Abstract
Alopecia areata (AA) is an autoimmune hair loss disease caused by a cell-mediated immune attack of the lower portion of the cycling hair follicle. Feeding mice 3–7 times the recommended level of dietary vitamin A accelerated the progression of AA in the graft-induced C3H/HeJ mouse model of AA. In this study, we also found that dietary vitamin A, in a dose dependent manner, activated the hair follicle stem cells (SCs) to induce the development and growth phase of the hair cycle (anagen), which may have made the hair follicle more susceptible to autoimmune attack. Our purpose here is to determine the mechanism by which dietary vitamin A regulates the hair cycle. We found that vitamin A in a dose-dependent manner increased nuclear localized beta-catenin (CTNNB1; a marker of canonical wingless-type Mouse Mammary Tumor Virus integration site family (WNT) signaling) and levels of WNT7A within the hair follicle bulge in these C3H/HeJ mice. These findings suggest that feeding mice high levels of dietary vitamin A increases WNT signaling to activate hair follicle SCs.
Keywords: Dietary vitamin A, hair cycling, hair follicle stem cell, WNT
Introduction
The hair follicle requires vitamin A; as both vitamin A deficiency and excess lead to alopecia (hair loss).1 The hair follicle cycles through three stages: growing phase (anagen), apoptosis phase (categen), and resting phase (telogen).2 Anagen can also be divided into three phases: development of the lower hair follicle (bulb; stages I–IIIa), development of the hair shaft (stages IIIb–V), and emergence of the hair shaft from the surface of the skin and its subsequent growth (stage VI).3 Alopecia occurs in all stages of the hair cycle, and different types of hair loss occur in different stages.
Cyclical alopecia during anagen develops with moderate increases in retinoids caused by blocking vitamin A storage in skin-specific acyl-CoA:diacylglycerol acyltransferase 1 (Dgat1tm2FarTg(KRT14-cre)1AMC) null mice.4 In contrast, telogen effluvium occurs during oral treatment with at least either 25 mg/day of the synthetic retinoid acitretin (Soriatane) and its parent compound Etretinate,5,6 or 0.5 mg/kg body weight/day of isotretinoin.7 Telogen effluvium occurs due to an arrest of the hair follicle in telogen and an anchorage defect that allows the hair to fall out.5 Follicular hyperkeratosis develops during vitamin A deficiency in rats,8,9 juvenile cattle,10 and humans.11–13 Time course studies in rats suggest that the hair follicle is the last organ affected by vitamin A deficiency,14 which may be why hair defects are rarely seen in vitamin A deficient humans. Combined, these studies suggest that different forms of alopecia occur when vitamin A is consumed at different doses.
Retinoic acid (RA) is the active metabolite of vitamin A, synthesized at or near its site of action, and can act in both autocrine and paracrine manners.15 RA acts primarily by binding its nuclear RA receptors (RARA, RARB, and RARG) and activating transcription.16 However, more recent studies suggest that RA can also activate kinase signaling pathways in the cytoplasm.17 One hair loss disease we previously examined is alopecia areata (AA). We found that feeding high (3–7 times recommended level) vitamin A accelerated disease; while feeding no vitamin A made the disease more severe.18 Similar to the eye, hair follicles are immune privileged and AA is argued to develop when that immune privilege collapses.19 AA develops when the anagen IIIa–VI hair follicle bulbs are attacked by cytotoxic CD8+NKG2D+ lymphocytes.20 This destruction of the hair follicle leads to a breakage of the hair shaft resulting in exclamation mark hairs, cadaver hairs, and hair loss.21,22 Vitamin A plays many roles in the immune system,23 which could be contributing to our previous results. In addition to these immune system effects, we found that feeding high vitamin A also altered the hair cycle.18 We found that high vitamin A increased the percentage of hair follicles in anagen IIIa–VI. The anagen bulb is primarily attacked in AA. Therefore, high vitamin A may have accelerated the disease by increasing anagen induction or extending the length of anagen, and therefore the amount of time the hair follicle could be attacked. This finding needs to be further examined in patients with AA, as most humans with AA have fewer hair follicles in anagen.24 We also reported that RA synthesis and signaling protein localization within the hair follicle was altered during the hair cycle,25,26 although few studies have examined how the hair cycle is regulated by vitamin A.
