Abstract
Objectives
To investigate premorbid sleep reactivity as a vulnerability to incident shift work disorder and related changes in depression and anxiety following a transition to a rotating shifts work schedule.
Methods
This is a longitudinal study with two waves of data collection. The community-based sample included normal sleeping non-shift workers (N=96; 62.5% female; 47.9±13.3 yo) without a lifetime history of insomnia or baseline excessive daytime sleepiness who transitioned to rotating shift work one year later. Participants reported demographic characteristics, trait sleep reactivity on the Ford Insomnia Response to Stress Test, depression symptoms on the Quick Inventory of Depression Symptomatology, and anxiety symptoms on the Beck Anxiety Inventory. Shift work disorder was determined based on significant sleep disturbance and/or excessive sleepiness in the context of working a rotating shifts schedule.
Results
Analyses revealed that the odds were over five times greater for highly sleep reactive individuals to develop shift work disorder after transitioning to rotating shifts (OR=5.59, p=.04). Nearly 90% of shift work disorder sufferers were accurately identified as high risk at 1-y prior to disease onset. Furthermore, individuals who developed SWD reported greater increases in symptoms of depression and anxiety. Finally, analyses revealed significant indirect effects wherein high sleep reactivity increased risk for SWD, which led to greater severity of anxiety and depression symptoms.
Conclusions
The FIRST accurately identifies a focused target population in which the premorbid psychobiological processes complicit in SWD onset and progression, as well as shift work-related depression and anxiety changes, can be better investigated, thus improving future preventative efforts.
Keywords: sleep reactivity, shift work disorder, FIRST, depression, anxiety, rotating shifts
1. Introduction
Labor statistics estimate that at least 2.5% of the US work force is scheduled on rotating shifts1, which include both rapid shifting (e.g., multiple changes in work hours during a week) and slow rotations (e.g., three weeks per shift schedule). Owing to the constant flux of rotating shifts, work-related sleep-wake schedules often conflict with internal circadian rhythms. Sleep-wake disturbances in response to a circadian challenge are highly variable between individuals2, 3, but a substantial subset of shift workers are unable to recalibrate the timing of their circadian clocks to their work-related sleep-wake schedules4, 5. Indeed, up to 26% of rotating shift workers develop shift work disorder (SWD)4, which is characterized by persistent and severe sleep disturbance during the sleep period and/or excessive sleepiness during the wake period6. In addition to sleep-wake complaints, shift workers report poorer mental health and lower quality of life7–9. To improve preventative efforts against SWD and comorbid psychiatric complaints, it is important to identify trait characteristics corresponding to adverse response to shift work-related challenges to the circadian system. Prior research has shown that normal sleepers with high sleep reactivity—i.e., a sensitive sleep system—are prone to transient sleep disturbance and increased wake time sleepiness in response to a single night of circadian misalignment10. However, no studies to date have compared normal sleepers with high versus low sleep reactivity in their risk for developing a circadian rhythm sleep disorder and associated psychiatric complaints.
Disruptions in sleep and wake patterns can reflect normative adjustment to shiftwork exposure and circadian misalignment11, 12. In comparison to normal sleep-wake characteristics of shift workers, SWD is marked by chronic and severe sleep-wake disturbances due to inability to sufficiently adjust to shiftwork11, 12. The cardinal features of SWD are sleep disturbance during the sleep period and/or excessive sleepiness during the wake period as a direct result of a mismatch between the endogenous timing of the circadian rhythm and the exogenous work-related sleep-wake schedule6. One previously identified trait characteristic predictive of shift work tolerance is diurnal preference, or morningness versus eveningness13, which has been linked to a polymorphism of the clock gene, PERIOD314, 15. Individuals with morningness preference, associated with the homozygotic PERIOD35/5 polymorphism14, 15, are less tolerant to shift work-related circadian disruption13 and sleep loss15. Much of the research on sequelae related to diurnal preference and the PERIOD3 gene following a circadian challenge largely focuses on the degree to which sleep loss impairs wake functioning12, 15, 16. However, prior research has also demonstrated a certain vulnerability to insomnia to be sensitive to circadian misalignment10, which may further elucidate trait characteristics that pre-dispose vulnerable individuals to sleep disturbance following a circadian challenge.
Sleep reactivity is a heritable pre-disposition to sleep disturbance and insomnia that manifests in a sleep system that is sensitive or “reactive” to stress17–21. Though prior research has shown highly reactive sleepers to be more sensitive to major life events19 and stimulant-use17 among other situational stressors, only one previous study has examined the effects of circadian disruption on good sleepers with robust versus sensitive sleep systems. In a laboratory setting, Bonnet and Arand10 found that good sleepers with high sleep reactivity—as evidenced by sensitivity to first night laboratory effects and caffeine intake—experienced significantly greater sleep disturbance and subsequent daytime sleepiness in response to a 6-hour phase advance than good sleepers with more robust sleep systems. Despite demonstrating the sensitivity of reactive sleepers in response to a phase advance, it is presently unclear whether these findings would translate outside of the laboratory. Further, it is not known whether highly reactive sleepers eventually adapt to circadian misalignment or if they continue to suffer.
