Abstract
Maldescent of the epididymo-testicular unit can occur as an isolated event or as a component of various syndromes. When part of a syndrome, crypto-epididymis is usually accompanied by other genital and/or extragenital features. Epididymis development is primarily regulated by androgens, and successful epididymo-testicular unit development and descent requires an intact hypothalamic-pituitary-gonadal axis. The developing gonadotropin-releasing hormone system is essential for epididymo-testicular descent and is highly sensitive to reduced fibroblast growth factor (FGF) signaling. Our understanding of the impact of FGFR1 in the process of epididymo-testicular descent has recently improved. At later stages of embryonic development, the undifferentiated epididymal mesenchyme is a specific domain for FGFR1 expression. The majority of individuals with syndromic crypto-epididymis, as well as individuals with isolated maldescent of the epididymo-testicular unit, exhibit some disturbance of FGF, FGFR1 and/or genes involved in hypothalamic-pituitary-gonadal axis regulation. However, the mechanisms underlying FGF dysregulation may differ between various syndromes.
Key Words: Androgens, Epididymo-testicular descent, FGF8, FGFR1, GnRH, Gubernaculum epididymis
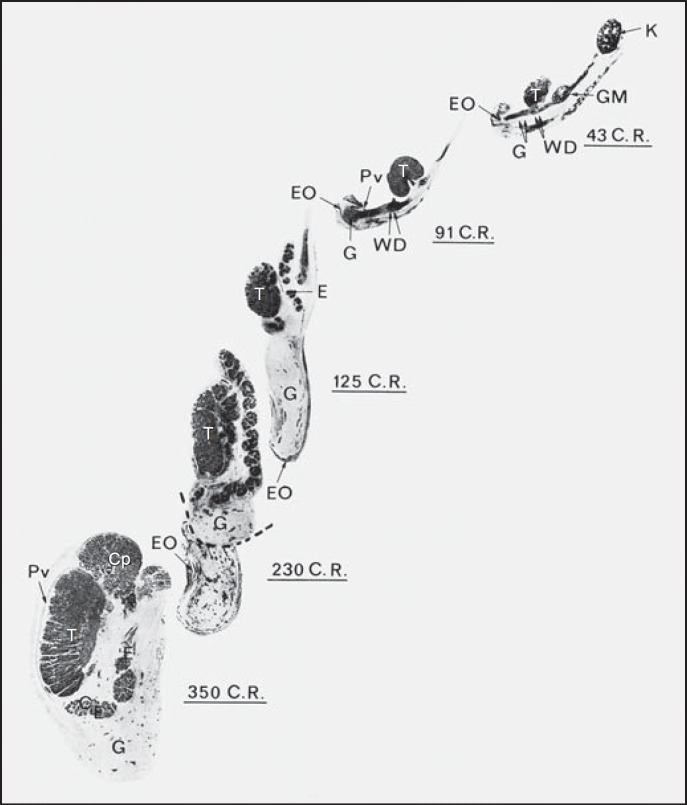
The process of epididymo-testicular descent occurs in many mammalian species [Bedford, 1978] and is governed by a variety of genetic, hormonal, anatomical, and environmental factors. Carried by the epididymis, each testis descends from the dorsal abdominal wall into the scrotum [Hadziselimovic, 1984]. A structure called the gubernaculum governs this descent of the epididymo-testicular unit [Hunter, 1762; Klaatsch, 1890]. The gubernaculum guides the epididymo-testicular unit such that it inserts proximally into the Wolffian duct and cauda epididymis (fig. 1) [Hadziselimovic and Herzog, 1993].
Fig. 1.
Sagittal sections of epididymo-testicular descent in humans of 43-350 mm crown-rump length [Hadziselimovic, 1983]. The gubernaculum (G) inserts proximally into the Wolffian duct (WD, double arrows) and cauda epididymis (CE). The epididymis (E) precedes the testis (T) throughout the entire process of descent into the scrotum. The processus vaginalis (Pv), external oblique fascia (EO), kidney (K), mesonephros (GM), head of the epididymis (Cp), and tail of the epididymis (CE) are indicated. The figure is reproduced from Hadziselimovic [1983] with permission.
