Abstract
BACKGROUND
The incidence of meningococcal disease is currently at historic lows in the United States; however, incidence remains highest among infants aged <1 year. With routine use of Haemophilus influenzae type b and pneumococcal vaccines in infants and children in the United States, Neisseria meningitidis remains an important cause of bacterial meningitis in young children.
METHODS
Data were collected from active, population- and laboratory-based surveillance for N meningitidis conducted through Active Bacterial Core surveillance during 2006 through 2012. Expanded data collection forms were completed for infant cases identified in the surveillance area during 2006 through 2010.
RESULTS
An estimated 113 cases of culture-confirmed meningococcal disease occurred annually among infants aged <1 year in the United States from 2006 through 2012, for an overall incidence of 2.74 per 100 000 infants. Among these cases, an estimated 6 deaths occurred. Serogroup B was responsible for 64%, serogroup C for 12%, and serogroup Y for 16% of infant cases. Based on the expanded data collection forms, a high proportion of infant cases (36/58, 62%) had a smoker in the household and the socioeconomic status of the census tracts where infant meningococcal cases resided was lower compared with the other Active Bacterial Core surveillance areas and the United States as a whole.
CONCLUSIONS
The burden of meningococcal disease remains highest in young infants and serogroup B predominates. Vaccines that provide long-term protection early in life have the potential to reduce the burden of meningococcal disease, especially if they provide protection against serogroup B meningococcal disease.
Meningococcal disease is a serious but rare infectious disease. In the United States, the incidence of meningococcal disease has declined since a peak of disease in the late 1990s; declines have been observed among all age groups and serogroups.1,2 In 2012, the incidence of meningococcal disease was at a historic low (0.15 per 100 000 persons)3; however, incidence remained highest among infants aged <1 year.1–3
Meningitis in infants and young children can be caused by a wide range of bacteria. In infants aged <2 months, group B Streptococcus causes most cases in the United States.4 Listeria monocytogenes is also seen in this age group. For infants and children aged 2 to 23 months, Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae also are important causes of bacterial meningitis and sepsis.4 With routine use of H influenzae type b and pneumococcal vaccines for infants and children in the United States, incidence due to both pathogens has been reduced, leaving N meningitidis as one of the most important causes of bacterial meningitis in infants and young children in the United States.4,5
This report describes the current epidemiology and burden of meningococcal disease in infants aged <1 year in the United States and potential risk factors for transmission to this vulnerable group. These data are key to informing future meningococcal disease vaccination strategies in the United States.
METHODS
Surveillance
Active, population- and laboratory-based surveillance for disease caused by N meningitidis was conducted from January 1, 2006, through December 31, 2012, as part of Active Bacterial Core surveillance (ABCs). ABCs is supported by the US Centers for Disease Control and Prevention (CDC) as part of its Emerging Infections Program Network, as described elsewhere.6 The surveillance area included California (3 San Francisco Bay area counties), Colorado (5 Denver area counties), Connecticut (statewide), Georgia (statewide), Maryland (statewide), Minnesota (statewide), New Mexico (statewide), Oregon (statewide), New York (15 Rochester and Albany area counties), and Tennessee (11 urban counties in 2002–2009, 20 counties in 2010–2012). In 2012, the total population under surveillance was 42.8 million, or 13.6% of the US population. Approximately 530 000 infants <1 year resided in the ABCs surveillance area during 2012.
A case of meningococcal disease was defined as isolation of N meningitidis from a normally sterile site (eg, blood or cerebrospinal fluid [CSF]) in a surveillance area resident. Epidemiologic and clinical information was abstracted from medical records. Outcome of illness was based on the patient's status at the time of hospital discharge; no information was collected on whether the death was attributed to N meningitidis infection. Information on assessment for sequelae before discharge and sequelae present at discharge are not routinely collected by ABCs. Regular laboratory audits were conducted to identify cases missed during routine surveillance.