Importantly, the impact of sleep reactivity may not be limited to work-related sleep-wake disturbances, but also to co-occurring changes in mood. Prior research has shown sleep problems to facilitate the relationship between sleep reactivity and depression, such that highly reactive sleepers are pre-disposed to sleep disturbance and insomnia, which, in turn, elevate risk for depression18, 19. As such, it is possible that high sleep reactivity constitutes a trait vulnerability to shift work-related changes in mood, and that SWD may facilitate this relationship. Despite evidence showing circadian desynchrony to elicit sleep-wake symptoms in highly reactive normal sleepers10, and that sleep reactivity increases risk for sleep problems and resultant mood difficulties18, the role of sleep reactivity in the development of SWD and shift work-related affective symptoms has not been explored. To address these gaps in the literature, we explored the role of trait sleep reactivity in the development of SWD and work-related changes in depression and anxiety following transition to rotating shifts.
Using the transition to rotating shift work as a naturalistic model of circadian misalignment, we examined premorbid sleep reactivity as a trait risk factor for developing SWD and related elevations in depression and anxiety. Rotating workers are unique in that their shifts are in constant flux, thus presenting a persistent challenge to the circadian system. As such, rotating shifts may confer a particularly robust challenge for individuals whose sleep systems are sensitive to such disruptions. We hypothesized that highly reactive sleepers would be at more vulnerable to developing SWD in comparison to non-reactive individuals. We also predicted that individuals with high sleep reactivity would experience greater increases in symptoms of depression and anxiety after transitioning to rotating shifts, and that SWD would mediate these relationships.
2. Methods
2.1 Participants
Data were collected from a large community sample in Southeastern Michigan from two similar protocols as part of the Evolutions of Pathways to Insomnia Cohort (EPIC) study, a 3-year NIMH-funded investigation (see Table 1 for sample characteristics). The EPIC study is a prospective investigation of a large community-based sample with no prior/current history of insomnia at baseline. Recruitment procedures and broader demographic statistics have been reported in detail elsewhere for both protocols18, 22. The present study analyzed data from participants who met the following inclusionary criteria: 1) no lifetime history of insomnia per DSM-IV based diagnostic criteria at baseline23, 2) no excessive daytime sleepiness at baseline, 3) not working rotating or night shift at baseline, and 4) transitioned to a rotating shifts schedule at 1-y follow-up. Rotating shiftwork status was based on self-report data, and frequency of rotations and timing of shifts were not reported. Ninety-six out of 5208 participants from both EPIC study protocols met inclusionary criteria.
Table 1.
Sample descriptive characteristics (N=96).
| Baseline work shift | |
| Day shift | N=55 |
| Evening shift | N=7 |
| Unemployed | N=34 |
| Age | M±SD: 38.9±16.1 |
| Sex (female) | N=60; 62.5% female |
| Race * | |
| White | 65.6% |
| Black | 19.8% |
| Asian | 6.3% |
| Middle Eastern/Indian | 4.2% |
| Other | 4.2% |
| Sleep ratings | |
| Weekday total sleep time | M±SD: 435 minutes ± 68 |
| Weekend total sleep time | M±SD: 477 minutes ± 76 |
| Sleep onset latency | M±SD: 24 minutes ± 17 |
| Wake after sleep onset | M±SD: 16 minutes ± 16 |
| Sleep quality | M±SD: 0.8 ± 0.6 – 'Fairly good' |
| Daytime sleepiness | |
| ESS total score | M±SD: 5.8±2.2 |
| Stress Exposure | |
| Number of events | M±SD: 1.8±1.7 |
| Depression | |
| QIDS | M±SD: 4.30±3.09 |
| Anxiety | |
| BAI | M±SD: 4.86±5.61 |
Notes:
Percentages do not equal 100% due to rounding.
ESS = Epworth Sleepiness Scale. Stress exposure assessed by number of endorsed items on the Social Readjustment Rating Scale. QIDS = Quick Inventory of Depressive Symptomatology. BAI = Beck Anxiety Inventory.
2.2 Procedure
Study protocols were approved by the Henry Ford Hospital institutional review board. All participants provided written informed consent prior to participating in the study. Data from both protocols were collected in three annual waves. One month prior to each annual follow-up (at Years 2 and 3), participants received email reminders. Each assessment took approximately 30 minutes to complete. For the present study, only two time-points were evaluated for each participant. Baseline assessment (Time 1) was defined as the time-point preceding a transition to rotating shift work (which could be either Year 1 or Year 2, depending on when the transition to rotating shifts occurred), and 1-y follow-up (Time 2) was defined as the year in which participants transitioned to rotating shifts (either Year 2 or Year 3). As such, between the two separate protocols, 53 eligible participants had baseline assessments at Year 1 and 1-y follow-up at Year 2, whereas the remaining 43 participants had baseline assessment at Year 2 and 1-y follow-up at Year 3 (this latter group had to meet `baseline' inclusionary criteria at both Years 1 and 2).
