Abstract
Background and Aim:
The glucocorticoid dexamethasone in a bolus dose of 8-10 mg followed by quarterly dose of 4 mg is commonly used during intracranial surgery so as to reduce oedema and vascular permeability. However, the detrimental hyperglycaemic effects of dexamethasone may override its potentially beneficial effects. The present prospective, randomised study aimed at comparing the degree and magnitude of hyperglycaemia induced by prophylactic administration of dexamethasone in patients undergoing elective craniotomy.
Materials and Methods:
Sixty American Society of Anaesthesiologist (ASA) grade-I and II patients were randomly assigned to three groups of 20 patients each. Group-I received dexamethasone during surgery for the first time. Group-II received dexamethasone in addition to receiving it pre-operatively, whereas Group-III (control group) patients were administered normal saline as placebo. Baseline blood glucose (BG) was measured in all the three groups before induction of anaesthesia and thereafter after every hour for 4 h and then two-hourly. Besides intra- and intergroup comparison of BG, peak BG concentration was also recorded for each patient. Statistical analysis was carried out with analysis of variance (ANOVA) and Student's t-test and value of P < 0.05 was considered statistically significant.
Results:
Baseline BG reading were higher and statistically significant in Group-II as compared with Group-I and Group-III (P < 0.05). However, peak BG levels were significantly higher in Group-I than in Group-II and III (P < 0.05). Similarly, the magnitude of change in peak BG was significantly higher in Group-I as compared to Group-II and III (P < 0.05).
Conclusion:
Peri-operative administration of dexamethasone during neurosurgical procedures can cause significant increase in BG concentration especially in patients who receive dexamethasone intra-operatively only.
Keywords: Dexamethasone, hyperglycaemic response, intracranial surgery
Introduction
Once thought to be totally incurable, neurosurgical tumours have been dealt with good success nowadays as a result of advancements in neurosurgical techniques and advent of newer anaesthetic drugs and equipment. Corticosteroids have been in use in neurosurgical practice for the past so many decades. Dexamethasone, a glucocorticoid, has been used extensively in the management of neurosurgical tumours. One of the most debatable issues concerning use of corticosteroids in this subset of population involves the benefit-to-risk ratio. The successful use of high-dose corticosteroids in neurological tumours was first described in October 1959, to a patient with a large recurrent glioblastoma. This therapeutic intervention resulted in complete resolution of the neurological deficit, which later propelled the introduction of dexamethasone as a treatment modality for tumour-related cerebral enema.[1] Dexamethasone has since become the standard pharmacological agent for treatment of cerebral enema associated with intracranial tumours.[2] The usual therapeutic regimen involves a bolus dose administration of 8-10 mg intravenously (IV) followed by 4 mg every six-hourly. This dosage often produces a significant improvement in intracranial dynamics, improving neurological function and reducing symptoms such as confusion and headache.[3,4]
Dexamethasone reduces tumour-associated oedema and vascular permeability. It has also been shown to reduce cerebral blood flow and blood volume and increase the fractional extraction of oxygen throughout the brain.[5] This leads to a reduction in intracranial pressure, ameliorating accompanying symptoms and prolonging useful neurologic function and life span in tumour patients.[6] Neurological improvement associated with dexamethasone can be dramatic, but the recent literature has highlighted its potential for negative physiological sequelae, which may have clinical relevance to its use in patients with brain tumours.[7,8] However, when dexamethasone is administered to neurosurgical patients for treatment of tumour-associated cerebral oedema, it may exacerbate brain injury in part as a result of increase in blood glucose (BG) concentrations. Dexamethasone has been shown to produce a significant rise in BG concentration in neurosurgical patients following even a single dose of 10 mg with no previous history of diabetes mellitus.[4,8,9] Hyperglycaemia in peri-operative patients has been identified as a risk factor for morbidity and mortality and in recent years there has been much research and debate on the association between glycaemic control and outcome after major surgery.[10] It has been shown to worsen neurological injury during both focal and global cerebral ischemia in both animal studies and in patients following stroke or cardiac arrest.[11,12] The present study was conducted to study the hyperglycaemic response to intra-operative dexamethasone administration in patients undergoing elective craniotomy and to compare it with those patients who may or may not be on preoperative dexamethasone, as well as to identify those patients who were at risk of hyperglycaemia during craniotomy and required treatment.
