Summary
Current obesity prevention strategies recommend increasing daily physical activity, assuming that increased activity will lead to corresponding increases in total energy expenditure and prevent or reverse energy imbalance and weight gain [1-3]. Such Additive total energy expenditure models are supported by exercise intervention and accelerometry studies reporting positive correlations between physical activity and total energy expenditure [4], but challenged by ecological studies in humans and other species showing that more active populations do not have higher total energy expenditure [5-8]. Here we test a Constrained total energy expenditure model, in which total energy expenditure increases with physical activity at low activity levels but plateaus at higher activity levels as the body adapts to maintain total energy expenditure within a narrow range. We compared total energy expenditure, measured using doubly labeled water, against physical activity, measured using accelerometry, for a large (n=332) sample of adults living in five populations [9]. After adjusting for body size and composition total energy expenditure was positively correlated with physical activity, but the relationship was markedly stronger over the lower range of physical activity. For subjects in the upper range of physical activity, total energy expenditure plateaued, supporting a Constrained total energy expenditure model. Body fat percentage and activity intensity appear to modulate the metabolic response to physical activity. Models of energy balance employed in public health [1-3] should be revised to better reflect the constrained nature of total energy expenditure and the complex effects of physical activity on metabolic physiology.
RESULTS
Models of Total Energy Expenditure & Physical Activity
The metabolic costs and health benefits of physical activity are well-established [1,2], but the long term effect of physical activity on total daily energy requirements is far less certain. The predominant view [1-3] assumes a dose dependent and additive effect of physical activity on total energy expenditure (kcal/day), with each increment of physical activity leading to a corresponding increase in total energy expenditure (Fig. 1). This Additive model is supported by studies showing positive correlations between total energy expenditure and accelerometry recordings of physical activity [4]. Moreover, the Additive total energy expenditure model of metabolic physiology has shaped public health strategies to combat the global rise in obesity, which typically propose increasing physical activity as a means to increase total energy expenditure and achieve a healthy weight and maintain energy balance [1-3].
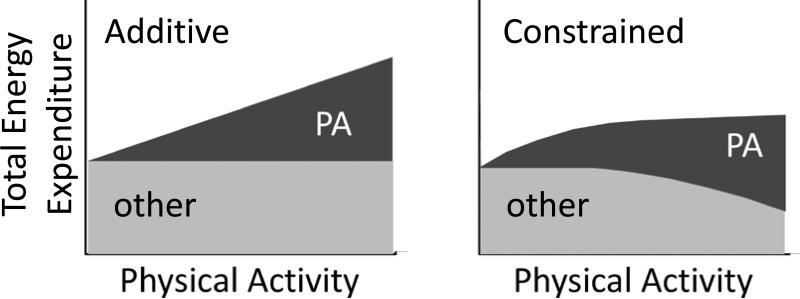
Figure 1.
Schematic of Additive total energy expenditure and Constrained total energy expenditure models. In Additive total energy expenditure models, total energy expenditure is a simple linear function of physical activity, and variation in physical activity energy expenditure (PA) determines variation in total energy expenditure. In Constrained total energy expenditure model, the body adapts to increased physical activity by reducing energy spent on other physiological activity, maintaining total energy expenditure within a narrow range.
A growing number of studies examining the long-term metabolic effects of exercise suggest that the relationship between physical activity and total energy expenditure is more complex than Additive models allow [5]. Rather than increasing total energy expenditure linearly in response to physical activity, individuals tend to adapt metabolically to increased physical activity, muting the expected increase in daily energy throughput [5,10-12]. These metabolic changes can be behavioral, such as sitting instead of standing, or fidgeting less, but they may also include reductions in other, non-muscular metabolic activity. For example, men and women enrolled in a long-term exercise study exhibited reduced basal metabolic rate at week 40 [11], and studies in healthy adult women have shown suppressed ovarian activity and lower estrogen production in response to moderate exercise [13]. Other species have also been shown to keep total energy expenditure remarkably constant in response to increased physical activity, reducing energy expenditure on growth [14], somatic repair [15,16], and basal metabolic rate [17,18], and even reducing lactation and cannibalizing nursing offspring [19], even when food is available ad libitum and total energy expenditure is well within maximum sustained levels [5, 14-19]. These observations are inconsistent with Additive models; instead, they favor a Constrained total energy expenditure model [5] in which energy allocation among physiological tasks responds dynamically to long-term shifts in physical activity, adapting to maintain total energy expenditure within some relatively narrow range (Fig. 1).
Constrained total energy expenditure may explain the remarkable degree of similarity in total energy expenditure among populations across a broad range of lifestyles. People in less socioeconomically developed populations, including subsistence farmers and traditional hunter-gatherers, have total energy expenditures similar to those in more developed populations [6,7] despite substantial differences in physical activity. Mammals living in the wild, including non-human primate species, have similar total energy expenditures to captive populations [8]. These population-level comparisons suggest that total energy expenditure is an evolved, species-specific trait that is homeostatically buffered against variation in habitual physical activity. It remains unclear, however, how the growing evidence for metabolic adaptation and metabolic constraint can be reconciled with accelerometry studies showing a positive correlation between physical activity and total energy expenditure [4]. Missing from these comparisons is an ecological study of total energy expenditure and physical activity collected simultaneously within a large, diverse sample, needed to characterize the relationship between variation in habitual levels of physical activity and total energy expenditure among individuals.
