Abstract
Cooperation among individuals depends, in large part, on a sense of fairness. Many cooperating non-human primates (NHPs) show inequity aversion, (i.e., negative responses to unequal outcomes), and these responses toward inequity likely evolved as a means to preserve the advantages of cooperative relationships. However, marmosets (Callithrix spp.) tend to show little or no inequity aversion, despite the high occurrence of prosociality and cooperative-breeding in callitrichid monkeys. Oxytocin [OXT] has been implicated in a wide variety of social processes, but little is known about whether OXT modulates inequity aversion toward others. We used a tray pulling task to evaluate whether marmosets would donate superior rewards to their long-term pairmate or an opposite-sex stranger following OXT, OXT antagonist, and saline treatments. We found that marmosets show inequity aversion, and this inequity aversion is socially- and sex-specific. Male marmosets show inequity aversion toward their pairmates but not strangers, and female marmosets do not show inequity aversion. OXT treatments did not significantly influence inequity aversion in marmosets. While OXT may modulate prosocial preferences, the motivations underlying cooperative relationships, such as inequity aversion, are multifaceted. More research is needed to evaluate the evolutionary origins, biological processes, and social contexts that influence complex phenotypes like inequity aversion. Inequity aversion can differ within species in important and distinct ways including between individuals who do and do not share a cooperative relationship. Overall, these findings support the view that inequity aversion is an important behavioural strategy for the maintenance of cooperative relationships.
Keywords: inequity aversion, cooperation, fairness, prosocial choice task, oxytocin, pro8 oxytocin, sex differences, pair-bonds, marmoset monkeys, Callithrix
INTRODUCTION
A sense of fairness between individuals is an important feature for the preservation of long-lasting cooperative relationships in primates. Maintaining fairness in cooperative relationships requires the ability to recognize and the motivation to refuse inequitable outcomes. There has been a long and rich interest in whether and to what extent primates understand differential reward outcomes. Nearly a century ago, it was first reported that young macaque monkeys trained to perform a response to receive a banana reward exhibited ‘disappointment’, ‘frustration’, and refusal when the banana reward was substituted with a less-preferred lettuce reward (Tinklepaugh, 1928). However, it wasn’t until recently that these individual reward contrast effects were studied in a social context involving differential reward outcomes between partners (Brosnan & De Waal, 2003). Specifically, Brosnan and de Waal found that capuchin monkeys actively refused lower preference cucumber rewards after witnessing partners receive higher preference grape rewards. The decrease in response frequency and refusal to accept less preferred rewards than what others receive is generally defined as inequity aversion (Fehr & Schmidt, 1999).
Inequity aversion is an important contextual feature that shapes cooperative behaviour in non-human primates (NHPs). NHPs show an extraordinary range of social decision making strategies across a wide collection of tasks aimed measuring inequity aversion including tray pulling, token exchanges, economic games, or naturalistic behaviours (Brosnan & de Waal, 2014). Many of these social decision making strategies reflect differences in cognitive and motivational capabilities, diversity in species-specific behaviours, and disparities in nuanced methodologies across experiments. The expression of cooperative behaviour, and, specifically, the aversion to inequity is intimately linked to the evolution of an organism’s social system. Inequity aversion likely evolved alongside cooperation as a means to optimize and maintain the best outcomes associated with cooperative relationships (Brosnan, 2011). Moreover, the presence of inequity aversion across many NHPs, and the early emergence of inequity aversion and parochialism in human children (Fehr, Bernhard, & Rockenbach, 2008), suggest a shared evolutionary mechanism for facilitating cooperative behaviours, and there is converging evidence to support this view. Specifically, NHPs that ordinarily cooperate in foraging or food-sharing situations (chimpanzees, capuchins, macaques) are often more sensitive to inequity (Brosnan & De Waal, 2003; Brosnan, Schiff, & De Waal, 2005; Brosnan, Talbot, Ahlgren, Lambeth, & Schapiro, 2010; Fletcher, 2008; Hopper, Lambeth, Bernacky, & Brosnan, 2013; Massen, Van den Berg, Spruijt, & Sterck, 2012; Takimoto, Kuroshima, & Fujita, 2010), than NHPs that do not regularly cooperate in foraging situations (squirrel monkeys and orangutans) (Bräuer, Call, & Tomasello, 2009; Talbot, Freeman, Williams, & Brosnan, 2011). Though not all studies show that chimpanzees and capuchins respond negatively to inequity (Bräuer et al., 2009; Silberberg, Crescimbene, Addessi, Anderson, & Visalberghi, 2009). Interestingly, callitrichids (marmosets and tamarins) and owl monkeys, species that exhibit biparental cooperation and form long-term pair bonds, are not as sensitive to inequity as one might expect given the prevalence of inequity aversion in species with cooperative relationships (Freeman et al., 2013; Katherine McAuliffe, Shelton, & Stone, 2014a; Neiworth, Johnson, Whillock, Greenberg, & Brown, 2009). This lack of inequity aversion in callitrichids suggests there is either an inability to recognize and/or respond to resource inequities, or that maintaining biparental cooperation may confer a greater benefit to the family group, (e.g., enhanced offspring survival), than losing a parental partner due to the avoidance of interactions that lead to minor inequities (Brosnan, 2011). Thus, investigating inequity aversion strategies in callitrichids serve as critical test of whether inequity aversion is a requisite for the formation and maintenance of cooperative relationships.
The neurohypophysial hormone, oxytocin (OXT), has been implicated as a key neuroendocrine substrate of many social processes (Heinrichs, von Dawans, & Domes, 2009; Insel, 2010; Johnson & Young, 2015). Of particular interest, OXT is critical for maternal-infant bonding and parental behaviour (Feldman, Gordon, Schneiderman, Weisman, & Zagoory-Sharon, 2010; Feldman, Weller, Zagoory-Sharon, & Levine, 2007), OXT modulates pair-bonding between opposite-sex partners (Cavanaugh, Mustoe, Taylor, & French, 2014; Smith, Agmo, Birnie, & French, 2010; Young & Wang, 2004), OXT influences prosocial decision making in primates (Brosnan, Talbot, et al., 2015; Chang, Barter, Ebitz, Watson, & Platt, 2012; Mustoe, Cavanaugh, Harnisch, Thompson, & French, 2015), and OXT has been emphasized as an important regulator of fairness, trust, cooperation, and competition in humans (De Dreu, 2012; Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005; Radke & De Bruijn, 2012; Zak, Stanton, & Ahmadi, 2007). While there is a general trend that OXT has mostly enhancing effects on sociality, there is increasing recognition and appreciation that the valence and magnitude of OXT effects are, in large part, context specific (Bartz, Zaki, Bolger, & Ochsner, 2011; van Anders, Goodson, & Kingsbury, 2013). It is also evident that these neuropeptide effects are highly diverse across species and social situations (Goodson, 2013). This multifaceted influence of OXT on social behaviour is especially apparent among social interactions that include social anxiety or social reward (Bethlehem, Baron-Cohen, van Honk, Auyeung, & Bos, 2014; Brosnan, Talbot, et al., 2015; Mustoe et al., 2015; Neumann & Slattery, 2015).
Marmoset monkeys (Callithrix spp.) offer important opportunities to explore the social and neuroendocrine factors that may regulate inequity aversion for a variety of reasons. First, marmosets are cooperatively-breeding NHPs, and in contrast to many other primates, marmosets are highly prosocial and form long-term male-female cooperative relationships (Agmo, Smith, Birnie, & French, 2012; Burkart, Fehr, Efferson, & van Schaik, 2007; Evans, 1983; Schaffner, Shepherd, Santos, & French, 1995). Second, New World monkeys (NWMs), including callitrichids, possess notably remarkable interspecific OXT and OXT receptor (OXTR) diversity relative to other mammals (Babb, Fernandez-Duque, & Schurr, 2015; Lee et al., 2011; Ren et al., 2015; Vargas-Pinilla et al., 2015; Wallis, 2012), and these varied OXT/OXTR systems potentially coevolved to modulate species-specific social behaviour such as social monogamy and paternal care (Ren et al., 2015; Vargas-Pinilla et al., 2015). Specifically, marmosets possess a modified OXT ligand with a leucine to proline (Pro8-OXT) substitution at the eighth amino acid position resulting in a significant change in the structure of the OXT ligand. These differences in OXT ligands allow for exploration between potential in vivo ligand-specificity of OXT, which may contribute to a broader evolutionary understanding of the effects of neuropeptides on behaviour. Parental and social behaviour in marmosets is highly amendable to OXT treatment. For instance, treatment with Pro8-OXT, but not the consensus mammalian ligand (Leu8-OXT), reduced both sociosexual behaviour and prosocial food sharing toward opposite-sex strangers (Cavanaugh et al., 2014; Mustoe et al., 2015). Additionally, OXT enhances responsiveness to infant stimuli in male marmosets (Saito & Nakamura, 2011; Taylor & French, 2015), social attractiveness (Cavanaugh, Huffman, Harnisch, & French, 2015), and basal OXT levels are synchronized with levels of affiliative behaviour (Finkenwirth, van Schaik, Ziegler, & Burkart, 2015). These findings highlight how OXT shapes the maintenance of cooperative social relationships (male-female pair bonds; parental-offspring bonds) in marmosets by reducing interest in opposite-sex strangers and enhancing interest toward infant stimuli, two characteristics that may preserve biparental cooperative relationships. However, the degree to which OXT regulates more complex sociocognitive decision-making processes, such as inequity aversion, has yet to be examined.