The following hierarchical definition was used to classify a single syndrome for cases: a patient was defined as having meningitis if a clinical diagnosis of meningitis had been entered into the patient's medical record or if N meningitidis was isolated from CSF, pneumonia if pneumonia was entered into the patient's medical record and N meningitidis was isolated from blood and pleural fluid, septic arthritis if N meningitidis was isolated from joint fluid, and isolated bacteremia if N meningitidis was isolated from blood and no localizing clinical syndrome was described.
Expanded Data Collection
Expanded case report forms were completed for cases of meningococcal disease in infants aged <1 year presenting from January 1, 2006, until December 31, 2010. The expanded chart review forms included variables for medical history (gestational age and weight, congenital anomalies and underlying conditions, sequelae at discharge) and social history (breastfeeding history, day care attendance, household smoking status, maternal and paternal age). Sites were encouraged to also review public health investigation records to improve the completeness of data collected on the expanded case report form. Additional information on medical and social history variables was collected from case birth certificates and by geocoding cases to collect socioeconomic data from the census tract where the case resided.
Eight of the 10 ABCs sites participated in the expanded chart review and data collection (Colorado, Georgia's Atlanta Metropolitan Statistical Area, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee). This study was determined to be nonhuman subject's research, exempt from human subject's regulations by the CDC Human Research Protection Office. This study also was determined to be exempt from human subjects' regulations by the participating ABCs surveillance sites. This study was reviewed and approved by the institutional review boards of the Georgia Metropolitan Statistical Areas, Maryland Department of Health and Mental Hygiene, and Johns Hopkins Bloomberg School of Public Health.
Laboratory Methods
Serogrouping of N meningitidis was performed at state public health laboratories, after which the isolates were sent to the CDC, where slide agglutination serogrouping and serogroup-specific real-time polymerase chain reaction (real-time PCR) were performed.7 If the CDC slide agglutination or PCR testing confirmed the state serogroup result, that result was used as the final serogroup. If the CDC slide agglutination and PCR both differed from the state serogroup, the CDC result was used as the serogroup. If an isolate was nonviable or contaminated on arrival at the CDC after sending 2 times, the result from the state laboratory was used.
Statistical Analyses
Incidence rates were calculated by using US census data for the ABCs sites. Population denominators by month of life for infants aged <1 year were calculated by obtaining US census estimates for the number of infants <1 year of age in the surveillance area for each year studied and dividing by 12. Missing race data were multiply imputed by using sequential regression imputation methods.8 Estimates of the number of cases and deaths in the 50 states were calculated, standardizing for race. For rate calculations, an ABCs site was included only if data were collected for the complete year. Oregon has reported higher rates of serogroup B and overall meningococcal disease because of hyperendemic serogroup B disease that was first reported in 1994.9,10 We verified that the Oregon clonal outbreak was not consistent with disease patterns in other states by comparing disease incidence in Oregon with the 50 states by using reports from the National Notifiable Diseases Surveillance System. From 2006 to 2012, Oregon reported a meningococcal disease incidence of 0.91 per 100 000 persons through the National Notifiable Diseases Surveillance System. The other 49 states reported incidence of 0.14 to 0.53 per 100 000 persons (CDC, unpublished data). Accordingly, incidence was standardized by race to the US population by using incidence excluding Oregon. Cases in Oregon were then added back in for the final standardized incidence rate and estimated annual burden of disease in the United States. For all other analyses, data presented includes cases from Oregon unless otherwise noted. The 95% confidence interval (CI) around the standardized rate was calculated by using a method derived from the relationship between Poisson distribution and the γ distribution.11 Incidence rates are reported as cases per 100 000 persons.
RESULTS
Surveillance
Between 2006 and 2012, 127 cases of meningococcal disease were identified in infants aged <1 year in the surveillance area for an average annual incidence of 3.30 cases per 100 000 infants (range 2.63–4.89). Incidence ranged from 1.11 per 100 000 infants in Connecticut to 10.45 cases per 100 000 infants in Oregon. The higher incidence observed in Oregon was driven by its hyperendemic serogroup B disease; 24 (71%) of the 34 infant cases reported from Oregon were serogroup B, compared with 58 (62%) of 93 infant cases in the other ABCs sites.