2.3 Measures
Shift Work Disorder
SWD criteria were based on the International Classification of Sleep Disorders-3rd Edition6 (ICSD-3), and were consistent with prior research using a large population-based sample4. Specifically, participants were required to meet criteria for sleep disturbance and/or excessive sleepiness that temporally corresponded to transitioning to a rotating shift work schedule based on self-reported sleep-wake symptoms. Sleep disturbance was established based on reported difficulty initiating and/or maintaining sleep, or having non-restorative sleep for at least 1 month. Additionally, daytime impairment secondary to these nocturnal symptoms had to be endorsed to meet criteria. Daytime impairment was assessed with the following item: `To what extent do you consider your sleep problem to interfere with your daily functioning (e.g. daytime fatigue, ability to function at work/daily chores, concentration, memory, mood, etc)?' with response options ranging from 0 “not at all” to 4 “very much.” Endorsement was defined as a response of 2 “somewhat” or higher. Excessive sleepiness was assessed using the Epworth Sleepiness Scale (ESS)24, 25, a self-report measure shown to distinguish between individuals with and without sleep disorders in clinical samples26 and the general population27. Higher scores on the ESS indicate more excessive sleepiness, and prior research has used a score of 10 or higher to reflect abnormal levels of sleepiness28, 29. As such, participants in the present study with ESS scores ≥ 10 were classified as having excessive sleepiness. Individuals with insomnia or excessive sleepiness at baseline were excluded from analyses, whereas participants who met criteria for either or both at Time 2, after transitioning to rotating shifts, were diagnosed with SWD.
Sleep reactivity
The present study examined baseline trait sleep reactivity, prior to new onset SWD. The Ford Insomnia Response to Stress Test (FIRST)29 is a self-report measure of sleep reactivity. Items on the FIRST ask respondents to rate the likelihood (1=Not, 2=Somewhat, 3=Moderately, and 4=Very likely) that they would experience sleep difficulties in reaction to nine hypothetical stressful situations (e.g., `after a stressful experience during the day,' `before an important meeting the next day'). Higher scores on the FIRST indicate greater sleep reactivity, and an as of yet unpublished study by our research team shows that scores of 16 and higher on the FIRST identifies individuals with reactive sleep systems accurately who are at elevated risk for future insomnia disorder. As such, in the present study, this cut-point was used to classify normal sleepers as having high versus low sleep reactivity.
Subjective sleep parameters
Participants estimated sleep parameters in response to the following items. Weekday total sleep time (TST): `Thinking about your average weekday, how long did you actually sleep each night?' with responses in hours and minutes. Weekend TST: `Thinking about your average weekend, how long did you actually sleep, each night?' with responses in hours and minutes. Sleep onset latency: `On average (including weekdays and weekends), how long does it take you to fall asleep (in the past month)?' with responses in minutes. Wake after sleep onset: `On average, how long does it take you to fall back asleep after waking up (during the past month)?' with responses in minutes. Sleep quality: `During the past month, how would you rate your sleep quality overall?' with responses ranging from 0 (Very good) to 3 (Very bad).
Stress exposure: Number of events
Stress exposure was based on the Social Readjustment Rating Scale-Revised (SRRS-R)30, an inventory of 52 stressful life events commonly reported by US adults. In the present study, we examined the total number of endorsed events for each participant reported for the year leading up to the transition to rotating shifts.
Depression
Participants also completed the 16-item version of the Quick Inventory of Depressive Symptomatology (QIDS)31, which quantifies depressive symptomatology on a 4-point (0-3) Likert-type scale with higher scores indicating greater depression severity. To reduce collinearity with insomnia, the sleep disturbance subscale of the QIDS was excluded. Total scores representing depression severity were used for testing substantive hypotheses, with higher scores indicating greater depression.
Anxiety
Participants self-reported anxiety levels using the Beck Anxiety Inventory (BAI)32, a 21-item questionnaire which measures the severity of common anxiety symptoms. Responses are rated on a 4-point (0-3) Likert-type scale with higher scores indicating greater anxiety severity.
2.4 Analysis plan
Logistic regression with maximum likelihood estimation was used to evaluate risk for SWD incidence at Time 2 as predicted by baseline sleep reactivity, while controlling for relevant demographic characteristics. Linear regression was used to test changes in depression and anxiety symptoms from baseline to 1-y follow-up. When testing mediation models, we first had to adjust parameter estimates for scaling differences between logistic and linear regression analyses as outlined by MacKinnon & Dwyer33. Next, we followed steps outlined by Fairchild and MacKinnon34 to test for mediation. Specifically, three regression models were conducted: 1) the direct effect of the predictor (sleep reactivity) on the outcome variable (depression or anxiety), 2) the effect of the predictor (sleep reactivity) on the proposed mediator (SWD; the α pathway), and finally 3) the effect of the mediator (SWD) on the outcome variable (depression or anxiety; the β pathway). The product of the α and β parameter estimates represent the indirect (i.e., mediated) effect of the predictor on the outcome variable. With regard to confidence intervals (CI) and significance testing of indirect pathways, traditional methods are relatively underpowered and yield inaccurate CIs given that mediated effects (i.e., products of two distributions) do not follow normal distributions34. Therefore, the CIs of the mediated effects were estimated using the PRODCLIN method35. This method does not assume a normal distribution, yields asymmetric CIs, and is thus more accurate than traditional significance tests36, 37. If the 95% CI for the indirect effect does not include zero, then significant mediation is inferred.