Although the gubernaculum is considered the key structure required for descent of the epididymo-testicular unit [Hutson et al., 2015], complete experimental transection of the gubernaculum does not prevent epididymo-testicular descent [Bergh et al., 1978]. Rather than gubernaculum elongation, it is actually the tail of the developing epididymis that enlarges and carries the testis towards the developing scrotum during the process of inguinal-scrotal descent (fig. 1). The epididymis precedes the testis throughout the entire descent process. Disturbances in epididymis development can result in a cryptorchid position of the unit [Rachmani et al., 2012; Hadziselimovic, 2015]. Maldescent can occur as an isolated event, or as part of a variety of syndromes. Crypto-epididymis as a syndrome component is usually accompanied by other genital and/or extragenital features that most often result from single-gene abnormalities.
Gonadotropin-releasing hormone (GnRH) neurons are essential for epididymo-testicular descent and onset as well as reproduction maintenance. Proper specification of GnRH cell fate, both prenatally and postnatally, relies on fibroblast growth factors (FGFs) [Rochester et al., 2012], and the developing GnRH system is highly sensitive to reduced levels of FGF signaling [Chung et al., 2008]. FGFs comprise a family of homologous polypeptide ligands that bind to their cognate fibroblast growth factor receptors (FGFRs). Various FGFs are classified as intracrine, paracrine or endocrine factors [Itoh et al., 2015]. Paracrine and endocrine FGFs are secreted signaling molecules that act via cell-surface FGFRs [Itoh et al., 2015]. FGF-mediated signaling is involved in mitogenesis, proliferation, differentiation, cellular migration, angiogenesis, and tissue injury repair.
Humans possess 22 FGF ligand genes comprising 6 subfamilies that differ in phylogeny and sequence homology. The ligand specificities of FGFR1, FGFR2 and FGFR3 are partially determined by alternative splicing within the C-terminal half of the third immunoglobulin loop of the extracellular FGF-binding domain [Kelleher et al., 2013]. Such alternative splicing creates a IIIb isoform (containing exon 8) that is preferentially expressed in epithelial cells and a IIIc isoform (containing exon 9) that is preferentially expressed in mesenchymal cells. The IIIb isoform preferentially binds FGF ligands that are secreted from adjacent mesenchyme, while the IIIc isoform usually binds ligands secreted from the adjacent epithelium [Kelleher et al., 2013]. The configuration of this paracrine arrangement has the benefit of obviating inadvertent autocrine stimulation [Kelleher et al., 2013].
Role of Gonadotropin-Releasing Hormone and FGFs
FGFR1 hypomorphy severely reduces the total number of GnRH neurons, thus inducing congenital hypogonadotropic hypogonadism (CHH), either alone or in association with other hypothalamic-pituitary deficiencies [Chung et al., 2008]. The largest study of CHH diagnosed during childhood found genetic causes for ∼30% of CHH cases, and reported that 39% of the cases were associated with malformations and syndromes, most frequently Kallmann syndrome and CHARGE syndrome [Vizeneux et al., 2013]. Both Kallmann syndrome and crypto-epididymis can reportedly be caused by FGFR1 mutation [Pitteloud et al., 2006].
Supporting the importance of GnRH in this developmental process, severe hypoplastic crypto-epididymis has been observed in transgenic mice with migratory arrest of GnRH neurons [Radovick et al., 1991], in hypogonadal mice lacking GnRH [Charlton et al., 1983], and in mice with loss-of-function mutations in the GnRH receptor gene [Pask et al., 2005]. Hypogonadal male mice lacking GnRH are cryptorchid but have a normal gubernaculum, and gonadotropin treatment leads to normal testes development and descent [Charlton et al., 1983]. In cryptorchid boys, GnRH treatment reportedly induces increased testosterone secretion and stimulates further epididymis development and completion of epididymo-testicular descent [Bica and Hadziselimovic, 1993]. Boys with successful descent of the epididymis and testis had a normal-sized epididymis, while the majority of nonresponders to hormonal treatment had a small and irregular epididymis [Bica and Hadziselimovic, 1993].
