Abstract
The prion paradigm has emerged as a unifying molecular principle for the pathogenesis of many age-related neurodegenerative diseases. This paradigm holds that a fundamental cause of specific disorders is the misfolding and seeded aggregation of certain proteins. The concept arose from the discovery that devastating brain diseases called spongiform encephalopathies are transmissible to new hosts by agents consisting solely of a misfolded protein, now known as the prion protein. Accordingly, “prion” was defined as a “proteinaceous infectious particle.” As the concept has expanded to include other diseases, many of which are not infectious by any conventional definition, the designation of prions as infectious agents has become problematic. We propose to define prions as “proteinaceous nucleating particles” to highlight the molecular action of the agents, lessen unwarranted apprehension about the transmissibility of noninfectious proteopathies, and promote the wider acceptance of this revolutionary paradigm by the biomedical community.
Keywords: Alzheimer’s disease, amyloid, cell-to-cell spreading, disease progression, Parkinson’s disease, tau, synuclein, proteopathy, transmission, prion
INTRODUCTION
The success of biomedicine in reducing mortality early in life has increasingly displaced the societal burden of disease to old age. One consequence of the lengthening life-expectancy is a rising prevalence of neurodegenerative diseases. These debilitating and largely intractable disorders are a growing source of suffering for the afflicted patients as well as their families and friends. Age-related neurodegenerative disorders include Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), and many others. Although they vary greatly in their clinical and pathological characteristics, at the molecular level the maladies share a fundamental pathogenic mechanism: the seeded aggregation of disease-specific proteins (Guo & Lee 2014, Jucker &Walker 2013, Prusiner 2012). The canonical model for the seeded aggregation of proteins is the prion paradigm (Prusiner 2012, 2013) (Figure 1), one of the most illuminating pathogenic principles to emerge in the past half century (Jucker & Walker 2013). Indeed, this mechanistic commonality among the proteopathies could soon unify and focus experimental and therapeutic approaches to these seemingly disparate disorders.
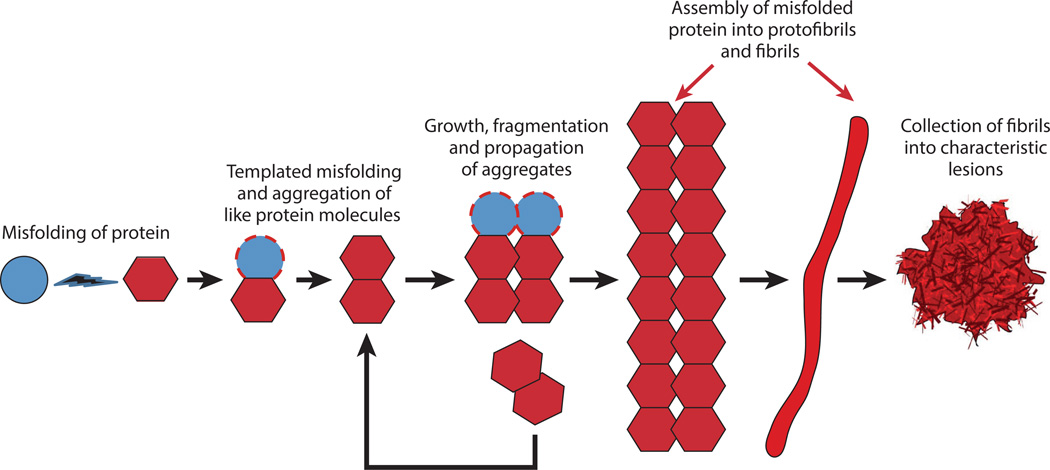
Figure 1.
The prion paradigm of seeded protein aggregation. A schematic diagram shows the hypothetical series of events leading from the misfolding and self-aggregation of protein molecules to the formation of characteristic lesions. The assemblies that act as seeds for the templated misfolding of other molecules can vary in size from small oligomers to large polymers. These seeds initiate and sustain the disease process and may be the agents by which the aggregates proliferate and spread within the nervous system. In addition, small, oligomeric assemblies have been identified as cytotoxic agents in several instances. The lesions that result from the seeding cascade can occur as intracellular inclusions (such as neurofibrillary tangles or Lewy bodies) or extracellular masses (such as amyloid plaques).
THE PRION PARADIGM
Pathogenic Protein Aggregates
The prion paradigm arose from decades of research on unusual diseases of animals and humans, known collectively as transmissible spongiform encephalopathies (TSEs) (Collins et al. 2004). In the 1930s, a TSE of sheep called scrapie was shown experimentally to exhibit atypical infectivity, i.e., a very long incubation period, the absence of inflammation, and no demonstrable microbial or viral agent (Fast & Groschup 2013). Later, several rare human spongiform encephalopathies were found to be transmissible to nonhuman primates (Gajdusek 1977). By the 1980s, Prusiner (1982, 1998) and colleagues had unambiguously demonstrated that the infectious agent consists solely of an abnormally folded protein, for which Prusiner coined the term “prion” (proteinaceous infectious particle). Prion diseases of humans include Creutzfeldt-Jakob disease, Gerstmann-Sträussler-Scheinker disease, kuru, and fatal insomnias; in nonhuman species, they include scrapie in sheep, chronic wasting disease of deer and elk, bovine spongiform encephalopathy (BSE), and others (Caughey et al. 2009, Prusiner 1998). Pathologically, the prion diseases are marked by spongiform change (vacuolation) in the brain, neuronal loss, astrocytosis, and the intracerebral buildup of misfolded prion protein (PrP) (DeArmond & Prusiner 1995; Parchi et al. 1999; Prusiner 1982, 1998) (Figure 2).