3. Results
3.1 Sample characteristics
Approximately half of the sample was classified as having high sleep reactivity at baseline (N=52/96). We observed 18 new cases of SWD after transitioning to rotating shifts, representing an incidence rate of 18.8% in the overall sample. We then compared normal sleepers and individuals with SWD on a number of sleep parameters and daytime impairment indices (see Table 2). Expectedly, individuals with SWD reported shorter TST on weekdays and weekends than good sleepers. When examining changes in TST from Time 1 to Time 2, individuals with SWD lost an hour of sleep on weekdays compared to just a half-hour on weekends. A group difference showing participants with SWD losing more sleep on weeknights than normal sleepers tended toward significance (p<.06). However, weekend-related sleep loss did not differ between groups (p=.44). As expected, large group differences were observed on ratings of sleep disturbance. Individuals with SWD reported average sleep onset latencies and wake time after sleep onset in the clinically significant range for sleep disturbance (>30 minutes; Table 2)38, whereas these parameters were within normal limits among asymptomatic rotating workers. Concordantly, subjective sleep quality ratings were more favorable in the normal sleeping group. Regarding impairment during the wake period, SWD sufferers reported greater severity of sleepiness, depression, and anxiety.
Table 2.
Comparing good sleepers and individuals with SWD on sleep ratings and daytime functioning at 1-y follow-up.
| Normal Sleepers M±SD | SWD M±SD | t(df), p-value, Cohen's d | |
|---|---|---|---|
| Weekday total sleep time | 408 ± 65 | 345 ± 101 | t(91)=2.97, p<.01, d=.74 |
| ΔWeekday total sleep time | −25 ± 68 | −62 ± 99 | t(91)=1.90, p<.06, d=.44 |
| Weekend total sleep time | 470 ± 76 | 404 ± 121 | t(91)=2.31, p=.02, d=.65 |
| ΔWeekend total sleep time | −8 ± 83 | −26 ± 80 | t(88)=.78, p=.44 |
| Sleep onset latency | 27 ± 24 | 54 ± 37 | t(89)=3.80, p<.001, d=.87 |
| Wake after sleep onset | 16 ± 14 | 42 ± 35 | t(91)=3.04, p<.01, d=.98 |
| Sleep quality | .83 ± .55 | 1.47 ± .72 | t(91)=4.10, p<.001, d=1.00 |
| Daytime sleepiness | 5.53 ± 2.31 | 8.06 ± 3.25 | t(91)=3.78, p<.001, d=.90 |
| Depression | 3.79 ± 2.92 | 7.27 ± 3.71 | t(85)=4.00, p<.001, d=1.04 |
| Anxiety | 3.79 ± 4.26 | 10.67 ± 9.33 | t(85)=4.46, p<.001, d=.95 |
Notes: Total sleep time, sleep onset latency, and wake after sleep onset are reported in minutes. ΔWeekday total sleep time represents the change in baseline weekday total sleep time to 1-y follow-up, with negative values indicating sleep loss on weeknights. ΔWeekend total sleep time represents the change in baseline weekend total sleep time to 1-y follow-up, with negative values indicating sleep loss on weekend nights. ESS = Epworth Sleepiness Scale. QIDS-SR16 = Quick Inventory of Depressive Symptomatology – Self-report 16 items (sleep items removed). BAI = Beck Anxiety Inventory.
3.2 Sleep reactivity as a risk factor for SWD
We began by identifying relevant covariates for estimating risk for SWD using logistic regression as predicted by age, gender, stress exposure, daytime sleepiness, depression, and anxiety. The overall model distinguished reliably between individuals with SWD and asymptomatic shift workers (χ2(5)=19.80, p<.01). Evaluation of the individual predictors revealed that greater daytime sleepiness at baseline increased the odds for SWD such that each 1-point increase in ESS scores corresponded to a 87% increase in the odds of developing SWD (b=.62, OR=1.87, 95% CI=1.18–2.95, p<.01). Additionally, the odds of developing SWD were 8% greater for men than women (b=−.08, OR=.93, 95% CI=.87–.99, p=.02). In contrast, age, stress exposure, and baseline depression and anxiety did not predict SWD-risk (all p-values > .05). Next, we estimated risk for SWD associated with being classified as having high versus low sleep reactivity (Table 3). Logistic regression revealed that the odds for highly reactive individuals developing SWD was over five times greater than the odds for low reactivity participants (OR=5.59, 95% CI=1.08–28.97, p=.04). Both gender (OR=.96, p=.03) and baseline sleepiness remained significant in the model (OR=1.49, p=.02). Reflecting the risk of high reactivity for incident SWD, 88.9% (N=16/18) of individuals with SWD were categorized as highly reactive at baseline. Further, 30.8% (16/52) of highly reactive baseline sleepers developed SWD, compared to just 4.5% (2/44) of the low reactivity group.
Table 3.