In this study, we evaluate Additive and Constrained total energy expenditure models in a large (n=332), mixed-sex (55% female), adult (age: 25 – 45y) human sample [9] drawn from five populations across Africa and North America (Ghana, South Africa, Seychelles, Jamaica, and United States; see Table S1 for sample characteristics). Total energy expenditure was measured using the doubly labeled water method. Resting metabolic rate was measured via respirometry. Physical activity was measured using wearable tri-axial accelerometers (reported as mean counts per minute per day, CPM/d); surveys were used to identify subjects employed in manual labor (Experimental Procedures). First, we used multivariate regression to examine the effects of anthropometric variables, population location, and physical activity on total energy expenditure and resting metabolic rate. We then used residuals from a multiple regression including anthropometrics and population location (Model 2, Table 1) to calculate adjusted total energy expenditure and adjusted resting metabolic rate, and investigate the relationship between physical activity and these size- and population-adjusted measures of expenditure.
Table 1.
Model parameters for multivariate analyses of total energy expenditure and total energy expenditureADJ.
| A. Total Energy Expenditure | MODEL 1 df=326, adj. r2=0.52 ±SE 383.7, p<0.001 | MODEL 2§ df=322, adj. r2=0.55 ±SE 368.2, p<0.001 | MODEL 3 df=292, adj. r2=0.59 ±SE 349.1, p<0.001 | MODEL 4 df=290, adj. r2=0.61 ±SE 341.8, p<0.001 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | β | ± SE | p | β | ± SE | p | β | ± SE | p | β | ± SE | p |
| (Intercept) | 1227.6 | 622.0 | * | 347.7 | 628.7 | −37.1 | 626.2 | −166.2 | 614.4 | |||
| FFM (kg) | 46.4 | 4.7 | **** | 42.2 | 5.3 | **** | 41.5 | 5.3 | **** | 40.5 | 5.2 | **** |
| Fat Mass (kg) | −5.0 | 2.5 | * | −2.1 | 2.9 | −0.9 | 2.9 | 0.4 | 2.9 | |||
| Height (cm) | −6.2 | 3.7 | * | 1.3 | 3.9 | 1.4 | 3.8 | 1.9 | 3.8 | |||
| Age (y) | 2.7 | 3.6 | 1.8 | 3.5 | 0.1 | 3.6 | −1.2 | 3.5 | ||||
| Sex (1=M, 0=F) | 6.5 | 88.8 | −14.4 | 95.2 | 60.2 | 95.5 | 39.9 | 94.0 | ||||
| Site: Ghana | - | - | - | - | - | - | ||||||
| Site: Jamaica | −374.0 | 73.6 | **** | −269.2 | 73.7 | **** | −273.1 | 73.3 | **** | |||
| Site: S Africa | −164.0 | 77.6 | ** | −122.5 | 76.4 | −111.2 | 76.4 | |||||
| Site: Seychelles | −100.8 | 73.1 | −39.7 | 78.6 | −56.8 | 78.9 | ||||||
| Site: US | −245.6 | 76.7 | *** | −181.1 | 80.7 | ** | −182.3 | 83.0 | ** | |||
| CPM/d | 1.1 | 0.2 | **** | 1.4 | 0.3 | **** | ||||||
| Manual Labor | 117.2 | 47.1 | ** | 114.2 | 46.2 | ** | ||||||
| Sedentary PA | 1.7 | 0.6 | *** | |||||||||
| Vigorous PA | −18.1 | 7.5 | ** | |||||||||
| B. Total Energy Expenditure ADJ§ | MODEL 5 df=330, adj. r2=0.07 ±SE 349.3, p<0.001 | MODEL 6 df=301, adj. r2=0.07 ±SE 348.3, p<0.001 | MODEL 7 df=300, adj. r2=0.09 ±SE 345.2, p<0.001 | MODEL 8 df=298, adj. r2=0.13 ±SE 338.0, p<0.001 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | β | ± SE | p | β | ± SE | p | β | ± SE | p | β | ± SE | p |
| (Intercept) | 2309.0 | 38.4 | **** | 2277.0 | 45.1 | **** | 2094.2 | 84.9 | **** | 1930.1 | 94.3 | **** |
| CPM/d | 0.9 | 0.2 | **** | 0.8 | 0.2 | **** | 1.0 | 0.2 | **** | 1.4 | 0.2 | **** |
| Manual Labor | 100.5 | 40.5 | ** | 104.4 | 40.2 | ** | 104.2 | 39.4 | *** | |||
| Body Fat % | 4.5 | 0.03 | ** | 5.2 | 1.7 | *** | ||||||
| Sedentary PA | 1.7 | 0.6 | *** | |||||||||
| Vigorous PA | −17.2 | 7.2 | ** | |||||||||
See methods for definitions of sedentary and vigorous physical activity (PA).
residuals from Model 2 used to calculated total energy expenditureADJ.
p values:
<0.001
0.001 – <0.01
0.01 – <0.05
0.05 – 0.10.
See also Figure S2, S3 and Tables S1, S3.