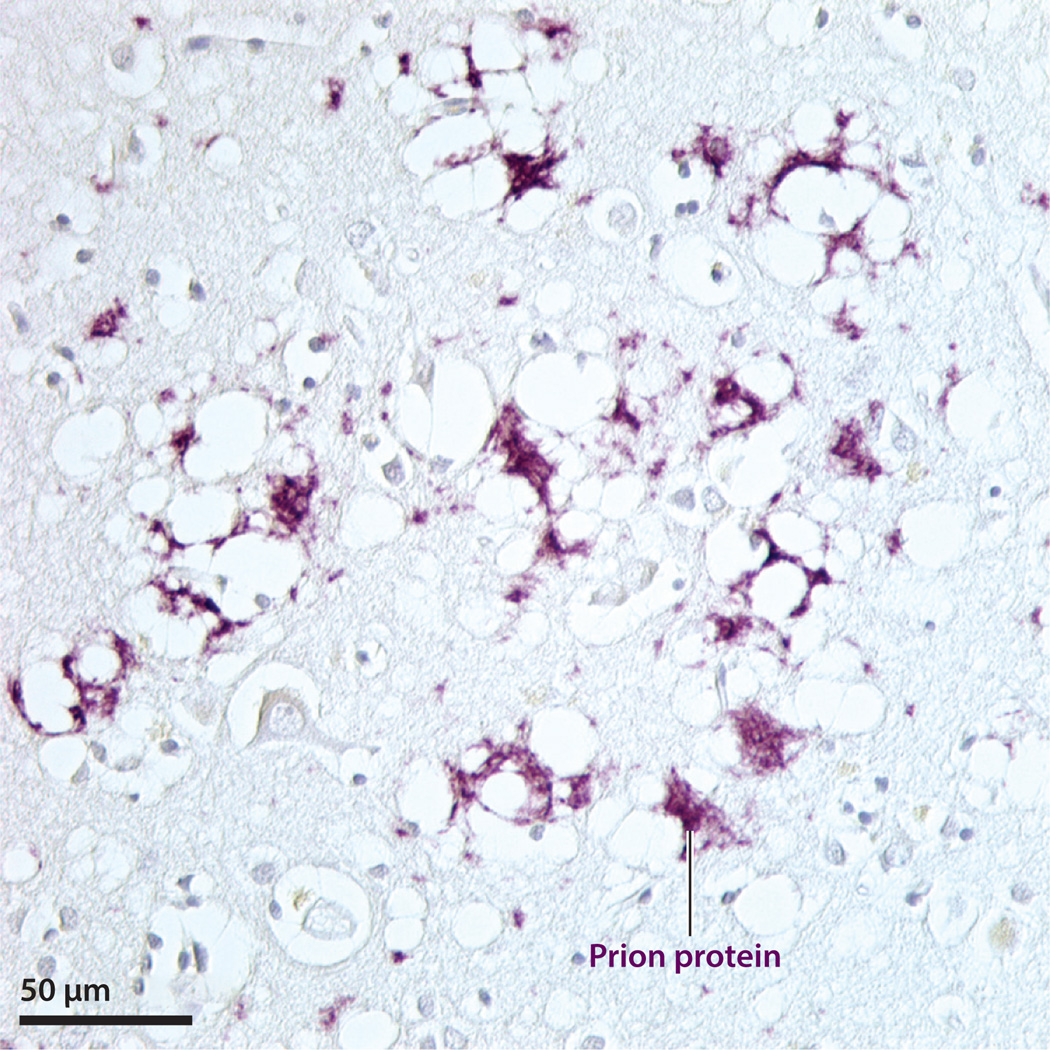
Figure 2.
Prion protein immunoreactivity (purple) and spongiform degeneration in the neocortex of a patient who had died of Creutzfeldt-Jakob disease. Nissl counterstain.
The prion diseases are best known for their unorthodox infectivity, but they are extraordinary in that they can also be heritable or idiopathic in origin. The key discovery that integrated these heterogeneous causes was the finding that the prion protein is constitutively generated by cells in the brain (Chesebro et al. 1985, Oesch et al. 1985) and elsewhere in the body (Oesch et al. 1985). Under normal circumstances, this cellular prion protein (often abbreviated PrPC) assumes a nonpathogenic three-dimensional conformation with little β-sheet. Disease arises when the prion protein folds into a shape that is abnormally enriched in β-sheet [PrP-scrapie (PrPSc)], in which state it induces other prion protein molecules to misfold and aggregate into small oligomers, protofibrils, and amyloid fibrils (Prusiner 1998, 2013). Amyloid—a generic term for abnormal masses of fibrillar protein (Sipe et al. 2014)—is inconsistently present in prion diseases, but an important molecular characteristic of misfolded prion protein is its enhanced potential to form amyloid (DeArmond & Prusiner 1995).
Although the nature of prion protein deposits varies from one manifestation of the disease to another, the essential disease mechanism is the seeded aggregation of the normally produced PrP. This process can be facilitated by mutations in the gene for PrP that render the protein more likely to misfold and aggregate (heritable); by the introduction of seeds of misfolded prion protein (i.e., prions; infectious); or, presumably, by the stochastic misfolding of PrP and subsequent formation and proliferation of endogenous seeds (idiopathic or sporadic). There is a long, clinically silent period during which the disease amplifies in the brain, followed by the appearance of characteristic signs and symptoms that progressively worsen and invariably lead to death (Johnson 2005). Experimental inoculation studies implicate neurons and immune cells in the spread of prions from the periphery to the brain (Aguzzi 2003). Within the brain, prions can be conveyed with high specificity along established anatomical pathways, indicative of neuronal transport mechanisms (Buyukmihci et al. 1983, Fraser 1982, Kimberlin &Walker 1986, Liberski et al. 2012).
Variations in the clinical and pathological characteristics of prion disease are influenced by differences in the multidimensional architecture of the prion assemblies, a phenomenon referred to as prion strains (Aguzzi et al. 2007, Collinge & Clarke 2007, Eisenberg & Jucker 2012, Gambetti et al. 2011, Prusiner 2013). Variant structural and functional synthetic prion strains can be generated by the experimental manipulation of cofactors during aggregation (Deleault et al. 2012, Zhang et al. 2014). Notably, the potential to form conformationally distinct proteinaceous strains is now considered a generic property of amyloid-forming proteins (Chiti & Dobson 2006, Eisenberg & Jucker 2012, Knowles et al. 2014, Tanaka et al. 2006). In addition, prions vary greatly in size, and small, soluble PrP assemblies are especially infectious (Silveira et al. 2005). Although the experimental enrichment of prions in brain homogenates is facilitated by their relative resistance to degradation by proteinase K, some prions are quite sensitive to this enzyme (Colby et al. 2010, Safar et al. 1998). Prions, thus, are not homogeneous, isomorphic particles but rather comprise a constellation of PrP assemblies that share the ability to structurally convert other PrP molecules.
Prion Infectivity
The term infectious commonly refers to the instigation of disease by an exogenous pathogenic agent that invades the body, often passing from one organism to another (see the section titled Perspective: Expanding the Prion Concept). In the case of prion infection, the particles enter the host and then incite disease by compelling normally generated host prion protein to similarly misfold, self-assemble, and form new prions (Prusiner 1998, 2013). Because the resulting assemblies consist of host-derived protein, the immune system fails to respond as it would to a truly foreign agent.
In humans, infectious prion disease is rare [approximately 3,400 total cases have been documented worldwide (Jucker & Walker 2013)]. Rather, most instances of human prion disease are idiopathic or genetic in origin. Furthermore, most infectious human prion disease has resulted from uncommon means of exposure, i.e., ritual cannibalism in kuru (approximately 2,700 cases) or internal exposure to contaminated medical instruments or biologics (Johnson 2005). In addition, as of the end of 2014, 229 cases of variant Creutzfeldt-Jakob disease (vCJD) appear to have resulted from consumption of beef contaminated with the prions of BSE or, secondarily (in 3 of the 229 cases), owing to transfusion of contaminated blood (for up-to-date statistics on vCJD, see http://www.cjd.ed.ac.uk/data.html). Fortunately, the wave of zoonotic human cases caused by infection with BSE prions has largely passed, and as a result of improved understanding of prions, infectious prion disease in humans has essentially disappeared (Brown et al. 2012).
