Abstract
Serum procalcitonin (ProCT) is elevated in response to bacterial infections, whereas high sensitivity C-reactive protein (hsCRP) is a nonspecific inflammatory marker that is increased by excess adipose tissue. We examined the efficacy of ProCT and hsCRP as biomarkers of periodontitis in the saliva and serum of patients with arthritis, which is characterized by variable levels of systemic inflammation that potentially can confound the interpretation of inflammatory biomarkers. Blood and unstimulated whole saliva were collected from 33 patients with rheumatoid arthritis (RA) and 50 with osteoarthritis (OA). Periodontal status was assessed by full mouth examination and patients were categorized as having no/mild, moderate or severe periodontitis by standard parameters. Salivary and serum ProCT and hsCRP concentrations were compared. BMI, diabetes, anti-inflammatory medications and smoking status were ascertained from the patient records. Differences between OA and RA in proportionate numbers of patients were compared for race, gender, diabetes, adiposity and smoking status. Serum ProCT was significantly higher in arthritis patients with moderate to severe and severe periodontitis compared with no/mild periodontitis patients. There were no significant differences in salivary ProCT or salivary or serum hsCRP in RA patients related to periodontitis category. Most of the OA and RA patients were middle aged or older, 28.9% were diabetic, 78.3% were overweight or obese, and slightly more than half were either current or past smokers. The OA and RA groups differed by race, but not gender; blacks and males were predominant in both groups. The OA and RA groups did not differ in terms of controlled or uncontrolled diabetes, smoking status or BMI. The RA patients had been prescribed more anti-inflammatory medication than the OA patients. Our results demonstrate that circulating ProCT is a more discriminative biomarker for periodontitis than serum hsCRP in patients with underlying arthritis. Any elevation in salivary and serum hsCRP due to periodontitis apparently was overshadowed by differences among these patients in factors that influence CRP, such as the extent of inflammation between RA and OA, the extent of adipose tissue, the use of anti-inflammatory medications and smoking status. Although our study showed no differences in salivary ProCT related to severity of periodontitis, this biomarker also may be useful with further refinement.
Keywords: C-reactive protein, gingivitis, osteoarthritis, periodontitis, procalcitonin, saliva
Periodontitis is a consequence of bacterial infection of the periodontium. Chronic, long-term periodontitis can result in mobility and loss of teeth. In a recent survey of adults in the United States (Eke et al. 2012a), 47% had periodontitis; 8.7% were mild cases, 30% moderate and 8.5% severe. The proportion with moderate or severe periodontitis was 64% in those 65 years and older. Males, blacks and Hispanics were affected disproportionately.
Moderate to severe periodontitis is a significant independent risk factor for coronary heart disease (Humphrey et al. 2008, D’Aiuto et al. 2013, Sharma et al. 2014), cerebrovascular disease (Jimenez et al. 2009) and earlier mortality from all causes (Garcia et al. 1998). In moderate to severe periodontitis, the non-keratinized epithelium of the gingival sulcus is damaged, which allows bacteria, their metabolic products and proteolytic enzymes released into the sulcus by leukocytes to enter the circulation in significant amounts. The transient bacteremia may last as long as 10–20 min after chewing or tooth brushing (Forner et al. 2006). Release of these substances and bacteria into the circulation evokes production of systemic inflammatory markers, some of which are biomarkers of cardiovascular disease (CVD). Treatment of moderate to severe periodontitis has been shown to lower the serum levels of these biomarkers of CVD (Payne et al. 2011, Caúla et al. 2014, Kumari et al. 2014), but evidence that such treatment improves CVD outcomes is weak (D'Aiuto et al. 2013).
Procalcitonin (ProCT) is produced by the thyroid gland and neuroendocrine cells of the lungs, where it is cleaved enzymatically to be secreted as calcitonin (Becker et al. 2004). Uncleaved ProCT is secreted by the thyroid in response to significant local and systemic bacterial infection. Many other tissues also produce ProCt in response to bacterial infection, but cannot cleave it and so secrete only ProCT (Müller et al. 2001). The increased serum ProCT stimulated by bacterial infection is at least an order of magnitude more than that evoked by inflammation of nonbacterial causes, including viral infection (Müller et al. 2001, Becker et al. 2004, 2008, 2010). Although C-reactive protein (CRP) reacts with the “C-fraction,” a polysaccharide derived from a strain of pneumococcus (Tillett and Francis 1930), it is produced in response to both bacterial infection and nonbacterial inflammation (Becker et al. 2008, 2010, Berbari et al. 2010), and by adipose tissue in the absence of inflammation (Brooks et al. 2010). Such non-bacterial sources of stimulation affect serum ProCT much less if at all (Becker et al. 2008, 2010, Sato et al. 2012, Wu et al. 2012). When the causative bacterial infection is controlled by antibiotics, serum ProCT returns to its normal range up to ten days sooner than serum CRP does (Becker et al. 2010). Accordingly, serum ProCT has been used to guide decisions concerning when to begin and end antibiotic therapy in adult intensive care unit patients (Soni et al. 2013) and in patients with respiratory tract infections (Soni et al. 2013, Müller et al. 2010). In addition, serum CRP is elevated not only in patients with rheumatoid arthritis (RA) (Wu et al. 2012), which has an inflammatory etiology, but also in patients with osteoarthritis (OA) (Chen et al. 2008, Stannus et al. 2013), which typically has a non-inflammatory etiology.
