Summary
Background
Hemostatic benefits of platelet transfusions in thienopyridine-treated acute coronary syndrome (ACS) patients may be compromised by residual metabolite in circulation.
Objectives
To estimate the earliest time after a prasugrel loading-dose when added platelets are no longer inhibited by prasugrel’s active metabolite.
Methods
Baseline platelet reactivity of healthy subjects (n = 25, 30 ± 5 years, 68% male) on ASA 325 mg was tested using maximum platelet aggregation (MPA, ADP 20 µm) and VerifyNow® P2Y12 and was followed by a 60 mg prasugrel loading-dose. At 2, 6, 12 and 24 h post-dose, fresh concentrated platelets from untreated donors were added ex-vivo to subjects’ blood, raising platelet counts by 0% (control), 40%, 60% and 80%. To estimate the earliest time when prasugrel’s active metabolite’s inhibitory effect on the added platelets ceases, platelet function in supplemented samples was compared across time-points to identify the time when effect of supplementation on platelet function stabilized (i.e. the increase in platelet reactivity was statistically similar to that at the next time-point).
Results
Supplemented samples showed concentration-dependent increases in platelet reactivity vs. respective controls by both MPA and VerifyNow® at all assessment time-points. For each supplementation level, platelet reactivity showed a sharp increase from 2 to 6 h but was stable (P = NS) between 6 and 12 h.
Conclusions
The earliest measured time when supplemented platelets were not inhibited by circulating active metabolite of prasugrel was 6 h after a prasugrel loading-dose. These findings may have important implications for prasugrel-treated ACS patients requiring platelet transfusions during surgery.
Keywords: acute coronary syndrome, antiplatelet, prasugrel, thienopyridine, transfusion
Introduction
Antiplatelet drugs are the mainstay therapy for the treatment of patients with acute coronary syndromes (ACS). The proven benefits in this population have led the American College of Cardiology/American Heart Association (ACC/AHA) to issue guidelines recommending dual antiplatelet therapy for ACS patients undergoing percutaneous coronary intervention (PCI) [1,2]. The current standard for dual antiplatelet therapy is the combination of aspirin and clopidogrel. Recent studies have demonstrated the benefits of earlier and more potent platelet inhibition for ACS patients undergoing PCI [3–6]. Following the TRITON study, prasugrel, a faster acting and more potent thienopyridine agent, has been incorporated into the ACC/AHA guidelines [1,2,7].
The interaction between prasugrel and P2Y12 adenosine diphosphate (ADP) receptors on the platelet is irreversible, rendering inhibited receptors unresponsive to ADP for the duration of the platelet’s lifespan [8].The irreversible nature of the platelet antagonism can be a double-edged sword: an advantage during chronic treatment because a missed dose may not have significant negative consequences, but a disadvantage for the ACS patients that require surgical intervention because they may be at increased risk for bleeding complications. Re-operation to control hemorrhage is almost six times more likely if patients receive dual antiplatelet therapy prior to coronary artery bypass grafting (CABG) and 20% of such patients require platelet transfusions [9]. The recommended intervention to minimize surgical bleeding risk is treatment cessation, allowing the physiological production of new platelets to replace the existing, inhibited platelet pool. The complete replacement of the platelet population typically takes 7–10 days to achieve [10]. Thus, for ACS patients requiring surgery after being treated with aspirin and prasugrel, the current ACC/AHA guidelines recommend delaying surgery for 7 days after discontinuation of prasugrel to allow for the dissipation of its antiplatelet effects [1,2]. In the absence of antiplatelet treatment, these patients may be vulnerable to cardiovascular events during this waiting period. It is therefore necessary in some cases to infuse platelets in order to accelerate the recovery of hemostatic potential following cessation of thienopyridine therapy. However, the timing of platelet infusion relative to the last dose of prasugrel is an important consideration. The plasma concentration of prasugrel’s active metabolite peaks approximately 30 min after a 60-mg loading-dose, with a mean Cmax of 475 ng mL−1 and a half-life of 7.4 h [11]. Transfusion of platelets when the active metabolite of prasugrel is still in circulation would inhibit the newly infused platelets and compromise their functionality.
The objective of the current study was to estimate the earliest time following a 60-mg prasugrel loading-dose when freshly added platelets were no longer inhibited by the presence of prasugrel’s active metabolite in circulation. A secondary goal was to investigate the degree to which platelet function of a prasugrel-treated subject could be normalized by adding fresh platelets, within 24 h of dosing.
Methods
Study population and design
This prospective study was carried out in healthy volunteers using an open-label, single-dose, adaptive design, which included two consecutive periods, A and B. The interval between parts A and B allowed for an interim analysis to review the adequacy of the selected assessment time-points for achieving the study objectives.
Healthy, non-smoking volunteers (‘subjects’, n = 25) aged 18–65 years underwent screening that included a medical history, physical examination, routine hematology and clinical chemistry analysis and 12-lead electrocardiogram. Individuals with a body weight outside the range of 60–120 kg, clinically significant abnormal test results or those taking medications were excluded. A separate group of healthy volunteers (‘donors’, n = 32) provided fresh platelets after undergoing the same screening process.