Luteinizing hormone (LH) also seems to play an important role, as LH receptor knockout mice exhibit bilateral cryptorchidism that can be corrected by testosterone replacement therapy. Specifically, this therapy reverses all of the morphological and gene expression changes in the knockout mice, except those related to insulin-like factor 3 (Insl3), suggesting that testosterone rather than INSL3 facilitates completion of testicular descent [Yuan et al., 2006]. Furthermore, in 66% of naturally cryptorchid mice, treatment with LH-releasing hormone reportedly induces epididymo-testicular descent, while increasing testosterone secretion and normalizing morphology of the underdeveloped cryptorchid epididymis [Hadziselimovic, 1981]. Notably, FGF8 mutation contributes to formation of the VATER/VACTERL association and is involved in cryptorchidism development [Zeidler et al., 2014].
Androgens and FGFR1
Androgens are the primary factors regulating epididymal development and function. However, a large body of evidence now suggests that growth factors also play important roles in epididymis regulation and maintenance. Over recent years, it has become clear that FGF signaling is involved in the development and normal functioning of male reproductive organs, such as the testis and epididymis [Cotton et al., 2008]. For example, Fgf10, is expressed in the mesenchyme of the lower Wolffian ducts and regulates epithelial growth of the seminal vesicles and prostate [Archambeault et al., 2009]. It has also been reported that testosterone treatment increases Fgf10 transcription in the seminal vesicles [Thomson and Cunha, 1999]. At later stages of epididymal development, FGFR1 is specifically expressed in the undifferentiated mesenchyme [Basilico and Moscatelli, 1992; Cotton et al., 2008]. Moreover, FGFR1 gene mutations have been described in cases of idiopathic hypogonadotropic hypogonadism and crypto-epididymis [Dodé et al., 2003; Pitteloud et al., 2006].
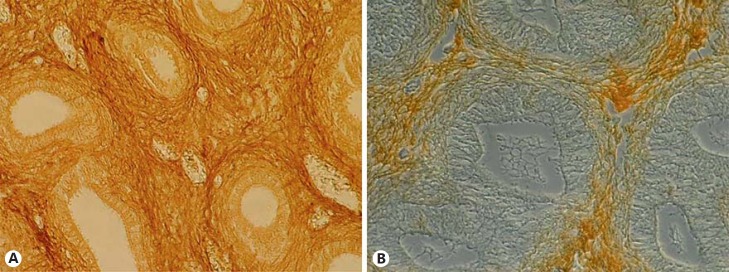
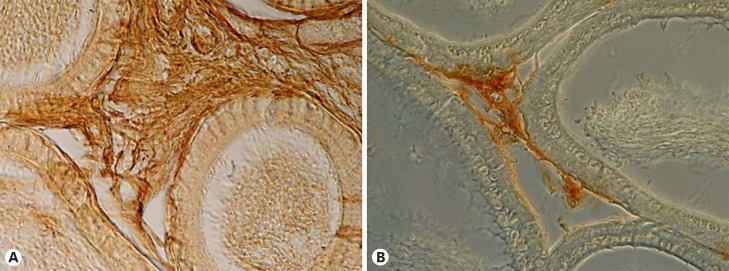
In 2010, we reported impaired FGFR1 expression in the undescended testis of unilateral cryptorchid boys [Hadziselimovic et al., 2010]. Additionally, decreased FGFR1 protein levels have been found in cryptorchid epididymides of both humans and rodents (fig. 2, 3). These findings support the involvement of FGFR1 in regulating epididymal mesenchyme development. It appears likely that the impaired FGFR1 protein secretion found in underdeveloped mesenchyme in cryptorchid humans and rodents contributes to the defective epididymis formation and the consequent undescended position (fig. 2, 3).
Fig. 2.
FGFR1 immunohistochemical staining of newborn descended (A) and undescended epididymis (B). Peroxidase staining with secondary antibodies (brown) appears less in the mesenchyme of the undescended epididymis. Additionally, the undescended epididymis was shorter and underdeveloped [Hadziselimovic, 1983].
Fig. 3.
FGFR1 immunohistochemical staining of descended (A) and undescended mouse epididymides (B). FGFR1 staining (brown) was consistent among all epididymal compartments, but there was a lesser degree of staining in the undescended epididymis. As in humans, the undescended epididymis was underdeveloped and shorter [Hadziselimovic, 1983].