Moderate to severe periodontitis has been linked to increased serum levels of either CRP (Christodoulides et al. 2007, Giannopoulou et al. 2012, Nakajima et al. 2010, Zhang et al. 2013, Bokhari et al. 2014, Caúla et al. 2014, Kumari et al. 2014, Arregoces et al. 2014, Leite et al. 2014, Kalburgi et al. 2014, Sharma et al. 2014), or ProCT (Bassim et al. 2008, Giannopoulou et al. 2012), which are two acute-phase inflammatory markers. Salivary CRP (Pederson et al. 1995, Christodoulides et al. 2007, Ouellet-Morin et al. 2011, Foley et al. 2012) and ProCT (Bassim et al. 2008) levels also have been shown to be higher in patients with moderate to severe periodontitis than in patients with an essentially healthy periodontium. Most studies of the association of periodontitis with increased serum or salivary ProCt and CRP have involved healthy, relatively young subjects. Further, Zhang et al. (2013) and Sharma et al. (2014) demonstrated that the mean body mass index (BMI) for patients with or without periodontitis was well below the threshold for being overweight.
The literature suggests that salivary and/or serum ProCT might be a better index of periodontitis than salivary or serum CRP in patients with systemic inflammatory conditions or who are older and tend to be overweight. Therefore, we investigated the association of salivary and serum levels of CRP and ProCT with moderate to severe periodontitis in OA and RA patients. Our hypothesis was that salivary and/or serum ProCT might be a more specific and, therefore, a better index of the extent or severity of periodontitis than salivary or serum CRP in patients with OA or RA.
Material and methods
Study population
We conducted a single site sub-study as part of a larger multicenter investigation (the parent study) to determine the relationship of periodontitis to RA (Mikuls et al. 2014). Most of the patients recruited from the Rheumatology Clinic of the Washington, DC, Department of Veterans Affairs Medical Center (DCVAMC) for the parent study participated in our sub-study as well. The design and informed consent forms for both the parent study and the sub-study were approved by the DCVAMC Human Subjects Subcommittee (IRB) and Research and Development Committee.
All patients with RA satisfied the 1987 American College of Rheumatology classification criteria (Arnett et al. 1988). Controls were patients with OA (physician and radiologic diagnosis). Patients with conditions for which prophylactic antibiotic treatment was indicated, e.g., history of bacterial endocarditis, were excluded. Patients with overt acute infection, e.g., upper respiratory tract, were not enrolled until at least 14 days after the infection had subsided. Patients with fewer than nine natural posterior teeth, not including third molars, were excluded.
Periodontal evaluation
The examiner (RSR) was trained for the procedures used to assess standard periodontitis characteristics (Mikuls et al. 2014) by the parent study periodontist (JBP). The prevalence and extent of periodontitis were evaluated in each patient by the following characteristics at six sites for each tooth: the presence or absence of bleeding with probing, the presence or absence of supragingival plaque, gingival recession relative to the cemento-enamel junction and periodontal probing depth.
For our sub-study, we categorized periodontal status as no periodontitis, mild periodontitis, moderate periodontitis and severe periodontitis based on the Center for Disease Control-American Academy of Periodontology (CDC-AAP) criteria (Eke et al. 2012b). We used these criteria for comparisons of biomarker concentrations across periodontitis severity categories. The severe periodontitis category using CDC-AAP criteria approximated the protocol definition of periodontitis that was used in the parent study (Mikuls et al. 2014).
We considered it likely that patients in the Rheumatology Clinic would have confounding factors for the assessment of periodontitis, hsCRP and ProCT. Therefore, we ascertained by review of medical records smoking habit, adiposity as estimated by BMI according to the 2015 website of the Centers for Disease Control, diabetes mellitus status, and the number of anti-inflammatory drugs prescribed at the time of enrollment. Smoking status categories were: never, current and past (last smoke 12 months or more ago). We considered diabetes to be inadequately controlled if the serum HgbA1c was consistently higher than 7.5% on the day of the periodontal examination and/or the preceding and following 15 days. We recorded the drugs by type: corticosteroids (e.g., dexamethasone, prednisone), nonsteroidal anti-inflammatory drugs (NSAIDS, e.g., ibuprofen, naproxen, aspirin), and disease-modifying anti-rheumatic drugs (DMARDs, e.g., methotrexate, adalimumab). Therefore, it was possible for the patients to have prescriptions for up to four types of anti-inflammatory drugs.
Sample collection and assays
Serum was collected from each patient on the day the study began, frozen in 1.5 ml aliquots and stored at −80° C. Unstimulated whole saliva was collected the same day using the method of Bassim et al. (2008). Both salivary and serum samples were collected between 10 AM and 12:30 PM; the patients had little or no opportunity to eat or to drink anything but water during the preceding 3–4 h. This arrangement minimized the effects of circadian rhythms on both serum hormone composition (Katz et al. 1975) and recent stimulation and unstimulated salivary flow rate and composition (Dawes and Ong 1973). The patient rinsed her/his mouth with tap water and expectorated into a disposable beaker. After waiting 1 min for saliva to begin to collect, the patient then at intervals of 30 to 60 sec expectorated into a 50 ml disposable centrifuge tube containing one drop of Tween 20® (Fisher Scientific, Fairlawn, NJ.) until 5 ml had been collected. The contents of the tube were swirled rapidly for 5 sec on a Vortex mixer (Fisher Scientific, Fairlawn, NJ), then centrifuged at 220 × g for 5 min to remove food particles, mucosal cells and other debris. The supernatant was drawn off, divided into 1.6 ml aliquots and stored at −80° C. Each aliquot of serum or saliva was thawed immediately before assay. Care was taken to assay samples for salivary and serum ProCT from a given patient at the same time.