Materials and Methods
After taking the Institute's Ethics Committee approval, 60 non-diabetic patients belonging to American Society of Anaesthesiologist (ASA) physical status I or II, of either sex, in the age range of 18-64 years scheduled for elective craniotomy were selected for this study. Patients with history of diabetes mellitus, pituitary or adrenal disease and patients on any medication affecting BG concentrations with the exception of dexamethasone were excluded from the study. Patients were randomly allocated to three groups of 20 patients each: Group-I: Patients not on dexamethasone before surgery but receiving it during surgery and continued after surgery; Group-II: Patients already receiving dexamethasone before surgery and continuing it during and after surgery; and Group-III: patients not receiving any dexamethasone but receiving saline as placebo.
No patient was given any premedication. All patients received a standardised general aesthetic technique and induction of anaesthesia was done with propofol 2 mg per kg body weight and morphine sulphate 100 µg per kg body weight. Muscle relaxation and tracheal intubation were facilitated with intubating dose of vecuronium bromide. Anaesthesia was maintained with 66% nitrous oxide in oxygen supplemented with halothane 0.5%-1% as an inhalational agent. Arterial and central venous catheterisation was done for continuous measurement of arterial and central venous pressures.
All patients taking dexamethasone preoperatively received dexamethasone 8 mg intravenously (IV) at induction of anaesthesia and continued it postoperatively as per protocol. All other patients received either dexamethasone or placebo intra-operatively at the same time. The decision to administer dexamethasone to patients not receiving it before surgery was taken on the basis of computerised tomographic (CT) documentation of brain enema or if significant enema was anticipated postoperatively.
BG analysis was performed with an Accucheck BG meter, which was calibrated daily. Finger prick capillary blood was used for estimation of BG. Baseline BG concentration was obtained just before induction of anaesthesia. Thereafter BG concentration was measured for 12 h, initially hourly for 4 h followed by two-hourly estimations.
Intra- and intergroup comparisons of BG concentration were made at each time interval and peak BG concentration was recorded for each patient to study the difference in baseline (pre-induction) BG concentration, differences in peak BG concentration, time course of BG concentration changes, correlation between the duration of surgery and peak BG concentration. The data thus obtained were analysed statistically using analysis of variance (ANOVA) and Student's t-test. A value of P < 0.05 was considered statistically significant.
Results
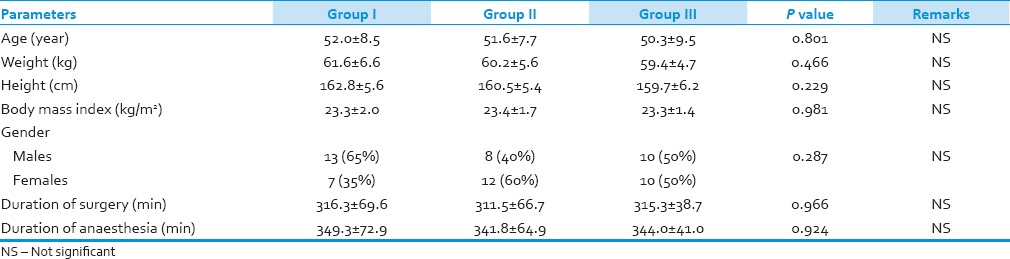
A total of 60 patients who underwent neurosurgical procedures were enrolled for the study and were randomly divided into three groups [Table 1]. The demographic profiles of the patients in all groups were comparable with regard to age, weight, height, body mass index and gender distribution. Distribution as per ASA status was similar in both groups and mean duration of surgery and anaesthesia was comparable in both groups and statistically non-significant (P > 0.05).