Statistical Models of Total Energy Expenditure
Anthropometric measurements explained just over half of the variation in total energy expenditure (df=326, adj. r2=0.52, p<0.001; Table 1, Model 1), with fat free mass the strongest single determinant. Adding a “study site” term to the model marginally improved the fit (df=322, adj. r2=0.55, p<0.001; Table 1, Model 2). Measures of physical activity (accelerometer CPM/d and manual labor employment) accounted for an additional 4% of the variation in total energy expenditure (df=292, adj. r2=0.59, p<0.001; Table 1, Model 3). Study site remained significant (Table 1, Model 3) indicating that differences in lifestyle among sites had measurable effects on total energy expenditure that were not wholly accounted for by accelerometry, anthropometry, and manual labor employment. Adding the term bodyweight×CPM/d to Model 3, to account for the greater metabolic cost of physical activity for larger individuals, did not affect the fit (adjusted r2) of the model, and the term was not a significant predictor of total energy expenditure (t(291)=−0.19, p=0.85). Similarly, substituting bodyweight×CPM/d for the CPM/d term in Model 3 did not affect the fit of the model. Adding measures of time spent in ‘sedentary’ (<100 CPM) and ‘vigorous’ (≥3960 CPM) physical activity improved the fit of the model to adj. r2 = 0.61 (Table 1, Model 4).
To examine the effects of physical activity on total energy expenditure, we calculated adjusted total energy expenditure (total energy expenditureADJ) from the residuals of Model 2 in Table 1, thereby controlling for the effects of fat free mass, fat mass, age, height, sex, and study site on total energy expenditure. Variation in total energy expenditureADJ with respect to physical activity was substantial; physical activity accounted for only 7% of the variation in total energy expenditureADJ (Table 1, Model 5,6). The mean coefficient of variation within CPM/d deciles (14±3%) was equivalent to the difference in mean total energy expenditureADJ between the 1st and 10th deciles (15%; see Table S2). The range of variation within any decile of CPM/d far exceeded the difference in median Adjusted total energy expenditure across the range of CPM/d. Results were similar across a range of approaches to control for potentially confounding effects of body size and other factors, such as employment in manual labor (see Figures S1, S2).
Size- and Population-Adjusted Total Energy Expenditure and Physical Activity
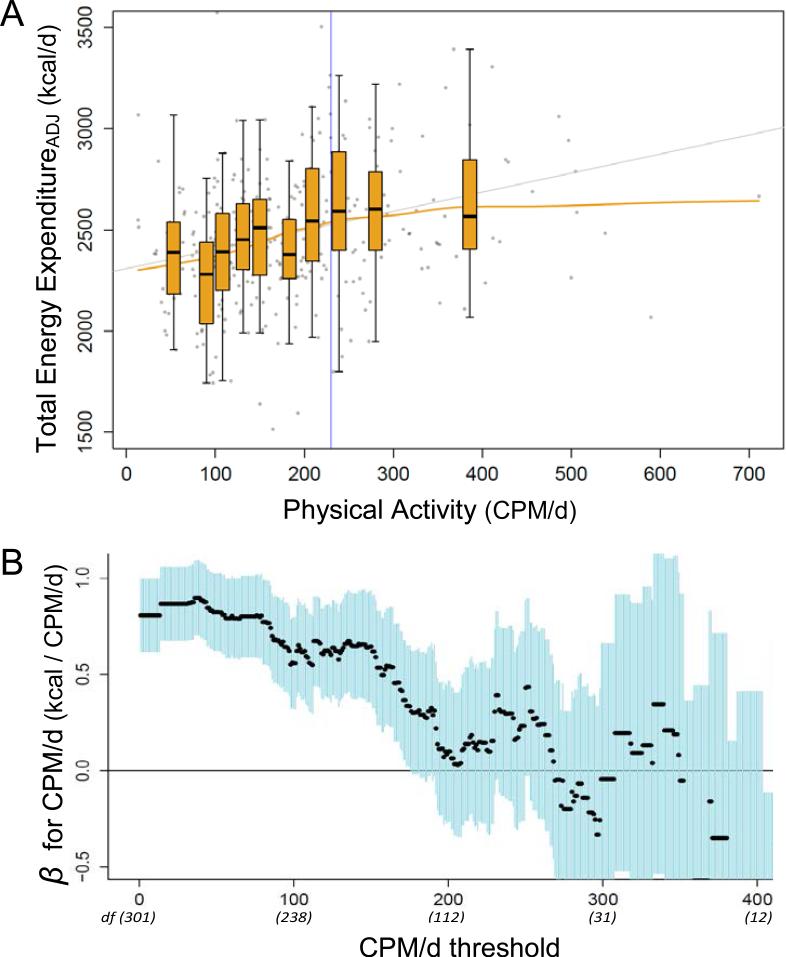
The effect of physical activity on total energy expenditureADJ was non-linear, with a plateau in daily energy expenditure over the upper four deciles (60th – 100th percentile) of CPM/d (Fig. 2A). This plateau was evident in the lowess regression and in the change in median total energy expenditureADJ over the range of CPM/d deciles (Fig. 2A). The slope of the lowess regression decreases markedly above 200 CPM/d, such that above 219 CPM/d each additional increment of 100 CPM/d is associated with less than 50 kcal/day increase in total energy expenditureADJ. We used two approaches to determine the activity level above which the effect of physical activity on total energy expenditureADJ was negligible.
Figure 2.
A. Total energy expenditureADJ (kcal/d) and physical activity (CPM/d) in the METS sample. Boxplots indicate medians and quartiles of total energy expenditureADJ for each decile of CPM/d, and are centered on the median CPM/d value for each decile. Lowess (yellow) and ordinary least squares (gray) regression lines are shown. The change point (230 CPM/d) for the change-point regression, indicated by the vertical blue line, marks the activity level at which the slope of the total energy expenditureADJ:CPM/d regression becomes indistinguishable from zero. Total energy expenditureADJ values for three subjects exceed 3500 and are not shown; see Fig. S1C. See also Table S2 and Figures S1 and S3. B. The effect of CPM/d on Total energy expenditureADJ for subjects above increasing CPM/d thresholds. Black circles show the β value for CPM/d for subjects above a given CPM/d threshold, blue lines represent ±standard error. Analyses include manual labor. Degrees of freedom (df) are given for major CPM/d thresholds.