The hair cycle is maintained through the regulation of stem cells (SCs) in the hair follicle bulge.27 Several factors regulate hair follicle SCs to ultimately regulate the hair cycle. Bone morphogenetic protein 2 (BMP2) from the subcutaneous fat, BMP4 from the dermis, BMP6 from the inner layer of the hair follicle, and several Wingless-related MMTV integration site (WNT) inhibitors within the SCs all act to keep hair follicle SCs in the quiescent state of telogen.28–30 Activation of these SCs occur when transforming growth factor beta 2 (TGFB2) and Noggin (NOG) inhibit BMP signaling and WNT ligands are secreted from the mesenchyme-derived dermal papilla, which sits below the SCs during telogen.31,32 WNT signaling then activates hair follicle SCs to induce the initial stages of anagen (anagen I).33,34 The canonical WNT signaling pathway involves the translocation of beta-catenin (CTNNB1) to the nucleus to activate transcription of target genes.35 WNT7A and WNT7B are 2 of the 19 members of the WNT-secreted protein family that are important for initiation of anagen and are both directly inhibited by BMP signaling.36,37 RA regulates members of both the WNT and TGFB/BMP families in other tissues,38–42 but it is unknown which of these factors is regulated by RA in the hair follicle.
Our purpose here is to determine the mechanism by which dietary vitamin A regulates hair follicle SCs to induce anagen initiation. We are using control samples from our previous vitamin A feeding study in C3H/HeJ mice.18 We found that both nuclear localized CTNNB1 and WNT7A were dose dependently increased by dietary vitamin A. These findings extend the knowledge of vitamin A signaling in skin SCs.
Materials and methods
Mice
To induce AA, recipient C3H/HeJ mice (The Jackson Laboratory, Bar Harbor, ME) received a dorsal skin graft from an older C3H/HeJ mouse that had spontaneously developed AA (sent from John P. Sundberg) as previously described.18 Control mice had their own dorsal skin removed and rotated 180° in a sham surgery. Recipient and control C3H/HeJ mice were fed an AIN93 Maintenance diet containing 0 (n = 8), 4 (n = 7), 12 (n = 9), or 28 (n = 8) IU vitamin A/g diet (Research Diets, Inc., New Brunswick, NJ) starting two weeks before these mice were grafted. The American Institute of Nutrition (AIN) recommends 4 IU vitamin A/g diet.43 The higher doses were based on vitamin A analysis of the diet fed mice in The Jackson Laboratory production facility, which contained 6–28 IU vitamin A/g diet. Samples from the control sham mice collected 15 weeks post grafting were used in this current study. The Ohio State University Institutional Animal Care and Use Committee (IACUC) approved all procedures (#2008A0044).
Immunohistochemistry (IHC)
IHC was performed using a three-antibody system in paraffin slides as described previously.25 The following antibodies were used: CTNNB1 (Millipore, Billerica, MA), WNT7A (Abcam, Cambridge, MA), WNT7B (Novus Biologicals, Littleton, CO), BMP4 (Vector Lab, Burlingame, CA), and TGFB2 (Santa Cruz, Biotechnology, Santa Cruz, CA). Biotinylated anti-rabbit and anti-mouse were used as secondary antibodies (Jackson Immunoresearch Laboratories, Inc., West Grove, PA) followed by a horseradish peroxidase-conjugated anti-biotin tertiary antibody (Bethyl Laboratories, Montgomery TX). The red 3-amino-9-ethylcarbazole plus enhancers (AEC+; Dako, Carpinteria, CA) substrate chromogen was used followed by counterstaining with Gils Hematoxylin III (Poly Scientific, Bay Shore, NY) and mounting in aquamount (Dako, Capinteria, CA). CTNNB1 sections were not counterstained to better visualize nuclear localization. Immunoreactivity (IR) was scored blinded on a 4-point scale with: 0 = negative; 1 = weak; 2 = moderate; 3 = strong; and 4 = very strong. An IHC level (IHCL) was then calculated for nuclear CTNNB1 for each telogen hair follicle by the following formula
The IHCL of all of the telogen hair follicles (19–64) were then averaged to get one IHCL per mouse. With this scoring method, the maximum IHCL is 4. The scoring system is modified from validated protocols.44–46 Blinded IHCLs for WNT7A, WNT7B, and TGFB were calculated similarly, but each hair follicle received one IR score and the formula summed the fraction of hair follicles with each IR score. A blinded IHC score of BMP4 was measured using the total number of BMP4 positive cells in the dermis around telogen hair follicles divided by the number of telogen hair follicles. Images were captured on an upright Olympus BX51 microscope with the DP71 camera (Olympus, Tokyo, Japan) and processed in Adobe Photoshop CS4 (San Francisco, CA).