Predicting shift work disorder and shift work-related changes in depression and anxiety (N=96).
| Predictor | Outcome | b (SE) | OR | χ 2 |
|---|---|---|---|---|
| Predicting incident SWD | 18.60* | |||
| FIRST ≥ 16 | SWD | 1.72 (.84)* | 5.59 | |
| Daytime sleepiness | SWD | .40 (.17)* | 1.49 | |
| Gender | SWD | −.04 (.02)* | .96 |
| Predictor | Outcome | b (SE) | b' (SE') | Pathway | α β |
|---|---|---|---|---|---|
| Depression mediation model | .13‡ | ||||
| Sleep reactivity | SWD | .16 (.06)* | .36 (.13) | α | |
| SWD | Depression | 1.65 (.80)* | .33 (.16) | β | |
| controlling for baseline depression | |||||
| .40‡ | |||||
| Anxiety mediation model | |||||
| Sleep reactivity | SWD | .16 (.06)* | .48 (.21) | α | |
| SWD | Anxiety | 6.43 (1.5)* | .81 (.19) | β | |
| controlling for baseline anxiety |
Notes: OR = odds ratio. b= beta. SE = standard error. b' = adjusted beta. SE' = adjusted standard error. χ2 = chi-square, an indicator of the overall model's ability to distinguish between SWD and normal sleepers. αβ = indirect effect parameter estimate.
= p-value < .05.
= the 95% confidence interval for the indirect parameter estimate does not overlap with zero, indicating significant mediation.
SWD = Shift work disorder. FIRST = Ford Insomnia Response to Stress Test. Daytime sleepiness measured using the Epworth Sleepiness Scale at baseline. Sleep reactivity assessed using total scores from the Ford Insomnia Response to Stress Test at baseline. Depression measured using the Quick Inventory of Depressive Symptomatology – Self-Report 16 items, with sleep items removed, at 1-year follow-up. Anxiety measured using the Beck Anxiety Inventory at 1-year follow-up.
3.3 SWD mediating sleep reactivity and depression
We then tested a mediation model wherein higher sleep reactivity increased risk for SWD, which led to greater depression symptom severity (Fig 1). Using linear regression to identify relevant covariates in predicting Time 2 depression symptoms, only baseline depression (b=.57, p<.001) was a significant predictor, whereas stress exposure, age, gender, and baseline anxiety and sleepiness were all non-significant (all p-values > .05). Given that nearly 90% of SWD sufferers were highly reactive at baseline (i.e., FIRST ≥16), we entered FIRST total scores as predictors in the following regression models to reduce redundancy between the FIRST and SWD status. Linear regression revealed that sleep reactivity did not have a direct effect on depression at 1-y follow-up (p=.77), when controlling for baseline depression (b=.67, p<.001). However, since testing the significance of an indirect effect does not require a direct effect39–43, we tested for a significant indirect pathway from sleep reactivity through SWD, to changes in depression (Table 3). Logistic regression revealed that higher reactivity scores predicted greater risk for SWD (α pathway: OR=1.18, p<.01) such that each 1-point increase on the FIRST corresponded to an 18% increase in the risk for SWD onset. We then estimated Time 2 depression using sleep reactivity and SWD status as predictors, while controlling for baseline depression. Linear regression analysis revealed that individuals with SWD reported greater depressive symptoms at 1-y follow-up (β pathway: b=1.65, p=.04), while controlling for baseline depression (b=.59, p<.001) and sleep reactivity (b=.01, p=.94). After adjusting for parameter estimates' scaling differences due to using linear and logistic regression33 (Table 3), the indirect effect parameter (i.e., αβ) was estimated to be 0.13 (SE=.08), and the 95% CI for the product of coefficients was estimated to be [0.002–0.322]. As the CI did not overlap with zero, these results indicated a significant indirect effect in which SWD mediated the influence of sleep reactivity on depression severity.
Fig 1.
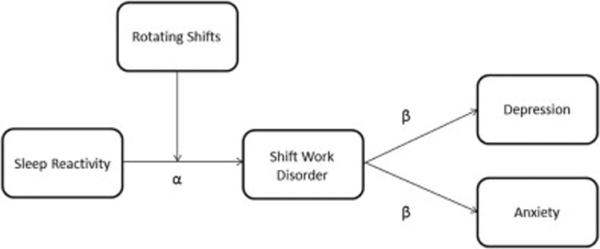
Sleep reactivity as a diathesis for shift work disorder and shift work-related depression and anxiety.
Notes: Figure depicts both a stress-diathesis model of SWD and the indirect pathways from sleep reactivity to depression and anxiety. Regarding the stress-diathesis model, sleep reactivity confers the diathesis and the transition to rotating shifts represents the stressor. Regarding the indirect pathways to depression and anxiety, sleep reactivity represents the trait vulnerability to SWD (α path), which is associated with increased depression and anxiety symptoms (β paths) among rotating workers.