Research on inequity aversion has long-favoured elucidating specific motivational contexts with a particular bias for studying individuals with established long-term cooperative relationships including related or highly familiar social partners or between mates with or without offspring (Brosnan & de Waal, 2014). However, it is also important to consider the role of inequity aversion between strangers with no established social history as these emerging relationships may be regulated by fundamentally different social motivations. In this study, we were interested in evaluating the role of OXT on inequity aversion between both long-term mates and opposite-sex strangers. To achieve these objectives, we investigated three primary aims in this study. 1) We sought to identify whether or not marmosets displayed inequity aversion or inequity tolerance in a food-sharing task across three separate inequity comparisons. 2) We examined whether inequity aversion and inequity tolerance varied depending on whether marmosets share a cooperative relationship (long-term pairmate) or no relationship (opposite-sex stranger). If inequity aversion is important for maintaining long-term cooperative social relationships in primates, then marmosets should display inequity aversion toward their long-term pairmate and inequity tolerance toward opposite-sex strangers. 3) Finally, we explored whether inequity aversion would be altered by OXT treatments. If inequity aversion is an important social feature of cooperative behaviour among marmosets, including pair-bond maintenance and biparental cooperation, then we would expect OXT to enhance inequity aversion toward their pairmate and enhance inequity tolerance and prosociality toward strangers. Thus, there may be an important trade-off for cooperatively-breeding primates, where inequity aversion would expectantly be socially-specific (increased toward pairmates), and this social-specificity would be enhanced by OXT, especially Pro8-OXT, and decreased by an OXT antagonist (OXTa).
METHODS
Study Subjects
We tested 8 individual marmosets (C. penicillata: three adult males and two adult females; C. jacchus: one adult male and two adult females). Marmosets were housed in female– male pairs at the Callitrichid Research Center (CRC) at the University of Nebraska at Omaha (UNO) for the duration of the project. Colony rooms at the CRC were maintained at a temperature range of 19.0 to 22.0 °C and a 12-h:12-h light–dark cycle. All housing enclosures were wire-meshed cages (0.9 × 0.8 × 2.0 m) and equipped with branches, nest boxes, and other assorted enrichment items (e.g., toys). All housing enclosures were furnished with barriers to prevent any visual contact between cages, though auditory and olfactory contact was likely. Marmosets were fed Zupreem® marmoset diet between 0700 and 0900 h and given fruits and varied proteins between 1400 and 1600 h. The weight of the individual marmosets across the duration of the experiment was between ~ 350 – 550 g. Dietary and husbandry details at the CRC can be reviewed (Schaffner et al., 1995). The UNO/UNMC IACUC approved all procedures for this study (#13-048-07), and the procedures also adhere to the ethical standards endorsed by the American Society of Primatologists.
Characterization of Marmoset Pairmates and Strangers
Marmoset pairmates were characterized as unrelated female-male dyads that cohabitated for a minimum of 2 months prior to testing. This duration is more than sufficient for marmosets to form normative behavioural and social characteristics consistent with long-term pair-bonds (Agmo et al., 2012). Marmosets were housed with their pair-bonded partner for the duration of the project. Marmoset strangers were characterized as unrelated female-male testing dyads that had no social history with each other. The marmosets participated with the same opposite-sex stranger throughout the duration of the study, but the marmoset strangers otherwise had no interaction with each other. Two of the four pair-bonded marmoset dyads were used in a previous study exploring the effects of OXT on ‘altruistic’ food-sharing in a tray-pulling task (Mustoe et al., 2015). No pairs had offspring.
Inequity Aversion Apparatus and Testing Procedures
The apparatus consisted of 2 sliding trays (one above the other) where only the donor marmoset was in position to grasp a tray handle and pull the tray similar to those used previously (Burkart et al., 2007; Jensen, Hare, Call, & Tomasello, 2006; Silk et al., 2005); consequently the decision to pull a tray and provision food was controlled exclusively by the donor marmoset. The trays consisted of one side that had food within reach of the donor only, and an adjacent side that had food accessible only for the partner (Figure 1). Null trials consisted of no food item present, but the donor was still able to pull the trays. Prior to start the testing procedures, marmosets, including those used in a previous prosocial tray pulling study, were trained to reach a minimum criterion of at least 10 correct tray pulls to reward themselves out of every 12 trials. For more training details see (Mustoe et al., 2015).
Figure 1.
View of the apparatus and testing setup between donor and recipient marmosets. The pictured trial depicts a qualitative inequity tray condition. In this case, when the donor pulls the tray, the recipient receives a higher quality reward (marshmallow) than the donor (cheerio: pictured in the donor’s left hand). Only the donor is able to reach and pull the tray to provide food rewards.
Each testing session included 15 individual trials consisting of multiple trials of tray conditions. The inequity tray conditions consisted of recipients receiving preferred reward outcomes relative to the donor in a quantitative inequity (reward > no reward) and qualitative inequity (marshmallow > cheerio) context. Equity tray conditions consisted of equal food rewards for both the donor and recipient, selfish tray conditions consisted of a food reward for the donor only, and null tray conditions consisted of no food reward for either the donor or recipient (Table 1). Preferences were confirmed based on choosing options at least 15 out of 20 trials. Marmosets showed a preference for marshmallow rewards over cheerio rewards, and both rewards were preferred over no reward. At the time of testing, the marmosets were maintained on their normative food schedules and diet and were not food-deprived. Testing occurred between ~ 10:30 and 12:30 h for each testing session (between morning and afternoon feedings), and 15 sessions were used to minimize over-consumption or fatigue toward food rewards. The size of the food items used for the project was half a standard cheerio and marshmallow pieces ~ 1.5 mm3. Each trial started after an experimenter showed the food items to the marmoset in each of the 4 possible tray food areas, and then placed the food on the appropriate tray positions. After the food items were placed, the experimenter simultaneously pushed both trays within reach of the donor. Donors were allowed to make only one choice per trial between a tray with a food item (inequity, equity, or selfish) and a tray without a food item. If the tray with the food items was pulled it was scored as a tray pull, and trays pulled without the food item were scored as incorrect tray pulls and excluded from analyses (incorrect tray pulls: 67/1536 total trials: 4%). If 30 s elapsed without a successful tray pull, the trial was scored as a ‘no pull.’ For the null tray conditions (where both trays had no food items present), all tray pulls were scored. The overall duration of an individual testing session with access to the trays ranged from ~ 5–15 minutes.
Table 1.
Different types of tray payout conditions for the donor and the recipient
| Outcome for Donor | Payout |
|---|---|
| Selfish | C,0; M,0 |
| Equity | C,C; M,M |
| Quantitative Inequity | 0,C; 0,M |
| Qualitative Inequity | C,M |
| Null | 0,0 |
0 = no food item; C = cheerio reward; M = marshmallow reward. First number in the condition is the payout for the donor and the second number is the payout for the recipient (donor, recipient). The location of the food items were counter-balanced between top and bottom trays.
Each marmoset served as a donor in all four treatment conditions (OXTa, saline, Leu8-OXT, Pro8-OXT) during testing with their pairmate, stranger, and alone. Strangers were opposite-sex partners with whom they had no visual familiarity outside of testing situations. All donors were also pairmate and stranger recipients in this study, thus they were trained/habituated to understand the outcome of both roles. All marmosets were tested as a recipient with a donor in all four treatment conditions. The order of OXT treatments and presence of partners was counterbalanced across the study. All testing sessions were video recorded. Tray pulling was scored in real time by experimenters blind to treatment conditions. Marmosets had high familiarity with all experimenters prior to testing.