Seven (6%) patients died; 5 patients who died had meningitis and 2 patients had isolated bacteremia. Eighty-eight (69%) cases were male. Seventeen (14%) were black, 94 (74%) were white, and 30 (24%) were reported as Hispanic. The median age was 5 months (range, 11–361 days). Five (4%) cases occurred in infants aged <1 month. In 53 (42%) cases, N meningitidis was isolated from the CSF, including 19 cases that had growth in both CSF and blood cultures. The remaining 55 cases had growth in blood cultures only (n = 51; 40%) or from joints (n = 4; 4%). Among the surviving cases, 102 (86%) were hospitalized; the median length of hospitalization was 7 days (range, 2–373 days). Seasonal variation occurred with the highest proportion of cases reported during the months of February to July (n = 82; 65%). Serogroup was available for all isolates: 82 (64%) were serogroup B, 15 (12%) serogroup C, 21 (16%) serogroup Y, 7 (6%) serogroup W, and 2 (2%) nongroupable.
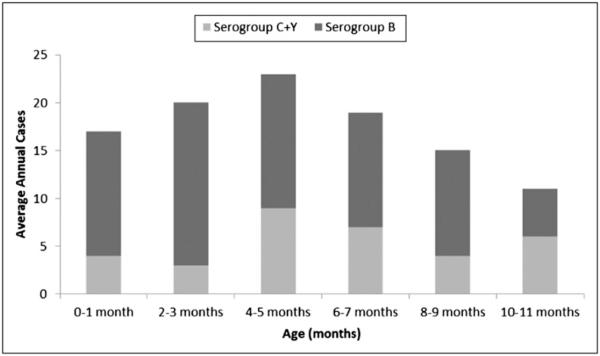
An estimated 113 cases of culture-confirmed meningococcal disease occurred annually among infants aged <1 year in the United States from 2006 to 2011, for an overall incidence of 2.74 per 100 000 infants (Table 1). Among these cases, an estimated 6 deaths occurred each year. Approximately 23% of infant meningococcal disease cases could be prevented with a serogroup C and Y vaccine if protection was achieved by age 4 months; a vaccine that protects against serogroup B by 4 months of age would have the potential to prevent ~37% of infant cases. A combined serogroup B, C, and Y vaccine would have the potential to prevent up to 63% of infant meningococcal disease cases in the United States (Fig 1).
TABLE 1.
Average Annual Incidence of Meningococcal Disease in Children <1 year: United States, 2006–2012
| Age, mo | Serogroup B |
Serogroup C |
Serogroup Y |
Totala |
|---|---|---|---|---|
| Incidence (95% CI) | Incidence (95% CI) | Incidence (95% CI) | Incidence (95% CI) | |
| 0–1 | 1.96 (0.98–3.52) | 0.02 (0.00–0.70) | 0.64 (0.17–1.71) | 2.81 (1.61–4.57) |
| 2–3 | 2.48 (1.37–4.16) | 0.29 (0.04–1.13) | 0.21 (0.01–1.05) | 3.45 (2.12–5.31) |
| 4–5 | 2.08 (1.10–3.60) | 0.39 (0.06–1.36) | 0.87 (0.28–2.06) | 3.34 (2.04–5.19) |
| 6–7 | 1.79 (0.87–3.29) | 0.39 (0.06–1.36) | 0.60 (0.16–1.62) | 2.95 (1.73–4.72) |
| 8–9 | 1.56 (0.74–2.95) | 0.39 (0.09–1.24) | 0.19 (0.00–1.03) | 2.34 (1.31–3.91) |
| 10–11 | 0.66 (0.19–1.72) | 0.12 (0.00–0.87) | 0.73 (0.21–1.86) | 1.54 (0.72–2.91) |
| Total | 1.76 (1.35–2.25) | 0.27 (0.13–0.49) | 0.54 (0.32–0.85) | 2.74 (2.22–3.34) |
ABCs cases from 2006 through 2012 directly standardized to the race distribution of the US population.