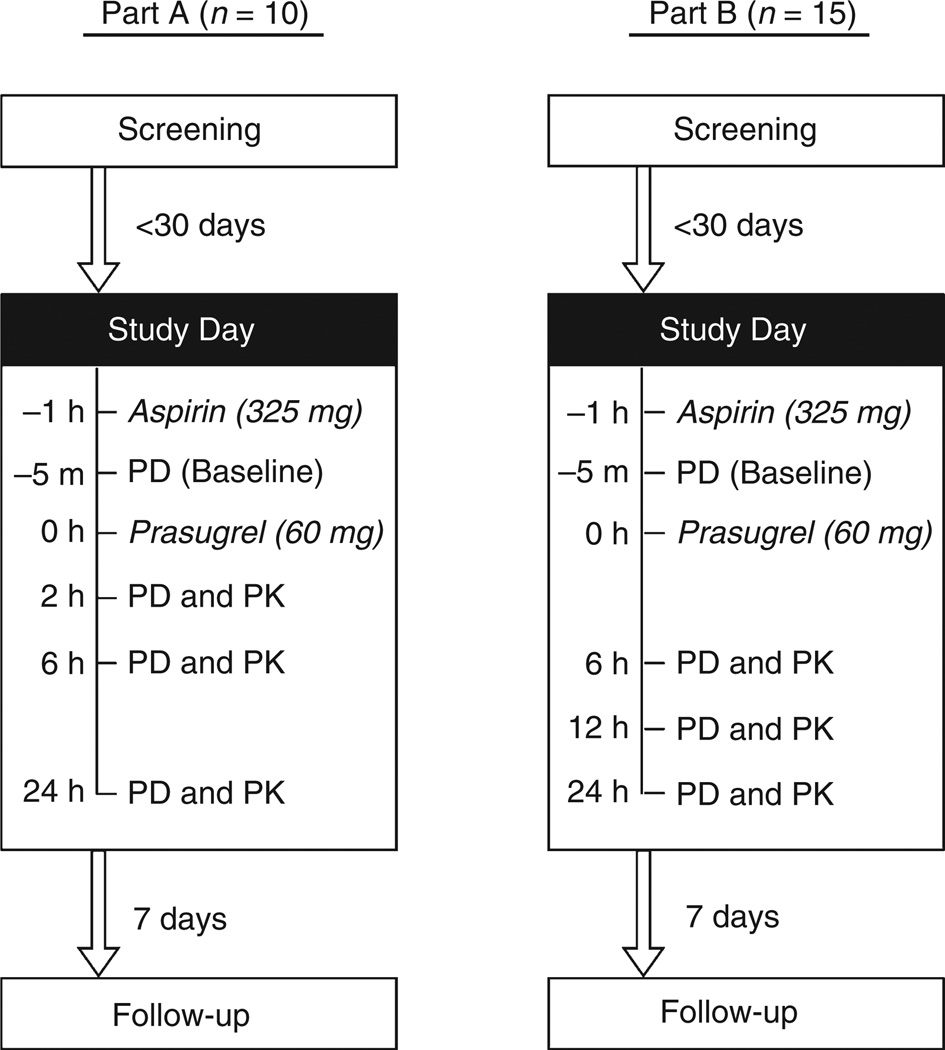
On the morning of the study day, subjects reported to the Mount Sinai Medical Center after an overnight fast. They received 325 mg of aspirin (one tablet taken with 150 mL of water) followed 1 h later by blood sampling for baseline platelet reactivity testing, using light transmission aggregometry (LTA) and VerifyNow® P2Y12 assay. A 60-mg loading-dose of prasugrel (six 10-mg tablets taken with 150 mL of water) was administered immediately after baseline blood collection, followed by two additional hours of fasting. Over the next 24 h, subjects’ blood samples were drawn at three time-points and donor-platelets added to them ex vivo in volumes calculated to raise the platelet counts by 40%, 60% and 80%. Platelet reactivity in the three supplemented and one non-supplemented control (0%) samples was then reassessed. The assessment time-points in Part A of the study were 2, 6 and 24 h after prasugrel dosing. Based on the preplanned interim analysis of Part A data, the 2 h time-point was assessed to be too close to dosing and substituted with 12 h in Part B (Fig. 1).
Fig. 1.
The study was conducted in two consecutive parts (A and B) that differed only in their post-treatment assessment time-points. PD, pharmacodynamic assessments (i.e. light transmission aggregometry and VerifyNow® P2Y12 assay); PK, pharmacokinetic assessment.
The primary objective was investigated by comparing platelet reactivity at each supplementation level (40%, 60% and 80%) across time-points (2 vs. 6 vs. 12 vs. 24 h) and identifying the time-point where platelet functional recovery stabilized (i.e. results were statistically similar to those from the next time-point). For the secondary objective of assessing the degree of platelet function restoration after prasugrel dosing, baseline (pretreatment) platelet reactivity was the reference comparator.