Oxytocin Treatments
Pro8-OXT (Anaspec, Fremont CA), Leu8-OXT (Sigma-Aldrich), and saline controls were administered intranasally consistent with procedures used previously in marmosets (Cavanaugh et al., 2014; Mustoe et al., 2015; Smith et al., 2010). For OXT treatments each animal received 50µg (~ 25 IU) of OXT/100 µ1 saline solution ~ 30 min before the beginning of each testing session. Intranasal administration of OXT in macaques and humans leads to increases in OXT concentrations in both plasma and CSF (Dal Monte, Noble, Turchi, Cummins, & Averbeck, 2014; Striepens et al., 2013), but see, (Leng & Ludwig, 2015), about important considerations of intranasal OXT administration. The OXTa (L-368,899; provided by Dr. Peter Williams, Merck) is a non-peptide antagonist with high affinity for OXT receptors (it also has a high affinity for AVPRla and AVPRlb) (Manning et al, 2012; Williams et al, 1994). The OXTa (L-368,899) has been shown to localize in both CSF and brain areas known to contain neurons with OXT receptors in rodents after oral administration (Boccia, Goursaud, Bachevalier, Anderson, & Pedersen, 2007). The OXTa was administered orally, in the animals’ home enclosure, at a dose of ~ 20 mg/kg in a preferred food item ~ 90 min before testing. Non OXTa-treated animals also received food controls in their homecage ~ 90 min before testing. To account for potential handling effects (e.g., anxiety and/or arousal) associated with the intranasal administration procedure, all animals were habituated to catching and handling procedures prior to the study. Animals that received oral OXTa treatments were also manually restrained and received 100 µ1 of intranasal saline −30 minutes before testing. Animals in all conditions underwent the same removal from homecage, handling, and intranasal procedures.
Inequity Comparisons and Data Analysis
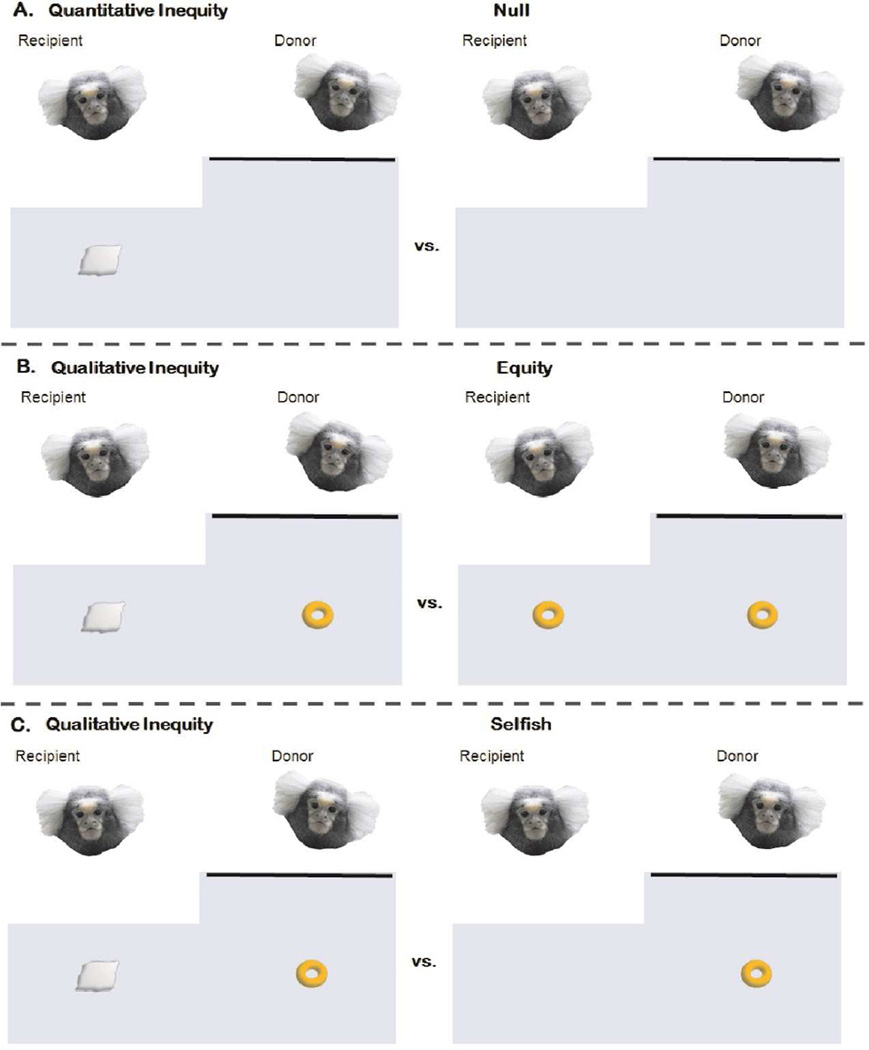
Because inequity aversion is likely context-dependent, we tested for inequity aversion based on relative rates (mean % of tray pulls per testing session) of tray pulling in three separate inequity comparisons, varying in the degree of inequity. 1) Quantitative inequity vs. null which measures inequity aversion as a consequence of prosociality or ‘altruistic’ food sharing, i.e., donor receives no reward; 2) Qualitative inequity vs. equity which measures inequity aversion relative to a mutually beneficial reward outcome where both receive the same rewards; and 3) Qualitative inequity vs. selfish which measures inequity aversion relative to cases when the donor receives a better reward (Figure 2). These comparisons are important insofar that the reward only varies between tray condition for the recipient and not the donor. This rules out differences based on donors’ motivations for their own reward; consequently, differences in donors’ tray pulling should reflect differences in motivation for the recipients’ reward. Therefore, with regard to the inequity comparisons (tray conditions), inequity aversion in marmosets occurred when the mean % of inequity tray pulls was significantly lower than equity or selfish tray pulls, and Inequity tolerance in marmosets occurred when there were no differences across inequity comparisons, or in cases where the inequity tray pulling was significantly higher than equity or selfish tray pulling. The separate selfish (C,0; M,0), equity (C,C; M,M) and qualitative inequity (0,C; 0;M) conditions (table 1) were each combined for analyses due to the limited number of individual trials.
Figure 2.
Differences in three separate inequity comparisons. A) Quantitative Inequity vs. Null (no reward) trays; B) Qualitative Inequity vs. Equity trays; C) Qualitative Inequity vs. Selfish trays. Marshmallows are more preferred than cheerios, so inequity aversion occurs when inequity trays are pulled significantly less frequently than null, equity, or selfish trays. Inequity tolerance occurs when there is no difference or inequity trays are pulled more frequently.
Individuals were tested in all tray conditions in a repeated-measures design (exposed to repeated OXT treatments and partner conditions). The data were analyzed using repeated-measures mixed ANOVA. Each ANOVA allowed for examining the rates of tray pulling based on a variety of variables including tray conditions (e.g., inequity vs. equity), partner familiarity (pairmate, stranger, alone), OXT treatment (OXTa, saline, Leu8-OXT, Pro8-OXT), sex, and the corresponding interactions among the variables with the tray conditions. An ANOVA was performed separately for each of the three inequity comparisons (Figure 2) to assess whether inequity trays are pulled more or less than equity or selfish trays. Effects sizes are reported as partial η2s. Because of the small repeated-measures sample size, the relatively high number of independent variables, and the measurement of tray pulling, the data were normalized for ANOVA analyses using a square root transformation. Square root transformation was preferred over a log transformation because of the presence of zeros in the data. Data presented in figures are untransformed tray pulling data.