3.4 SWD mediating sleep reactivity and anxiety
We then tested a mediation model in which higher sleep reactivity increased risk for SWD, which led to increased anxiety symptoms (Fig 1). Using linear regression to identify relevant covariates in predicting Time 2 anxiety symptoms, only baseline anxiety was shown to be a significant predictor (b=.54, p<.001). Similar to the depression model, sleep reactivity did not predict Time 2 anxiety (p=.82). We then tested for an indirect effect in which sleep reactivity led to shift work-related sleep problems that, in turn, increased anxiety symptoms. As previously established, higher reactivity predicted SWD-risk (α pathway: b=.16, OR=1.18, 95% CI=1.04–1.33, p<.01). Next, we regressed Time 2 anxiety on sleep reactivity and SWD status, while controlling for baseline anxiety. Analyses revealed that SWD was significantly associated with BAI scores (β pathway: b=6.43, p<.001), such that individuals with SWD reported greater anxiety severity. Baseline anxiety remained a significant predictor (b=.58, p<.001), whereas sleep reactivity was not (p=.47). After adjusting for parameter estimates' scaling differences (Table 3), the indirect effect parameter was estimated to be 0.40 (SE=.22) and the distribution of the product of coefficients' 95% CI was [0.043–0.891]. As the CI did not overlap with zero, these results indicated a significant indirect effect in which SWD mediated sleep reactivity and shift work-related anxiety.
4. Discussion
Using the transition to rotating shift work as a naturalistic challenge to the circadian system, this investigation sought to use a trait measure of sleep reactivity to identify normal sleepers at elevated risk for SWD and shift work-related changes in depression and anxiety. Rotating workers with high sleep reactivity were at substantially elevated risk for SWD. Importantly, results also indicated that highly reactive sleepers experienced escalations in depression and anxiety symptoms, but that these relationships were attributable to work-related changes in sleep-wake experiences. Together, these findings offer empirical support for high sleep reactivity conferring a trait vulnerability to SWD and related changes in affective symptoms.
The overall incidence rate of SWD at 1-y follow-up was 18.8%. Normal sleepers with high reactivity are at elevated risk for SWD, and nearly 90% of those who developed the sleep disorder evidenced high premorbid sleep reactivity. When comparing high versus low reactivity individuals at baseline, over 30% of highly reactive sleepers developed SWD compared to less than 5% of participants with low reactivity. Importantly, sleep reactivity was a more robust predictor of SWD than male gender and baseline daytime sleepiness. These findings are consistent with a prior laboratory study examining the effects of circadian phase advance on participants with robust sleep systems versus transient situational insomnia10. Our results add to the literature by demonstrating this effect using a more ecologically valid design, as well as demonstrating that desynchrony between the circadian system and work-related sleep patterns result in symptomatology that evolves beyond transient sleep-wake disturbance into clinical SWD. Even so, the mechanisms underlying the relationship between sleep reactivity and SWD remain unclear.
Borrowing from the insomnia literature, a mechanism by which highly reactive individuals develop and perpetuate sleep disturbances center on the cognitive processes that promote wakefulness during the sleep period19, 21, 44. Importantly, prior research offers corroborating neurobiological underpinnings consistent with this phenomenon. Specifically, Nofzinger and colleagues45 showed that wake-promoting brain regions in insomniacs showed smaller metabolic decreases when transitioning from wakefulness to sleep. As such, sleep reactivity and corresponding wake-promoting cognitive processes may reflect a neurological deficit in which the arousal mechanisms do not sufficiently abate, thus manifesting in tendency for wakefulness prior to and during the sleep period. Therefore, similar to the sensitivity to psychosocial stressors and stimulants among reactive sleepers10, a circadian challenge and resultant deviation from the endogenous timing of sleep onset may exacerbate a deficient neurological down-regulation of wake-promoting brain activity, resulting in marked wakefulness during the sleep period and subsequent evolution into SWD.
Given the psychiatric morbidities common among shift workers and SWD sufferers4, 9, we evaluated whether sleep reactivity predicted shift work-related changes in depression and anxiety. Contrary to our hypotheses, baseline sleep reactivity did not directly correspond to changes in depression or anxiety. However, consistent with the insomnia literature18, there were significant indirect pathways in which high sleep reactivity increased risk for SWD, which, in turn, was associated with elevated symptom severity in both depression and anxiety (Fig 1). When comparing asymptomatic shift workers and SWD sufferers at 1-year follow-up, we observed that individuals with SWD obtained significantly shorter sleep on week nights and weekends compared to asymptomatic shift workers. Further, workers with SWD reported greater sleep loss on weeknights from baseline to 1-y follow-up, as well as more prolonged wakefulness prior to and during the sleep period. These differences were related to poorer sleep quality and greater sleepiness among those with SWD. The observed elevations in depressed and anxious mood among participants with inadequate and inefficient sleep and related daytime drowsiness are consistent with a large body of research highlighting the detrimental effects of poor sleep on psychological wellbeing18, 46–48, thus highlighting the importance of identifying individuals pre-disposed to developing sleep and circadian rhythm disorders.