Includes other serogroups (eg, serogroup W, nongroupable).
FIGURE 1.
Average annual cases of meningococcal disease by month of life and serogroup, United States, 2006–2012. ABCs cases from 2006 to 2012 are directly standardized to the race distribution of the US population.
Expanded Data Collection
Expanded case report forms were completed for 81 (99%) of 82 cases, birth certificate data were available for 79 (96%) cases, and census tract data were available for all 82 cases in infants <1 year from 2006 to 2010 in the participating sites. Most cases occurred among healthy full-term infants. Gestational age and birth weight were available for 78 cases; the median gestational age was 38 weeks (range 29–42 weeks) and the median birth weight was 3192 g (range 1389–4960 g). Three infants were reported to have congenital anomalies or abnormal conditions at birth (intrauterine growth restriction and drug exposure, congenital cytomegalovirus infection, and meningomyelocele/spina bifida).
Data on social history variables were less complete. Among cases with available data, 8 (19.5%) of 41 cases had a history of current breastfeeding; an additional 11 cases had a history of ever being breastfed. On the birth certificate, 35% of infants were breastfed at birth. Daycare attendance was reported by 17 (26%) of 65 cases. A high proportion of cases (36/58, 62%) had a smoker in the household. The median household size was 4 persons (range, 2–14), with a median of 2 adults (range, 1–9) and 2 children (range, 0–8). Most cases (67/75, 89%) reported no sequelae at discharge. Of those patients who survived, 2 patients had skin necrosis, 3 patients had seizures, 2 patients had hearing loss, and 1 additional patient failed an audiology screen but final audiology results were not available. Six cases had unknown or insufficient information to determine if sequelae were present.
The median maternal age was 22.9 years (range, 14.0–39.7 years) and the median paternal age was 27.0 years (range, 17–54 years). Thirty-four percent of case mothers had less than a high school education, 34% were high school or equivalent graduates, and 23% had some college or higher-level education. The principal source of payment for the delivery was Medicaid for 53%, private insurance for 13%, self-pay for 6%, and unknown for 28%.
Socioeconomic indicators from the census tract where the cases resided were compared with other census tracts in the ABCs areas and the United States as a whole (Table 2). In general, the census tracts in which the cases resided had lower socioeconomic status than the other ABCs areas and the United States as a whole.
TABLE 2.
Socioeconomic Measures for Persons Residing in Census Tracts With Infant Meningococcal Disease Cases Compared With All US Census Tracts and Census Tracts in Participating ABCs Areas, 2006–2010 American Community Survey 5-Year Estimates
| Case Census Tracts, Median | United States, All Census Tracts | Participating ABCs Area Census Tracts | |
|---|---|---|---|
| Persons aged ≥ 16 who are unemployed, % | 8.6 | 7.9 | 7.4 |
| Median household income | $41 831 | $51 914 | $55 815 |
| Households with income <$25 000, % | 29.1 | 23.5 | 20.7 |
| Households with income ≥$200 000, % | 0.9 | 4.2 | 4.6 |
| All persons living below US poverty line, % | 15.3 | 13.8 | 12.0 |
| Persons aged ≥25 y with less than a high school education, % | 13.4 | 14.9 | 13.6 |
| Persons aged ≥25 y with a Bachelor's degree or higher, % | 17.8 | 27.9 | 27.8 |
| homes worth ≥$500 000, % | 1.7 | 12.5 | 10.5 |
| Homes worth ≥$750 000, % | 0.1 | 5.1 | 3.7 |
| All households containing >1 person per room, % | 2.3 | 3.1 | 2.1 |
| Census tract population living in a rural area, % | 0 | 22.4 | 19.0 |
| GINI indexa of income inequality | 0.405 | 0.467 | 0.425 |
A measurement of the income distribution of a country's residents. This number, which ranges between 0 and 1 and is based on residents' net income, helps define the gap between the rich and the poor, with 0 representing perfect equality and 1 representing perfect inequality.