RESULTS
Quantitative Inequity vs. Null
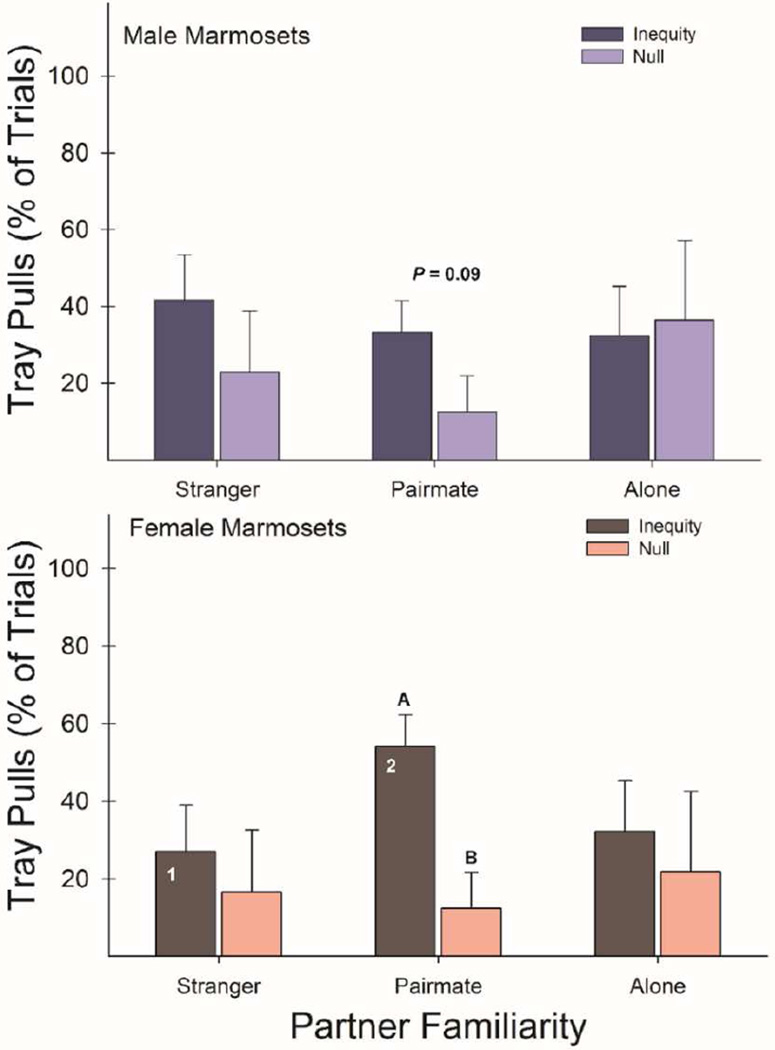
Marmosets pulled the inequity tray more frequently than they pulled null trays (F1,6 = 9.91, P = 0.02, η2 = 0.62), suggesting an inequity tolerance and a general prosociality toward others. This inequity tolerance, i.e., preference to pull the inequity tray more than the null trays, was strongest when tested with their pairmate, compared to testing with strangers and testing alone (F2,12 = 8.76, P < 0.01, η2 = 0.59). However, the preference of inequity trays over null trays did not significantly vary based on the sex of the partner (F1,6 = 0.59, P = 0.47, η2 = 0.09). Females pulled inequity trays more frequently when tested with their pairmate (t3 = 4.40, P = 0.02), and males pulled inequity trays marginally more frequently when tested their pairmate (t3 = 2.39, P = 0.09) (Figure 3). When examining the frequency of inequity tray pulling specifically, there was a significant partner by sex interaction (F2,12 = 3.84, P = 0.05, η2 = 0.39), where females showed more frequent inequity tray pulling when tested with their pairmate than strangers (t3 = 3.26, P = 0.04) (Figure 3). This inequity tolerance in marmosets did not significantly change across OXT treatment conditions (F3,18 = 0.17, P = 0.92, η2 = 0.03) (Figure S1).
Figure 3.
Quantitative inequity vs. null comparison: mean + S.E. (untransformed) % trials with tray pulls by the donor marmosets in inequity and null (no reward) conditions between strangers, long-term pairmates, and alone without a partner present across all OXT conditions. Group means labeled with letters (A & B) and/or numbers (1 & 2) represent significant post-hoc mean differences = P < 0.05 two-tailed. Males = top; females = bottom panel.
Qualitative Inequity vs. Equity
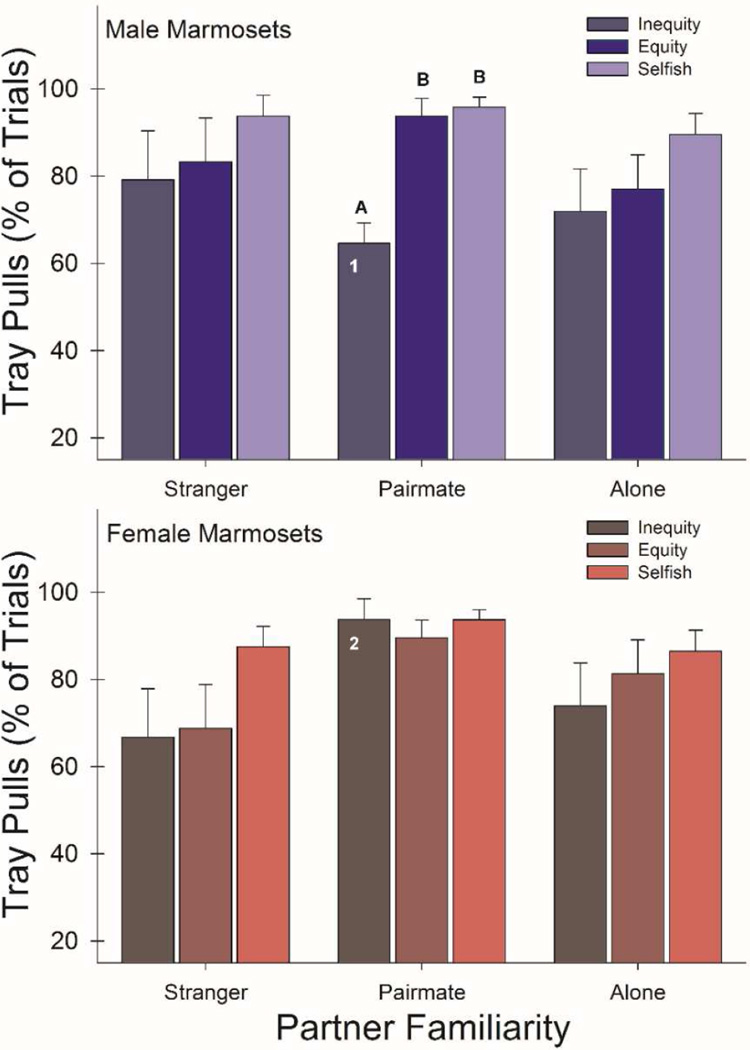
Marmoset donors pulled the inequity trays significantly less often than the equity trays (F1,6 = 67.85, P < 0.01, η2 = 0.92), indicating inequity aversion. This inequity aversion, i.e., preference for pulling the equity trays more than the inequity trays, was significantly higher for males than females (F1,6 = 24.97, P < 0.01, η2 = 0.81), but this overall preference did not significantly vary by partner familiarity alone (F2,12 = 1.11, P = 0.36, η2 = 0.16). However, there was a significant three-way interaction for the tray condition, sex, and partner (F2,12 = 5.32, P = 0.02, η2 = 0.47), with male marmosets showing inequity aversion only when tested with their pairmate (t3 = 6.88, P < 0.01) (Figure 4). The frequency of inequity tray pulling varied by partner familiarity and sex (F2,12 = 3.14, P = 0.08, η2 = 0.34), indicating that males pulled the inequity trays when tested with their pairmate significantly less often than females did (t6 = 4.09, P < 0.01) (Figure 4). Furthermore, the frequency of equity tray pulling was higher when tested with their pairmate for both males and females (F2,12 = 4.14, P = 0.04, η2 = 0.41). Overall, this inequity aversion in marmosets did not significantly change across OXT treatment conditions (F3,18 = 0.13, P = 0.94, η2 = 0.02) (Figure S2).
Figure 4.
Qualitative inequity vs equity and Qualitative inequity vs selfish comparisons: mean + S.E. (untransformed) % trials with tray pulls by the donor marmosets in inequity, equity, and selfish conditions between strangers, long-term pairmates, and alone without a partner present across all OXT conditions. Group means labeled with letters (A & B) and/or numbers (1 & 2) represent significant post-hoc mean differences = P < 0.05 two-tailed. Males = top; females = bottom panel.
Qualitative Inequity vs. Selfish
Marmosets pulled the inequity trays significantly less often than the selfish trays (F1,6 = 16.30, P < 0.01, η2 = 0.73), which also suggests a form of inequity aversion. This inequity aversion, i.e., preference for pulling the selfish trays more than the inequity trays, was not socially- (F2,12 = 0.21, P = 0.81, η2 = 0.04) or sex-specific (F1,6 = 1.33, P = 0.29, η2 = 0.18), and there was no significant three-way interaction between tray, partner familiarity, and sex (F2,12 = 2.22, P = 0.15, η2 = 0.27). However, there was an overall sex by partner interaction for tray pulling of inequity and selfish trays together (F2,12 = 3.85, P = 0.05, η2 = 0.39), suggesting the same the pattern of the inequity aversion being limited to only males when tested with their pairmate (t3 = 4.42, P = 0.02) (Figure 4). As with the other inequity comparisons, the inequity aversion in marmosets did not significantly differ by OXT treatment (F3,18 = 0.15, P = 0.93, η2 = 0.02) (Figure S2). All data are available in supplemental Table S1.