In nonhuman species, infectious prion disease is much more common than in humans. Scrapie in sheep—the first form of the disease to be identified (Fast & Groschup 2013, Johnson 2005) and the first to be experimentally transmitted—remains endemic in Great Britain and many other parts of the world (Fast & Groschup 2013). In addition, chronic wasting disease is a particularly infectious prion disease of free-ranging cervids that is spreading rapidly in North America (Saunders et al. 2012, Williams 2005). The reasons for the facile transmissibility of some types of prion disease are not yet entirely clear.
Unconventional infectivity was the feature of prions that ultimately led to the discovery of templated protein transformation as a new biological principle. However, as we discuss below, ambiguities associated with the definition of prions as infectious particles have dissuaded many researchers from fully adopting the prion paradigm as a framework within which other diseases can be understood, as many of these diseases are not, by any customary definition, infectious.
PRION-LIKE SEEDING AND ALZHEIMER’S DISEASE
Alzheimer’s Disease
Intriguing clinicopathological similarities between the prion diseases and other neurodegenerative disorders have not escaped the notice of researchers (Gajdusek 1977, Prusiner 1984). Notable among these disorders is AD, a condition characterized clinically by a slow but inexorable decline in cognitive function, and histologically by intracerebral senile plaques and neurofibrillary tangles (Holtzman et al. 2011). Senile plaques are heterogeneous lesions consisting principally of extracellular masses of fibrillar amyloid-β (Aβ) peptide, whereas neurofibrillary tangles are intracellular bundles of fibrillar tau protein (Holtzman et al. 2011, Nelson et al. 2012) (Figure 3). Other lesions can occur in the AD brain, such as cerebral Aβ-amyloid angiopathy, and comorbid conditions are frequent in the elderly (Nelson et al. 2012); however, plaques and tangles remain the defining histopathological features of AD, and current evidence favors the aggregation of Aβ as the earliest pathognomonic signature of the disease (Bateman et al. 2012, Holtzman et al. 2011, Jack et al. 2010, Selkoe 2012). Tauopathy, although downstream of Aβ in the ensuing cascade of events (Hardy & Selkoe 2002), contributes importantly to the cognitive deficits that characterize AD (Holtzman et al. 2011, Nelson et al. 2012).
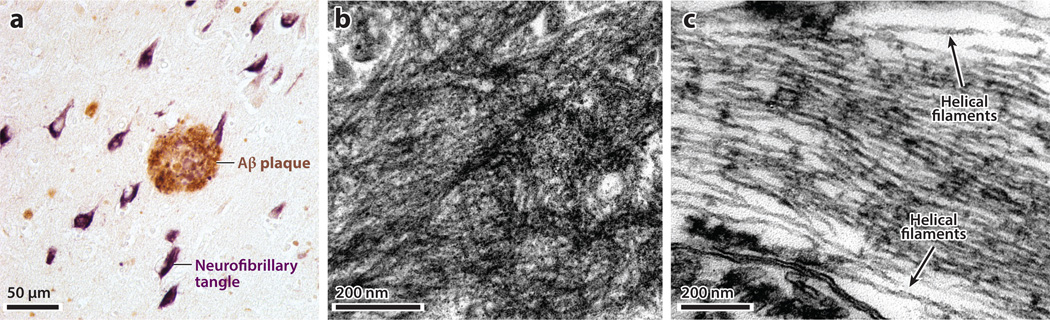
Figure 3.
Senile [amyloid-β (Aβ)] plaque and neurofibrillary (tau) tangles in Alzheimer’s disease. (a) Light micrograph of hippocampal tissue that has been dual-immunostained for Aβ (brown in an Aβ plaque) and hyperphosphorylated tau (purple in neurofibrillary tangles). (b) Electron micrograph of a mass of extracellular amyloid fibrils in an Aβ plaque. (c) Electron micrograph of intraneuronal fibrillar polymers of tau protein, the ultrastructural components of neurofibrillary tangles. Labels denote two paired helical filaments.
Seeding Amyloid β
In the 1970s and 1980s, when many scientists still thought that the spongiform encephalopathies were caused by an unorthodox slow virus, two research groups undertook experiments to test the hypothesis that AD, like the spongiform encephalopathies, was transmissible. A team headed by D. Carleton Gajdusek performed intracerebral injections of brain homogenates from patients with AD (and several other neurodegenerative diseases) into nonhuman primates. The results were inconclusive (Goudsmit et al. 1980). In Great Britain, Harry Baker, Rosalind Ridley, and colleagues injected AD brain homogenates intracerebrally into marmosets (Callithrix jacchus) and found an increase in senile plaque load after an incubation period of approximately 6–7 years (Baker et al. 1993). The researchers concluded that the extract was capable of inducing plaque formation, but the inductive agent remained uncertain.
With the introduction of Aβ precursor protein (APP) transgenic mouse models of cerebral Aβ deposition in the mid-1990s, we initiated a series of experiments in which we injected Aβ-rich brain extracts from AD patients or aged APP transgenic mice into APP transgenic hosts (Eisele et al. 2009, 2010, 2014; Fritschi et al. 2014a,b; Hamaguchi et al. 2012; Heilbronner et al. 2013; Kane et al. 2000; Langer et al. 2011; Meyer-Luehmann et al. 2006; Rosen et al. 2012; Walker et al. 2002; Ye et al. 2015). These studies, and those of other laboratories (Duran-Aniotz et al. 2013, 2014; Morales et al. 2012; Stöhr et al. 2012, 2014; Watts et al. 2011, 2014), have demonstrated that Aβ plaques, cerebral Aβ angiopathy, and related pathologies such as neuritic changes and neuroinflammation can be induced to form by the prion-like seeding of Aβ aggregation. Seeded induction of Aβ deposition in young, APP transgenic hosts is possible even with subattomolar amounts of brain-derived Aβ (Fritschi et al. 2014b) and with aggregates of pure, synthetic Aβ (Stöhr et al. 2012, 2014). However, as with PrP, the specific biological seeding potency of aggregated, synthetic Aβ is much less than that of similar amounts of brain-derived Aβ. The reasons for this discrepancy remain unclear; the inclusion of certain cofactors during aggregation can enhance the seeding potential of synthetic prions (Deleault et al. 2012, Wang et al. 2010, Zhang et al. 2014), suggesting that cofactors also might augment the potency of Aβ seeds.