Table 1.
Demographic profile of all the groups
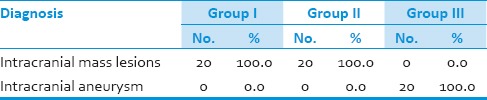
Various indications for neurosurgical procedures are enumerated in Table 2. A total of 60 patients had undergone elective craniotomy. Twenty patients each from Group-I and II had undergone surgery for intracranial mass lesions, whereas Group-III patients underwent surgery for intracranial aneurysm. Group-III patients were administered normal saline as placebo before induction of anaesthesia [Table 2].
Table 2.
Various indications for craniotomy in the three groups
Baseline BG level in Group-I as well as in Group-III was significantly lower than in Group-II (P = 0.05) and the overall difference (ANOVA) was also significant (P < 0.05) [Table 3, Figure 1]. BG level after 1 h was significantly higher in Group-II than in Group-III (P < 0.05). But there was no significant difference between Group-I and Group-II, Group-I and Group-III, and the overall difference was also not significant (P > 0.05). After 2 h interval, BG concentration was significantly higher in Group-I than in Group-III (P < 0.05). But there was no significant difference between Group-I and Group-II and Group-II and Group-III, and the overall difference was also not significant (P > 0.05). At 3 h interval, BG concentration was significantly higher in Group-I than in Group-III and Group-II than Group-III, and the overall difference was also significant (P < 0.05). BG level after 4 h was significantly higher in Group-I than Group-III and Group-II than Group-III, and the overall difference was also significant (P = 0.05). BG level after 6 h was significantly higher in Group-I than Group-II, Group-I than Group-III and Group-II than Group-III, and the overall difference was also significant (P < 0.05). After an 8-h interval, BG level was significantly higher in Group-I than Group-II, Group-I than Group-III and Group-II than Group-III, and the overall difference was also significant (P < 0.05). BG level after 10 h was significantly higher in Group-I than Group-II, Group-I than Group-III and Group-II than Group-III, and the overall difference was also significant (P < 0.05). At 12-h interval, BG concentration was significantly higher in Group-I than Group-II, Group-I than Group-III and Group-II than Group-III, and the overall difference was also significant (P < 0.05).
Table 3.
Comparison of BG concentration (mg/dl) between the three groups at different time intervals (mean±SD)
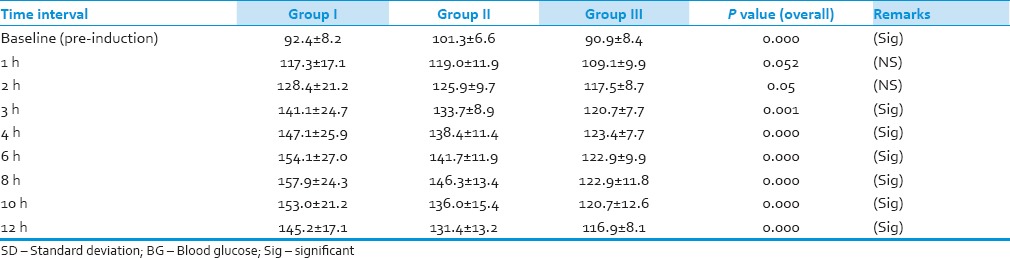
Figure 1.