Müllerian-Inhibiting Substance and INSL3
Nef and Parada [1999] reported normal epididymis development in Insl3-deficient cryptorchid mice, with no impairment of descent. In contrast, we found that Insl3 mutant mice showed absent smooth musculature around the epididymal duct, which resulted in a high intra-abdominal undescended position [Hadziselimovic and Adham, 2007]. This finding supports the importance of an intact epididymis for descent of the epididymo-testicular unit. Emmen et al. [2000] reported that Insl3 is not essential for Wolffian duct growth, and that the Müllerian-inhibiting substance (MIS) does not influence gubernaculum growth. Moreover, mice with mutations of Mis and the MIS receptor, and mice with intrauterine immunization against MIS show normal epididymo-testicular descent and normal scrotum development [Picar et al., 1983; Behringer et al., 1994; Bartlett et al., 2002]. Although MIS has no obvious effect on testicular descent in mice, it is still considered to potentially affect human descent [Hutson et al., 2015]. Those authors investigated boys with androgen insensitivity and found testicle localization in the inguinal region, indicating that the ‘first phase’ of descent is androgen independent and MIS dependent [Clarnette et al., 1997]. In contrast, abdominal testes were reported in 86% of patients showing a complete female phenotype, with a decreasing incidence corresponding with increasing masculinization [Barthold et al., 2000]. Thus, in patients with complete androgen insensitivity, testicular position correlates with the genital phenotype, supporting a major role for androgens in epididymo-testicular descent.
Syndromes Associated with Crypto-Epididymis
A large number of syndromes are associated with maldescent of the epididymo-testicular unit (table 1). Hypogonadism is also established in many of these conditions, often based on central or hypothalamic-pituitary deficit [Pinsky et al., 1999]. In majority of studied syndromes, affected individuals with crypto-epididymis also exhibit various forms of mental retardation (table 1). The EGR4, FMR2 (AFF2), and VCX3A genes encode proteins involved in signaling pathways that contribute to intellectual and cognitive functions. Thus, the impaired expressions of these genes in cryptorchid boys with high infertility risk could explain the increased odds ratio for the low IQ observed among boys with undescended testes [Hadziselimovic et al., 2014].
Table 1.
Some syndromes with crypto-epididymis and hypogonadotropic hypogonadism
| Frequent | Occasional |
|---|---|
| Aarskog-Scott (faciogenital dysplasia)* | Ablepharon-Macrostomia |
| Apert* | Anophthalmia-Esophageal-Genital |
| C syndrome (Opitz trigonocephaly)* | Ataxia-Telangiectasia* |
| Carpenter* | Beckwith-Wiedemann* |
| Cockayne* | Bird-Headed Dwarfism (Seckel and others)* |
| Cryptophthalmos (Fraser)* | Blepharochalasis* |
| De Lange* | Bloom* |
| Dubowitz* | Börjeson-Forssman-Lehmann* |
| Ellis-van Creveld* | Branchial Arch* |
| Fanconi panmyelopathy* | Chudley Mental Retardation* |
| Fetal-face syndrome (Robinow)* | Chudley* |
| FG syndrome (Opitz-Kaveggia)* | Cohen* |
| Fryns* | Gardner-Silengo-Wachtel |
| Goeminne | Gorlin (Basal Cell Nevus)* |
| Lenz-Majewski* | Hallerman-Streiff* |
| Lissencephaly* | Herrmann-Opitz* |
| Meier-Gorlin* | Ieshima* |
| Micro* | Jarcho-Levin* |
| N syndrome* | Johanson-Blizzard* |
| Neu-Laxova* | Lenz Microphthalmia* |
| Oculocerebrorenal* | Leopard* |
| Prader-Willi* | Limb/Pelvis-Hypoplasia/Aplasia* |
| Roberts* | Lowry* |
| Rothmund-Thompson* | Marden-Walker* |
| Rubenstein-Taybi* | McKusick-Kaufman* |
| Silver-Russell* | Noonan* |
| Urioste* | Norrie disease |
| XK aprosencephaly* | Oculocerebal (Cross)* |
| X-linked mental retardation, gynecomastia, and hypogonadism* | Opitz G/BBB Compound* |
| Oto-Palatal-Digital-Type II* | |
| Pallister-Hall* | |
| Perlman* | |
| Peters-Plus* | |
| Pfeiffer* | |
| Pterygium* | |
| Saethre-Chotzen* | |
| Scarf* | |
| Simpson-Golabi-Behmel* | |
| Sprintzen-Goldberg* | |
| Ulnar-Mammary* | |
| Urban-Rogers-Meyer* | |
| Van Benthem* | |
| Wiedemann* | |
| X-linked congenital adrenal hypoplasia* |
Syndromes associated with different degrees of mental retardation, adapted according to Pinsky et al. [1999].