First, we iteratively removed subjects at low CPM/d values and evaluated the effect of physical activity for subjects above increasing CPM/d thresholds (Methods). Fig. 2B shows the effect (β) of CPM/d on total energy expenditureADJ, in a model including manual labor, at increasing CPM/d thresholds. For the n=143 subjects above a threshold of CPM/d=176 the effect of CPM/d on total energy expenditureADJ is non-significant and its standard error includes zero (β=0.31±0.32, p=0.33; Fig 2B). For the n=99 subjects above a threshold of CPM/d=216, a model including both CPM/d and manual labor fails to achieve significance (adj. r2=0.02, p=0.12). There was no measurable effect of physical activity on total energy expenditureADJ above this threshold.
Second, we used change-point regression to estimate the CPM/d value at which the slope of total energy expenditureADJ on CPM/d changes from positive to zero (Experimental Procedures). The change-point was 230 CPM/d (95%CI 44 – 428), consistent with the iterative CPM/d threshold analysis (Fig. 2A,B). For the n=92 subjects above the change-point, the relationship between physical activity and total energy expenditureADJ is indistinguishable from zero (slope: 0.21±0.35; p=0.54). The change-point regression also captured a marginally greater amount of variance in total energy expenditureADJ (df=304; adj. r2=0.09, p<0.001) than linear regression (adj. r2=0.07, p<0.001, Table 1, Model 5,6).
Resting Metabolic Rate and Activity Energy Expenditure
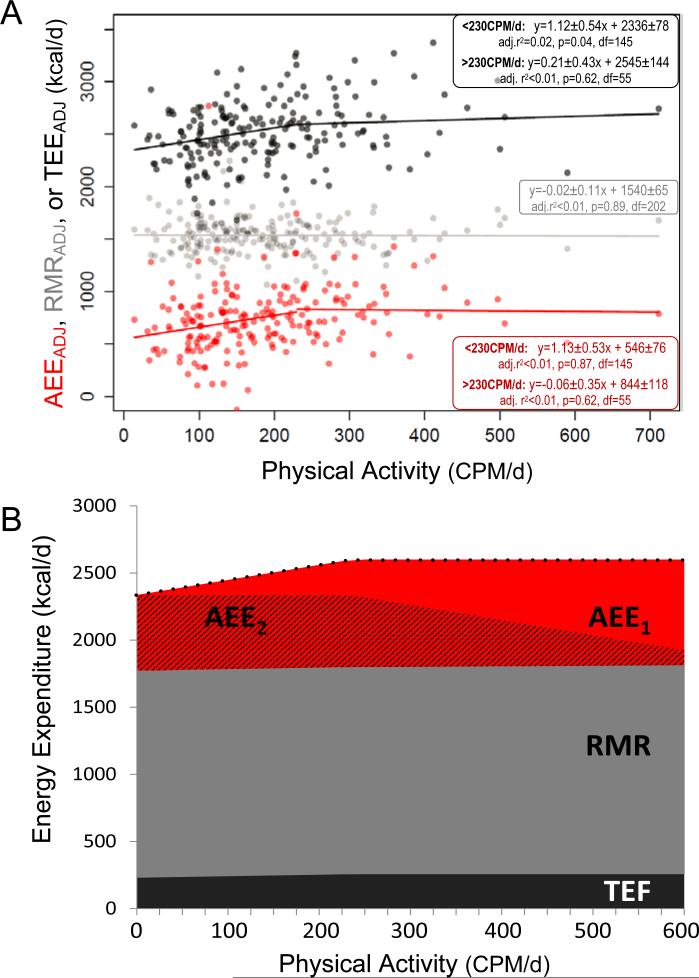
To further investigate metabolic response to variation in habitual physical activity levels, we examined two components of total energy expenditureADJ: adjusted resting metabolic rate (resting metabolic rateADJ) and adjusted activity energy expenditure (activity energy expenditureADJ). Activity energy expenditureADJ was calculated as (0.9total energy expenditureADJ – resting metabolic rateADJ). Resting metabolic rateADJ was not correlated with physical activity (t(202)=−0.14, β=−0.02±0.11, p=0.89; Fig. 3A). Like total energy expenditureADJ, activity energy expenditureADJ increased over the low and middle range of physical activity but plateaued above ~230 CPM/d (Fig. 3A). Notably, the activity energy expenditureADJ vs physical activity regression had a significantly non-zero intercept (621.8±44.3, t(202)=14.0, p<0.001). That is, activity energy expenditureADJ, the component of total energy expenditure generally thought to reflect physical activity, was estimated at ~600 kcal/d (~27% of total energy expenditure) when physical activity assessed by accelerometry was 0 CPM/d. The intercept remains significantly greater than zero (545.7±76.4, t(145)=5.27, p<0.001) even when the analysis is limited to subjects with physical activity values below the 230 CPM/d plateau point, where the activity energy expenditureADJ vs physical activity slope is greatest. Similar results were obtained when examining raw (i.e., unadjusted) total energy expenditure, resting metabolic rate, and activity energy expenditure values, and for a range of models controlling for effects of body size and composition (see Supplemental Experimental Procedures).
Figure 3.
