]