Kallmann syndrome is a classic example of a congenital syndrome associated with hypothalamus-pituitary-gonadal axis impairment. Clinical findings also indicate that some developmental defects observed in CHARGE syndrome may be caused by insufficient FGF signaling levels. For example, CHARGE syndrome shows substantial clinical overlap with 22q11.2 deletion and Kallmann syndrome, which are both linked to reduced FGF signaling [Randall et al., 2009; Scambler, 2010; Corsten-Janssen et al., 2013; Miraoui et al., 2013]. Mutations in the same exon of FGFR2 are observed in Crouzon, Jackson-Weiss and Pfeiffer syndrome [Meyers et al., 1996]. Mutations elsewhere in the same gene also present with a common phenotype - for example, in Crouzon syndrome with mutations in either exon IIIa or in IIIc [Meyers et al., 1996]. Moreover, disorders such as Apert's syndrome are associated with incorrect FGF ligand binding and inappropriate FGFR autocrine activation [Kelleher et al., 2013].
The discovery of mutations in FGFR1 and FGF8 in CHH indicates a previously unappreciated role of FGF8-FGFR1 signaling in GnRH neuron ontogeny [Miraoui et al. 2013]. Fgf8 hypomorphic mice show a lack of GnRH neurons in the hypothalamus, demonstrating the exquisite sensitivity of the GnRH neuronal population to FGF8 signaling, which appears to be androgen dependent [Gnanapragasam et al., 2002]. More recent studies establish FGF8 as a critical morphogen for GnRH neuron fate specification and for olfactory system development [Miraoui et al., 2013]. Using expression data from diverse organisms, Miraoui et al. [2013] identified a cluster of genes that are similarly expressed and regulated during development, which are collectively referred to as the ‘FGF8 synexpression’ group. These genes show spatiotemporal expression patterns similar to that of FGF8, as well as act as enhancers or inhibitors, specifically modulating the signaling efficiency of FGF8 through FGFR1. Investigating FGFR1 mutations in CHH has provided pivotal data, challenging the traditional monogenic view of this disorder and guiding the transition towards more complex genetic models [Pitteloud et al., 2007].
Five additional gene mutations in FGF17, IL17RD, DUSP6, SPRY4, and FLRT3 have now been observed in CHH [Miraoui et al., 2013]. Moreover, the mechanisms underlying FGF dysregulation may differ among different syndromes.
Conclusion
Many cases of syndromic crypto-epididymis, as well as a majority of isolated cases, have either a disturbance of FGFs, FGFR1, FGFR3, and/or a disturbance of the genes involved in regulating the hypothalamic-pituitary-gonadal axis in common [Hadziselimovic et al., 2009; Hadziselimovic et al., 2010]. Normally, muscle development requires signaling by members of the FGF family and their downstream effector EGR1 [Nentwich et al., 2009]. Deficient expression of EGR4 and EGR1 was observed in cryptorchid boys [Hadziselimovic et al., 2009]. Thus, the observed decrease in FGF expression may result in decreased EGR gene expression, inducing CHH and impaired epididymal mesoderm development, resulting in maldescent of the epididymal-testicular union.