Aβ seeds and prions share several other similarities. In vitro, Aβ has been shown to transfer from neuron to neuron (Domert et al. 2014, Nath et al. 2012, Song et al. 2014), and in vivo, the deposition of Aβ ramifies systematically through the brain, suggestive of neuron-mediated spread along anatomical pathways (Hamaguchi et al. 2012, Ye et al. 2015). As in the case of prions, Aβ can form strain-like variants in vitro (Mehta et al. 2013; Meinhardt et al. 2009; Nilsson et al. 2007; Paravastu et al. 2008, 2009; Petkova et al. 2005) and in vivo (Heilbronner et al. 2013; Lu et al. 2013; Meyer-Luehmann et al. 2006; Rosen et al. 2010, 2011; Stöhr et al. 2014; Watts et al. 2014). Seeding-competent Aβ multimers also exist in a range of sizes (Langer et al. 2011). As for prions (Silveira et al. 2005), small, soluble Aβ seeds are particularly potent, and these, unlike larger, fibrillar assemblies, are readily neutralized by proteinase K (Langer et al. 2011). Furthermore, Aβ aggregation in the brain can be triggered by seeds delivered to the peritoneal cavity (Eisele et al. 2010, 2014), and stainless steel wires coated with Aβ-rich brain extract are able to induce plaque formation following implantation in the brains of APP transgenic mice (Eisele et al. 2009). Finally, recent evidence indicates that Aβ seeds, like prions, are resistant to inactivation by formaldehyde (Fritschi et al. 2014a).
It is important to note that these experiments have not induced AD per se, which to date has only been identified in humans (Heuer et al. 2012, Jucker 2010). Rather, they have demonstrated that, at the molecular level, the mechanisms that impel the formation of Aβ plaques and cerebral Aβ angiopathy are virtually identical to those underlying the misfolding and proliferation of PrP-prions.
Seeding Tau Protein
Tau protein is abundant in neurons and normally serves to stabilize microtubules (Avila et al. 2004, Lee et al. 2001). In many brain disorders, including AD, tau becomes hyperphosphorylated and abnormally polymerizes into intracellular neurofibrillary tangles (Lee et al. 2001, Spillantini & Goedert 2013) (Figure 3). Tauopathy can result from autosomal dominant mutations in the tau-coding MAPT gene on human chromosome 17, but more often it occurs as a nonspecific response of neurons to various kinds of insult (Nelson et al. 2012). In AD, tauopathy is considered to be a subsequent, but critically important, reaction to Aβ abnormalities in the pathogenic cascade (Hardy & Selkoe 2002, Holtzman et al. 2011, Nelson et al. 2012).
Like the seeded aggregation of Aβ, the pathogenesis of tauopathy now appears to fit firmly into the prion paradigm. When brain extracts containing aggregated tau are injected into the brains of young, tau transgenic host mice, tauopathy is induced that propagates systematically from the injection site to axonally connected areas (Ahmed et al. 2014; Clavaguera et al. 2009, 2013), indicative of neuronal uptake, transport, and release of tau seeds (Frost et al. 2009, Sanders et al. 2014, Wu et al. 2013). The intracerebral injection of brain extracts from different human tauopathies instigates tau lesions in mice that resemble those in the corresponding human diseases (Boluda et al. 2015, Clavaguera et al. 2013), suggestive of proteopathic tau strains (Sanders et al. 2014). Additionally, tauopathy can be induced in the brain by tau seeds administered intraperitoneally (Clavaguera et al. 2014). Tau seeds also assume a range of sizes, the most potent of which are small and soluble (Lasagna-Reeves et al. 2012). Unlike Aβ aggregation in vivo, which has not yet been seeded in wild-type mice and is relatively weakly stimulated by synthetic Aβ seeds, tauopathy is inducible in wild-type mice and (in tau transgenic mice) by recombinant tau fibrils (Clavaguera et al. 2013, Iba et al. 2013, Lasagna-Reeves et al. 2012, Peeraer et al. 2015).
The induction of tau lesions by exogenous seeds has identified structurally variant tau as the culpable agent, but tauopathy results—exclusively, as far as we now know—from the endogenous generation of tau seeds. To establish a model of endogenous spreading of tau lesions, two laboratories have used mice in which the expression of a pathogenic human tau transgene is mainly restricted to projection neurons of the entorhinal cortex (de Calignon et al. 2012, Liu et al. 2012). In this paradigm, tau abnormalities first emerge in the entorhinal cortex, followed by a time-dependent expansion of lesions to axonally connected areas (de Calignon et al. 2012, Liu et al. 2012). These experiments, in the context of longstanding evidence for the expansion of tau lesions along neuronal pathways in AD (Arnold et al. 1991, Braak & Braak 1995, Saper et al. 1987), implicate normal neuronal transport mechanisms in the proliferation of neurofibrillary pathology. More recently, advanced in vivo imaging of structural and pathological features has disclosed a general involvement of the connectome in AD and other neurodegenerative processes (Bero et al. 2011, Iturria-Medina et al. 2014, Raj et al. 2015, Zhou et al. 2012).
PRION-LIKE SEEDING AND OTHER NEURODEGENERATIVE DISEASES
Most age-related neurodegenerative disorders involve the abnormal accumulation of disease-specific proteins, often in the form of histopathologically appreciable amyloid lesions (Guo & Lee 2014, Jucker &Walker 2013). In PD, dementia with Lewy bodies, and multiple system atrophy, misfolded α-synuclein assembles into fibrillar inclusions called Lewy bodies and Lewy neurites (Goedert et al. 2013) (Figure 4). Similar to other proteinaceous deposits, these lesions can be seeded in the brains of young, α-synuclein transgenic mice by the intracerebral injection of brain extracts containing aggregated α-synuclein (Luk et al. 2012b, Mougenot et al. 2012). Recombinant α-synuclein fibrils also are able to induce α-synuclein aggregation in cellular models (Aulić et al. 2014, Luk et al. 2009, Volpicelli-Daley et al. 2011) and in α-synuclein transgenic mice or wild-type mice (Luk et al. 2012a,b; Masuda-Suzukake et al. 2013). α-Synucleinopathy also has been induced in nonhuman primates by intracerebrally delivered brain extract from PD patients (Recasens et al. 2014).