The study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of Mount Sinai Medical Center. A written informed consent was obtained from each subject and donor before initiating any study-related procedures.
Blood sampling
Subjects
Venous blood samples for pharmacodynamic studies were collected in 3.2% sodium citrate tubes at baseline, and at 2, 6 and 24 h post-dose in Part A and 6, 12 and 24 h post-dose in Part B (Fig. 1).
Pharmacokinetic (PK) blood samples for the measurement of prasugrel’s active metabolite were collected in pre-chilled EDTA tubes at the same time-points as the pharmacodynamic samples, except at baseline when metabolite concentrations were not measured. Within 30 s of collection, 25 µL of 500 mm 3′-methoxyphenacyl bromide in acetonitrile was added to the PK samples to derivatize the active metabolite. Following immediate centrifugation at 2000 g × 15 min at 2–8 °C, plasma from the PK samples was stored at −70 °C until shipment on dry ice to a central laboratory for analysis. Concentrations of prasugrel’s active metabolite in plasma were determined using validated liquid chromatography methods and tandem mass spectrometric detection, as previously described [12].
Donors (treatment-naïve donor-platelets)
Venous blood samples for fresh donor-platelets were collected from non-treated, healthy volunteers (three per subject) into acid citrate dextrose (ACD) and 3.2% sodium citrate tubes. The ACD samples were used for the isolation of platelets and the citrated samples provided the platelet-poor plasma (PPP) for their resuspension.
Citrated blood samples were spun once (2000 g × 10 min at room temperature) and the PPP from the three donors pooled. The ACD samples underwent a two-step centrifugation. The first spin (175 g for 10 min at room temperature) produced platelet-rich plasma (PRP). To avoid unintended platelet activation during the second, faster centrifugation, 10 µL mL−1 of prostaglandin E1 (Cayman Chemical, Ann Arbor, MI, USA) was added to the ACD PRP to a final concentration of 10 nm. The second centrifugation (1000 g for 15 min) yielded a platelet pellet and supernatant PPP. The ACD PPP was aspirated and the platelet pellet resuspended in one-tenth original volume of citrated PPP, yielding concentrated donor-platelets with cell counts of approximately 2500 × 103 µL−1. Reactivity of the donor-platelets was measured using LTA after adjusting the cell count in tested samples to approximately 250 × 103 µL−1 with citrated PPP.
Supplementation of subjects-blood with donor-platelets
As the active metabolite of prasugrel in collected blood is stable for only a very brief period, donor-platelets were added to the treated subjects’ blood within 30 s of its collection. The volumes of donor-platelet suspension needed to raise the platelet counts in subjects’ blood samples by 40%, 60% and 80% were calculated using cell counts in the blood samples and the donor-platelet suspension. At each time-point, one sample (0%) was kept non-supplemented and served as a reference control. The concentration of donor-platelets was maintained at levels that allowed limiting the volume of added solution to < 10% of the blood, thereby minimizing sample dilution. After gentle mixing the samples were kept at room temperature for 30 min before measuring platelet counts and platelet reactivity.
Platelet reactivity
Platelet aggregation was assessed using two different methodologies.
Light transmission aggregometry (LTA): platelet aggregation studies were carried out in PRP using conventional LTA (Chrono-log aggregometer model 570VS, Chrono-log Corporation, Havertown, PA, USA). Briefly, supplemented whole blood, prepared as described above, was allowed to stand for 30 min after supplementation and then centrifuged to obtain PRP (175 g × 10 min) and PPP (2000 g × 10 min). After 3 min of incubation at 37 °C, aggregation was induced in the PRP samples using 20 µm ADP. Peak per cent aggregation over the next 6 min was recorded as maximum platelet aggregation (MPA).
VerifyNow® P2Y12: tests were performed on supplemented whole blood in Greiner vacuette tubes according to the manufacturer’s instructions (Accumetrics, San Diego, CA, USA). As with the LTA, supplemented blood samples were allowed to stand at room temperature for 30 min prior to testing. All tests were carried out using P2Y12 cartridges and the device reported data recorded as P2Y12 Reaction Units (PRU), ‘baseline’ platelet aggregation (BASE) and % inhibition.
Statistical analysis
The primary pharmacodynamic parameter was MPA to 20 µm ADP. The pharmacodynamic parameters were listed and summarized using standard descriptive statistics (mean, 90% confidence intervals) separately by each time-point and each quantity of freshly added platelets for the combined (Part A and B) study.
After testing for normality of distribution, the analyses were carried out using the paired t-test or Wilcoxon signed-ranks test as appropriate, with Bonferroni correction for multiple comparisons. The statistical significance was set at the nominal P < 0.05 level. All analyses were performed using stata 12 software (StataCorp, College Station, TX, USA).