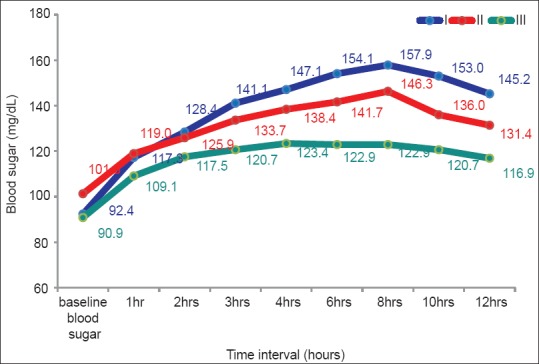
Comparison of BG concentration (mg/dl) between the three groups at different intervals of time
Change from baseline to peak BG concentration was significantly higher in Group-I than Group-II and III, and was statistically significant (P < 0.05). [Table 4] This change was also higher in Group-II than Group-III and on statistical analysis the value was significant (P < 0.05).
Table 4.
Baseline and peak BG concentration (mg/dl) in the three groups (mean±SD)

Discussion
Corticosteroids are presently used in routine practice in patients undergoing neurosurgical tumours as treatment for tumour-related cerebral oedema.[2] Use of dexamethasone in large doses leads to significant increase in BG concentration following even a single dose of 10 mg dexamethasone and it may exacerbate brain injury as a result of hyperglycaemia even in patients without a history of diabetes mellitus.[4,8,9]
It was observed in our study that baseline BG concentration was higher in Group-II (101.3 ± 6.6 mg/dl) as compared with Group-III (90.9 ± 8.4 mg/dl) and Group-I (92 ± 8.2). We also observed that a single dose of dexamethasone (8 mg IV) given intra-operatively only (Group-I) produced a significant increase in BG concentrations. During subsequent time intervals, although a statistically significant increase in BG concentration was observed in all three groups, however, the magnitude of change was larger in Group-I, with a statistically significant larger peak BG concentration (167.7 ± 19.5) and a greater change from baseline to peak BG concentration (75.3 ± 17.8) than the other two groups. Patients in this group also reached the peak BG level at an average of 6-8 h after commencement of surgery. BG concentration also increased over time in those who received placebo, but the magnitude of change was less than that observed in those receiving dexamethasone.
Our results correlate with the study of Jeffrey et al.[13] who observed that a single dose of dexamethasone (10 mg IV) produced significant changes in the BG concentration. In addition, the glucose concentration was still increasing at the end of 240-min study period. They also reported increase in BG concentrations over time in those who received placebo with lesser change in the magnitude than observed in those receiving dexamethasone. Our observations are also consistent with Michael and Pirjo[14] who observed that patients who were not taking dexamethasone before surgery but received it intra-operatively and postoperatively had the largest peak BG concentrations from baseline (pre-induction) values when compared with the other two groups. It is interesting that in our study Group-I had larger BG concentrations than Group-II, even though both groups received the same dose of dexamethasone during the study. This was also observed by Michael and Pirjo[14] who attributed it to the development of some tolerance to hyperglycaemic effect of dexamethasone with its chronic use. Another possibility is that the adrenal response to stress is suppressed in patients already taking dexamethasone thereby obtunding the response of surgical stress to endogenous steroids.
Previously it was recommended that BG concentrations greater than 200 mg/dl should be avoided; however, increasing evidence is now supporting lower goal glucose concentrations. Wass and Lanier[15] in their comprehensive review also recommended that in patients without a history of glucose intolerance, target glucose concentrations under 150 mg/dl seem a reasonable goal. In the advent of data supporting strict glycaemic control, one should attempt to identify factors that lead to hyperglycaemia, especially when caring for patients at increased risk of cerebral ischemia. It is clear from our investigation that a single 8-mg intravenous dose of dexamethasone, administered intra-operatively, increases the likelihood significant increase in the BG concentration, possibly requiring treatment.