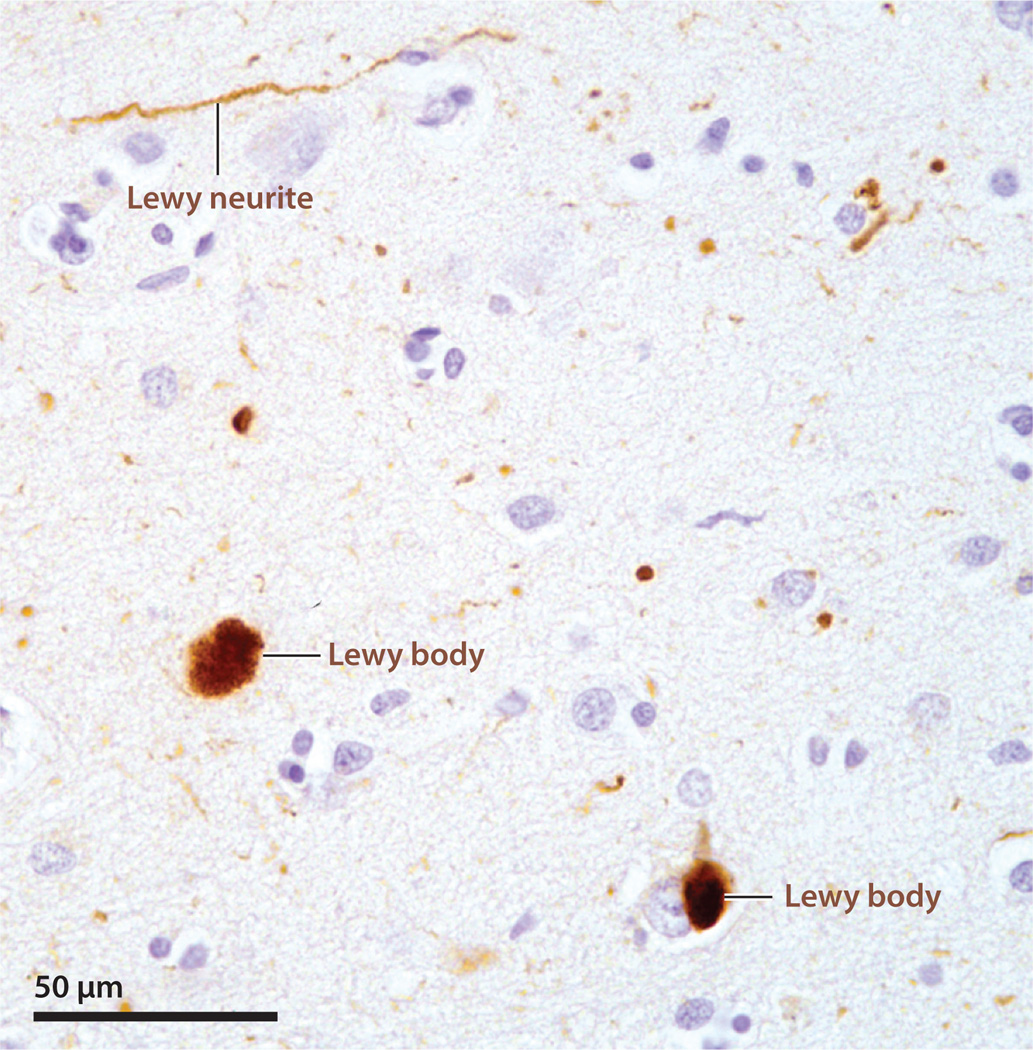
Figure 4.
α-Synuclein-immunoreactive lesions (brown) in the neocortex of a patient with Lewy body disease. The labels indicate Lewy bodies and a Lewy neurite. Nissl counterstain.
Within the brain, seeded α-synuclein pathology appears to progress through anatomically linked regions (Luk et al. 2012a,b; Masuda-Suzukake et al. 2013) and is associated with a progressive neurodegenerative disorder that mimics key aspects of PD (Luk et al. 2012a,b; Masuda-Suzukake et al. 2014; Mougenot et al. 2012; Recasens et al. 2014). α-Synuclein aggregates from patients with multiple system atrophy transmit a fatal brain disease to susceptible mice (Watts et al. 2013). Interestingly, recombinant α-synuclein fibrils cross-seed tau fibrillization (Guo et al. 2013), and the efficacy of cross-seeding is governed by strain-like variations in the α-synuclein seeds (Guo et al. 2013). Similarly, aggregates of α-synuclein in the brains of PD patients exhibit differential proteinase K cleavage patterns, indicative of variant molecular conformations of α-synuclein in the human brain (Guo et al. 2013). In addition, fetal brain cells transplanted into PD patients can eventually develop Lewy pathology, suggesting that α-synuclein in the transplanted cells is impelled to misfold and aggregate by α-synuclein seeds from the host (Kordower et al. 2008, Li et al. 2008).
Numerous other proteopathies are also likely to originate and progress by the prion-like induction of protein aggregation (Jucker & Walker 2013). Among these are ALS and FTD, which occupy two poles of a spectrum of loosely related brain disorders (Rademakers et al. 2012, Van Langenhove et al. 2012). Proteins implicated in ALS/FTD spectrum disorders include tau, superoxide dismutase-1 (SOD1), TAR-DNA binding protein-43 (TDP-43), fused in sarcoma (FUS), C9orf72, heterogeneous nuclear ribonucleoproteins, and others (Al-Chalabi et al. 2012, Bennion Callister & Pickering-Brown 2014, Cruts et al. 2013, Kim et al. 2013, King et al. 2012, Li et al. 2013, Ling et al. 2013, Rademakers et al. 2012, Van Langenhove et al. 2012). These proteins are prone to aggregation, and several incorporate aggregation-prone stretches of amino acids called prion-like domains, which are present in some RNA-binding proteins (Han et al. 2012, Kato et al. 2012, King et al. 2012). The reversible acquisition of cross-β architecture and aggregation of RNA-binding proteins can help cells to survive stressful conditions by downregulating protein function, but if the assemblies convert to a more persistent amyloid-like state, they become harmful to the cell (King et al. 2012, Mizielinska et al. 2014). In ALS and FTD, the lesions appear to spread systematically among brain regions (Braak et al. 2013, Brettschneider et al. 2014, Ravits & La Spada 2009), suggestive of the transport of a prion-like agent (Braak et al. 2013, Cushman et al. 2010, Grad & Cashman 2014, Ling et al. 2013, Udan & Baloh 2011). TDP-43 aggregation can be induced by cognate TDP-43 seeds in vitro (Furukawa et al. 2011, Nonaka et al. 2013). Cell culture studies also confirm the seeded aggregation and cell-to-cell propagation of both SOD1 (Grad & Cashman 2014, Grad et al. 2014, Munch et al. 2011) and huntingtin, the protein linked to Huntington’s disease (Pecho-Vrieseling et al. 2014, Ren et al. 2009). The existing data thus underscore the likelihood that the prion principle applies to the instigation and propagation of protein aggregation in an extraordinary variety of neurodegenerative diseases.
Many key issues remain unresolved. The mechanisms by which abnormal protein aggregates injure tissue can vary within and among diseases, and these mechanisms are poorly understood. We also need to learn more about the molecular structure of the pathogenic proteins, the diversity of protein architecture, and the factors that govern molecular shape. How the aggregates emerge and how they are normally removed are also critical issues, as these processes present potential therapeutic targets. Finally, whereas it is increasingly apparent that prion-like seeded aggregation of proteins is fundamental to these diseases, it is imperative to identify exogenous or endogenous risk factors that can influence the likelihood that proteins will misfold and progressively accumulate.