Statistics
Comparison among experimental groups was analyzed by one-way analysis of variance, followed by Tukey’s post hoc tests when appropriate using Statistical Package for the Social Sciences, v21 (IBM; Armonk, NY). P value < 0.05 is defined as statistical significance.
Results
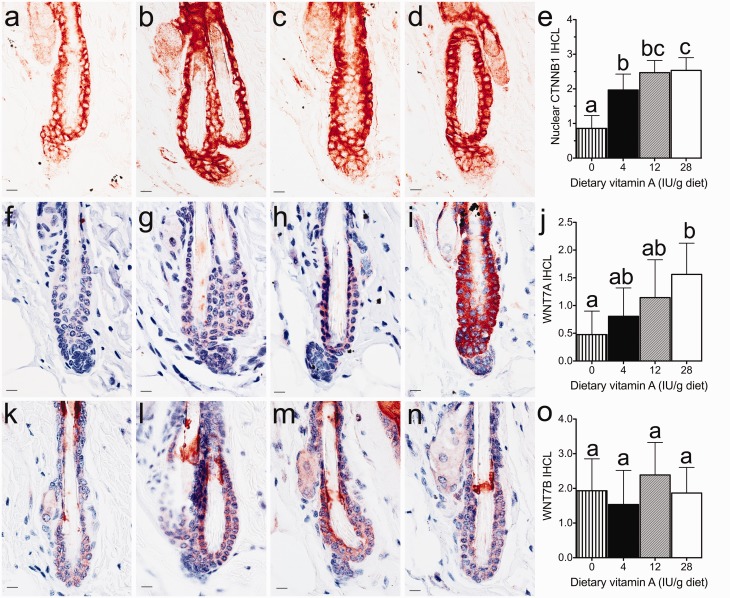
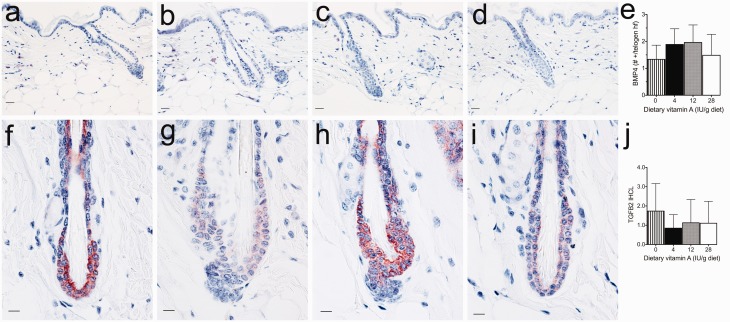
To determine the mechanisms by which vitamin A regulates hair follicle SCs, we examined several factors known to regulate the hair cycle by IHC in mice fed various levels of vitamin A. Nuclear CTNNB1 is an important downstream component and marker of the active canonical WNT signaling pathway. We found that dietary vitamin A dose dependently increased the nuclear localization of CTNNB1 in C3H/HeJ mice with a significant increase occurring with as little as 4 IU vitamin A/g diet (P < 0.001) and an additional significant increase with 28 IU vitamin A/g diet (Figure 1(a–e)). WNT7A IR also increased with dietary vitamin A in a dose-dependent manner with C3H/HeJ mice fed 28 IU vitamin A/g diet being significantly greater than mice fed no vitamin A (P < 0.05, Figure 1(f–j)). WNT7B IR was not altered by the level of dietary vitamin A (Figure 1(k–o)). Dietary vitamin A also did not significantly alter either BMP4 or TGFB2 (Figure 2).
Figure 1.