A. Total energy expenditureADJ, resting metabolic rateADJ, and activity energy expenditureADJ (kcal/d) versus physical activity (CPM/d) for the subset of subjects (n=204) with measured resting metabolic rate. Ordinary least squares regressions are shown. Resting metabolic rateADJ is not correlated with physical activity, nor are total energy expenditureADJ or activity energy expenditureADJ among subjects with physical activity above 230 CPM/d. B. Components of total energy expenditure (dotted line) modeled as a function of physical activity, using relationships shown in panel A. Resting metabolic rate is constant (1540 kcal/d). Below the change-point of 230 CPM/d, total energy expenditure = 1.12 CPM/d + 2336; above 230 CPM/d, total energy expenditure is constant (2600 kcal/d). The thermic effect of food (TEF) is calculated as 10%total energy expenditure. Activity energy expenditure (red) calculated as 0.9total energy expenditure – resting metabolic rate, is divided into two components. Activity energy expenditure1 (AEE1, solid red) increases with physical activity in a dose-dependent manner as 1.13CPM/d, the slope of the Adjusted energy expenditure vs physical activity regression for subjects below 230 CPM/d in panel A. Activity energy expenditure2 (AEE2, hatched red) is the remainder of activity energy expenditure, calculated as activity energy expenditure2 = activity energy expenditure – activity energy expenditure1. See also Figures S1 and S3.
We modeled two components of activity energy expenditure (Fig. 3B). Activity energy expenditure1 is the component directly linked to physical activity in a dose-dependent manner, and is calculated using the slope of the activity energy expenditure vs physical activity regression for CPM/d<230 (Fig. 3A). When CPM/d=0, activity energy expenditure1=0, and each increment of physical activity incurs a corresponding increase in activity energy expenditure1. Activity energy expenditure2 is the remainder of activity energy expenditure, calculated by subtracting activity energy expenditure1 from activity energy expenditure. Activity energy expenditure2 decreases with physical activity above 230 CPM/d, absorbing increases in activity energy expenditure1 while total energy expenditure plateaus (Fig. 3B).
DISCUSSION
Metabolic Response to Variation in Habitual Physical Activity
Our analyses of total energy expenditure and physical activity support a Constrained total energy expenditure model. Rather than increasing linearly, in the dose-dependent manner predicted by Additive total energy expenditure models, the relationship between physical activity and total energy expenditureADJ plateaued over the upper range of CPM/d, representing n=92 to 99 subjects, roughly 30% of the dataset (Fig. 2; Fig. 3; Table S2). While physical activity must incur an immediate energy cost (activity energy expenditure1), compensatory changes in energy expended on other activities (activity energy expenditure2) apparently negated the additive effect of additional physical activity on total energy expenditure among individuals above ~230 CPM/d.
The physiological activities comprising activity energy expenditure2, and adapting to high levels of habitual physical activity, are not immediately evident. One hypothesis is that activity energy expenditure2 reflects muscle activity that is not readily recorded via accelerometry (e.g., postural efforts against gravity, fidgeting). These activities have been shown to contribute substantially to total energy expenditure [20-22] and their reduction may contribute to metabolic adaptation [23]. However, the magnitude of activity energy expenditure2 for sedentary subjects (~600 kcal/d) exceeds the estimated daily cost of standing, fidgeting, and peripheral limb movement [20-22] that would be missed using our accelerometry protocol, suggesting that muscular activity cannot solely account for activity energy expenditure2.
We hypothesize that non-muscular physiological activity contributes substantially to activity energy expenditure2 and its adaptation to physical activity. Human studies and non-human animal models show that energy allocation across a broad range physiological tasks, including reproductive activity and somatic maintenance [5,13–19], may be reduced when physical activity increases, resulting in decreased activity energy expenditure2. Indeed, such physical activity-induced reduction in activity energy expenditure2 could potentially contribute to the beneficial health effects of exercise, reducing energy expenditure on inflammation and detrimental immune system activity [24]. Non-muscular contribution to activity energy expenditure2 could also explain why inactive subjects confined to bedrest exhibit physical activity levels (i.e., the ratio of total energy expenditure/basal metabolic rate) of 1.2 – 1.4, above the value of 1.1 predicted by Additive total energy expenditure models [25]).
The mechanisms determining the total energy expenditure set-point and regulating activity energy expenditure2 in response to physical activity and the specific changes in energy expenditure are a critical target for future research. Food availability, and particularly the ratio of food availability to physical activity, may be an important developmental signal in determining an individual's total energy expenditure set-point [5]. In support of this hypothesis, subjects with greater body fat percentage, which can be considered a long-term signal integrating food energy availability and habitual physical activity, exhibited marginally higher total energy expenditureADJ across all physical activity levels (Table 1, Models 7 and 8; see Fig. S3). Activity intensity may also play a signaling role, given the positive and negative effects of sedentary and vigorous activity bouts, respectively, on total energy expenditure (Table 1, Models 4 and 8). Activity intensity could potentially modulate activity energy expenditure2 via its effect on fatigue, for example by promoting postural behaviors that save energy (e.g., sitting instead of standing; see ref. 23), or via myokine signaling [26].