Inequity Aversion across Callithrix spp
We also evaluated whether there were significant differences in inequity aversion between the two species Callithrix penicillata (n = 5) and Callithrix jacchus (n = 3). Overall, the difference in species did not significantly interact with tray pulling in any of the three inequity comparisons. In the quantitative inequity vs. null comparison, we found that there was no species interaction with the finding that marmosets pull inequity trays more frequently than the null trays (F1,6 = 0.13, P = 0.74, η2 = 0.02), and there was no significant species by partner familiarity interaction (F2,12 = 0.27, P = 0.76, η2 = 0.14) or OXT interaction (F3,18 = 0.13, P = 0.74, η2 = 0.04) on this inequity tolerance.
In the qualitative inequity vs. equity comparison, we found that there was no species interaction with the finding that marmosets pulled the inequity trays less frequently than the equity trays (F1,6 = 0.51, P = 0.50, η2 = 0.08), and there was no significant species by partner familiarity interaction (F2,12 = 0.44, P = 0.65, η2 = 0.18) or OXT interaction (F3,18 = 1.30, P = 0.31, η2 = 0.07) on this inequity aversion.
Finally, in the qualitative vs. selfish comparison, we found that there was no species interaction with the finding that marmosets pulled the inequity trays less frequently than the selfish trays (F1,6 = 0.11, P = 0.75, η2 = 0.01), and there was no significant species by partner familiarity interaction (F2,12 = 1.52, P = 0.24, η2 = 0.20) or OXT interaction (F3,18 = 1.35, P = 0.30, η2 = 0.18) on this inequity aversion. Overall, these data show that both species of Callithrix exhibit the same frequency and pattern of tray pulling and inequity aversion strategies across all three of the inequity comparisons.
DISCUSSION
The expression of inequity aversion in marmosets depends on the magnitude and type of inequity and the level of familiarity between partners. Specifically, when donor marmosets were presented with the option of pulling the tray and receiving no food reward while their partner received a food reward, the donors’ pulled these quantitative inequity trays equally or more frequently than the null trays; but when donor marmosets were presented with the option to pull trays to receive a qualitatively less-preferred food reward than their partner, they pulled these qualitative inequity tray significantly less than the equity or selfish trays. These responses to inequity also varied based on the relationship with the partner and the sex of the donor marmoset. Specifically, male donors displayed greater reduction in pulling qualitative inequity trays when tested with their pairmate, while female donors showed no difference in the rate of pulling qualitative inequity trays when tested with their pairmate. In general, both males and females were inequity tolerant toward strangers, though females were less so. Despite the social-specificity of these responses to inequity, OXT treatment did not influence rates of inequity tray pulling and inequity aversion toward others. These findings suggest that marmosets are both inequity tolerant (prosocial) and inequity averse depending on the type of inequity and the social context including familiarity and sex of the social partner.
Callitrichids fill an important gap in studying the potential requisite of inequity aversion in maintaining cooperative relationships across primates. Like humans, callitrichids rely on cooperative breeding strategies—i.e., the dependence on help from the father and siblings—to successfully raise offspring. While marmosets are cooperative-breeders, male and female marmosets exhibit different breeding strategies (Yamamoto et al., 2014). Specifically, subordinate females within a family rarely become breeders, and disperse with much higher frequency than males, leading females to exhibit more aggression and less affiliative behaviour. Males, on the other hand, are much more likely to display affiliative behaviour and consequently form strong social bonds within the natal group. Males are also more likely to fill natal group breeding vacancies than females. Consequently, females rely on more competitive strategies while males rely on more cooperative strategies (Díaz-Muñoz et al., 2014; Yamamoto et al., 2014). Furthermore, marmoset families with more male helpers are more prosocial than marmoset families with more female helpers, and infant care experience enhanced prosociality in males but not females (Burkart, 2015). Thus, if inequity aversion were a behavioural strategy to maintain cooperative relationships in marmosets, we would expect that males would be more inequity averse than females given male marmosets’ stronger propensity to maintain cooperative relationships. Our findings support this view, as only male donors were inequity averse, and this inequity aversion was only toward their pairmate.
Studying the relevance of biological and social contexts and inequity aversion across species is challenging because species-specific behaviours require different methodological approaches. However, one key factor that appears to influence prosociality and inequity aversion across most primates is the relationship history and familiarity of the social partners. The specific context of the social relationship between individuals is an important feature because inequity aversion appears to emerge alongside cooperative relationships, and the properties of cooperative social interactions vary greatly depending on whether partners are maintaining (related or long-term mates) or forming/avoiding new cooperative relationships (novel or unfamiliar social partners). Aside from our study, only a few studies have directly addressed the influence of relationship type on prosociality. (de Waal, Leimgruber, & Greenberg, 2008)), demonstrated that capuchins show increased prosociality toward kin relative to nonkin, and this prosociality decreased as familiarity of the partner decreased, suggesting that individuals behave more prosocially toward more closely-bonded individuals. There is also evidence suggesting from some species that these preferences are regulated by OXT. For instance, OXT is only correlated with affiliative behaviour between individuals who share social bonds (Crockford et al., 2013; Wittig et al., 2014), and there is greater OXT synchrony with affiliative behaviour between marmosets who share stronger social bonds (Finkenwirth et al., 2015). With regard to unfamiliar social relationships, macaques will reward strangers (Chang, Winecoff, & Platt, 2011), and OXT treatment increases the proportion of trials in which sharing with strangers occurs (Chang et al., 2012). Studies of callitrichids have demonstrated that prosociality is higher toward both pairmates (Burkart et al., 2007; Cronin, Schroeder, & Snowdon, 2010; Stevens, 2010) and opposite-sex strangers (Mustoe et al., 2015) across a variety of food-sharing and social contexts. The conditions in which individuals are willing to behave prosocially change across different relationship types, and these differences in cooperative motivations can, in turn, influence whether or not individuals are inequity averse across different experiments. Overall, the key social distinction that may account for the differences in prosociality and inequity aversion strategies in marmosets in the current study and Mustoe et al., 2015, compared to others (Burkart et al., 2007; Freeman et al., 2013), is that our marmosets lived in exclusive male-female pairs with no offspring while other studies have investigated marmosets that live in larger family groups. Thus while callitrichids may be predisposed to behave prosocially, the social context of these behavioural strategies, and the importance of food sharing within the family may be different with and without the presence of offspring. Overall, more research is needed to disentangle the role of active or previous infant care experience and family living vs. pair-living on marmoset prosociality and cooperation.
The patterns of prosocial preferences among primates vary considerably both across species and within species (Brosnan, 2011; Brosnan and de Waal, 2014; Cronin, 2012; McAuliffe and Thornton, 2015). These differences raise important questions about whether primates are primed to behave prosocially based on their social structure and/or whether specific social and biological cues enhance or inhibit the expression of prosociality toward others. One approach to study inequity aversion is to compare similarities and differences in prosociality and inequity aversion across species while attempting to control for species, social, and other experimental differences. For example, in a comparative study that examined levels of prosociality across many group-living primates using a standardized experimental procedure (‘group-service paradigm’), the extent to which a primate species exhibited alloparental care was the strongest predictor of ‘proactive prosociality’ (Burkart et al., 2014). Additionally, in another study that examined the importance of cooperative-breeding and biparental cooperation on inequity aversion, marmosets, owl monkeys, and squirrel monkeys were found to be inequity tolerant, yet there were subtle species and sex differences that support the view that social structures based on pair-bonding and biparental care lead to inequity tolerance in primates (Freeman et al., 2013). The comparative method is an important and informative approach to evaluate the evolutionary origins of specific social traits. Taken together, these studies show that primate species that engage in biparental and alloparental cooperation develop specific biological and social mechanisms that lead to prosocial phenotypes.
While there are many benefits to using a comparative approach to understand the origins of social phenotypes in primates, it is widely viewed and accepted that comparisons of prosociality across experiments are hindered by differing methodologies and measures (Cronin, 2012; Tan, Kwetuenda, & Hare, 2015). The variability in the type of effort and exchange, such as tray pulling in the presence of real food rewards compared token exchanges (and the corresponding ecological validity), can each influence the presence of inequity aversion (Brosnan & de Waal, 2014). For example, capuchins show sensitivity to the magnitude of the inequity, where prosociality decreases as the discrepancies in the rewards increases (Brosnan, Houser, et al., 2010). However when it comes to the cost and effort to obtain rewards, responses to inequity vary considerably across New World monkeys both in high and low cost/effort situations (Freeman et al., 2013; Katherine McAuliffe et al., 2015; Katherine McAuliffe, Shelton, & Stone, 2014b; Neiworth et al., 2009; Sheskin, Ashayeri, Skerry, & Santos, 2014). Importantly, these prosocial responses inherently generate and lead to inequity as well (such as the case represented in our quantitative inequity comparison). For example, capuchin monkeys were given a choice between the option of receiving one food item and their partner receiving one food item (equal reward option) versus receiving one food item and their partner receiving three food items (the option could be considered both ‘prosocial’ and ‘inequity’), the capuchins preferred the equal reward option (Fletcher, 2008). Importantly, this preference occurred only when a partner was present, thus showing these capuchins were inequity averse based on a social comparison and not a ‘frustration’ effect of not being able to receive the better reward (i.e., individual contrast). In the current study, our findings suggest that responses to inequity were unlikely due to a frustration effect when tested alone, i.e., marmosets did not refuse to reward themselves when presented with the lower-quality reward. Instead the data suggest that, in the cases where there was a qualitative inequity aversion, donor marmosets choose to forego their own food reward in order to prevent their pairmate, but not strangers, from receiving the better reward. This inequity aversion occurred in spite of a minor short-term cost of missing a food reward for both the donor and their mate, which is likely to occur in the context of maintaining a beneficial long-term cooperative relationship.