Dietary vitamin A dose dependently altered nuclear localized CTNNB1 and WNT7A in telogen hair follicles of C3H/HeJ mice. Immunohistochemistry was performed with antibodies against CTNNB1 (a–e), WNT7A (f–j), and WNT7B (k–o) in dorsal skin from C3H/HeJ mice fed 0 (a, f, k), 4 (b, g, l), 12 (c, h, m), and 28 (d, i, n) IU vitamin A/g diet. Blinded IHC score for CTNNB1 and WNT7A, WNT7B were calculated as the immunoreactivity (IR) measured on a 0–4 scale multiplied by the fraction of cells within the telogen hair follicle and the fraction of telogen hair follicles. Mean ± SD. Different letters are significantly different, P < 0.05. Bar = 10.1 μM
Figure 2.
Dietary vitamin A did not significantly altered BMP4 or TGFB2 in telogen hair follicles of C3H/HeJ mice. Immunohistochemistry was performed with antibodies against BMP4 (a–e) and TGFB2 (f–j) in dorsal skin from C3H/HeJ mice fed 0 (a, f), 4 (b, g), 12 (c, h), and 28 (d, i) IU vitamin A/g diet. A blinded IHC score of BMP4 was measured using the total number of BMP4 positive cells in the dermis around telogen hair follicles divided by the number of telogen hair follicles. Blinded IHC score for TGFB2 was calculated as the immunoreactivity (IR) measured on a 0–4 scale multiplied by the fraction of cells within the telogen hair follicle and the fraction of telogen hair follicles. Mean ± SD. Bar = 10.1 μM
Discussion
Vitamin A is important for the development and homeostasis of many tissues, including skin and hair.8,47,48 Our previous study found that dietary vitamin A-induced anagen in a dose-dependent manner in C3H/HeJ mice.18 Increased anagen was also seen in skin specific Dgat1tm2FarTg(KRT14-cre)1AMC null mice, which have elevated retinol and RA levels.4 In our current study, we explored the mechanism by which dietary vitamin A regulates hair cycling. We found that dietary vitamin A increased WNT signaling, as indicated by nuclear CTNNB1, and the ligand WNT7A. Thus, high vitamin A increases the percentage of anagen hair follicles by increasing WNT signaling in the bulge SCs to induce the onset of anagen.
We also observed that the alteration pattern of WNT7A expression by dietary vitamin A and that of CTNNB1 nuclear localization was similar in C3H/HeJ mice. However, the magnitude of change for CTNNB1 nuclear localization was greater. This suggests that other signals in addition to WNT7A may play a role in directing CTNNB1 to the nucleus. While WNT7A and WNT7B are essential to activate hair follicle bugle SCs,36,37 there are 19 members of the WNT secretion protein family that play different roles during hair follicle development and cycling.49 Future studies could analyze different WNTs and WNT inhibitors.
The doses of vitamin A used in this study were 3–7 times the AIN recommendation.43 In humans, consumption of vitamin A at 3–7 times the recommendation is at or above the established upper limit and potentially toxic.50 But most mice consume these high levels on a regular basis because most standard unpurified diets contain extra vitamin A. Dr. Napoli calls the amount of vitamin A in unpurified diets “Copious”; and his group recently showed that these diets are causing lasting effects that impede vitamin A research.51 According to the National Health and Nutrition Examination Survey (NHANES), most Americans consume the recommended level of dietary vitamin A through diet alone. But a significant number of Americans receive 2–7 times the recommended level of vitamin A through supplements.52 These high levels of dietary vitamin A are on top of the retinoids that are delivered directly to the skin and hair via cosmetic products.
The interactions between RA and WNT signaling are tissue dependent.53,54 During skeletal myogenesis, osteogenesis, and adipogenesis, RA-activated WNT signaling.38,40 Yet during limb development, RA inhibited Wnt7a.42 Our study shows that RA is activating WNT7A and WNT signaling within the adult skin in vivo. Several Wnts and WNT inhibitors were also increased in the skin of E16.5 and E18.5 Cyp26b1 null embryos,55 suggesting that RA regulates WNTs in the developing hair follicle as well. Future studies are needed to determine whether RA is acting directly on WNT7A within the hair follicle.