Serum and salivary ProCT were assayed in triplicate using the Kryptor® method (Steinbach et al. 2004) in the DCVAMC Endocrinology Laboratory. This method uses an antibody that is specific to the ProCt whole molecule and with the aminopeptide, but not the CC-P1 peptide, removed; the assay has a sensitivity of 0.0020 ng/ml (Becker et al. 2008). Salivary high sensitivity CRP (hsCRP) was assayed using an EIA (Salivary CRP ELISA kit; Salimetrics, Inc., State College, PA) in the DCVAMC Laboratory. Serum hsCRP was performed by nephelometry as part of the parent study. Data are expressed as ng/ml.
Data analysis
For each biomarker, two pair-wise comparisons were made based on periodontitis status: severe and moderate/severe vs. no/mild disease. To optimize study power and because comparisons based on arthritis status (RA vs. OA) showed no significant difference for any of the biomarkers examined (data not shown), arthritis groups were combined for subsequent analyses. Statistical analysis was performed using the non-parametric Wilcoxon rank sum test or the Chi Squared test. Differences were considered significant at p ≤ 0.05.
Results
Demographics
Of the 83 patients, 27 exhibited severe periodontitis, 44 exhibited moderate periodontitis, none exhibited mild periodontitis and 12 exhibited no periodontitis.
The age, race and gender distribution of our sub-study patients overall and by the arthritis group are given in Table 1. The OA and RA groups differed by race, but not by age or gender. Blacks (African Americans) and males were predominant in both groups, and most of the patients were middle aged or older. The distributions of diabetes and adiposity among the patients are shown in Table 2. Overall, 28.9% of the study patients had a diagnosis of diabetes and 78.3% were either overweight or obese. The OA and RA groups did not differ by number of patients with diabetes, controlled vs. uncontrolled diabetes, or BMI category.
Table 1.
Demographics
| Group | Gender | Age | Race | ||||
|---|---|---|---|---|---|---|---|
| Male | Female | Mean ± SD | Range | Black | White | Others | |
| OA | 36 | 14 | 54.3 ± 10.6 | 30–88 | 42 | 6 | 2 |
| RA | 21 | 12 | 58.4 ± 11.0 | 38–80 | 19 | 11 | 3 |
| Totals | 57 | 26 | 55.9 ± 10.9 | 30–88 | 61 | 17 | 5 |
OA, osteoarthritis; RA, rheumatoid arthritis. “Others” are Hispanic and Asian/Pacific Islander. One OA person in the “Others” column is both Hispanic and Black. OA and RA differed by race (X2, p = 0.028), but not gender, (X2, p = 0.65) or age (Wilcoxon Rank Sum Test, p = 0.140)
Table 2.
Number of patients in categories of body mass index and diabetes by arthritis type
| Diabetes | BMI estimate of excess weight | ||||||
|---|---|---|---|---|---|---|---|
| Arthritis type | Controlleda | Total | Normal | Overweight | Obese | Total, obeseb | |
| Yes | No | 18.5–24.9b | 25.0–29.9 | > 29.9 | Overweight | ||
| OA (% of OA) | 11 (22.0) | 5 (10.0) | 16 (32.0) | 11 (22.0) | 14 (28.0) | 25 (50.0) | 39 (78.0) |
| RA (% of RA) | 5 (15.2) | 3 (9.1) | 8 (24.2) | 7 (21.2) | 9 (27.3) | 17 (51.6) | 26 (78.8) |
| Totals (% of total) | 16 (19.3) | 8 (9.6) | 24 (28.9) | 18 (21.7) | 23 (27.7) | 42 (50.6) | 65 (78.3) |
BMI, body mass index. OA, osteoarthritis; RA, rheumatoid arthritis.
Diabetes was considered to be controlled when the HbA1c value was ≤ 7.5%.
BMI range. OA and RA did not differ by number of patients with diabetes (X2, p = 0.446), controlled and uncontrolled diabetes (X2, p = 0.716), or BMI category (X2, p = 0.991).
The types of anti-inflammatory medications prescribed and the smoking status of the patients are shown in Table 3. The RA patients were prescribed far more anti-inflammatory medications than the OA patients. The OA and RA groups did not differ by smoking status.
Table 3.
Number of patients in categories of anti-inflammatory medications and smoking status by arthritis type
| Number of patients/types of anti-inflammatory drugsa |
Smoking status number of patients who smoke(d) |
|||||
|---|---|---|---|---|---|---|
| Arthritis type | 0 Drugs | 1 Drug | 2–4 Drugs | Never | Pastb | Currently |
| OA (% of OA) | 42 (84.0) | 8 (16.0) | 0 (00.0) | 22 (44.0) | 10 (20.0) | 18 (36.0) |
| RA (% of RA) | 8 (24.2) | 12 (36.4) | 13 (39.4) | 16 (48.5) | 10 (30.3) | 7 (21.2) |
| Totals (% of total) | 50 (60.2) | 20 (24.1) | 13 (15.7) | 38 (45.8) | 20 (24.1) | 25 (30.1) |
OA, osteoarthritis; RA, rheumatoid arthritis.
Patients had current prescriptions for up to four types of anti-inflammatory drugs at enrollment: corticosteroids (dexamethasone, prednisone), NSAIDS (ibuprofen, naproxen), chemotherapeutic (methotrexate), and biological (adalimumab).