Limitations
One important limitation of this study is its cross-sectional design. While the available data from prospective studies support a Constrained total energy expenditure model [5], it would be useful to investigate the relationships between total energy expenditure and physical activity examined here within subjects as physical activity was increased over several months, in a longitudinal design. Further, as discussed above, accelerometery is an imperfect measure of physical activity and energy expended in physical activity, which undoubtedly adds to the variance in total energy expenditureADJ with respect to physical activity (Fig. 1A & S3). Another limitation is the absence of resting metabolic rate measurements for subjects at the Jamaica study site, which reduces the sample size for calculating resting metabolic rate and activity energy expenditure. We also lack measurements of the thermic effect of food and must rely on estimates here for calculating activity energy expenditure. Finally, we lack biomarker data to test hypotheses regarding the role of non-muscular physiological activity in modulating activity energy expenditure2.
Bridging Ecological and Experimental Studies of Total Energy Expenditure
The Constrained total energy expenditure model evaluated here provides a unifying framework for seemingly contradictory results from previous studies examining physical activity and total energy expenditure. For studies with large samples that include both high- and low-physical activity individuals [4], physical activity is expected to have a significant positive effect on total energy expenditure due to the effect of physical activity on total energy expenditure in low- to moderate-physical activity individuals (Fig. 2). Similarly, intervention studies that increase physical activity in sedentary subjects are expected to see an increase in total energy expenditure, at least over the short-term (~20 weeks; ref. 5,10-12). However, metabolic adaptation to long-term changes in physical activity will blunt the relationship between habitual physical activity levels and total energy expenditure. As a result, comparing industrialized populations with more active traditional populations [6,7], or animal populations in the wild with those in captivity [8], may not reveal differences in total energy expenditure despite clear differences in physical activity.
The relationship between physical activity and total energy expenditure demonstrated in the large, diverse human sample here is both more variable and more complex than current Additive total energy expenditure models allow. Regardless of the preferred statistical model, physical activity accounts for only ~7 – 9% of the variation in total energy expenditure after controlling for anthropometric variables and population location. Energy balance models focusing solely on the effect of physical activity on total energy expenditure while ignoring the interdependent and dynamic role of other organ systems will miss a large portion of the variation in daily energy requirements, and may provide a biased measure of total energy expenditure. As shown here, Additive total energy expenditure approaches will tend to underestimate the effect of physical activity on total energy expenditure at low to moderate levels of activity, and overestimate the effect of physical activity at higher activity levels (Fig. 2B). Further, using activity energy expenditure or the ratio of total energy expenditure / basal metabolic rate (i.e., physical activity level) to assess physical activity will overestimate energy expenditure on activity for subjects at habitually low physical activity levels by pooling activity energy expenditure1 with activity energy expenditure2 (Fig. 4), which we suggest includes non-muscular physiological activity. Adopting a Constrained total energy expenditure model for physical activity [5] and parsing activity energy expenditure into activity energy expenditure1 and activity energy expenditure2 will improve the accuracy of energy balance models and advance public health strategies for mitigating the global epidemic of metabolic disease.
EXPERIMENTAL PROCEDURES
Data Collection
Subjects were enrolled as part of the Modeling the Epidemiological Transition Study, METS [9]. Institutional permissions and subjects’ informed consent were obtained prior to data collection. Height and weight were measured using a stadiometer and digital scale, respectively, and self-reported age was recorded. Total energy expenditure was measured for each subject for 7 d using the doubly labeled water (DLW) method [27]. Subjects ingested 1.8 g of 10% H2 18O and 0.12 g 99.9% 2H2O per kg body water. Urine samples collected prior to dosing, 4 hours after dosing, and 7 days after dosing, were analyzed for isotope enrichment at the Stable Isotope Core Laboratory at University of Wisconsin, Madison, WI, USA. The CO2 production was calculated using equation 6.6 in ref. 27, and energy expenditure was calculated using the modified Weir equation, with respiratory exchange ratio determined from dietary records. Surveys were used to identify subjects employed in manual labor.
Resting metabolic rate was measured via respirometry (MaxIIa indirect calorimeter, AEI Technologies, Aurora, IL, USA; SensorMedics, Viasys Health Care, Waukegan, IL, USA) in the morning, after an overnight fast. Subjects were supine during resting metabolic rate measurements, which lasted 30 minutes. Both oxygen consumption and CO2 production were monitored; data from the first 10 minutes of each measurement was discarded. Due to equipment failure, resting metabolic rate data from the Jamaican study site had to be discarded prior to analysis; Jamaican subjects are not represented in resting metabolic rate or activity energy expenditure analyses here.
Physical activity was measured using wearable tri-axial accelerometer (Actical, Phillips Respironics, Bend, OR, USA) [9]. Subjects were asked to wear the accelerometers continuously for 8 days coinciding with total energy expenditure measurement, and to remove the devices only for swimming, showering, or bathing. Days were considered valid for analysis only if the devices were worn ≥62% of maximal available wear time, and subjects were only included in analyses of physical activity if they recorded a minimum of 4 valid days. Wear time did not covary with measured physical activity levels: there are no differences among the deciles of physical activity (CPM/d) in wear time (ANOVA: F(9,322)=0.423, p=0.922)). For analyses of physical activity intensity (Table 1, Model 4 & 8), physical activity was defined as “sedentary” (<100 CPM) or “vigorous” (≥3960 CPM) using published cut-points [28,29]. Following the National Center for Health Statistics [30], “sedentary” and “vigorous” physical activity intensity (Table 1, Model 4 & 8) is the total time in minutes accumulated in 10- minute intervals. Following prior conventions, we allowed for up to 2 minutes of below- or above-threshold count activity before considering the bout to be ended [30].