In conclusion, peri-operative administration of dexamethasone to neurosurgical patients causes significant increase in BG concentration. The hyperglycaemic response is found to be more in patients who receive dexamethasone intra-operatively only. In view of the above findings, it is recommended that all neurosurgical patients should have regular blood sugar monitoring when taking dexamethasone and receive treatment if necessary.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Galicich JH, French LA. Use of dexamethasone in the treatment of cerebral edema resulting from brain tumors and brain surgery. Am J Pract Dig Treat. 1961;12:169–74. [PubMed] [Google Scholar]
- 2.Mclelland S, Long DM. Genesis of the use of corticosteroids in the treatment and prevention of brain edema. Neurosurgery. 2008;62:965–8. doi: 10.1227/01.neu.0000318183.25783.77. [DOI] [PubMed] [Google Scholar]
- 3.Hockey B, Leslie K, Williams D. Dexamethasone for intracranial neurosurgery and anesthesia. J Clin Neurosci. 2009;16:1389–93. doi: 10.1016/j.jocn.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Hans P, Vanthuyne A, Dewandre PY, Brichant JF, Bonhomme V. Blood glucose concentration profile after 10 mg dexamethasone in non-diabetic and type 2 diabetic patients undergoing abdominal surgery. Br J Anaesth. 2006;97:164–70. doi: 10.1093/bja/ael111. [DOI] [PubMed] [Google Scholar]
- 5.Leenders KL, Beaney RP, Brooks DJ, Lammertsma AA, Heather JD, McKenzie CG. Dexamethasone treatment of brain tumor patients. Effects on regional cerebral blood flow, blood volume and oxygen utilization. Neurology. 1985;36:1400–3. doi: 10.1212/wnl.35.11.1610. [DOI] [PubMed] [Google Scholar]
- 6.Heiss JD, Papavassiliou E, Merrill MJ, Nieman L, Knightly JJ, Walbridge S, et al. Mechanism of dexamethasone suppression of brain tumor-associated vascular permeability in rats. J Clin Invest. 1996;98:1400–8. doi: 10.1172/JCI118927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGirt MJ, Chaichana KL, Gathinji M, Attenello F, Than K, Ruiz AJ, et al. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery. 2008;63:286–91. doi: 10.1227/01.NEU.0000315282.61035.48. [DOI] [PubMed] [Google Scholar]
- 8.Lukins MB, Manninen PH. Hyperglycaemia in patients administered dexamethasone for craniotomy. Anesth Analg. 2005;100:1129–33. doi: 10.1213/01.ANE.0000146943.45445.55. [DOI] [PubMed] [Google Scholar]
- 9.Pasternak JJ, McGregor DG, Lanier WL. Effect of single-dose dexamethasone on blood glucose concentration in patients undergoing craniotomy. J Neurosurg Anesthesiol. 2004;16:122–5. doi: 10.1097/00008506-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Lipshutz AK, Gropper MA. Perioperative glycemic control. Anaesthesiology. 2009;110:408–21. doi: 10.1097/ALN.0b013e3181948a80. [DOI] [PubMed] [Google Scholar]
- 11.Longstreth WT, Jr, Diehr P, Cobb LA, Hanson RW, Blair AD. Neurologic outcome and blood glucose levels during out-of-hospital cardiopulmonary resuscitation. Neurology. 1986;36:1186–91. doi: 10.1212/wnl.36.9.1186. [DOI] [PubMed] [Google Scholar]
- 12.Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208–14. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey JP, Diana GM, William LL. Effect of single-dose dexamethasone on blood glucose concentration in patients undergoing craniotomy. J Neurosurg Anesthesiol. 2004;16:122–5. doi: 10.1097/00008506-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Michael BL, Pirjo HM. Hyperglycemia in patients administered dexamethasone for craniotomy. Anesth Analg. 2005;100:1129–33. doi: 10.1213/01.ANE.0000146943.45445.55. [DOI] [PubMed] [Google Scholar]
- 15.Wass CT, Lanier WL. Glucose modulation of ischemic brain injury: Review and clinical recommendations. Mayo Clin Proc. 1996;71:801–12. doi: 10.1016/S0025-6196(11)64847-7. [DOI] [PubMed] [Google Scholar]