Past, Patient quit smoking ≥ 1 year prior to enrollment.
OA and RA differed by the number of patients in the categories of number of anti-inflammatory drugs prescribed (X2, p < 0.00001), but not by smoking status (X2, p = 0.300).
Assay results
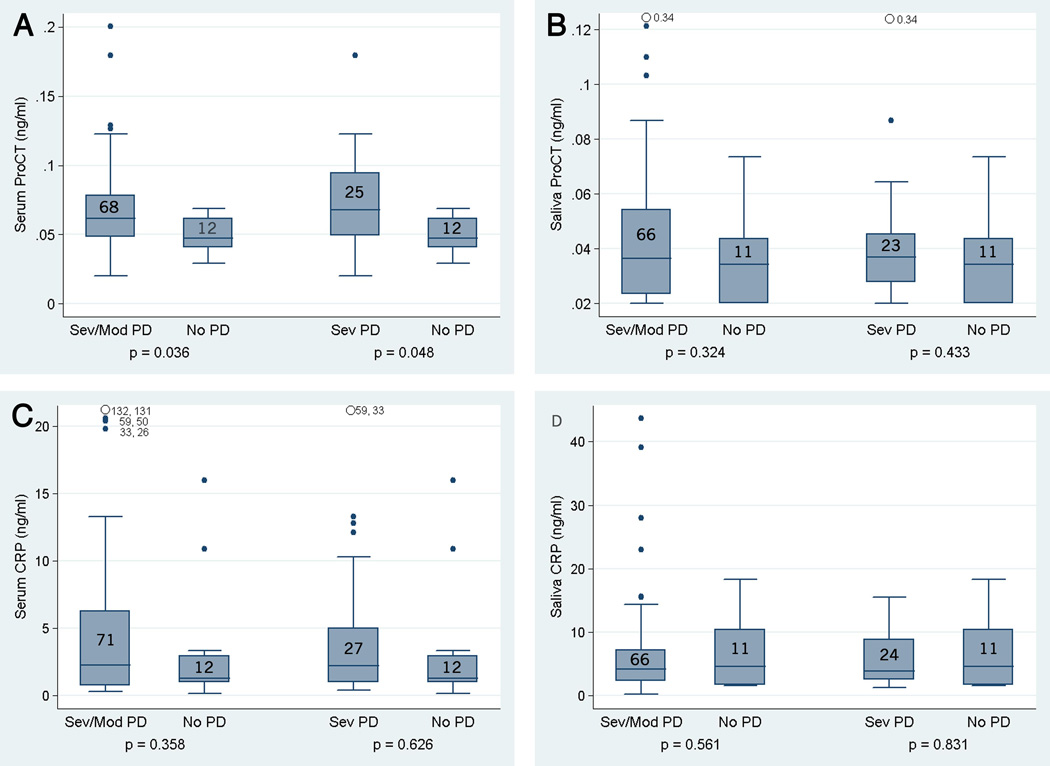
The serum ProCT level was significantly higher in the moderate/severe group (p = 0.036) and in patients with severe periodontitis (p = 0.048) compared to the No/Mild group (Fig. 1A). We found no significant differences in salivary ProCT (Fig. 1B) or serum (Fig. 1C) or salivary hsCRP levels (Fig. 1D) among patients in any of the periodontitis categories. The mean serum ProCT level was significantly higher in the patients with diabetes (0.0835 ± 0.0425) than in the nondiabetic patients (0.0586 ± 0.0425, p = 0.007), but the salivary ProCt level was not (0.0407 ± 0.0246 vs. 0.0389 ± 0.0269, p = 0.527).
Fig. 1.
Box and whisker plots showing biomarker concentrations (ng/ml) based on moderate/severe periodontitis (Mod/Sev PD) and severe periodontitis (Sev PD) vs. no periodontitis (No PD). Bottoms and tops of boxes reflect first and third quartiles, respectively. Bands inside boxes reflect median values and ends of whiskers reflect approximately 3/2 of quartiles. Individual values outside the whiskers are shown with dots and numbers to reflect the range used in the data set. Numbers inside the boxes = n for that group. Significance of each comparison was computed using the Wilcoxon rank sum test. A, serum ProCT; B, salivary ProCT; C, serum hsCRP; D, salivary hsCRP.
Discussion
That most of the patients had moderate to severe periodontitis is not surprising, because prevalence of periodontitis has been shown to increase with age (Eke et al. 2012a) and to be more prevalent in patients with RA (Dissick et al. 2010, Mikuls et al. 2014).
Our findings differed from those of Bassim et al. (2008). We found that the salivary ProCT level was neither higher than the serum ProCT nor were there significant differences in salivary ProCT based on periodontitis severity. Plausible causes for this difference include differences in the ProCT assays (ELISA vs. Kryptor) and the possible inflammatory impact of underlying diabetes, because all patients other than controls in the study by Bassim et al. (2008) were diabetic compared to only 28.9% of patients in our study. It is interesting that the serum ProCT was significantly higher in patients with diabetes in our study, but the salivary ProCT was not. Possible reasons for this difference include the different case definitions used for periodontitis, the substantially smaller number of patients enrolled in the earlier study and the limited number of study participants with no or no/mild periodontitis in both studies. Another potential variable was that some of the samples in our study had been stored briefly at 4° C prior to centrifugation and freezing. Therefore, we examined deterioration during storage in the refrigerator in additional samples from 12 of the patients. The means of the sample aliquots frozen for 15 min, one day, one week and three weeks after collection were 0.0505, 0.0572, 0.0472 and 0.0556 ng/ml, respectively. The coefficient of variation of these means was 0.0758 and the mean of the sample coefficients of variation was 0.141. These results indicate that ProCT is stable in whole saliva for up to three weeks at 4° C. In addition, CRP has been reported to be stable in saliva for up to 8 h at room temperature (Ouellet-Morin 2011).