Data Analysis
We analyzed the association between total energy expenditure and physical activity, assessed via accelerometry as mean CPM per day (CPM/d), using several approaches. We began by using multivariate regression to investigate the relative effects of anthropometric variables (fat free mass, fat mass, height, age, and sex) and behavioral or lifestyle variables (accelerometry CPM/d, employment in manual labor, and location) on total energy expenditure, using linear regression in R [31]. By far the strongest anthropometric correlate of total energy expenditure was fat free mass; fat mass and height were marginally, negatively correlated with total energy expenditure, and age and sex had no effect (Table 1, Model 1). To examine the effect of physical activity on total energy expenditure while controlling for anthropometric effects, we calculated adjusted total energy expenditure, total energy expenditureADJ, for each subject by adding residuals from the total energy expenditure~fat free mass+fat mass+ height+age+sex+study site regression to mean total energy expenditure (Model 2 in Table 1; see Supplemental Experimental Procedures). Total energy expenditureADJ was used for subsequent analyses in the main text. We similarly calculated an adjusted resting metabolic rate, resting metabolic rateADJ, by adding residuals from the resting metabolic rate~fat free mass+fat mass+height+age+sex+study site regression to mean resting metabolic rate, and calculated an adjusted activity energy expenditure, activity energy expenditureADJ= 0.9total energy expenditureADJ – resting metabolic rateADJ. We also tested a range of other models correcting for anthropometric and other effects on total energy expenditure and resting metabolic rate, as well as raw (unadjusted values) of total energy expenditure, resting metabolic rate, and activity energy expenditure; results were nearly identical to those reported in the main text (see Supplemental Experimental Procedures and Figures S1,S2).
To examine the shape of the relationship between physical activity and total energy expenditure and compare Additive and Constrained total energy expenditure models, we fit three different regression models to the scatterplot of total energy expenditureADJ against CPM/d. First, we fit a robust Locally Weighed Regression (LOWESS) curve [32] using the lowess function in R [31], with f=2/3, iter=5. This nonparametric model allows studying non-linear relationships between continuous variables (e.g., physical activity and total energy expenditure) without assumptions about the shape of the underlying function. Second, to test the fit of a linear, Additive total energy expenditure model, we estimated the linear correlation, via Pearson's correlation coefficient, between total energy expenditureADJ and physical activity (Table 1, Model 5,6). We used a modified version of this approach for the CPM/d threshold analysis (Fig. 1B): we evaluated the effect of CPM/d and manual labor on total energy expenditureADJ via linear regression for all subjects with CPM/d values above a threshold CPM/d=i, and iterated this analysis over the range of CPM/d thresholds i = (1,2,3...500). The resulting set of β, standard error, and model adjusted r2 values were examined with respect to CPM/d threshold (Fig. 1B). Lastly, we used change-point regression to estimate the association between physical activity and total energy expenditureADJ, controlling for manual labor employment. This model is similar to the Constrained total energy expenditure model which predicts a plateau in the physical activity:total energy expenditure relationship at higher activity levels (Fig. 1) and allows the estimation of a change point, from increasing linear/additive to flat/plateau. The change point was estimated using a computer intensive grid search approach [33], which has been shown to more flexible than the standard method based on maximum likelihood estimation [34]. Bootstrap simulations were applied to calculate the standard error of the change point estimator [35]. We applied an F-like test, based on an approximate permutation test, using a computer intensive algorithm as described in the literature to formally test whether the Constrained total energy expenditure model (piece-wise regression model) was preferred over the Additive total energy expenditure model (traditional linear regression) [36].
Supplementary Material
Acknowledgements
The authors would like to acknowledge the site-specific clinic staff members responsible for this data collection, as well as the 332 participants. Three anonymous reviewers provided comments that improved this paper. METS is funded in part by the National Institutes of Health (1R01DK80763).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
HP, LD, and AL conceived the study. LD, JP-R, PB, TEF, EVL, and AL collected data. HP, RD-A, LD, RSC, DAS, and AL analyzed data. HP, RD-A, LD, PB, RSC, DAS, and AL wrote the manuscript.
Supplemental Information
Supplemental information includes Supplemental Experimental Procedures, two tables, and four figures, and can be found online.
References
- 1.World Health Organization Obesity and Overweight. 2014 Fact sheet No. 311, Available: http://www.who.int/mediacentre/factsheets/fs311/en/
- 2.World Health Organization . Global recommendations on physical activity for health. World Health Organization; Geneva: 2010. p. 60. [PubMed] [Google Scholar]
- 3.F.A.O./W.H.O./U.N.U. Human energy requirements. FAO Food and Nutrition Technical Report Series 1. 2001 http://www.fao.org/docrep/007/y5686e/y5686e00.htm#Contents.