Prosocial behaviour can vary across multiple domains and should be viewed as contextual rather than as simply present or absent. Differences in task and methodological design, such as task complexity and cost, can substantially alter whether and to what degree individuals respond prosocially (Burkart, Rueth, & Stanyon, 2013; House, Henrich, Brosnan, & Silk, 2012; House, Silk, Lambeth, & Schapiro, 2014). For instance, tasks that require greater attentional demand toward others can inhibit prosocial responses (Burkart et al., 2013). Moreover, different reward payouts can also influence prosocial responses consistent with what we found in this study. Specifically, when donors do not receive rewards they are much more tolerant of the inequity. And when donors do receive rewards, they are much more averse to the inequity. Importantly, in all inequity comparisons, the effects are socially specific suggesting that the donors are attending to who the recipient is, and the donor’s familiarity with the recipient is an important context for prosociality. Additionally, other important social factors including personality and social rank can influence whether individuals respond prosocially (Brosnan, Hopper, et al., 2015; Price & Brosnan, 2012), and the relative importance of these social factors can considerably vary from species to species. Ultimately, prosocial responses occur through multiple processes both within and across species, some of which may be modified by OXT (Brosnan, Talbot, et al., 2015; Chang et al., 2012; Mustoe et al., 2015) while others may not (this study).
While we found differences in inequity aversion based on differences in social context, we failed to find an effect of OXT on inequity aversion. Inequity aversion and tolerance are important social features for marmoset pairmates, but it is likely that these social decisions following inequity reflect an emergent property of multiple social behaviours. Marmosets exhibit a highly flexible behavioural repertoire for the formation and maintenance of long-term pairbonds and biparental cooperation, and these specific social behaviours are highly amendable to OXT treatments. Marmosets only show inequity aversion toward others in specific situations (e.g., males pulling trays that result in better rewards for their pairmate less frequently). In light of the lack of an OXT effect on inequity aversion in our paradigm, it may be that the influence of OXT on inequity aversion is restricted to social interactions that result in differential outcomes for partners that are more extreme than those in our study, or it may be that the influence of OXT on inequity aversion is limited to species that are far more sensitive to inequity, such as capuchin monkeys, chimpanzees, and humans. Studies on humans have established a link between OXT treatment and an increased willingness to cooperate, to act generously, and an increased expectation that others cooperate (Barraza, McCullough, Ahmadi, & Zak, 2011; Israel, Weisel, Ebstein, & Bornstein, 2012; Zak et al., 2007). However, the enhancement of prosociality by OXT is not universal, as other work has shown that OXT reduces sensitivity to fairness based on perspective-taking and generosity, and more generally, OXT reduces conformity to ‘social norms’ (Radke & De Bruijn, 2012). These studies in humans strongly reinforce the view that the relationship between OXT and prosociality is highly sensitive to social context, especially with regard to the relationship quality between social partners (De Dreu, 2012; Everett, Faber, Crockett, & De Dreu, 2015). The overarching link among studies of OXT and human cooperation is the role of OXT in enhancing group-level cooperation, though there is considerable debate about the robustness of these OXT studies (Leng & Ludwig, 2015; Nave, Camerer, & McCullough, 2015; Van IJzendoorn & Bakermans-Kranenburg, 2012; Walum, Waldman, & Young, 2015). It is alternatively possible the our OT treatment did not produce a big effect because OT administered intranasally may not abundantly penetrate the brain; though large doses may have strong peripheral effects. While this study cannot speak directly to which central or peripheral mechanism OT modifies behaviour, previous studies using this intranasal OT procedure in marmosets have shown socially-specific effects of intranasal OT treatment across multiple social realms (Cavanaugh et al., 2015, 2014; Mustoe et al., 2015; Smith et al., 2010; Taylor & French, 2015). However, considerably more experimental research is needed in both human and animal populations, to establish whether and how administration of OXT reliably affects how individuals maintain cooperative relationships across a diversity of social interactions and species.
CONCLUSIONS
Whether cooperative strategies in primates are a consequence of physiological, psychological, or ecological factors and to what extent these findings inform us about the evolution of human cooperation is a stimulating topic under current debate (Brosnan, 2011; Burkart et al., 2014; Jaeggi, Burkart, & Van Schaik, 2010; K. McAuliffe & Thornton, 2015; Trumble, Jaeggi, & Gurven, 2015). In captive marmosets, there is a clear willingness or tolerance to reward others in a low-cost task without the expectation to receive an immediate reward for themselves (Burkart et al., 2007; Mustoe et al., 2015), but less is known about when marmosets are unwilling to behave prosocially. Our study was the first to directly assess the role of OXT and relationship type on inequity aversion, and we demonstrate important contextual features that influence responses to inequity in marmosets. However, there are some important limitations worth noting. Like many NHP and OXT studies, the sample size was small, the marmosets in this study lived in pairs instead of family groups, and the marmosets had no parental experience. These differences limit the generalizability across other primate studies examining prosociality and cooperation, such as the work using the ‘group-service paradigm’ to study species-level differences in prosociality (Burkart et al., 2014). However, these findings contribute to our understanding of the intraspecific social and neuroendocrine underpinnings of prosociality among marmosets (Burkart, 2015; Burkart et al., 2007; Mustoe et al., 2015). The majority of studies on prosocial behaviour are limited to inequity and prosociality in food sharing situations, and studying prosociality across different behavioural contexts such as grooming, parental care, territorial maintenance, helping behaviour, etc., is needed. Finally, it is critical to interpret social behaviour in a species-specific way; therefore evaluating inequity aversion across a wide variety of cooperating and non-cooperating species (including non-mammalian species such as birds and fish, for example, Wascher & Bugnyar, 2013) can only enrich and advance our understanding for the evolutionary origins and implications of inequity aversion, cooperation, and social disparities.
Supplementary Material
We tested whether social familiarity and oxytocin (OXT) influence inequity aversion in marmosets.
Marmosets showed inequity aversion that was context specific and sex specific.
Inequity aversion was only present between males and their pair-bonded partners.
OXT treatment did not influence responses to inequity in marmosets.
Inequity aversion is important for maintaining specific cooperative relationships.