ProCT levels in our study and CRP levels (Ouellet-Morin 2011, Foley et al. 2012) were lower in saliva and the CRP level was lower in gingival crevicular fluid (Kumari et al. 2014) than they were in serum collected at the same time. CRP is produced in the liver in response to significant inflammation (Ouellet-Morin et al. 2011), whereas ProCT is produced in many tissues, and by leukocytes and macrophages in response to bacterial infection (Müller et al. 2001). This suggests that both CRP and ProCt are diluted by the watery component of salivary secretion despite any contribution of ProCT by either the salivary glands or inflammatory cells in the gingiva.
Serum CRP and ProCT were compared in relation to periodontitis in two studies in which the subjects were considered to be healthy. Ziebolz et al. (2007) assessed these and other inflammatory biomarkers in samples of plasma from blood donors. Both CRP and ProCT levels were below or barely above the level of detection in nearly all subjects and not significantly higher in response to periodontitis. Moderate to severe periodontitis was uncommon in this study, however, which likely reflects the younger age of study participants (mean 25.7 ± 5, range 18–54 years). Giannopoulou et al. (2012) reported that both serum CRP and ProCT levels were elevated in cases of periodontitis, but CRP fell during the six months following periodontal therapy, while the ProCT level fell during the first two months, then increased steadily during the next four months. The latter study suggests that serum ProCT might be more useful than CRP for monitoring the recurrence of periodontitis
Bassim et al. (2008) found both salivary and serum ProCT levels to be higher, and we found the serum ProCT level to be higher, with advanced periodontitis in small groups of patients with diabetes and arthritis. Salivary ProCT seems worthy of further study, because it may prove more convenient for the patient to provide than serum samples. Its usefulness as a biomarker for monitoring the course of periodontitis may be limited by the smaller area of tissue infection compared to those of sepsis or bacterial pneumonia. Further refinement of the salivary ProCT assay, e.g., sample filtration, may help to establish a threshold for early detection of recurrence of moderate to severe periodontitis in individual patients. It then would be useful to examine whether the salivary ProCT assay might complement other biomarkers or self-reported symptoms that have been shown to identify individuals with periodontitis (Coburn et al. 2015). Nonetheless, the results of our study partially support our hypothesis that ProCT would be a more discriminative biomarker for periodontitis than hsCRP in patients with arthritis, because the circulating, but not salivary, levels of ProCT were higher in arthritis patients with periodontitis than in arthritis patients without periodontitis. As anticipated, there was marked variability of the hsCRP values. This suggests that elevations of salivary and serum hsCRP owing to periodontitis were overshadowed by differences in factors that can influence CRP levels among these patients, such as the different levels of inflammation associated with RA and OA, and the differences observed in adipose tissue, use of anti-inflammatory medications and smoking status.
Corticosteroids have the potential to affect two of the standard procedures used to diagnose periodontitis. They decrease inflammation and thus bleeding on probing. Long term use of corticosteroids causes osteopenia, which might affect alveolar bone loss or the radiologic interpretation of alveolar bone loss; however, neither accelerated marginal bone loss nor loss of attachment occurred in one study (von Wowern et al. 1992). Oral naproxen administration decreased all periodontitis parameters measured except gingival bleeding compared to placebo controls (Aras et al. 2007). The anti-TNF-alpha activities of the biologicals, adalimubab and infliximab, caused either increased (Pers et al. 2008) or decreased inflammation (Kobyashi et al. 2014), but both drugs retarded the progression of periodontal destruction. In a review of smoking and periodontitis, Nociti et al. (2015) concluded that "…smokers present increased susceptibility to periodontitis and greater severity of progression. Paradoxically, the signs and symptoms of periodontitis are less pronounced in smokers than in non-smokers."
Although the anti-inflammatory action of medications and smoking can suppress some of the diagnostic parameters of periodontitis, this does not appear to have been an important confounder in our study; all but twelve patients had moderate to severe periodontitis. Reduction of inflammation should decrease the production of CRP, however, and this has been shown to be the case with corticosteroids (Renvert et al. 2009), adalimumab (Strober et al. 2014), and naproxen (Tarp et al. 2012), but not with methotrexate (Kingsley et al. 2012). Oddly, hsCRP levels reportedly are higher in smokers than in nonsmokers (Kleber 2015),
Our findings suggest that a complex pattern of co-morbid factors contributed to the lack of significant effects of periodontitis on circulating and salivary hsCRP, but not serum ProCT, in the patients with OA and RA. Serum ProCT has been shown to be superior to CRP as a marker for large-scale bacterial infection, such as sepsis or pneumonia, in patients with OA and RA (Sato et al. 2012, Wu et al. 2012); this suggests that the superiority of serum ProCT as a marker of periodontitis in patients with arthritis in our study might be generalized to patients with RA and other chronic systemic inflammatory diseases among the general public.