Results
Twenty-five healthy subjects (68% male) received aspirin and prasugrel in the study (10 in Part A and 15 in Part B) with an average age of 30 ± 5 years and average BMI of 24.6 ± 2.4 kg m−2. Thirty-two healthy donors (35 ± 10 years old, 31% male) provided blood samples for the preparation of donor-platelets. No serious adverse events occurred during this study. There were five mild adverse events in total, of which only one, mild contusion in Part A, was judged to be drug related by the study physician.
Data from the common time-points of Part A and Part B (i.e. 6 and 24 h) were virtually identical, confirming the reproducibility of the methodology employed and that analyses of the combined dataset were unlikely to be confounded by study period. All analyses presented were performed on the combined database.
Platelet counts
Table 1 shows mean platelet counts in whole blood before and after the addition of donor-platelets. In the control blood samples, mean platelet counts were steady at each time-point during the 24-h period following prasugrel dosing. In the supplemented samples, mean platelet counts increased consistently and by the approximate intended percentages.
Table 1.
Whole blood platelet counts (× 103 µL−1)
| Platelet supplementation | Baseline (n = 25) | 2 h (n = 10) | 6 h (n = 25) | 12 h (n = 15) | 24 h (n = 25) |
|---|---|---|---|---|---|
| 0% | 221.2 (34.4) | 222.7 (42.3) | 222.7 (34.2) | 221.1 (32.3) | 221.8 (36.2) |
| 40% | – | 300.1 (42.6) | 310.5 (47.3) | 310.9 (47.5) | 307.6 (46.0) |
| 34.8% | 39.4% | 40.6% | 38.7% | ||
| 60% | – | 337.6 (53.3) | 351.3 (54.2) | 350.3 (50.4) | 350.7 (50.0) |
| 51.6% | 57.7% | 58.4% | 58.1% | ||
| 80% | – | 394.7 (67.4) | 400.6 (56.4) | 393.9 (56.2) | 392.8 (56.2) |
| 77.2% | 79.9% | 78.2% | 77.1% |
Data presented as mean (standard deviation). Italics indicate increase from corresponding 0% (non-supplemented) values.
Platelet reactivity
Donor-platelets
Aggregation of concentrated donor-platelets in response to ADP was lower than that of normal, healthy platelets under standard test conditions. This finding is consistent with the diminished reactivity reported in platelet concentrates prepared in blood-banks for clinical use. The mean MPA of donor-platelets in our study at 2, 6, 12 and 24 h was between 47.9% and 55.1%, comparable to the reactivity of 2–3-day-old blood bank platelet concentrates.
Control (0%) samples
At baseline (1 h after aspirin dosing but before prasugrel administration) the mean platelet reactivities were 65% MPA and 292.1 PRU. By 2 h after prasugrel dose, these values had dropped to 7.0% and 4.0, respectively (Tables 2 and 3). Over the next 24 h a time-dependent increase in platelet function was observed in the unsupplemented control samples, which was significant between 2–24 h and 6–24 h (P < 0.01 for both MPA and PRU comparisons). This natural increase in function is likely to represent the physiologic release of new platelets into the circulation. Between 2 and 24 h, the MPA went from 7.0% to 14.7%, an increase of about 8% points or approximately 12% of the baseline MPA (65%). This is close to the percentage of new platelets expected to be released into circulation every day in a healthy human subject. A similar pattern of platelet function recovery was observed with the VerifyNow® assay, but with smaller changes. This could partly be due to lower sensitivity of VerifyNow® at extreme values.
Table 2.
Maximum platelet aggregation measured by light transmission aggregometry
| Platelet supplementation | Time-point (h) | Mean MPA (%) | 90% CI | P-value | |
|---|---|---|---|---|---|
| Pretreatment baseline | 0 | 65.0 | 61.1, 68.9 | ||
| Post-treatment | |||||
| 0% | 2 | 7.0* | 3.4, 10.6 | } | NS |
| 6 | 10.8* | 9.2, 12.5 | } | NS | |
| 12 | 11.6* | 9.7, 13.5 | } | NS | |
| 24 | 14.7* | 12.6, 16.8 | |||
| 40% | 2 | 11.4* | 7.9, 15.0 | } | 0.010 |
| 6 | 18.9† | 16.7, 21.0 | |||
| 12 | 19.5† | 17.0, 22.1 | } | NS | |
| 24 | 24.4† | 21.8, 27.0 | } | 0.010 | |
| 60% | 2 | 14.6† | 10.6, 18.5 | } | 0.008 |
| 6 | 23.6† | 21.4, 25.9 | |||
| 12 | 25.1† | 22.0, 28.2 | } | NS | |
| 24 | 31.6† | 28.5, 34.7 | } | 0.004 | |
| 80% | 2 | 19.9† | 15.6, 24.2 | } | NS |
| 6 | 28.1† | 26.0, 30.2 | |||
| 12 | 28.5† | 25.5, 31.5 | } | NS | |
| 24 | 36.2† | 33.2, 39.3 | } | 0.004 |
P < 0.05 vs. baseline.