DISCUSSION
During the study period, the incidence of meningococcal disease was at historic lows in the United States (0.15 per 100 000 persons),3 however incidence remained highest among infants aged <1 year (2.74 per 100 000 infants). In the United States, ~60% of disease among infants aged <1 year is caused by serogroup B,1,2 which is not contained in recently licensed meningococcal vaccines for infants and toddlers.
Meningococcal disease can be devastating; 5% to 10% of children with meningococcal disease do not survive and another 10% to 20% experience long-term sequelae, such as hearing loss, limb loss, and neurologic deficits.12
Infants may be at increased risk for meningococcal disease for several reasons. The infant immune system is immature, which increases the risk for infections in general; traditional risk factors for meningococcal disease (ie, complement component deficiencies and asplenia) are rarely identified in infants, and typically present later in life. Other risk factors that are more generally associated with an increased risk for meningococcal disease include close contact with a case, living in crowded conditions, active and passive smoking, and antecedent viral infections.13–18 Our findings are consistent with the results of previous studies and indicate these risk factors also increase risk of disease in infants.14,19–21 In particular, our study found a high proportion of infant meningococcal disease cases with exposure to cigarette smoke (62%), compared with 35% of children in the United States who live in homes where residents or visitors smoke in the home on a regular basis.22 In our study, we also found that the median age of the case mothers was lower than the average age at first birth for US mothers in 2006 (22.9 years versus 25.0 years),23 and the socioeconomic status of the census tracts where infant meningococcal cases resided was lower compared with the other ABCs areas and the United States as a whole. Low socioeconomic status is often considered a marker for other risk factors (eg, smoking and household crowding),24,25 but lower maternal age may be associated with increased contact of infant cases with adolescents or young adults who are the main carriers of N meningitis and serve as reservoirs of transmission to other age groups.21,26
Three meningococcal conjugate vaccines have been licensed for use in infants and toddlers in the United States, protecting against 2 of the 3 major serogroups that cause disease (serogroups C and Y, but not B). The Advisory Committee on Immunization Practices recommends vaccination of infants and toddlers who are at increased risk for disease, but does not recommend routine vaccination of all infants.27 This decision was based on the current low burden of disease and the low proportion of meningococcal cases that are preventable with vaccines that do not protect against serogroup B, which limits the potential impact of a routine meningococcal vaccination program in infants in the United States. A multicomponent serogroup B vaccine (Bexsero; Novartis, Basel, Switzerland) was recently licensed in Europe, Australia, and Canada and is currently under consideration for inclusion in infant immunization programs in these countries. Two vaccines to prevent serogroup B meningococcal disease are in late-stage clinical development in the United States; however, licensure of a vaccine that protects against serogroup B in infants in the United States is likely several years away.
The meningococcal conjugate vaccines currently available in the United States and the vaccines for serogroup B currently under development require multiple doses early in life to provide protection to infants. Because the largest burden of disease in <1-year-olds occurs in children 0 through 6 months of age, many will be too young to have received the minimum 2 or 3 doses of vaccine that likely will be needed to prevent disease. Consideration of other programmatic interventions may be necessary to protect these youngest infants because direct protection of most infants with these vaccines is not possible. These could potentially include a maternal vaccination program with a vaccine that protects against serogroup B and continuing to improve vaccination coverage with 2 doses of conjugate meningococcal vaccine in adolescents who are the main carriers of N meningitidis. Serogroup B vaccines have the potential to offer an important advance in the fight against meningococcal disease. However, research is needed to better understand how to optimally implement these vaccination programs.
The overall burden of meningococcal disease in the United States is substantially lower than in other countries. However, the epidemiology of meningococcal disease is dynamic, and close monitoring of trends is needed to accurately assess the benefit of adding meningococcal vaccines to the routine infant schedule. Vaccines that provide long-term protection early in life have the potential to reduce the burden of meningococcal disease, especially if they provide protection against serogroup B meningococcal disease.