In summary, we demonstrated here that dietary vitamin A activates hair follicle SCs dose dependently through the activation of WNT signaling. Our next step is to study the mechanism by which retinoids regulate WNT. We will also establish the healthy range of retinoids for the hair follicle, which could provide possible knowledge for maintaining healthy hair in the normal population and determine effective doses of retinoids in treating other hair loss diseases.
ACKNOWLEDGEMENTS
The authors thank Charles J. Johnson and Adam Owens for feeding the mice and Kathleen A. Silva for coordinating the transfer of mice for the surgeries. This work was supported by the National Institutes of Health (AR052009 to HBE, AR056635 and AR052710 to JPS), National Alopecia Areata Foundation (HBE), The Virginia Vivian Endowment Fund (LS), and the T. Kline Hamilton Endowment Fund (LS).
Author contributions
All authors participated in the design, interpretation of the studies, and analysis of the data and review of the manuscript; LS and HBE conducted research; LS and HBE analyzed data; LS and HBE wrote the manuscript; JPS provided C3H/HeJ mice for the surgeries and edited the manuscript; HBE had primary responsibility for final content. All authors read and approved the final manuscript.
REFERENCES
- 1.Everts HB, Silva KA, Montgomery S, Suo L, Menser M, Valet AS, King LE, Ong DE, Sundberg JP. Retinoid metabolism is altered in human and mouse cicatricial alopecia. J Invest Dermatol 2013; 133: 325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chase HB, Rauch H, Smith VW. Critical stages of hair development and pigmentation in the mouse. Physiolog Zool 1951; 24: 1–7. [DOI] [PubMed] [Google Scholar]
- 3.Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol 2001; 117: 3–15. [DOI] [PubMed] [Google Scholar]
- 4.Shih MY, Kane MA, Zhou P, Yen CL, Streeper RS, Napoli JL, Farese RV., Jr Retinol esterification by DGAT1 is essential for retinoid homeostasis in murine skin. J Biol Chem 2009; 284: 4292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berth-Jones J, Hutchinson PE. Novel cycle changes in scalp hair are caused by etretinate therapy. Br J Dermatol 1995; 132: 367–75. [DOI] [PubMed] [Google Scholar]
- 6.Gupta AK, Goldfarb MT, Ellis CN, Voorhees JJ. Side-effect profile of acitretin therapy in psoriasis. J Am Acad Dermatol 1989; 20: 1088–93. [DOI] [PubMed] [Google Scholar]
- 7.Kmiec ML, Pajor A, Broniarczyk-Dyla G. Evaluation of biophysical skin parameters and assessment of hair growth in patients with acne treated with isotretinoin. Postepy Dermatol Alergol 2013; 30: 343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A vitamin. J Exp Med 1925; 42: 753–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolbach SB, Howe PR. Epithelial repair in recovery from vitamin A deficiency – an experimental study. J Exp Med 1933; 57: 511–U186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldwin TJ, Rood KA, Kelly EJ, Hall JO. Dermatopathy in juvenile Angus cattle due to vitamin A deficiency. J Vet Diagn Invest 2012; 24: 763–6. [DOI] [PubMed] [Google Scholar]
- 11.Bleasel NR, Stapleton KM, Lee MS, Sullivan J. Vitamin A deficiency phrynoderma: due to malabsorption and inadequate diet. J Am Acad Dermatol 1999; 41: 322–4. [DOI] [PubMed] [Google Scholar]
- 12.Girard C, Dereure O, Blatiere V, Guillot B, Bessis D. Vitamin A deficiency phrynoderma associated with chronic giardiasis. Pediatr Dermatol 2006; 23: 346–9. [DOI] [PubMed] [Google Scholar]
- 13.Ocon J, Cabrejas C, Altemir J, Moros M. Phrynoderma: a rare dermatologic complication of bariatric surgery. J Parenter Enteral Nutr 2012; 36: 361–4. [DOI] [PubMed] [Google Scholar]
- 14.Anzano MA, Lamb AJ, Olson JA. Growth, appetite, sequence of pathological signs and survival following the induction of rapid, synchronous vitamin A deficiency in the rat. J Nutr 1979; 109: 1419–31. [DOI] [PubMed] [Google Scholar]