Acknowledgments
We thank John Peter Kokkinos for the salivary and serum procalcitonin and salivary hsCRP assays. This research was supported by the Department of Veterans Affairs, Federal funds (Grant # UL1TR000101, previously UL1RR031975) from the National Center for Research Resources (NCRR), National Institutes of Health (NIH), through the Clinical and Translational Science Awards Program (CTSA), a trademark of DHHS, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise,” and The Rheumatology Research Foundation Disease Targeted Research Institute.
Footnotes
Declaration of interests: The authors report no conflict of interest. The authors alone are responsible for the content and writing of this paper.
References
- Aras H, Cağlayan F, Güncü GN, Berberoğlu A, Kilinç K. Effect of systemically administered naproxen sodium on clinical parameters and myeloperoxidase and elastase-like activity levels in gingival crevicular fluid. J. Periodontol. 2007;78:868–873. doi: 10.1902/jop.2007.060412. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries F, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Jr, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GH. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthr. Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Arregoces FE, Uriza CL, Porras JV, Camargo MB, Morales AR. Relation between ultra-sensitive C-reactive protein, diabetes and periodontal disease in patients with and without myocardial infarction. Arg. Bras. Endocrinol. Metab. 2014;58:362–368. doi: 10.1590/0004-2730000002899. [DOI] [PubMed] [Google Scholar]
- Bassim CW, Redman RS, DeNucci DJ, Becker KL, Nylén ES. Salivary procalcitonin and periodontitis in diabetes. J. Dent. Res. 2008;87:630–634. doi: 10.1177/154405910808700707. [DOI] [PubMed] [Google Scholar]
- Becker KL, Nylén ES, White JC, Müller B, Snider RH., Jr Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J. Clin. Endocrinol. Metab. 2004;89:1512–1525. doi: 10.1210/jc.2002-021444. [DOI] [PubMed] [Google Scholar]
- Becker KL, Snider RH, Nylén ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit. Care Med. 2008;36:941–952. doi: 10.1097/CCM.0B013E318165BABB. [DOI] [PubMed] [Google Scholar]
- Becker KL, Snider RH, Jr, Nylén ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br. J. Pharmacol. 2010;159:253–264. doi: 10.1111/j.1476-5381.2009.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari E, Mabry T, Tsaras G, Erwin PJ, Spangehl M, Murad MH, Steckleberg J, Osmon D. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J. Bone Joint Surg. Am. 2010;92:2102–2109. doi: 10.2106/JBJS.I.01199. [DOI] [PubMed] [Google Scholar]
- Bokhari SA, Khan AA, Butt AK, Hanif M, Izhar M, Tatakis DN, Ashfaq M. Periodontitis in coronary heart disease patients: strong association between bleeding on probing and systemic biomarkers. J. Clin. Periodontol. 2014;41:1048–1054. doi: 10.1111/jcpe.12284. [DOI] [PubMed] [Google Scholar]
- Brooks GC, Blaha MJ, Blumenthal RS. Relation of C-reactive protein to abdominal adiposity. Am. J. Cardiol. 2010;106:56–61. doi: 10.1016/j.amjcard.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Caúla AL, Lira-Junior R, Tinoco EMB, Fischer RG. The effects of periodontal therapy on cardiovascular risk markers: a 6-month randomized clinical trial. J. Clin. Periodontol. 2014;41:875–882. doi: 10.1111/jcpe.12290. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. BMI: about BMI (CDC Website) 2015 [Google Scholar]
- Chen HC, Shah S, Stabler TV, Li YJ, Kraus VB. Biomarkers associated with clinical phenotypes of hand osteoarthritis in a large multigenerational family: the CARRIAGE family study. Osteoarthr. Cart. 2008;16:1054–1059. doi: 10.1016/j.joca.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulides N, Floriano PN, Miller CS, Ebersole JL, Mohanty S, Dharshan P, Griffin M, Lennart A, Ballard KL, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV, McDevitt JT. Lab-on a chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann. NY Acad. Sci. 2007;1098:411–428. doi: 10.1196/annals.1384.035. [DOI] [PubMed] [Google Scholar]
- Coburn BW, Sayles HR, Payne JB, Redman RS, Markdt JC, Beatty MW, Griffiths GR, McGowan DJ, Mikuls TR. Performance of self-reported measures for periodontitis in rheumatoid arthritis and osteoarthritis. J. Periodontol. 2015;86:16–26. doi: 10.1902/jop.2014.140339. [DOI] [PubMed] [Google Scholar]
- D'Aiuto F, Orlandi M, Gunsolley JC. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J. Clin. Periodontol. 2013;40(Suppl. 14):S85–S105. doi: 10.1111/jcpe.12061. [DOI] [PubMed] [Google Scholar]
- Dawes C, Ong BY. Circadian rhythms in the concentrations of protein and the main electrolytes in human unstimulated saliva. Arch. Oral Biol. 1973;18:1233–1242. doi: 10.1016/0003-9969(73)90035-6. [DOI] [PubMed] [Google Scholar]