WHAT'S KNOWN ON THIS SUBJECT
Meningococcal disease is a serious but rare infectious disease. In 2012, the incidence of meningococcal disease was at a historic low in the United States; however, incidence remained highest among infants aged <1 year.
WHAT THIS STUDY ADDS
This report describes the epidemiology and burden of meningococcal disease in infants aged <1 year in the United States and potential risk factors for transmission to this vulnerable group. These data are key to informing future meningococcal disease vaccination strategies.
ACKNOWLEDGMENTS
We thank the following ABCs staff: Pam Daily, Joelle Nadle, and Gretchen Rothrock (California); Deborah Aragon, Steve Burnite, Shaun Cosgrove, Jennifer Sadlowski, Allison Wheeler, and Benjamin White (Colorado); Matt Cartter, Carmen Marquez, and Michelle Wilson (Connecticut); Wendy Baughman, Ashley Moore, and Stephanie Thomas (Georgia); Terresa Carter, Rosemary Hollick, and Kathleen Shutt (Maryland); Richard Danila, Kelly Gall, Brenda Jewell, Billie Juni, Christine Lees, Catherine Lexau, Beth Shade, and Lori Triden (Minnesota); Joseph Bareta (New Mexico); Nancy Spina (New York State); Jamie Thompson (Oregon); and Brenda Barnes (Tennessee). We also thank Gayle Langley, Emily Weston, and Karrie-Ann Toews (of the CDC's ABCs program).
FUNDING: The Active Bacterial Core surveillance sites received funding from the Centers for Disease Control and Prevention Emerging Infections Program cooperative agreement CI05-026. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: Dr Harrison has consulted for Sanofi Pasteur, Merck, GSK, Novartis, and Pfizer; received grants from Sanofi Pasteur; and received payment for lectures including service on speakers bureaus from Novartis. Dr Schaffner is an occasional consultant to Sanofi Pasteur, Pfizer, and Dynavax, and is a member of the Data Safety Monitoring Board for Merck for experimental vaccine clinical trials. The other authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
- 1.Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50(2):184–191. doi: 10.1086/649209. [DOI] [PubMed] [Google Scholar]
- 2.Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2013;62(RR-2):1–28. [PubMed] [Google Scholar]
- 3.Report ABCS . Emerging Infections Program Network, Neisseria meningitidis, 2012. Centers for Disease Control and Prevention; [Accessed November 20, 2014]. 2013. Available at: www.cdc.gov/abcs/reports-findings/survreports/mening12.html. [Google Scholar]
- 4.Thigpen MC, Whitney CG, Messonnier NE, et al. Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med. 2011;364(21):2016–2025. doi: 10.1056/NEJMoa1005384. [DOI] [PubMed] [Google Scholar]
- 5.Schuchat A, Robinson K, Wenger JD, et al. Active Surveillance Team. Bacterial meningitis in the United States in 1995. N Engl J Med. 1997;337(14):970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 6.Schuchat A, Hilger T, Zell E, et al. Active Bacterial Core Surveillance Team of the Emerging Infections Program Network. Active bacterial core surveillance of the emerging infections program network. Emerg Infect Dis. 2001;7(1):92–99. doi: 10.3201/eid0701.010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mothershed EA, Sacchi CT, Whitney AM, et al. Use of real-time PCR to resolve slide agglutination discrepancies in serogroup identification of Neisseria meningitidis. J Clin Microbiol. 2004;42(1):320–328. doi: 10.1128/JCM.42.1.320-328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raghunathan TELJ, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27(1):85–95. [Google Scholar]
- 9.Diermayer M, Hedberg K, Hoesly F, et al. Epidemic serogroup B meningococcal disease in Oregon: the evolving epidemiology of the ET-5 strain. JAMA. 1999;281(16):1493–1497. doi: 10.1001/jama.281.16.1493. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Serogroup B meningococcal disease—Oregon, 1994. MMWR Morb Mortal Wkly Rep. 1995;44(7):121–124. [PubMed] [Google Scholar]