- 15.Duester G. Retinoid signaling in control of progenitor cell differentiation during mouse development. Semin Cell Dev Biol 2013; 24: 694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giguere V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature 1987; 330: 624–9. [DOI] [PubMed] [Google Scholar]
- 17.Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res 2013; 54: 1761–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan FJ, Silva KA, Johnson CJ, King BL, Szatkiewicz JP, Kamdar SP, Ong DE, Napoli JL, Wang J, King LE, Jr, Whiting DA, McElwee KJ, Sundberg JP, Everts HB. Endogenous retinoids in the pathogenesis of alopecia areata. J Invest Dermatol 2013; 133: 334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilhar A, Paus R, Kalish RS. Lymphocytes, neuropeptides, and genes involved in alopecia areata. J Clin Invest 2007; 117: 2019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, de Jong A, Harel S, DeStefano GM, Rothman L, Singh P, Petukhova L, Mackay-Wiggan J, Christiano AM, Clynes R. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med 2014;20:1043–9. [DOI] [PMC free article] [PubMed]
- 21.Gilhar A, Etzioni A, Paus R. Medical progress alopecia areata. N Engl J Med 2012; 366: 1515–25. [DOI] [PubMed] [Google Scholar]
- 22.McElwee KJ, Gilhar A, Tobin DJ, Ramot Y, Sundberg JP, Nakamura M, Bertolini M, Inui S, Tokura Y, King LE, Duque-Estrada B, Tosti A, Keren A, Itami S, Shoenfeld Y, Zlotogorski A, Paus R. What causes alopecia areata?: Section Editors: Ralf Paus, Manchester/Lubeck and Raymond Cho, San Francisco. Exp Dermatol 2013; 22: 609–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raverdeau M, Mills KHG. Modulation of T cell and innate immune responses by retinoic acid. J Immunol 2014; 192: 2953–8. [DOI] [PubMed] [Google Scholar]
- 24.Horenstein MG, Bacheler CJ. Follicular density and ratios in scarring and nonscarring alopecia. Am J Dermatopathol 2013; 35: 818–26. [DOI] [PubMed] [Google Scholar]
- 25.Everts HB, Sundberg JP, King LE, Jr., Ong DE. Immunolocalization of enzymes, binding proteins, and receptors sufficient for retinoic acid synthesis and signaling during the hair cycle. J Invest Dermatol 2007; 127: 1593–604. [DOI] [PubMed] [Google Scholar]
- 26.Everts HB, King LE, Jr., Sundberg JP, Ong DE. Hair cycle-specific immunolocalization of retinoic acid synthesizing enzymes Aldh1a2 and Aldh1a3 indicate complex regulation. J Invest Dermatol 2004; 123: 258–63. [DOI] [PubMed] [Google Scholar]
- 27.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990; 61: 1329–37. [DOI] [PubMed] [Google Scholar]
- 28.Chen CC, Chuong CM. Multi-layered environmental regulation on the homeostasis of stem cells: the saga of hair growth and alopecia. J Dermatol Sci 2012; 66: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol 2006; 126: 1459–68. [DOI] [PubMed] [Google Scholar]
- 30.Hsu YC, Fuchs E. A family business: stem cell progeny join the niche to regulate homeostasis. Nat Rev Mol Cell Biol 2012; 13: 103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oshimori N, Fuchs E. Paracrine TGF-beta signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 2012; 10: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, McMahon AP, Paus R. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol 1999; 1: 158–64. [DOI] [PubMed] [Google Scholar]
- 33.Choi YS, Zhang Y, Xu M, Yang Y, Ito M, Peng T, Cui Z, Nagy A, Hadjantonakis AK, Lang RA, Cotsarelis G, Andl T, Morrisey EE, Millar SE. Distinct functions for WNT/beta-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell 2013; 13: 720–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009; 4: 155–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 2012; 13: 767–79. [DOI] [PubMed] [Google Scholar]