Though we believe the present study adds to the literature on risk for SWD, our findings should be interpreted in the context of some methodological limitations. First, we must acknowledge the potential threats to validity due to the use of self-report instruments. Clinical interviews are the gold standard for assessing sleep disorders, and the ICSD-36 recommends using sleep logs and actigraphy-based assessments as collateral measurements to inform SWD diagnoses whenever possible. Future studies replicating these results utilizing clinician-diagnosed SWD are needed to ensure validity of these findings in a clinical population. Related, biomarkers of circadian timing were not collected. Second, though participants reported working on rotating shifts, neither the specific timing of shifts nor the frequency of their rotations were recorded. Therefore, it is unclear from our findings which specific characteristics of rotating shifts (e.g., frequency, timing of shift, shift length, direction of rotation) are most disruptive to sleep and wake patterns for highly reactive sleepers. Future research may benefit from examining the characteristics of rotating shiftwork to which highly reactive sleepers are sensitive. Along these lines, future research is necessary to test if these results are generalizable to individuals transitioning to other types of shift schedules including early morning, split, extended, or night shifts. Unique to rotating shifts is the persistent challenge to the circadian system corresponding to shift rotation that may not grant the opportunity for sufficient realignment of the circadian system, therefore exploration of sleep reactivity as a diathesis in SWD among non-rotating shift workers is warranted. Finally, we must acknowledge our limited sample size, which may have limited our statistical power. It is notable, however, that we were able to detect these effects in a modestly sized study sample. Even so, we recommend that future investigations examining SWD and sleep reactivity utilize larger samples, findings from which are likely to be more generalizable and less susceptible to type II errors.
5. Conclusions
The present study demonstrated high sleep reactivity to be an important premorbid vulnerability to incident SWD and elevations in depression and anxiety in response to desynchrony between endogenous circadian timing and exogenous sleep patterns among rotating shift workers. By demonstrating the capability of pre-morbid sleep reactivity in identifying these at-risk individuals prior to work-related circadian disruption, preventative care and early detection may be appropriately directed. Moreover, identifying a targeted population intolerant of shift work allows for the identification of new premorbid biomarkers associated with circadian rhythm sleep disorders in response to challenges to the circadian system, such as deficient down-regulation of wake-promoting areas of the brain. Finally, future investigators may consider collecting assays of circadian biomarkers and circadian genes for comparison between high versus low reactivity sleepers, which may offer insight into the biological processes underlying the relationship between sleep reactivity and SWD.
Supplementary Material
Highlights.
We studied 96 individuals transitioning to rotating shift work
Sleep reactivity is a vulnerability to shift work disorder
Reactive sleepers are more depressed and anxious after starting shift work
Shift work disorder mediates relationship between sleep reactivity and mood
Acknowledgments
Funding/Support Acknowledgment: This study was supported by an NIMH Grant (R01 MH082785; PI: Drake) and an investigator initiated research award from Merck & Co (PI: Drake).
This study was supported by an NIH Grant R01 MH082785 to Dr. Christopher L. Drake. Dr. Drake has severed as consultant for Teva; has received research support from Merck and Teva; and has served on speakers bureau for Jazz, Purdue, and Teva.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The other authors have indicated no financial conflicts of interest.
5 References
- 1.McMenamin TM. Time to work: recent trends in shift work and flexible schedules, A. Monthly Labor Review. 2007;130:3–15. [Google Scholar]
- 2.Walsh JK, Schweitzer PK, Sugerman JL, Muehlbach MJ. Transient Insomnia Associated with a 3-Hour Phase: Advance of Sleep Time and Treatment with Zolpidem. Journal of Clinical Psychopharmacology. 1990;10:184–9. [PubMed] [Google Scholar]
- 3.Bonnet MH, Dexter J, Gillin JC, et al. The use of triazolam in phase-advanced sleep. Neuropsychopharmacology. 1988 doi: 10.1016/0893-133x(88)90021-8. [DOI] [PubMed] [Google Scholar]
- 4.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. SLEEP. 2004;27:1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 5.Sack R, Blood M, Lewy A. Melatonin rhythms in night shift workers. Sleep. 1992;15:434–41. doi: 10.1093/sleep/15.5.434. [DOI] [PubMed] [Google Scholar]
- 6.Medicine AAoS . International classification of sleep disorders—third edition (ICSD-3) Darien, Illinois: 2014. [Google Scholar]
- 7.Axelsson J, Åkerstedt T, Kecklund G, Lowden A. Tolerance to shift work—how does it relate to sleep and wakefulness? International archives of occupational and environmental health. 2004;77:121–9. doi: 10.1007/s00420-003-0482-1. [DOI] [PubMed] [Google Scholar]
- 8.Bambra C, Whitehead M, Sowden A, Akers J, Petticrew M. “A hard day's night?” The effects of Compressed Working Week interventions on the health and work-life balance of shift workers: a systematic review. Journal of epidemiology and community health. 2008;62:764–77. doi: 10.1136/jech.2007.067249. [DOI] [PubMed] [Google Scholar]
- 9.Bjorvatn B, Dale S, Hogstad-Erikstein R, Fiske E, Pallesen S, Waage S. Self-reported sleep and health among Norwegian hospital nurses in intensive care units. Nursing in critical care. 2012;17:180–8. doi: 10.1111/j.1478-5153.2012.00504.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet MH, Arand DL. Situational insomnia: consistency, predictors, and outcomes. SLEEP. 2003;26:1029–37. doi: 10.1093/sleep/26.8.1029. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz JR, Roth T. Shift Work Sleep Disorder. Drugs. 2006;66:2357–70. doi: 10.2165/00003495-200666180-00007. [DOI] [PubMed] [Google Scholar]