Statement of Ethics
The Institutional Review Board and the Independent Ethics Committee of the Children's Clinic Liestal approved all aspects of this study, which were in accordance with the Declaration of Helsinki. Approval was provided for research involving the use of material (data records or biopsy specimens) that had been collected for non-research purposes.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Archambeault DR, Tomaszewski J, Joseph A, Hinton BT, Yao HH. Epithelial-mesenchymal crosstalk in Wolffian Duct and fetal testis cord development. Genesis. 2009;47:40–48. doi: 10.1002/dvg.20453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barthold JS, Kumasi-Rivers K, Upadhyay J, Shekarriz B, Imperato-Mcginley J. Testicular position in the androgen insensitivity syndrome: implications for the role of androgens in testicular descent. J Urol. 2000;164:497–501. [PubMed] [Google Scholar]
- 3.Bartlett JE, Lee SM, Mishina Y, Behringer RR, Yang N, et al. Gubernacular development in Müllerian inhibiting substance receptor-deficient mice. BJU Int. 2002;1:113–118. doi: 10.1046/j.1464-4096.2001.00783.x. [DOI] [PubMed] [Google Scholar]
- 4.Basilico C, Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- 5.Bedford M. Anatomical evidence for epididymis as a prime mover in the evolution of the scrotum. Am J Anat. 1978;152:483–507. doi: 10.1002/aja.1001520404. [DOI] [PubMed] [Google Scholar]
- 6.Behringer RR, Finegold MJ, Cate RL. Müllerian-inhibiting substances function during mammalian sexual development. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 7.Bergh A, Helander H.F, Wahlqvist L. Studies on factors governing testicular descent in the rat-Partic larly the role of gubernaculum testis. Inter J Androl. 1978;1:342–356. [Google Scholar]
- 8.Bica DTG, Hadziselimovic F. The behavior of epididymis, processus vaginalis, and testicular descent in cryptorchid boys treated with buserelin. Eur J Pediatr 152 Suppl. 1993;2:S38–S42. doi: 10.1007/BF02125436. [DOI] [PubMed] [Google Scholar]
- 9.Charlton HM, Halpin DM, Iddon C, Rosie R, Levy G, et al. The effects of daily administration of single and multiple injections of gonadotropin-releasing hormone on pituitary and gonadal function in the hypogonadal (hpg) mouse. Endocrinology. 1983;113:535–544. doi: 10.1210/endo-113-2-535. [DOI] [PubMed] [Google Scholar]
- 10.Chung WC, Moyle SS, Tsai PS. Fibroblast growth factor 8 signaling through fibroblast growth factor receptor 1 is required for the emergence of gonadotropin-releasing hormone neurons. Endocrinology. 2008;149:4997–5003. doi: 10.1210/en.2007-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarnette TD, Sugita Y, Hutson JM. Genital anomalies in human and animal models reveal the mechanisms and hormones governing testicular descent. Br J Urol. 1997;79:99–112. doi: 10.1046/j.1464-410x.1997.25622.x. [DOI] [PubMed] [Google Scholar]
- 12.Corsten-Janssen N, Saitta SC, Hoefsloot LH, McDonald-McGinn DM, Driscoll DA, et al. More clinical overlap between 22q11.2 deletion syndrome and CHARGE syndrome than often anticipated. Mol Syndromol. 2013;4:235–245. doi: 10.1159/000351127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotton LM, O'Bryan MK, Hinton BT. Cellular signaling by fibroblast growth factors (FGFs) and their receptors (FGFRs) in male reproduction. Endocr Rev. 2008;29:193–216. doi: 10.1210/er.2007-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodé C, Levilliers J, Dupont JM, De Paepe A, Le Dû N, et al. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet. 2003;33:463–465. doi: 10.1038/ng1122. [DOI] [PubMed] [Google Scholar]