- Dissick A, Redman RS, Jones M, Rangan BV, Reimold A, Griffiths GR, Mikuls TR, Amdur RL, Richards JS, Kerr GS. Association of periodontitis with rheumatoid arthritis: a pilot study. J. Periodontol. 2010;81:223–230. doi: 10.1902/jop.2009.090309. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012a;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Eke P, Page R, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J. Periodontol. 2012b;83:1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JD, 3rd, Sneed JD, Steinhubl SR, Kolasa j, Ebersole JL, Lin Y, Kryscio RJ, McDevitt JT, Campbell CL, Miller CS. Oral fluids that detect cardiovascular disease biomarkers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012;114:207–214. doi: 10.1016/j.oooo.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J. Clin. Periodontol. 2006;33:401–407. doi: 10.1111/j.1600-051X.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- Garcia RI, Krall EA, Vokonas PS. Periodontal disease and mortality from all causes in the VA Dental Longitudinal Study. Ann. Periodontol. 1998;3:339–349. doi: 10.1902/annals.1998.3.1.339. [DOI] [PubMed] [Google Scholar]
- Giannopoulou C, Cappuyns I, Cancela J, Cionca N, Mombeli A. Effect of photodynamic therapy, diode laser, and deep scaling on cytokine and acute-phase protein levels in gingival crevicular fluid of residual periodontal pockets. J. Periodontol. 2012;83:1018–1027. doi: 10.1902/jop.2011.110281. [DOI] [PubMed] [Google Scholar]
- Humphrey LL, Buckley DI, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J. Gen. Int. Med. 2008;23:2079–2086. doi: 10.1007/s11606-008-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez M, Krall EA, Garcia RI, Vokonas PS, Dietrich T. Periodontitis and incidence of cerebrovascular disease in men. Ann. Neurol. 2009;66:505–512. doi: 10.1002/ana.21742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalburgi V, Sravya L, Warad S, Vijayalaxmi K, Sejal P, Hazeil DJ. Role of systemic markers in periodontal diseases: a possible inflammatory burden and risk factor for cardiovascular disease? Ann. Med. Health. Sci. Res. 2014;4:388–392. doi: 10.4103/2141-9248.133465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz FH, Romfh P, Smith JA, Roper EF, Barnes JS, Boyd JB. Diurnal variation of plasma aldosterone, cortisol and renin activity in supine man. J. Clin. Endocrinol. Metab. 1975;40:125–134. doi: 10.1210/jcem-40-1-125. [DOI] [PubMed] [Google Scholar]
- Kingsley GH, Kowalczyk A, Taylor H, Ibrahim F, Packham JC, McHugh NJ, Mulherin DM, Kitas GD, Chakravarty K, Tom BD, O'Keefe AG, Maddison PJ, Scott DL. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology (Oxford) 2012;51:1368–1377. doi: 10.1093/rheumatology/kes001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber ME, Siekmeier R, Delgado G, Grammer TB, Winkelmann BR, Schamagl H, Boehm BO, März W. C-reactive protein and lipoprotein-associated phospholipase A2 in smokers and nonsmokers of the Ludwigshafen Risk Cardiovascular Health Study. Adv. Exp. Med. Biol. 2015;832:15–23. doi: 10.1007/5584_2014_6. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Yokoyama T, Ito S, Kobayashi D, Yamagata A, Okada M, Oofusa K, Narita i, Murasawa A, Nakazono K, Yoshie H. Periodontal and serum protein profiles in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitor adalimumab. J. Periodontol. 2014;85:1480–1488. doi: 10.1902/jop.2014.140194. [DOI] [PubMed] [Google Scholar]
- Kumari M, Pradeep AR, Priyanka N, Kalra N, Naik SB. Crevicular and serum levels of monocyte chemoattractant protein-4 and high-sensitivity C-reactive protein in periodontal health and disease. Arch. Oral Biol. 2014;59:645–653. doi: 10.1016/j.archoralbio.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Leite AC, Carneiro VM, Guimarães MC. Effects of periodontal therapy on C-reactive protein and HDL in serum of subjects with periodontitis. Rev. Bras. Cir. Cardiovasc. 2014;29:69–77. doi: 10.5935/1678-9741.20140013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuls TR, Payne JB, Yu F, Thiele GM, Reynolds RJ, Cannon GW, Markt J, McGowan D, Kerr GS, Redman RS, Reimold A, Griffiths G, Beatty M, Gonzalez SM, Bergman DA, Hamiliton BC, III, Erickson AR, Sokolove J, Robinson WH, Walker C, Chandad F, O'Dell JR. Periodontitis and Porphryomonas gingivalis in patients with rheumatoid arthritis. Arthr. Rheum. 2014;66:1090–1100. doi: 10.1002/art.38348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B, White JC, Nylén ES, Snider RH, Jr, Becker KL, Habener JF. Ubiquitous expression of the calcitonin-1 gene in multiple tissues in response to sepsis. J. Clin. Endocrinol. Metab. 2001;86:396–404. doi: 10.1210/jcem.86.1.7089. [DOI] [PubMed] [Google Scholar]
- Müller F, Christ-Crain M, Bregenzer T, Krause M, Zimmerli W, Mueller B, Schuetz P. Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia. Chest. 2010;138:121–129. doi: 10.1378/chest.09-2920. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Honda T, Domon H, Okui T, Kajita K, Ito H, Takahashi N, Maskawa T, Tabeta K, Yamazaki K. Periodontitis-associated up-regulation of systemic inflammatory mediator level may increase the risk of coronary artery disease. J. Periodont. Res. 2010;45:116–122. doi: 10.1111/j.1600-0765.2009.01209.x. [DOI] [PubMed] [Google Scholar]