- 12.Drake C, Wright KP Jr, editors. Shift Work, Shift-Work Disorder, and Jet Lag. 5 ed Elsevier; St. Louis, MO: 2011. [Google Scholar]
- 13.Hilliker N, Muehlbach M, Schweitzer P, Walsh J. Sleepiness/alertness on a simulated night shift schedule and morningness-eveningness tendency. Sleep. 1992;15:430–3. doi: 10.1093/sleep/15.5.430. [DOI] [PubMed] [Google Scholar]
- 14.Archer SN, Robilliard DL, Skene DJ, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–5. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 15.Dijk D-J, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep medicine reviews. 2010;14:151–60. doi: 10.1016/j.smrv.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Viola AU, Archer SN, James LM, et al. PER3 polymorphism predicts sleep structure and waking performance. Current Biology. 2007;17:613–8. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 17.Drake CL, Jefferson C, Roehrs T, Roth T. Stress-related sleep disturbance and polysomnographic response to caffeine. Sleep Medicine. 2006;7:567–72. doi: 10.1016/j.sleep.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drake CL, Pillai V, Roth T. Stress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomnia. SLEEP. 2013;37:1295–304. doi: 10.5665/sleep.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillai V, Roth T, Mullins HM, Drake CL. Moderators and mediators of the relationship between stress and insomnia: stressor chronicity, cognitive intrusion, and coping. SLEEP. 2013;37:1199–208. doi: 10.5665/sleep.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalmbach DA, Pillai V, Arnedt JT, Drake CL. Identifying at-risk individuals for insomnia disorder using the Ford Insomnia Response to Stress Test. SLEEP under review. doi: 10.5665/sleep.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández-Mendoza J, Vela-Bueno A, Vgontzas AN, et al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosomatic medicine. 2010;72:397–403. doi: 10.1097/PSY.0b013e3181d75319. [DOI] [PubMed] [Google Scholar]
- 22.Pillai V, Roth T, Drake C. The Nature of Stable Insomnia Phenotypes. SLEEP. 2014 doi: 10.5665/sleep.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Association AP . DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision: American Psychiatric Association. 2000. [Google Scholar]
- 24.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 25.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 26.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth sleepiness scale: failure of the MSLT as a gold standard. Journal of sleep research. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 27.Punjabi NM, Bandeen-Roche K, Young T. Predictors of objective sleep tendency in the general population. Sleep. 2003;26:678–83. doi: 10.1093/sleep/26.6.678. [DOI] [PubMed] [Google Scholar]
- 28.Walsleben JA, Kapur VK, Newman AB, et al. Sleep and reported daytime sleepiness in normal subjects: the Sleep Heart Health Study. SLEEP. 2004;27:293–8. doi: 10.1093/sleep/27.2.293. [DOI] [PubMed] [Google Scholar]
- 29.Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. SLEEP. 2004;27:285–92. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 30.Hobson CJ, Kamen J, Szostek J, Nethercut CM, Tiedmann JW, Wojnarowicz S. Stressful life events: A revision and update of the social readjustment rating scale. International Journal of Stress Management. 1998;5:1–23. [Google Scholar]
- 31.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of consulting and clinical psychology. 1988;56:893. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 33.MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Evaluation review. 1993;17:144–58. [Google Scholar]
- 34.Fairchild AJ, MacKinnon DP. A general model for testing mediation and moderation effects. Prevention Science. 2009;10:87–99. doi: 10.1007/s11121-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tofighi D, MacKinnon DP. RMediation: An R package for mediation analysis confidence intervals. Behavior Research. 2011;43:692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological methods. 2002;7:83. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate behavioral research. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lichstein K, Durrence H, Taylor D, Bush A, Riedel B. Quantitative criteria for insomnia. Behaviour research and therapy. 2003;41:427–45. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Lynch JG, Chen Q. Reconsidering Baron and Kenny: Myths and truths about mediation analysis. Journal of consumer research. 2010;37:197–206. [Google Scholar]
- 40.Rucker DD, Preacher KJ, Tormala ZL, Petty RE. Mediation analysis in social psychology: Current practices and new recommendations. Social and Personality Psychology Compass. 2011;5:359–71. [Google Scholar]
- 41.Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication monographs. 2009;76:408–20. [Google Scholar]
- 42.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prevention Science. 2000;1:173–81. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological methods. 2002;7:422. [PubMed] [Google Scholar]
- 44.Kalmbach DA, Pillai V, Arnedt JT, Drake C. Something about FIRST cutoffs. p. 20XX. [Google Scholar]
- 45.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. American Journal of Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 46.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biological psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 47.Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. Journal of affective disorders. 1997;42:209–12. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- 48.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. Journal of affective disorders. 2011;135:10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