PRION-LIKE PROCESSES BEYOND THE NERVOUS SYSTEM
The reach of the prion paradigm extends well beyond neurodegenerative diseases, and now includes systemic amyloidoses (Lladó et al. 2010, Murakami et al. 2014, Westermark & Westermark 2010, Xing et al. 2001) and possibly some forms of cancer (Ano Bom et al. 2012, Forget et al. 2013, Rangel et al. 2014, Xu et al. 2011). Although we focus this review on disease mechanisms, the discovery that prion-like processes govern heritable traits in certain fungi opened a rich and productive field of research that has yielded invaluable insights into the biology of prions (Krauss & Vorberg 2013, Liebman & Chernoff 2012, Newby & Lindquist 2013, Prusiner 2013, Sugiyama & Tanaka 2014, Tuite 2013, Wickner et al. 2013). Prion-like processes also appear to influence functional protein aggregation in such phenomena as memory formation (Raveendra et al. 2013, Si et al. 2003), peptide storage (Maji et al. 2009), and the innate immune response (Cai et al. 2014, Hou et al. 2011) and inflammation (Franklin et al. 2014). The prion paradigm thus has evolved from a limited mechanistic explanation for atypical infectivity to become a compelling principle of biological information transfer.
PERSPECTIVE: EXPANDING THE PRION CONCEPT
The discovery of prions as transmissible agents has enriched our understanding of molecular communication in health and disease. In many instances, however, diseases caused by structurally aberrant proteins do not have an infectious source; rather, they emerge endogenously when specific proteins misfold and accumulate within the organism in the absence of exogenous seeds. Critical insights into this phenomenon will emerge from a deeper comprehension of the molecular mechanisms that regulate inductive protein folding (Jucker & Walker 2011, 2013; Walker & LeVine 2012), regardless of whether the seed is exogenous or endogenous in origin. An important challenge for the field is to establish the factors that govern the origination and persistence of endogenous seeds. For example, an intriguing possibility is that somatic mutations influence the generation, architecture, or persistence of seeds (Frank 2014, McConnell et al. 2013). Epigenetic influences also may contribute, such as age-related changes in DNA (CpG) methylation (Horvath 2013). Another likely factor is the integrity of cellular proteostasis; a decline in protein disposal with age, for instance, is one means by which misfolded proteins could accumulate beyond the threshold for irreversible autopropagation (Balch et al. 2008).
Unfortunately, acceptance of the prion paradigm in the general context of disease has been hindered by the ambiguous and somewhat disconcerting connotations of the term “infectious” as a defining feature of prions. As defined by Stedman’s Medical Dictionary, infectious (“Capable of being transmitted by infection, with or without actual contact”) is essentially synonymous with contagious (“communicable or transmissible by contact with the sick or their fresh secretions or excretions”) and transmissible [“Capable of being transmitted (carried across) from one person to another, as a transmissible disease, an infectious or contagious disease”] (Stedman 2012, pp. 857, 388, 1705). In this prevailing sense of the term, there is no evidence that AD, PD, Huntington’s disease, or any of a number of other proteopathies are infectious diseases (Irwin et al. 2013, Jucker &Walker 2013); to imply otherwise could unjustifiably stigmatize patients and distress caregivers who already are suffering under the burden of disease (Hardy & Revesz 2012).
Accordingly, sound scientific and ethical reasons exist for using the word prion judiciously. However, prion is a useful, descriptive, and firmly established term in the biomedical literature, and it is increasingly invoked as a comprehensive designation for the concept of self-propagation of proteinaceous aggregates (Jucker & Walker 2013). We propose that the time has come to modify the definition to encompass the full scope of the prion paradigm in biology and to accommodate the many instances in which proteopathic diseases lack an infectious origin. The fundamental molecular mechanism that unifies the templating capacity of prion-like proteins is the nucleated transformation of protein conformation. In this spirit, we suggest that “prion” should be defined broadly as a “proteinaceous nucleating particle” (rather than a “proteinaceous infectious particle”). This revised definition does not deny the infectivity of PrP-prions or the potential infectious instigation of certain other proteopathies such as AA amyloidosis (Murakami et al. 2014, Westermark & Westermark 2010) or transthyretin amyloidosis (Lladó et al. 2010). Rather, the designation of prions as proteinaceous nucleating particles more fully embodies the true scope of prion-like processes in biology and allows the prudent inclusion of noninfectious proteopathies under the umbrella of the prion concept. This expanded and refined definition could help to obviate unnecessary confusion and concern about the communicability of noninfectious proteopathies and speed acceptance of this important paradigm within the biomedical community.
Acknowledgments
We gratefully acknowledge helpful discussions with Harry LeVine III, Amarallys Cintron, Frank Baumann, Yvonne Eisele, and the members of our laboratories. We are also indebted to Jeromy Dooyema for technical assistance and to Simone Eberle for editorial support. This work was made possible by grants from the Competence Network on Degenerative Dementias (BMBF- 01GI0705), ALZKULT (BMBF-031A198A), and from the US National Institutes of Health (AG040589, RR000165, OD11132, and AG025688), the CART Foundation, and anonymous foundations. The authors would also like to acknowledge the generous support of the MetLife Foundation.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Lary C. Walker, Email: lary.walker@emory.edu.
Mathias Jucker, Email: mathias.jucker@uni-tuebingen.de.
LITERATURE CITED
- Aguzzi A. Prions and the immune system: a journey through gut, spleen, and nerves. Adv. Immunol. 2003;81:123–171. doi: 10.1016/s0065-2776(03)81004-0. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Heikenwalder M, Polymenidou M. Insights into prion strains and neurotoxicity. Nat. Rev. Mol. Cell Biol. 2007;8:552–561. doi: 10.1038/nrm2204. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Cooper J, Murray TK, Garn K, McNaughton E, et al. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: The pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 2014;127:667–683. doi: 10.1007/s00401-014-1254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Chalabi A, Jones A, Troakes C, King A, Al-Sarraj S, van den Berg LH. The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathol. 2012;124:339–352. doi: 10.1007/s00401-012-1022-4. [DOI] [PubMed] [Google Scholar]