- Nociti FH, Jr, Casati MZ, Duarte PM. Current perspective of the impact of smoking on the progression and treatment of periodontitis. Periodontology 2000. 2015;67:187–210. doi: 10.1111/prd.12063. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Danese A, Williams B, Arseneault L. Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain Behav. Immun. 2011;25:640–646. doi: 10.1016/j.bbi.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Payne JB, Golub LM, Stoner JA, Lee HM, Reinhardt RA, Sorsa T, Slepian MJ. The effect of subantimicrobial-dose-doxycycline on serum biomarkers of systemic inflammation: a randomized, double-masked, placebo-controlled clinical trial. J. Am. Dent. Assoc. 2011;142:262–273. doi: 10.14219/jada.archive.2011.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson ED, Stanke SR, Whitener SJ, Sebastiani PT, Lamberts BL, Turner DW. Salivary levels of alpha-2-macroglobulin, alpha-1-antitrypsin, C-reactive protein, cathepsin G and elastase in humans with or without destructive periodontal disease. Arch. Oral Biol. 1995;40:1151–1155. doi: 10.1016/0003-9969(95)00089-5. [DOI] [PubMed] [Google Scholar]
- Pers JO, Saraux A, Pierre R, Youinou P. Anti-TNF-alpha immunotherapy is associated with increased gingival inflammation without clinical attachment loss in subjects with rheumatoid arthritis. J. Periodontol. 2008;79:1645–1651. doi: 10.1902/jop.2008.070616. [DOI] [PubMed] [Google Scholar]
- Renvert S, Lindahl C, Roos-Jansåker AM, Lessem J. Short-term effects of an anti-inflammatory treatment on clinical parameters and serum levels of C-reactive protein and proinflammatory cytokines in subjects with periodontitis. J. Periodontol. 2009;80:892–900. doi: 10.1902/jop.2009.080552. [DOI] [PubMed] [Google Scholar]
- Sato H, Tanabe N, Murasawa A, Otaki Y, Sakai T, Sugaya T, Ito S, Otani H, Abe A, Ishikawa H, Nakazono K, Kuroda T, Nakano M, Narita I. Procalcitonin is a specific marker for detecting bacterial infection in patients with rheumatoid arthritis. J. Rheumatol. 2012;39:1517–1523. doi: 10.3899/jrheum.111601. [DOI] [PubMed] [Google Scholar]
- Sharma A, Astekar M, Metgud R, Soni A, Verma M, Patel S. A study of C-reactive protein, lipid metabolism and peripheral blood to identify a link between periodontitis and cardiovascular disease. Biotech. & Histochem. 2014;89:577–582. doi: 10.3109/10520295.2014.918280. [DOI] [PubMed] [Google Scholar]
- Soni NJ, Samson DJ, Galaydick JL, Vats V, Pitrak DL, Aronson N. Procalcitonin-guided antibiotic therapy: a systematic review and meta-analysis. J. Hosp. Med. 2013;8:530–540. doi: 10.1002/jhm.2067. [DOI] [PubMed] [Google Scholar]
- Stannus OP, Blizzard JG, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann. Rheum. Dis. 2013;72:535–540. doi: 10.1136/annrheumdis-2011-201047. [DOI] [PubMed] [Google Scholar]
- Steinbach G, Rau B, Debard AL, Javourez JF, Bienvenu J, Ponzio A, Bonfà A, Hubi W, Demnant T, Külpmann WR, Bucholz J, Schumann G. Multicenter evaluation of a new immunoassay for procalcitonin measurement on the Kryptor system. Clin. Chem. Lab. Med. 2004;42:440–449. doi: 10.1515/CCLM.2004.077. [DOI] [PubMed] [Google Scholar]
- Strober BE, Poulin Y, Teller C, Wang Y, Williams DA, Goldblum OM. Changes in C-reactive protein in patients with moderate-to-severe psoriasis switched to adalimumab therapy after suboptimal response to etanercept, methotrexate or phototherapy. J. Eur. Acad. Dermatol. Venereol. 2014;28:1701–1706. doi: 10.1111/jdv.12372. [DOI] [PubMed] [Google Scholar]
- Tarp S, Bartels EM, Bliddal H, Furst DE, Boers M, Danneskiold-Samsøe B, Rasmussen M, Christensen R. Effect of nonsteroidal anti-inflammatory drugs on the C-reactive protein level in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthr. Rheum. 2012;64:3511–3521. doi: 10.1002/art.34644. [DOI] [PubMed] [Google Scholar]
- Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J. Exp. Med. 1930;52:561–571. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wowern N, Klausen B, Olgaard K. Steroid-induced bone loss in relation to marginal periodontal changes. J. Clin. Periodontol. 1992;19:182–186. doi: 10.1111/j.1600-051x.1992.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Wu JY, Lee SH, Shen CJ, Hsieh YC, Yo PH, Cheng HY, Chan RC, Lee CC, Chang SS. Use of serum procalcitonin to detect bacterial infection in patients with autoimuune diseases: a systematic review and meta-analysis. Arthr. Rheum. 2012;64:3034–3042. doi: 10.1002/art.34512. [DOI] [PubMed] [Google Scholar]
- Zhang X, Meng H, Sun X, Xu L, Zhang L, Shi D, Feng X, Lu R, Chen Z. Elevation of vitamin D-binding protein levels in the plasma of patients with generalized aggressive periodontitis. J. Periodont. Res. 2013;48:74–79. doi: 10.1111/j.1600-0765.2012.01505.x. [DOI] [PubMed] [Google Scholar]
- Ziebolz D, Jager GC, Hornecker E, Mausberg RF. Periodontal findings and blood analysis of blood donors: a pilot study. J. Contemp. Dent. Pract. 2007;8:43–50. [PubMed] [Google Scholar]