Acknowledgments
We wish to thank Heather Jensen and Liz Gunkelman for the excellent care of the marmosets; Kevin Barton for assembly of the apparatus used in this study; and Breanna Thompson, Rachel Stein, Brittney Meays, and Chelsea Rock for their contributions and participation with marmoset training and related data collection. We would also like to thank Jack Taylor and Michelle Huffman for comments and discussion on previous versions of this manuscript, and the anonymous reviewers for their thoughtful comments and suggestions. This research was supported by funds from N.I.H. [R01-HD042882] awarded to JAF and UNO-Graduate Research and Creative Activity Award to ACM. The funding sources had no role in the design, analysis, writing, preparation, or decision to submit this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agmo A, Smith AS, Birnie AK, French JA. Behavioral characteristics of pair bonding in the black tufted-ear marmoset (Callithrix penicillata) Behaviour. 2012;149(3–4):3–4. doi: 10.1163/156853912X638454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb PL, Fernandez-Duque E, Schurr TG. Oxytocin Receptor Gene Sequences in Owl Monkeys and Other Primates Show Remarkable Interspecific Regulatory and Protein Coding Variation. Molecular Phylogenetics and Evolution. 2015 doi: 10.1016/j.ympev.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Barraza JA, McCullough ME, Ahmadi S, Zak PJ. Oxytocin infusion increases charitable donations regardless of monetary resources. Hormones and Behavior. 2011;60(2):148–151. doi: 10.1016/j.yhbeh.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bethlehem RA, Baron-Cohen S, van Honk J, Auyeung B, Bos PA. The oxytocin paradox. Frontiers in Behavioral Neuroscience. 2014:8. doi: 10.3389/fnbeh.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia ML, Goursaud A-PS, Bachevalier J, Anderson KD, Pedersen CA. Peripherally administered non-peptide oxytocin antagonist, L368,899, accumulates in limbic brain areas: A new pharmacological tool for the study of social motivation in non-human primates. Hormones and Behavior. 2007;52(3):344–351. doi: 10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräuer J, Call J, Tomasello M. Are apes inequity averse? New data on the token-exchange paradigm. American Journal of Primatology. 2009;71(2):175–181. doi: 10.1002/ajp.20639. [DOI] [PubMed] [Google Scholar]
- Brosnan SF. A hypothesis of the co-evolution of cooperation and responses to inequity. Frontiers in Neuroscience. 2011:5. doi: 10.3389/fnins.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan SF, De Waal FB. Monkeys reject unequal pay. Nature. 2003;425(6955):297–299. doi: 10.1038/nature01963. [DOI] [PubMed] [Google Scholar]
- Brosnan SF, de Waal FB. Evolution of responses to (un) fairness. Science. 2014;346(6207):1251776. doi: 10.1126/science.1251776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan SF, Hopper LM, Richey S, Freeman HD, Talbot CF, Gosling SD, Schapiro SJ. Personality influences responses to inequity and contrast in chimpanzees. Animal Behaviour. 2015;101:75–87. doi: 10.1016/j.anbehav.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan SF, Houser D, Leimgruber K, Xiao E, Chen T, de Waal FB. Competing demands of prosociality and equity in monkeys. Evolution and Human Behavior. 2010;31(4):279–288. [Google Scholar]
- Brosnan SF, Schiff HC, De Waal FB. Tolerance for inequity may increase with social closeness in chimpanzees. Proceedings of the Royal Society of London B: Biological Sciences. 2005;272(1560):253–258. doi: 10.1098/rspb.2004.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan SF, Talbot C, Ahlgren M, Lambeth SP, Schapiro SJ. Mechanisms underlying responses to inequitable outcomes in chimpanzees, Pan troglodytes. Animal Behaviour. 2010;79(6):1229–1237. doi: 10.1016/j.anbehav.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan SF, Talbot CF, Essler JL, Leverett K, Flemming T, Dougall P, Zak PJ. Oxytocin reduces food sharing in capuchin monkeys by modulating social distance. Behaviour. 2015 http://doi.org/10.1163/1568539X-00003268. [Google Scholar]
- Burkart JM. Opposite effects of male and female helpers on social tolerance and proactive prosociality in callitrichid family groups. Scientific Reports. 2015:5. doi: 10.1038/srep09622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart JM, Allon O, Amici F, Fichtel C, Finkenwirth C, Heschl A others. The evolutionary origin of human hyper-cooperation. Nature Communications. 2014:5. doi: 10.1038/ncomms5747. [DOI] [PubMed] [Google Scholar]
- Burkart JM, Fehr E, Efferson C, van Schaik CP. Other-regarding preferences in a non-human primate: Common marmosets provision food altruistically. Proceedings of the National Academy of Sciences. 2007;104(50):19762–19766. doi: 10.1073/pnas.0710310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart JM, Rueth K, Stanyon R. Preschool children fail primate prosocial game because of attentional task demands. PloS One. 2013;8(7):e68440. doi: 10.1371/journal.pone.0068440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Huffman MC, Harnisch AM, French JA. Marmosets treated with oxytocin are more socially attractive to their long-term mate. Frontiers in Behavioral Neuroscience. 2015 doi: 10.3389/fnbeh.2015.00251. http://doi.org/10.3389/fnbeh.2015.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Mustoe AC, Taylor JH, French JA. Oxytocin facilitates fidelity in well-established marmoset pairs by reducing sociosexual behavior toward opposite-sex strangers. 2014;49:1–10. doi: 10.1016/j.psyneuen.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proceedings of the National Academy of Sciences. 2012;109(3):959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SW, Winecoff AA, Platt ML. Vicarious reinforcement in rhesus macaques (Macaca mulatta) Frontiers in Neuroscience. 2011:5. doi: 10.3389/fnins.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford C, Wittig RM, Langergraber K, Ziegler TE, Zuberbühler K, Deschner T. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proceedings of the Royal Society B: Biological Sciences. 2013;280(1755):20122765. doi: 10.1098/rspb.2012.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin KA. Prosocial behaviour in animals: the influence of social relationships, communication and rewards. Animal Behaviour. 2012;84(5):1085–1093. [Google Scholar]
- Cronin KA, Schroeder KK, Snowdon CT. Prosocial behaviour emerges independent of reciprocity in cottontop tamarins. Proceedings of the Royal Society B: Biological Sciences. 2010;277(1701):3845–3851. doi: 10.1098/rspb.2010.0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Turchi J, Cummins A, Averbeck BB. CSF and Blood Oxytocin Concentration Changes following Intranasal Delivery in Macaque. PloS One. 2014;9(8):e103677. doi: 10.1371/journal.pone.0103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK. Oxytocin modulates cooperation within and competition between groups: an integrative review and research agenda. Hormones and Behavior. 2012;61(3):419–428. doi: 10.1016/j.yhbeh.2011.12.009. [DOI] [PubMed] [Google Scholar]
- de Waal FB, Leimgruber K, Greenberg AR. Giving is self-rewarding for monkeys. Proceedings of the National Academy of Sciences. 2008;105(36):13685–13689. doi: 10.1073/pnas.0807060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S. The pair-bond of the common marmoset, Callithrix jacchus jacchus: an experimental investigation. Animal Behaviour. 1983;31(3):651–658. [Google Scholar]
- Everett JA, Faber NS, Crockett MJ, De Dreu CK. Economic games and social neuroscience methods can help elucidate the psychology of parochial altruism. Frontiers in Psychology. 2015:6. doi: 10.3389/fpsyg.2015.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr E, Bernhard H, Rockenbach B. Egalitarianism in young children. Nature. 2008;454(7208):1079–1083. doi: 10.1038/nature07155. [DOI] [PubMed] [Google Scholar]
- Fehr E, Schmidt KM. A theory of fairness, competition, and cooperation. Quarterly Journal of Economics. 1999:817–868. [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology. 2010;35(8):1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science. 2007;18(11):965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Finkenwirth C, van Schaik C, Ziegler TE, Burkart JM. Strongly bonded family members in common marmosets show synchronized fluctuations in oxytocin. Physiology & Behavior. 2015;151:246–251. doi: 10.1016/j.physbeh.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher GE. Attending to the outcome of others: Disadvantageous inequity aversion in male capuchin monkeys (Cebus apella) American Journal of Primatology. 2008;70(9):901–905. doi: 10.1002/ajp.20576. [DOI] [PubMed] [Google Scholar]
- Freeman HD, Sullivan J, Hopper LM, Talbot CF, Holmes AN, Schultz-Darken N, Brosnan SF. Different Responses to Reward Comparisons by Three Primate Species. PloS One. 2013;8(10):e76297. doi: 10.1371/journal.pone.0076297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. Deconstructing sociality, social evolution and relevant nonapeptide functions. Psychoneuroendocrinology. 2013;38(4):465–478. doi: 10.1016/j.psyneuen.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Frontiers in Neuroendocrinology. 2009;30(4):548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hopper LM, Lambeth SP, Bernacky BJ, Brosnan SF. The ontogeny of social comparisons by rhesus macaques (Macaca mulatta) 2013 [Google Scholar]
- House BR, Henrich J, Brosnan SF, Silk JB. The ontogeny of human prosociality: behavioral experiments with children aged 3 to 8. Evolution and Human Behavior. 2012;33(4):291–308. [Google Scholar]