P < 0.05 vs. baseline and corresponding 0% (non-supplemented) sample.
Table 3.
P2Y12 reaction units (PRU) as measured by VerifyNow® P2Y12 assay
| Platelet supplementation | Time-point (h) | Mean PRU | 90% CI | P-value | |
|---|---|---|---|---|---|
| Pretreatment baseline | 0 | 292.1 | 274.4, 309.9 | ||
| Post-treatment | |||||
| 0% | 2 | 4.0* | 2.0, 6.0 | } | NS |
| 6 | 6.6* | 3.6, 9.6 | } | 0.007 | |
| 12 | 9.0* | 3.2, 14.8 | } | NS | |
| 24 | 11.8* | 7.9, 15.7 | |||
| 40% | 2 | 52.3* | 28.0, 76.6 | } | NS |
| 6 | 60.0† | 48.0, 71.9 | |||
| 12 | 79.5† | 60.8, 98.3 | } | NS | |
| 24 | 98.2† | 85.3, 111.1 | } | 0.009 | |
| 60% | 2 | 78.5† | 52.9, 104.1 | ||
| 6 | 98.8† | 86.5, 111.1 | } | NS | |
| 12 | 112.1† | 92.9, 131.4 | } | NS | |
| 24 | 138.2† | 123.6, 152.7 | } | 0.001 | |
| 80% | 2 | 96.8† | 66.9, 126.7 | ||
| 6 | 127.9† | 115.2, 140.5 | } | NS | |
| 12 | 137.4† | 118.1, 156.7 | } | Ns | |
| 24 | 163.0† | 150.0, 176.0 | } | 0.005 |
P < 0.05 vs. baseline.
P < 0.05 vs. baseline and corresponding 0% (non-supplemented) sample.
Cessation time of prasugrel’s active metabolite’s inhibitory effect
The addition of 40%, 60% and 80% donor-platelets to blood samples from aspirin and prasugrel-treated subjects resulted in concentration-dependent increases in platelet aggregation. These supplementation effects became greater with passing time (Table 2).
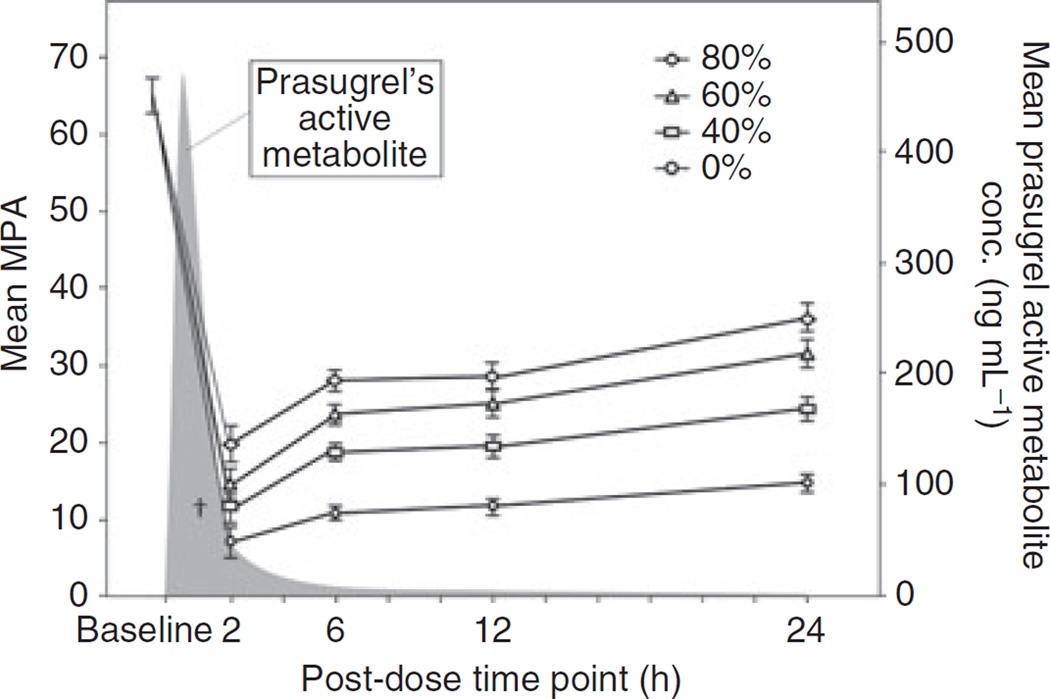
The increases in MPA after supplementation were significantly greater at 6 h compared with the respective 2 h increases (Fig. 2, P < 0.01 for 40% and 60%). The relatively lower MPA increases at 2 h suggest that the plasma concentration of prasugrel’s active metabolite at 2 h post-dose was sufficient to exert inhibitory effects on the freshly added platelets. Between 6 and 12 h, the increases in MPA following platelet supplementation did not vary much (P = NS for all), suggesting prasugrel’s active metabolite concentrations were too low by 6 h to inhibit the donor-platelets. Results from the 24 h time-point again showed significant improvements in MPA vs. the corresponding 12 h results, probably due to the combined effect of added donor-platelets and new platelets released by the megakaryocytes.