- 11.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16(7):791–801. doi: 10.1002/(sici)1097-0258(19970415)16:7<791::aid-sim500>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Meningococcal vaccines: WHO position paper, November 2011. Wkly Epidemiol Rec. 2011;86(47):521–539. [PubMed] [Google Scholar]
- 13.Cartwright KA, Jones DM, Smith AJ, Stuart JM, Kaczmarski EB, Palmer SR. Influenza A and meningococcal disease. Lancet. 1991;338(8766):554–557. doi: 10.1016/0140-6736(91)91112-8. [DOI] [PubMed] [Google Scholar]
- 14.Fischer M, Hedberg K, Cardosi P, et al. Tobacco smoke as a risk factor for meningococcal disease. Pediatr Infect Dis J. 1997;16(10):979–983. doi: 10.1097/00006454-199710000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Moore PS, Harrison LH, Telzak EE, Ajello GW, Broome CV. Group A meningococcal carriage in travelers returning from Saudi Arabia. JAMA. 1988;260(18):2686–2689. [PubMed] [Google Scholar]
- 16.Stanwell-Smith RE, Stuart JM, Hughes AO, Robinson P, Griffin MB, Cartwright K. Smoking, the environment and meningococcal disease: a case control study. Epidemiol Infect. 1994;112(2):315–328. doi: 10.1017/s0950268800057733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephens DS, Hajjeh RA, Baughman WS, Harvey RC, Wenger JD, Farley MM. Sporadic meningococcal disease in adults: results of a 5-year population-based study. Ann Intern Med. 1995;123(12):937–940. doi: 10.7326/0003-4819-123-12-199512150-00007. [DOI] [PubMed] [Google Scholar]
- 18.Stuart JM, Cartwright KA, Dawson JA, Rickard J, Noah ND. Risk factors for meningococcal disease: a case control study in south west England. Community Med. 1988;10(2):139–146. [PubMed] [Google Scholar]
- 19.Yusuf HR, Rochat RW, Baughman WS, et al. Maternal cigarette smoking and invasive meningococcal disease: a cohort study among young children in metropolitan Atlanta, 1989–1996. Am J Public Health. 1999;89(5):712–717. doi: 10.2105/ajph.89.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray RL, Britton J, Leonardi-Bee J. Second hand smoke exposure and the risk of invasive meningococcal disease in children: systematic review and meta-analysis. BMC Public Health. 2012;12:1062. doi: 10.1186/1471-2458-12-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–861. doi: 10.1016/S1473-3099(10)70251-6. [DOI] [PubMed] [Google Scholar]
- 22.Schuster MA, Franke T, Pham CB. Smoking patterns of household members and visitors in homes with children in the United States. Arch Pediatr Adolesc Med. 2002;156(11):1094–1100. doi: 10.1001/archpedi.156.11.1094. [DOI] [PubMed] [Google Scholar]
- 23.Mathews TJ, Hamilton BE. [Accessed May 14, 2013];Delayed childbearing: more women are having their first child later in life. NCHS Databrief. 2009 Available at: www.cdc.gov/nchs/data/databriefs/db21.htm. [PubMed]
- 24.Jackson LA, Wenger JD. Laboratory-based surveillance for meningococcal disease in selected areas, United States, 1989–1991. MMWR CDC Surveill Summ. 1993;42(2):21–30. [PubMed] [Google Scholar]
- 25.Rosenstein NE, Perkins BA, Stephens DS, et al. The changing epidemiology of meningococcal disease in the United States, 1992–1996. J Infect Dis. 1999;180(6):1894–1901. doi: 10.1086/315158. [DOI] [PubMed] [Google Scholar]
- 26.Caugant DA, Høiby EA, Magnus P, et al. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiol. 1994;32(2):323–330. doi: 10.1128/jcm.32.2.323-330.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC) Infant meningococcal vaccination: Advisory Committee on Immunization Practices (ACIP) recommendations and rationale. MMWR Morb Mortal Wkly Rep. 2013;62(3):52–54. [PMC free article] [PubMed] [Google Scholar]