- 36.Kandyba E, Leung Y, Chen YB, Widelitz R, Chuong CM, Kobielak K. Competitive balance of intrabulge BMP/WNT signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proc Natl Acad Sci U S A 2013; 110: 1351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kandyba E, Kobielak K. Wnt7b is an important intrinsic regulator of hair follicle stem cell homeostasis and hair follicle cycling. Stem Cells 2014; 32: 886–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Liu Y, Zhang R, Wang X, Huang F, Yan Z, Nie M, Huang J, Wang Y, Wang Y, Chen L, Yin L, He B, Deng Z. All-trans retinoic acid modulates bone morphogenic protein 9-induced osteogenesis and adipogenesis of preadipocytes through BMP/Smad and Wnt/beta-catenin signaling pathways. Int J Biochem Cell Biol 2014; 47: 47–56. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Suzuki K, Nakagata N, Mihara K, Matsumaru D, Ogino Y, Yashiro K, Hamada H, Liu Z, Evans SM, Mendelsohn C, Yamada G. Retinoic acid signaling regulates sonic hedgehog and bone morphogenetic protein signalings during genital tubercle development. Birth Defects Res B Dev Reprod Toxicol 2012; 95: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy KAM, Porter T, Mehta V, Ryan SD, Price F, Peshdary V, Karamboulas C, Savage J, Drysdale TA, Li SC, Bennett SAL, Skerjanc IS. Retinoic acid enhances skeletal muscle progenitor formation and bypasses inhibition by bone morphogenetic protein 4 but not dominant negative beta-catenin. BMC Biol 2009; 7: 67–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez-Leon J, Merino R, Macias D, Ganan Y, Santesteban E, Hurle JM. Retinoic acid regulates programmed cell death through BMP signalling. Nat Cell Biol 1999; 1: 125–6. [DOI] [PubMed] [Google Scholar]
- 42.Stratford T, Logan C, Zile M, Maden M. Abnormal anteroposterior and dorsoventral patterning of the limb bud in the absence of retinoids. Mech Dev 1999; 81: 115–25. [DOI] [PubMed] [Google Scholar]
- 43.Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents – final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993; 123: 1939–51. [DOI] [PubMed] [Google Scholar]
- 44.Peng C, Zhang X, Wang Y, Li L, Wang Q, Zheng J. Expression and prognostic significance of wnt7a in human endometrial carcinoma. Obstet Gynecol Int 2012; 2012: 134962–134962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shamonki MI, Kligman I, Shamonki JM, Schattman GL, Hyjek E, Spandorfer SD, Zaninovic N, Rosenwaks Z. Immunohistochemical expression of endometrial L-selectin ligand is higher in donor egg recipients with embryonic implantation. Fertil Steril 2006; 86: 1365–75. [DOI] [PubMed] [Google Scholar]
- 46.Wong SC, Lo ES, Chan AK, Lee KC, Hsiao WL. Nuclear beta catenin as a potential prognostic and diagnostic marker in patients with colorectal cancer from Hong Kong. Mol Pathol 2003; 56: 347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zouboulis CC. Retinoids – which dermatological indications will benefit in the near future? Skin Pharmacol Appl Skin Physiol 2001; 14: 303–15. [DOI] [PubMed] [Google Scholar]
- 48.Everts HB. Endogenous retinoids in the hair follicle and sebaceous gland. Biochim Biophys Acta 2012; 1821: 222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev 2001; 107: 69–82. [DOI] [PubMed] [Google Scholar]
- 50.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc 2001; 101: 294–301. [DOI] [PubMed] [Google Scholar]
- 51.Obrochta KM, Kane MA, Napoli JL. Effects of diet and strain on mouse serum and tissue retinoid concentrations. PLoS ONE 2014; 9: e99435–e99435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park SY, Murphy SP, Martin CL, Kolonel LN. Nutrient intake from multivitamin/mineral supplements is similar among users from five ethnic groups: the Multiethnic Cohort Study. J Am Diet Assoc 2008; 108: 529–33. [DOI] [PubMed] [Google Scholar]
- 53.Beildeck ME, Gelmann EP, Byers SW. Cross-regulation of signaling pathways: an example of nuclear hormone receptors and the canonical Wnt pathway. Exp Cell Res 2010; 316: 1763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu QH, Kopp JB. Retinoid and TGF-beta families: crosstalk in development, neoplasia, immunity, and tissue repair. Sem Nephrol 2012; 32: 287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okano J, Levy C, Lichti U, Sun HW, Yuspa SH, Sakai Y, Morasso MI. Cutaneous retinoic acid levels determine hair follicle development and downgrowth. J Biol Chem 2012; 287: 39304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]