Fig. 2.
Maximum platelet aggregation (MPA, mean ± SE) to 20 µm ADP using light transmission aggregometry (left Y-axis) and prasugrel active metabolite concentrations (right Y-axis) over 24 h following a prasugrel loading-dose. Platelet aggregation was measured in aspirin and prasugrel-treated subjects’ blood with (40%, 60% and 80%) and without (0%) the addition of fresh platelets. Aggregation values in all supplemented samples are higher than respective controls (0%, P ≤ 0.01) except †, but lower than baseline (P < 0.01). Aggregation values in supplemented samples at 6 h are higher than respective values at 2 h (P < 0.05 for 40% and 60%), but similar to those at 12 h (P = NS for all). Prasugrel active metabolite was not measured before the 2 h time-point; results are taken from an earlier study [11].
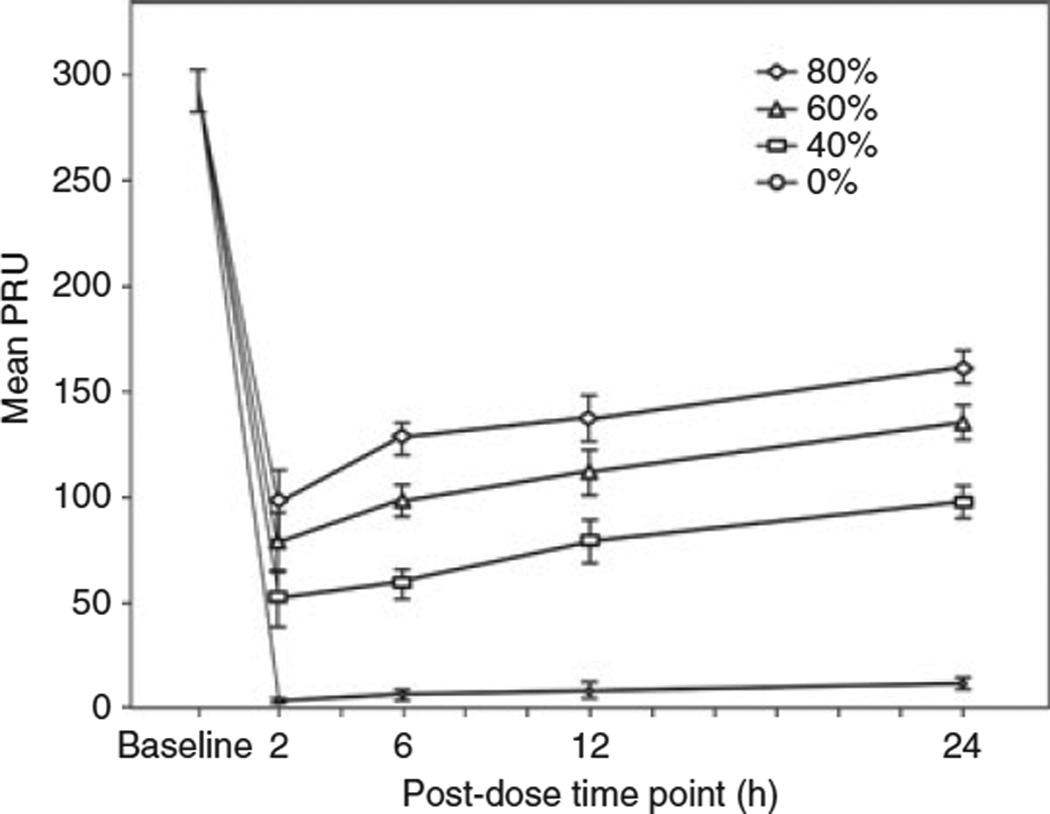
Data from the VerifyNow® P2Y12 assay showed a similar pattern to the LTA results, a concentration-dependent recovery in platelet reactivity that increased with time within each supplementation level (Fig. 3). Unlike MPA, the PRU results of supplemented samples did not differ significantly between 2 and 6 h (Table 3).
Fig. 3.
Platelet reactivity measured by VerifyNow® P2Y12 assay (PRU) over a 24-h period following a prasugrel loading dose. Platelet reactivity is shown in aspirin and prasugrel-treated subjects’ samples with (40%, 60% and 80%) and without (0%) the addition of fresh platelets. PRU values in supplemented samples are higher than respective controls (P < 0.01), but lower than baseline (P < 0.01). Within each supplementation level, the results are statistically similar between 2 and 6, and 6 and 12 h time-points, but significantly higher at 24 h (P<0.05 vs. 2, 6 and 12 h).