- 4.Plasqui G, Bonomi AG, Westerterp KR. Daily physical activity assessment with accelerometers: new insights and validation studies. Obes. Rev. 2013;14:451–62. doi: 10.1111/obr.12021. [DOI] [PubMed] [Google Scholar]
- 5.Pontzer H. Constrained total energy expenditure and the evolutionary biology of energy balance. Exer. Sport. Sci. Rev. 2015;43:110–116. doi: 10.1249/JES.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 6.Dugas LR, Harders R, Merrill S, Ebersole K, Shoham DA, Rush EC, Assah FK, Forrester T, Durazo-Arvizu RA, Luke A. Energy expenditure in adults living in developing compared with industrialized countries: a meta-analysis of doubly labeled water studies. Am. J. Clin. Nutr. 2011;93:427–441. doi: 10.3945/ajcn.110.007278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pontzer H, Raichlen DA, Wood BM, Mabulla AZ, Racette SB, Marlowe FW. Hunter-gatherer energetics and human obesity. PLoS ONE. 2012;7:e40503. doi: 10.1371/journal.pone.0040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pontzer H, Raichlen DA, Gordon AD, Schroepfer-Walker KK, Hare B, O'Neill MC, Muldoon KM, Dunsworth HM, Wood BM, Isler K, et al. Primate energy expenditure and life history. Proc. Nat'l. Acad. Sci. USA. 2014;111:1433–7. doi: 10.1073/pnas.1316940111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luke A, Bovet P, Forrester TE, Lambert EV, Plange-Rhule J, Schoeller DA, Dugas LR, Durazo-Arvizu RA, Shoham D, Cooper RS, et al. Protocol for the modeling the epidemiologic transition study: a longitudinal observational study of energy balance and change in body weight, diabetes and cardiovascular disease risk. BMC Public Health. 2011;11:927. doi: 10.1186/1471-2458-11-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Med. Sci. Sports Exerc. 2001;33:S521–7. doi: 10.1097/00005768-200106001-00023. [DOI] [PubMed] [Google Scholar]
- 11.Westerterp KR, Meijer GA, Janssen EM, Saris WH, Ten Hoor F. Long-term effect of physical activity on energy balance and body composition. Br. J. Nutr. 1992;68:21–30. doi: 10.1079/bjn19920063. [DOI] [PubMed] [Google Scholar]
- 12.Thomas DM, Bouchard C, Church T, Slentz C, Kraus WE, Redman LM, Martin CK, Silva AM, Vossen M, Westerterp K, et al. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes. Rev. 2012;13:835–4. doi: 10.1111/j.1467-789X.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison PT. Energetics and reproductive effort. Am. J. Hum. Biol. 2003;15:342–51. doi: 10.1002/ajhb.10152. [DOI] [PubMed] [Google Scholar]
- 14.Perrigo G, Bronson FH. Foraging effort, food intake, fat deposition and puberty in female mice. Biol. Repro. 1983;29:455–463. doi: 10.1095/biolreprod29.2.455. [DOI] [PubMed] [Google Scholar]
- 15.Weirsma P, Selman C, Speakman JR, Verhulst S. Birds sacrifice oxidative protection for reproduction. Proc. R. Soc. Lond. B. (Suppl) 2004;271:S360–S363. doi: 10.1098/rsbl.2004.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weirsma P, Verhulst S. Effects of intake rate on energy expenditure, somatic repair and reproduction of zebra finches. J. Exp. Biol. 2005;208:4091–4098. doi: 10.1242/jeb.01854. [DOI] [PubMed] [Google Scholar]
- 17.Bautista LM, Tinbergen J, Wiersma P, Kacelnik A. Optimal foraging and beyond: How starlings cope with changes in food availability. Am. Nat. 1998;152:543–561. doi: 10.1086/286189. [DOI] [PubMed] [Google Scholar]
- 18.Deerenberg C, Overkamp GJF, Visser GH, Daan S. Compensation in resting metabolism for experimentally increased activity. J. Comp. Physiol. B. 1998;168:507–512. [Google Scholar]
- 19.Perrigo G. Breeding and feeding strategies in deer mice and house mice when females are challenged to work for their food. Animal Behav. 1987;35:1298–1316. [Google Scholar]
- 20.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 21.Levine JA. Non-exercise activity thermogenesis. Proc. Nutr. Soc. 2003;62:667–679. doi: 10.1079/PNS2003281. [DOI] [PubMed] [Google Scholar]
- 22.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J. Clin. Invest. 1986;78:1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melanson EL, Keadle SK, Donnelly JE, Braun B, King NA. Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Med. Sci. Sports Exerc. 2013;45:1600–1609. doi: 10.1249/MSS.0b013e31828ba942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverman MN, Deuster PA. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus. 2014;4:20140040. doi: 10.1098/rsfs.2014.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pontzer H. Energy expenditure in humans and other primates: a new synthesis. Ann. Rev. Anthrop. 2015;44:169–187. [Google Scholar]
- 26.Pedersen BK, Febbraio MA. Muscle, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012;8:457Y65. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 27.I.A.E.A. Human Health Series 3, Assessment of Body Composition and Total Energy Expenditure in Humans Using Stable Isotope Techniques. IAEA; Vienna: 2009. [Google Scholar]
- 28.Colley RC, Tremblay MS. Moderate and vigorous physical activity intensity cut-points for the Actical accelerometer. J. Sports Sci. 2011;29:783–9. doi: 10.1080/02640414.2011.557744. [DOI] [PubMed] [Google Scholar]
- 29.Wong SL, Colley R, Connor Gorber S, Tremblay M. Actical accelerometer sedentary activity thresholds for adults. J. Phys. Act. Health. 2011;8:587–591. doi: 10.1123/jpah.8.4.587. [DOI] [PubMed] [Google Scholar]
- 30.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 31.Team RC. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna), Version 3.1.0. 2013 [Google Scholar]
- 32.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 1979;74:829–836. [Google Scholar]
- 33.Lerman PM. Fitting segmented regression models by grid search. Appl. Stat. 1980;29:77–84. [Google Scholar]
- 34.Hinkley DV. Inference in two-phase regression. J. Am. Stat. Assoc. 1971;66:736–43. [Google Scholar]
- 35.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat. Sci. 1986;1:54–75. [Google Scholar]
- 36.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statist. Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.