- Ano Bom APD, Rangel LP, Costa DCF, de Oliveira GAP, Sanches D, et al. Mutant p53 aggregates into prion-like amyloid oligomers and fibrils: implications for cancer. J. Biol. Chem. 2012;287:28152–28162. doi: 10.1074/jbc.M112.340638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb. Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Aulić S, Le TTN, Moda F, Abounit S, Corvaglia S, et al. Defined α-synuclein prion-like molecular assemblies spreading in cell culture. BMC Neurosci. 2014;15:69. doi: 10.1186/1471-2202-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol. Rev. 2004;84:361–384. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Evidence for the experimental transmission of cerebral β-amyloidosis to primates. Int. J. Exp. Pathol. 1993;74:441–454. [PMC free article] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennion Callister J, Pickering-Brown SM. Pathogenesis/genetics of frontotemporal dementia and how it relates to ALS. Exp. Neurol. 2014;262(Part B):84–90. doi: 10.1016/j.expneurol.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boluda S, Iba M, Zhang B, Raible KM, Lee VMY, Trojanowski JQ. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol. 2015;129:221–237. doi: 10.1007/s00401-014-1373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Braak H, Brettschneider J, Ludolph AC, Lee VM, Trojanowski JQ, Del Tredici K. Amyotrophic lateral sclerosis—a model of corticofugal axonal spread. Nat. Rev. Neurol. 2013;9:708–714. doi: 10.1038/nrneurol.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J, Del Tredici K, Irwin DJ, Grossman M, Robinson JL, et al. Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD) Acta Neuropathol. 2014;127:423–439. doi: 10.1007/s00401-013-1238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Brandel JP, Sato T, Nakamura Y, MacKenzie J, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg. Infect. Dis. 2012;18:901–907. doi: 10.3201/eid1806.120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyukmihci N, Goehring-Harmon F, Marsh RF. Neural pathogenesis of experimental scrapie after intraocular inoculation of hamsters. Exp. Neurol. 1983;81:396–406. doi: 10.1016/0014-4886(83)90271-6. [DOI] [PubMed] [Google Scholar]
- Cai X, Chen J, Xu H, Liu S, Jiang QX, et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156:1207–1222. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B, Baron GS, Chesebro B, Jeffrey M. Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions. Annu. Rev. Biochem. 2009;78:177–204. doi: 10.1146/annurev.biochem.78.082907.145410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B, Race R, Wehrly K, Nishio J, Bloom M, et al. Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain. Nature. 1985;315:331–333. doi: 10.1038/315331a0. [DOI] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. PNAS. 2013;110:9535–9540. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Hench J, Lavenir I, Schweighauser G, Frank S, et al. Peripheral administration of tau aggregates triggers intracerebral tauopathy in transgenic mice. Acta Neuropathol. 2014;127:299–301. doi: 10.1007/s00401-013-1231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby DW, Wain R, Baskakov IV, Legname G, Palmer CG, et al. Protease-sensitive synthetic prions. PLOS Pathog. 2010;6:e1000736. doi: 10.1371/journal.ppat.1000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science. 2007;318:930–936. doi: 10.1126/science.1138718. [DOI] [PubMed] [Google Scholar]
- Collins SJ, Lawson VA, Masters CL. Transmissible spongiform encephalopathies. Lancet. 2004;363:51–61. doi: 10.1016/S0140-6736(03)15171-9. [DOI] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, Van Langenhove T, van der Zee J, Van Broeckhoven C. Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum. Trends Neurosci. 2013;36:450–459. doi: 10.1016/j.tins.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Cushman M, Johnson BS, King OD, Gitler AD, Shorter J. Prion-like disorders: blurring the divide between transmissibility and infectivity. J. Cell Sci. 2010;123:1191–1201. doi: 10.1242/jcs.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeArmond SJ, Prusiner SB. Etiology and pathogenesis of prion diseases. Am. J. Pathol. 1995;146:785–811. [PMC free article] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleault NR, Walsh DJ, Piro JR, Wang F, Wang X, et al. Cofactor molecules maintain infectious conformation and restrict strain properties in purified prions. PNAS. 2012;109:E1938–E1946. doi: 10.1073/pnas.1206999109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domert J, Rao SB, Agholme L, Brorsson AC, Marcusson J, et al. Spreading of amyloid-β peptides via neuritic cell-to-cell transfer is dependent on insufficient cellular clearance. Neurobiol. Dis. 2014;65:82–92. doi: 10.1016/j.nbd.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Duran-Aniotz C, Morales R, Moreno-Gonzalez I, Hu PP, Fedynyshyn J, Soto C. Aggregate-depleted brain fails to induce Aβ deposition in a mouse model of Alzheimer’s disease. PLOS ONE. 2014;9:e89014. doi: 10.1371/journal.pone.0089014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Aniotz C, Morales R, Moreno-Gonzalez I, Hu PP, Soto C. Brains from non-Alzheimer’s individuals containing amyloid deposits accelerate Aβ deposition in vivo. Acta Neuropathol. Commun. 2013;1:76. doi: 10.1186/2051-5960-1-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Bolmont T, Heikenwalder M, Langer F, Jacobson LH, et al. Induction of cerebral β-amyloidosis: intracerebral versus systemic Aβ inoculation. PNAS. 2009;106:12926–12931. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Fritschi SK, Hamaguchi T, Obermüller U, Füger P, et al. Multiple factors contribute to the peripheral induction of cerebral β-amyloidosis. J. Neurosci. 2014;34:10264–10273. doi: 10.1523/JNEUROSCI.1608-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Obermüller U, Heilbronner G, Baumann F, Kaeser SA, et al. Peripherally applied Aβ-containing inoculates induce cerebral β-amyloidosis. Science. 2010;330:980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148:1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast C, Groschup MH. Classical and atypical scrapie in sheep and goats. In: Zou WQ, Gambetti P, editors. Prions and Diseases, Vol. 2: Animals, Humans and the Environment. New York: Springer; 2013. pp. 15–44. [Google Scholar]
- Forget KJ, Tremblay G, Roucou X. p53 Aggregates penetrate cells and induce the co-aggregation of intracellular p53. PLOS ONE. 2013;8:e69242. doi: 10.1371/journal.pone.0069242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SA. Somatic mosaicism and disease. Curr. Biol. 2014;24:R577–R581. doi: 10.1016/j.cub.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat. Immunol. 2014;15:727–737. doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser H. Neuronal spread of scrapie agent and targeting of lesions within the retino-tectal pathway. Nature. 1982;295:149–150. doi: 10.1038/295149a0. [DOI] [PubMed] [Google Scholar]
- Fritschi SK, Cintron A, Ye L, Mahler J, Bühler A, et al. Aβ seeds resist inactivation by formaldehyde. Acta Neuropathol. 2014a;128:477–484. doi: 10.1007/s00401-014-1339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschi SK, Langer F, Kaeser SA, Maia LF, Portelius E, et al. Highly potent soluble amyloid-β seeds in human Alzheimer brain but not cerebrospinal fluid. Brain. 2014b;137:2909–2915. doi: 10.1093/brain/awu255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y, Kaneko K, Watanabe S, Yamanaka K, Nukina N. A seeding reaction recapitulates intracellular formation of Sarkosyl-insoluble transactivation response element (TAR) DNA-binding protein-43 inclusions. J. Biol. Chem. 2011;286:18664–18672. doi: 10.1074/jbc.M111.231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek DC. Unconventional viruses and the origin and disappearance of kuru. Science. 1977;197:943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Gambetti P, Cali I, Notari S, Kong Q, Zou WQ, Surewicz WK. Molecular biology and pathology of prion strains in sporadic human prion diseases. Acta Neuropathol. 2011;121:79–90. doi: 10.1007/s00401-010-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- Goudsmit J, Morrow CH, Asher DM, Yanagihara RT, Masters CL, et al. Evidence for and against the transmissibility of Alzheimer disease. Neurology. 1980;30:945–950. doi: 10.1212/wnl.30.9.945. [DOI] [PubMed] [Google Scholar]