Degree of platelet function normalization
The addition of fresh donor-platelets to blood samples from aspirin and prasugrel-treated subjects significantly increased platelet reactivity in the recipient blood. This concentration-dependent effect was observed as early as 2 h post-dose (Tables 2 and 3). Aggregation in each supplemented sample was significantly higher than in its corresponding control, with both LTA and VerifyNow® measurements (P < 0.05 for all except 40% level at 2 h). The greatest effect was observed at 24 h with 80% supplementation, reaching 56.4% (± 13.0) of baseline MPA and 56.2% (± 11.1) of baseline PRU. However, even with such large increases, platelet reactivity in the supplemented samples remained significantly lower than the subject’s pretreatment baseline values (P < 0.01 for both). This was to be expected because the reactivity of donor platelets was lower than the subjects’ baseline to begin with.
Plasma prasugrel active metabolite concentrations
All 25 subjects were included in the pharmacokinetic analyses. Mean (± SD) plasma concentrations of prasugrel’s active metabolite after 2, 6, 12 and 24 h of prasugrel dosing were 42.4 (± 11.0), 4.5 (± 1.0), 2.1 (± 0.5) and 0.7 (± 0.2) ng mL−1, respectively (Fig. 2).
Discussion
Acute coronary syndrome patients requiring surgical intervention after PCI often receive platelet infusions to counter the effects of antiplatelet therapy and reduce the risk of bleeding. However, residual concentrations of the antiplatelet drugs or their active metabolites still in circulation could blunt the desired benefits of these infusions by exerting their inhibitory effects on the freshly added platelets. The earliest time-point following a prasugrel loading-dose at which infused platelets are not compromised by the active metabolite of prasugrel has not been shown.
In the present study we observed that the addition of fresh platelets to blood samples of healthy subjects treated with 325 mg aspirin and 60 mg prasugrel produced concentration-dependent increases in platelet reactivity as early as 2 h after the loading dose of prasugrel. These improvements in platelet function were significantly muted compared with the recovery seen after 6 and 12 h of dosing. This indicates that the circulating levels of prasugrel’s active metabolite at 2 h (42.4 ± 11 ng mL−1) are high enough to significantly inhibit the freshly added platelets. From 6 to 12 h, the improvements in platelet reactivity achieved by the addition of fresh platelets did not change markedly, no matter the supplementation level. Stabilization of the effect of platelet supplementation by 6 h indicates that prasugrel’s active metabolite concentrations recorded at this time (4.5 ± 1 ng mL−1) are no longer at levels where they could significantly inhibit the newly added platelets. These findings are significant for patients on prasugrel therapy who need urgent surgery. Our results suggest that in order to maximize the benefits of platelet transfusions in prasugrel-treated patients, the infusions should be administered at least 6 h after a loading dose. Platelets infused after 6 h would exhibit maximum reactivity, and therefore maximum effectiveness in reducing the risk of bleeding. Before 6 h, the benefit of fresh platelets may be significantly diminished by residual prasugrel active metabolite.
The secondary objective of our study was to investigate the degree to which platelet function could be restored by adding fresh platelets, within 24 h of a 325-mg aspirin and 60-mg prasugrel dose. We found that the maximum recovery achieved was with 80% platelet supplementation at the 24 h post-dose time-point, when platelet reactivity was restored to 56% of baseline. In a similar study, Li et al. [13] reported that90%fresh platelets were needed to restore ADP aggregation after a week of clopidogrel (75 mg) and aspirin (81 mg) dosing. Certain design and methodological differences make a direct comparison between the two studies difficult. Besides using maintenance dosing and a lower concentration of ADP to induce aggregation, that study used platelet-richplasma instead of concentrated platelets. The timing of the platelet supplementation and testing in relation to the last dose is also different.
In clinical practice, transfusion of one unit of platelet concentrate will typically increase the platelet count by approximately 5–10 × 103 µL−1, which equates to an increase in cell count of 30% with one unit of apheresis platelets in a patient with 200 × 103 µL−1 platelets [14]. However, even with all the pieces of information, including the findings from our study, it remains difficult to predict how many units of platelets would be required to obviate a patient’s risk of bleeding. This limitation is due to the lack of a known platelet function threshold that can be clearly linked to the risk of bleeding. It seems logical that the relationship between the risk of bleeding and platelet reactivity is a continuous one, rather than one governed by a cut-off. It may therefore not be necessary to restore platelet function completely back to normal in order to provide hemostatic support.
Study limitations
The objectives of our study were investigated using ex vivo supplementation of donor-platelets to treated blood from healthy volunteers. A more ideal study design for this purpose would involve subjects receiving infusions of fresh platelets followed by in vitro assessment of platelet function in their blood samples. However, administering platelet infusions to healthy volunteers could have serious health consequences and would not be ethical. Also, the relatively large intervals between the time-points may have prevented a more precise estimation of the earliest time when prasugrel active metabolite ceases to affect freshly added platelets.