- 15.Emmen JM, McLuskey A, Adham IM, Engel W, Grootegoed JA, Brinkmann AO. Hormonal control of gubernaculum development during testis descent: gubernaculum outgrowth in vitro requires both insulin-like factor and androgen. Endocrinology. 2000;141:4720–4727. doi: 10.1210/endo.141.12.7830. [DOI] [PubMed] [Google Scholar]
- 16.Gnanapragasam VJ, Robson CN, Neal DE, Leung HY. Regulation of FGF8 expression by the androgen receptor in human prostate cancer. Oncogene. 2002;21:5069–5080. doi: 10.1038/sj.onc.1205663. [DOI] [PubMed] [Google Scholar]
- 17.Hadziselimovic F. Pathogenesis of cryptorchidism. In: Kogan SJ, Hafez ESE, editors. Pediatric Andrology. The Hague: Martinus Nijhoff; 1981. pp. 147–162. [Google Scholar]
- 18.Hadziselimovic F. Cryptorchidism Management and Implications. Berlin: Springer; 1983. [Google Scholar]
- 19.Hadziselimovic F. Mechanism of testicular descent. Urol Res. 1984;12:155–157. doi: 10.1007/BF00255914. [DOI] [PubMed] [Google Scholar]
- 20.Hadziselimovic F. Re: Regulation of testicular descent. Pediatr Surg Int. 2015;31:689–692. doi: 10.1007/s00383-015-3714-z. [DOI] [PubMed] [Google Scholar]
- 21.Hadziselimovic F, Adham I. Insulin 3-like hormone and its role in epididymo-testicular descent. Int Braz J Urol. 2007;33:407–411. doi: 10.1590/s1677-55382007000300015. [DOI] [PubMed] [Google Scholar]
- 22.Hadziselimovic F, Herzog B. The development and descent of the epididymis. Eur J Pediatr 152 Suppl. 1993;2:S6–S9. doi: 10.1007/BF02125424. [DOI] [PubMed] [Google Scholar]
- 23.Hadziselimovic F, Hadziselimovic NO, Demougin P, Krey G, Hoecht B, Oakeley EJ. EGR4 is a master gene responsible for fertility in cryptorchidism. Sex Dev. 2009;3:253–263. doi: 10.1159/000249147. [DOI] [PubMed] [Google Scholar]
- 24.Hadziselimovic F, Hadziselimovic NO, Demougin P, Oakeley EJ. Decreased expression of genes associated with memory and X-linked mental retardation in boys with non-syndromic cryptorchidism and high infertility risk. Mol Syndromol. 2014;5:76–80. doi: 10.1159/000357931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadziselimovic NO, de Geyter C, Demougin P, Oakeley EJ, Hadziselimovic F. Decreased expression of FGFR1, SOS1, RAF1 genes in cryptorchidism. Urol Int. 2010;84:353–361. doi: 10.1159/000288242. [DOI] [PubMed] [Google Scholar]
- 26.Hunter J. Observations on the state of the testis, in the foetus, and on the hernia congenital. In: Hunter W, editor. Medical Commentaries. London: Hamilton; 1762. pp. 75–90. [Google Scholar]
- 27.Hutson JM, Li R, Southwell BR, Newgreen D, Cousinery M. Regulation of testicular descent. Pediatr Surg Int. 2015;31:317–325. doi: 10.1007/s00383-015-3673-4. [DOI] [PubMed] [Google Scholar]
- 28.Itoh N, Ohta H, Konishi M. Endocrine FGFs: evolution, physiology, pathophysiology, and pharmacotherapy. Front Endocrinol (Lausanne) 2015;6:154. doi: 10.3389/fendo.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelleher FC, O'Sullivan H, Smyth E, McDermott R, Viterbo A. Fibroblast growth factor receptors, developmental corruption and malignant disease. Carcinogenesis. 2013;34:2198–2205. doi: 10.1093/carcin/bgt254. [DOI] [PubMed] [Google Scholar]
- 30.Klaatsch H. Über den Descensus testiculorum. Morphologisches Jahrbuch. 1890;16:587–646. [Google Scholar]
- 31.Meyers GA, Day D, Goldberg R, Daentl DL, Przylepa KA, et al. FGFR2 exon IIIa and IIIc mutations in Crouzon, Jackson-Weiss, and Pfeiffer syndromes: evidence for missense changes, insertions, and a deletion due to alternative RNA splicing. Am J Hum Genet. 1996;58:491–498. [PMC free article] [PubMed] [Google Scholar]
- 32.Miraoui H, Dwyer AA, Sykiotis GP, Plummer L, Chung W, et al. Mutations in FGF17, IL17RD, DUSP6, SPRY4, and FLRT3 are identified in individuals with congenital hypogonadotropic hypogonadism. Am Hum Genet. 2013;92:725–743. doi: 10.1016/j.ajhg.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nef S, Parada LF. Cryptorchidism in mice mutant for Insl3. Nat Genet. 1999;22:295–299. doi: 10.1038/10364. [DOI] [PubMed] [Google Scholar]