- House BR, Silk JB, Lambeth SP, Schapiro SJ. Task design influences prosociality in captive chimpanzees (Pan Troglodytes) 2014 doi: 10.1371/journal.pone.0103422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65(6):768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel S, Weisel O, Ebstein RP, Bornstein G. Oxytocin, but not vasopressin, increases both parochial and universal altruism. Psychoneuroendocrinology. 2012;37(8):1341–1344. doi: 10.1016/j.psyneuen.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Jaeggi AV, Burkart JM, Van Schaik CP. On the psychology of cooperation in humans and other primates: combining the natural history and experimental evidence of prosociality. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365(1553):2723–2735. doi: 10.1098/rstb.2010.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K, Hare B, Call J, Tomasello M. What’s in it for me? Self-regard precludes altruism and spite in chimpanzees. Proceedings of the Royal Society B: Biological Sciences. 2006;273(1589):1013–1021. doi: 10.1098/rspb.2005.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. Neurobiological mechanisms of social attachment and pair bonding. Current Opinion in Behavioral Sciences. 2015;3:38–44. doi: 10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lee AG, Cool DR, Grunwald WC, Neal DE, Buckmaster CL, Cheng MY, Parker KJ. A novel form of oxytocin in New World monkeys. Biology Letters. 2011;7(4):584–587. doi: 10.1098/rsbl.2011.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Intranasal oxytocin: myths delusions. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. Journal of Neuroendocrinology. 2012;24(4):609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massen JJ, Van den Berg LM, Spruijt BM, Sterck EH. Inequity aversion in relation to effort and relationship quality in long-tailed Macaques (Macaca fascicularis) American Journal of Primatology. 2012;74(2):145–156. doi: 10.1002/ajp.21014. [DOI] [PubMed] [Google Scholar]
- McAuliffe K, Chang LW, Leimgruber KL, Spaulding R, Blake PR, Santos LR. Capuchin monkeys, Cebus apella, show no evidence for inequity aversion in a costly choice task. Animal Behaviour. 2015;103:65–74. [Google Scholar]
- McAuliffe K, Shelton N, Stone L. Does effort influence inequity aversion in cotton-top tamarins (Saguinus oedipus)? Animal Cognition. 2014a;17(6):1289–1301. doi: 10.1007/s10071-014-0764-x. [DOI] [PubMed] [Google Scholar]
- McAuliffe K, Shelton N, Stone L. Does effort influence inequity aversion in cotton-top tamarins (Saguinus oedipus)? Animal Cognition. 2014b;17(6):1289–1301. doi: 10.1007/s10071-014-0764-x. [DOI] [PubMed] [Google Scholar]
- McAuliffe K, Thornton A. The psychology of cooperation in animals: an ecological approach. Journal of Zoology. 2015;295(1):23–35. [Google Scholar]
- Mustoe AC, Cavanaugh J, Harnisch AM, Thompson BE, French JA. Do marmosets care to share? Oxytocin treatment reduces prosocial behavior toward strangers. Hormones and Behavior. 2015;71:83–90. doi: 10.1016/j.yhbeh.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave G, Camerer C, McCullough M. Does oxytocin increase trust in humans? A critical review of research. Perspectives on Psychological Science. 2015 doi: 10.1177/1745691615600138. [DOI] [PubMed] [Google Scholar]
- Neiworth JJ, Johnson ET, Whillock K, Greenberg J, Brown V. Is a sense of inequity an ancestral primate trait? Testing social inequity in cotton top tamarins (Saguinus oedipus) Journal of Comparative Psychology. 2009;123(1):10. doi: 10.1037/a0012662. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Slattery DA. Oxytocin in general anxiety and social fear: A translational approach. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.004. Retrieved from http://www.sciencedirect.com/science/article/pii/S0006322315004783. [DOI] [PubMed] [Google Scholar]
- Price SA, Brosnan SF. To each according to his need? Variability in the responses to inequity in non-human primates. Social Justice Research. 2012;25(2):140–169. [Google Scholar]
- Radke S, De Bruijn ER. The other side of the coin: oxytocin decreases the adherence to fairness norms. Frontiers in Human Neuroscience. 2012:6. doi: 10.3389/fnhum.2012.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Lu G, Moriyama H, Mustoe AC, Harrison E, French JA. Genetic Diversity in Oxytocin Ligands and Receptors in New World Monkeys. Plos One. 2015:e0125775. doi: 10.1371/journal.pone.0125775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Nakamura K. Oxytocin changes primate paternal tolerance to offspring in food transfer. Journal of Comparative Physiology A. 2011;197(4):329–337. doi: 10.1007/s00359-010-0617-2. [DOI] [PubMed] [Google Scholar]
- Schaffner CM, Shepherd RE, Santos CV, French JA. Development of heterosexual relationships in wied’s black tufted-ear marmosets (Callithrix kuhli) American Journal of Primatology. 1995;36(3):185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Sheskin M, Ashayeri K, Skerry A, Santos LR. Capuchin monkeys (Cebus apella) fail to show inequality aversion in a no-cost situation. Evolution and Human Behavior. 2014;35(2):80–88. [Google Scholar]
- Silberberg A, Crescimbene L, Addessi E, Anderson JR, Visalberghi E. Does inequity aversion depend on a frustration effect? A test with capuchin monkeys (Cebus apella) Animal Cognition. 2009;12(3):505–509. doi: 10.1007/s10071-009-0211-6. [DOI] [PubMed] [Google Scholar]
- Silk JB, Brosnan SF, Vonk J, Henrich J, Povinelli DJ, Richardson AS, Schapiro SJ. Chimpanzees are indifferent to the welfare of unrelated group members. Nature. 2005;437(7063):1357–1359. doi: 10.1038/nature04243. [DOI] [PubMed] [Google Scholar]
- Smith AS, \AAgmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Hormones and Behavior. 2010;57(2):255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JR. Donor payoffs and other-regarding preferences in cotton-top tamarins (Saguinus oedipus) Animal Cognition. 2010;13(4):663–670. doi: 10.1007/s10071-010-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, Hurlemann R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific Reports. 2013:3. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto A, Kuroshima H, Fujita K. Capuchin monkeys (Cebus apella) are sensitive to others’ reward: an experimental analysis of food-choice for conspecifics. Animal Cognition. 2010;13(2):249–261. doi: 10.1007/s10071-009-0262-8. [DOI] [PubMed] [Google Scholar]
- Talbot CF, Freeman HD, Williams LE, Brosnan SF. Squirrel monkeys’ response to inequitable outcomes indicates a behavioural convergence within the primates. Biology Letters. 2011:rsbl20110211. doi: 10.1098/rsbl.2011.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Kwetuenda S, Hare B. Preference or paradigm? Bonobos show no evidence of other-regard in the standard prosocial choice task. Behaviour. 2015;152(3–4):521–544. [Google Scholar]
- Taylor JH, French JA. Oxytocin and vasopressin enhance responsiveness to infant stimuli in adult marmosets. Hormones and Behavior. 2015;75:154–159. doi: 10.1016/j.yhbeh.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinklepaugh OL. An experimental study of representative factors in monkeys. Journal of Comparative Psychology. 1928;8(3):197. [Google Scholar]
- Trumble BC, Jaeggi AV, Gurven M. Evolving the neuroendocrine physiology of human and primate cooperation and collective action. Phil. Trans. R. Soc. B. 2015;370(1683):20150014. doi: 10.1098/rstb.2015.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Anders SM, Goodson JL, Kingsbury MA. Beyond “oxytocin= good”: neural complexities and the flipside of social bonds. Archives of Sexual Behavior. 2013;42(7):1115. doi: 10.1007/s10508-013-0134-9. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ. A sniff of trust: meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology. 2012;37(3):438–443. doi: 10.1016/j.psyneuen.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Vargas-Pinilla P, Paixão-Côrtes VR, Paré P, Tovo-Rodrigues L, Vieira CM, de AG, Xavier A others. Evolutionary pattern in the OXT-OXTR system in primates: Coevolution and positive selection footprints. Proceedings of the National Academy of Sciences. 2015;112(1):88–93. doi: 10.1073/pnas.1419399112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis M. Molecular evolution of the neurohypophysial hormone precursors in mammals: Comparative genomics reveals novel mammalian oxytocin and vasopressin analogues. General and Comparative Endocrinology. 2012;179(2):313–318. doi: 10.1016/j.ygcen.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Walum H, Waldman ID, Young LJ. Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wascher CA, Bugnyar T. Behavioral responses to inequity in reward distribution and working effort in crows and ravens. PloS One. 2013;8(2):e56885. doi: 10.1371/journal.pone.0056885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PD, Anderson PS, Ball RG, Bock MG, Carroll L, Chiu S-HL, Erb JM. 1-(((7, 7-Dimethyl-2 (S)-(2 (S)-amino-4-(methylsulfonyl) butyramido) bicyclo [2.2. 1] heptan-1 (S)-yl) methyl) sulfonyl)-4-(2-methylphenyl) piperazine (L-368,899): an orally bioavailable, non-peptide oxytocin antagonist with potential utility for managing preterm labor. Journal of Medicinal Chemistry. 1994;37(5):565–571. doi: 10.1021/jm00031a004. [DOI] [PubMed] [Google Scholar]
- Wittig RM, Crockford C, Deschner T, Langergraber KE, Ziegler TE, Zuberbühler K. Food sharing is linked to urinary oxytocin levels and bonding in related and unrelated wild chimpanzees. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1778):20133096. doi: 10.1098/rspb.2013.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nature Neuroscience. 2004;7(10):1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007;2(11):e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.