Conclusions
Platelet supplementation up to 2 h after prasugrel loading-dose is partially inhibited by circulating active metabolite of prasugrel. In patients needing surgery after receiving prasugrel, the best option for minimizing the risk of perioperative bleeding would be to wait for the effects of antiplatelet medications to resolve. In cases where urgent surgery is needed, administration of platelet concentrates may be most effective 6 h after the loading-dose of prasugrel, although partial reversal of prasugrel effects could be obtained earlier.
Acknowledgments
The authors thank S. Palencia, NP (MSSM) for her support in this project and Wei W. Z. Zhang (Eli Lilly) for statistical analysis. This work was supported by funding from Daiichi Sankyo Company, Ltd and Eli Lilly and Company.
Footnotes
Addendum
M. U. Zafar: study design and execution, data analysis and manuscript preparation. C. Santos-Gallego, D. A. Vorchheimer, J. F. Viles-Gonzalez, S. Elmariah and C. Giannarelli: study execution and manuscript preparation. S. Sartori: statistical analysis. D. S. Small and J. A. Jakubowski: study concept, design, analysis and manuscript preparation. V. Fuster: study concept and manuscript preparation. J. J. Badimon: study concept and design, and manuscript preparation.
References
- 1.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, et al. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 2.Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Antman EM, Califf RM, Chavey WE, 2nd, Hochman JS, Levin TN. 2011 ACCF/AHA focused update of the Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;57:1920–1959. doi: 10.1016/j.jacc.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Koul S, Smith JG, Schersten F, James S, Lagerqvist B, Erlinge D. Effect of upstream clopidogrel treatment in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J. 2011;32:2989–2997. doi: 10.1093/eurheartj/ehr202. [DOI] [PubMed] [Google Scholar]
- 4.Cuisset T, Frere C, Quilici J, Morange PE, Nait-Saidi L, Carvajal J, Lehmann A, Lambert M, Bonnet JL, Alessi MC. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol. 2006;48:1339–1345. doi: 10.1016/j.jacc.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 5.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 6.Wiviott SD, Trenk D, Frelinger AL, O’Donoghue M, Neumann FJ, Michelson AD, Angiolillo DJ, Hod H, Montalescot G, Miller DL, Jakubowski JA, Cairns R, Murphy SA, McCabe CH, Antman EM, Braunwald E. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation. 2007;116:2923–2932. doi: 10.1161/CIRCULATIONAHA.107.740324. [DOI] [PubMed] [Google Scholar]
- 7.Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, Antman EM TRITON-TIMI 38 investigators. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373:723–731. doi: 10.1016/S0140-6736(09)60441-4. [DOI] [PubMed] [Google Scholar]
- 8.Asai F, Jakubowski JA, Naganuma H, Brandt JT, Matsushima N, Hirota T, Freestone S, Winters KJ. Platelet inhibitory activity and pharmacokinetics of prasugrel (CS-747) a novel thienopyridine P2Y12 inhibitor: a single ascending dose study in healthy humans. Platelets. 2006;17:209–217. doi: 10.1080/09537100600565551. [DOI] [PubMed] [Google Scholar]
- 9.Yende S, Wunderink RG. Effect of clopidogrel on bleeding after coronary artery bypass surgery. Crit Care Med. 2001;29:2271–2275. doi: 10.1097/00003246-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Price MJ, Walder JS, Baker BA, Heiselman DE, Jakubowski JA, Logan DK, Winters KJ, Li W, Angiolillo DJ. Recovery of platelet function after discontinuation of prasugrel or clopidogrel maintenance dosing in aspirin-treated patients with stable coronary disease. J Am Coll Cardiol. 2012;59:2338–2343. doi: 10.1016/j.jacc.2012.02.042. [DOI] [PubMed] [Google Scholar]
- 11.Small DS, Li YG, Ernest CS, 2nd, April JH, Farid NA, Payne CD, Winters KJ, Rohatagi S, Ni L. Integrated analysis of pharmacokinetic data across multiple clinical pharmacology studies of prasugrel, a new thienopyridine antiplatelet agent. J Clin Pharmacol. 2011;51:321–332. doi: 10.1177/0091270010367429. [DOI] [PubMed] [Google Scholar]
- 12.Farid NA, McIntosh M, Garofolo F, Wong E, Shwajch A, Kennedy M, Young M, Sarkar P, Kawabata K, Takahashi M, Pang H. Determination of the active and inactive metabolites of prasugrel in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:169–179. doi: 10.1002/rcm.2813. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Hirsh J, Xie C, Johnston MA, Eikelboom JW. Reversal of the anti-platelet effects of aspirin and clopidogrel. J Thromb Haemost. 2012;10:521–528. doi: 10.1111/j.1538-7836.2012.04641.x. [DOI] [PubMed] [Google Scholar]
- 14.Practice parameter for the use of fresh-frozen plasma, cryoprecipitate, and platelets. Fresh-Frozen Plasma, Cryoprecipitate, and Platelets Administration Practice Guidelines Development Task Force of the College of American Pathologists. JAMA. 1994;271:777–781. [PubMed] [Google Scholar]