- 34.Nentwich O, Dingwell KS, Nordheim A, Smith JC. Downstream of FGF during mesoderm formation in Xenopus: the roles of Elk-1 and Egr-1. Dev Biol. 2009;336:313–326. doi: 10.1016/j.ydbio.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 35.Pask AJ, Kanasaki H, Kaiser UB, Conn PM, Janovick JA, et al. A novel mouse model of hypogonadotrophic hypogonadism: N-ethyl-N-nitrosourea-induced gonadotropin-releasing hormone receptor gene mutation. Mol Endocrinol. 2005;19:972–981. doi: 10.1210/me.2004-0192. [DOI] [PubMed] [Google Scholar]
- 36.Picar JY, Tran D, Vigier B, Josso N. Persistence of Müllerian ducts in male rabbits by passive immunization against anti-Müllerian hormone during fetal life (in French) C R Seances Acad Sci III. 1983;297:567–570. [PubMed] [Google Scholar]
- 37.Pinsky L, Erickson R, Schimke RN. Genetic Disorders of Human Sexual Development. New York: Oxford University Press; 1999. [Google Scholar]
- 38.Pitteloud N, Acierno JS, Jr, Meysing A, Eliseenkova AV, Ma J, et al. Mutations in fibroblast growth factor receptor 1 cause both Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA. 2006;103:6281–6286. doi: 10.1073/pnas.0600962103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, et al. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest. 2007;117:457–463. doi: 10.1172/JCI29884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rachmani E, Zachariou Z, Snyder H, Hadziselimovic F. Complete testis-epididymis fusion anomaly: a typical association with cryptorchid testis. Urol Int. 2012;89:355–357. doi: 10.1159/000342665. [DOI] [PubMed] [Google Scholar]
- 41.Radovick S, Wray S, Lee E, Nicols DK, Nakayama Y, et al. Migratory arrest of gonadotropin-releasing hormone neurons in transgenic mice. Proc Natl Acad Sci USA. 1991;88:3402–3406. doi: 10.1073/pnas.88.8.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Randall V, McCue K, Roberts C, Kyriakopoulou V, Beddow S, et al. Great vessel development requires biallelic expression of Chd7 and Tbx1 in pharyngeal ectoderm in mice. J Clin Invest. 2009;119:3301–3310. doi: 10.1172/JCI37561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rochester JR, Chung WC, Hayes TB, Tsai PS. Opposite-sex housing reactivates the declining GnRH system in aged transgenic male mice with FGF signaling deficiency. Am J Physiol Endocrinol Metab. 2012;303:E1428–E1439. doi: 10.1152/ajpendo.00289.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scambler PJ. 22q11 deletion syndrome: a role for TBX1 in pharyngeal and cardiovascular development Pediatr Cardiol. 2010;31:378–390. doi: 10.1007/s00246-009-9613-0. [DOI] [PubMed] [Google Scholar]
- 45.Thomson AA, Cunha GR. Prostatic growth and development are regulated by FGF10. Development. 1999;126:3693–3701. doi: 10.1242/dev.126.16.3693. [DOI] [PubMed] [Google Scholar]
- 46.Vizeneux A, Hilfiger A, Bouligand J, Pouillot M, Brailly-Tabard S, et al. Congenital hypogonadotropic hypogonadism during childhood: presentation and genetic analyses in 46 boys. PLoS One. 2013;8:e77827. doi: 10.1371/journal.pone.0077827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan FP, Lin DX, Rao CV, Lei ZM. Cryptorchidism in LhrKO animals and the effect of testosterone-replacement therapy. Hum Reprod. 2006;21:936–942. doi: 10.1093/humrep/dei433. [DOI] [PubMed] [Google Scholar]
- 48.Zeidler C, Woelfle J, Draaken M, Mughal SS, Große G, et al. Heterozygous FGF8 mutations in patients presenting cryptorchidism and multiple VATER/VACTERL features without limb anomalies. Birth Defects Res A Clin Mol Teratol. 2014;100:750–759. doi: 10.1002/bdra.23278. [DOI] [PubMed] [Google Scholar]