- Grad LI, Cashman NR. Prion-like activity of Cu/Zn superoxide dismutase: implications for amyotrophic lateral sclerosis. Prion. 2014;8:33–41. doi: 10.4161/pri.27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad LI, Yerbury JJ, Turner BJ, Guest WC, Pokrishevsky E, et al. Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosome-dependent and -independent mechanisms. PNAS. 2014;111:3620–3625. doi: 10.1073/pnas.1312245111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, et al. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell. 2013;154:103–117. doi: 10.1016/j.cell.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JL, Lee VMY. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat. Med. 2014;20:130–138. doi: 10.1038/nm.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi T, Eisele YS, Varvel NH, Lamb BT, Walker LC, Jucker M. The presence of Aβ seeds, and not age per se is critical to the initiation of Aβ deposition in the brain. Acta Neuropathol. 2012;123:31–37. doi: 10.1007/s00401-011-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TW, Kato M, Xie S, Wu LC, Mirzaei H, et al. Cell-free formation of RNA granules: Bound RNAs identify features and components of cellular assemblies. Cell. 2012;149:768–779. doi: 10.1016/j.cell.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Hardy J, Revesz T. The spread of neurodegenerative disease. N. Engl. J. Med. 2012;366:2126–2128. doi: 10.1056/NEJMcibr1202401. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Heilbronner G, Eisele YS, Langer F, Kaeser SA, Novotny R, et al. Seeded strain-like transmission of β-amyloid morphotypes in APP transgenic mice. EMBO Rep. 2013;14:1017–1022. doi: 10.1038/embor.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer E, Rosen RF, Cintron A, Walker LC. Nonhuman primate models of Alzheimer-like cerebral proteopathy. Curr. Pharm. Des. 2012;18:1159–1169. doi: 10.2174/138161212799315885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci. Transl. Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VMY. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J. Neurosci. 2013;33:1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, Abrams JY, Schonberger LB, Leschek EW, Mills JL, et al. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol. 2013;70:462–468. doi: 10.1001/jamaneurol.2013.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturria-Medina Y, Sotero RC, Toussaint PJ, Evans AC Alzheimer’s Dis. Neuroimaging Initiat. Epidemic spreading model to characterize misfolded proteins propagation in aging and associated neurodegenerative disorders. PLOS Comput. Biol. 2014;10:e1003956. doi: 10.1371/journal.pcbi.1003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RT. Prion diseases. Lancet Neurol. 2005;4:635–642. doi: 10.1016/S1474-4422(05)70192-7. [DOI] [PubMed] [Google Scholar]
- Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat. Med. 2010;16:1210–1214. doi: 10.1038/nm.2224. [DOI] [PubMed] [Google Scholar]
- Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 2011;70:532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, et al. Evidence for seeding of β-amyloid by intracerebral infusion of Alzheimer brain extracts in β-amyloid precursor protein-transgenic mice. J. Neurosci. 2000;20:3606–3611. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Han TW, Xie S, Shi K, Du X, et al. Cell-free formation of RNA granules: Low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149:753–767. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim NC, Wang YD, Scarborough EA, Moore J, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495:467–473. doi: 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin RH, Walker CA. Pathogenesis of scrapie (strain 263K) in hamsters infected intracerebrally, intraperitoneally or intraocularly. J. Gen. Virol. 1986;67(Part 2):255–263. doi: 10.1099/0022-1317-67-2-255. [DOI] [PubMed] [Google Scholar]
- King OD, Gitler AD, Shorter J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 2012;1462:61–80. doi: 10.1016/j.brainres.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles TP, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014;15:384–396. doi: 10.1038/nrm3810. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body–like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Krauss S, Vorberg I. Prions ex vivo: what cell culture models tell us about infectious proteins. Int. J. Cell Biol. 2013;2013:704546. doi: 10.1155/2013/704546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer F, Eisele YS, Fritschi SK, Staufenbiel M, Walker LC, Jucker M. Soluble Aβ seeds are potent inducers of cerebral β-amyloid deposition. J. Neurosci. 2011;31:14488–14495. doi: 10.1523/JNEUROSCI.3088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Kiritoshi T, et al. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci. Rep. 2012;2:700. doi: 10.1038/srep00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VMY, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Li YR, King OD, Shorter J, Gitler AD. Stress granules as crucibles of ALS pathogenesis. J. Cell Biol. 2013;201:361–372. doi: 10.1083/jcb.201302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberski PP, Hainfellner JA, Sikorska B, Budka H. Prion protein (PrP) deposits in the tectum of experimental Gerstmann-Stra üssler-Scheinker disease following intraocular inoculation. Folia Neuropathol. 2012;50:85–88. [PubMed] [Google Scholar]
- Liebman SW, Chernoff YO. Prions in yeast. Genetics. 2012;191:1041–1072. doi: 10.1534/genetics.111.137760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. 2013;79:416–438. doi: 10.1016/j.neuron.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, et al. Trans-synaptic spread of tau pathology in vivo. PLOS ONE. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lladó L, Baliellas C, Casasnovas C, Ferrer I, Fabregat J, et al. Risk of transmission of systemic transthyretin amyloidosis after domino liver transplantation. Liver Transplant. 2010;16:1386–1392. doi: 10.1002/lt.22174. [DOI] [PubMed] [Google Scholar]
- Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, Tycko R. Molecular structure of β-amyloid fibrils in Alzheimer’s disease brain tissue. Cell. 2013;154:1257–1268. doi: 10.1016/j.cell.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012a;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VMY. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 2012b;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Song C, O’Brien P, Stieber A, Branch JR, et al. Exogenous α-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. PNAS. 2009;106:20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maji SK, Perrin MH, Sawaya MR, Jessberger S, Vadodaria K, et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Kubo M, Shimozawa A, et al. Pathological alpha-synuclein propagates through neural networks. Acta Neuropathol. Commun. 2014;2:88. doi: 10.1186/s40478-014-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell MJ, Lindberg MR, Brennand KJ, Piper JC, Voet T, et al. Mosaic copy number variation in human neurons. Science. 2013;342:632–637. doi: 10.1126/science.1243472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AK, Rosen RF, Childers WS, Gehman JD, Walker LC, Lynn DG. Context dependence of protein misfolding and structural strains in neurodegenerative diseases. Biopolymers. 2013;100:722–730. doi: 10.1002/bip.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J, Sachse C, Hortschansky P, Grigorieff N, Fändrich M. Aβ(1–40) fibril polymorphism implies diverse interaction patterns in amyloid fibrils. J. Mol. Biol. 2009;386:869–877. doi: 10.1016/j.jmb.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, et al. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- Mizielinska S, Grönke S, Niccoli T, Ridler CE, Clayton EL, et al. C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science. 2014;345:1192–1194. doi: 10.1126/science.1256800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales R, Duran-Aniotz C, Castilla J, Estrada LD, Soto C. De novo induction of amyloid-β deposition in vivo. Mol. Psychiatry. 2012;17:1347–1353. doi: 10.1038/mp.2011.120. [DOI] [PubMed] [Google Scholar]
- Mougenot AL, Nicot S, Bencsik A, Morignat E, Verchere J, et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol. Aging. 2012;33:2225–2228. doi: 10.1016/j.neurobiolaging.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Munch C, O’Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. PNAS. 2011;108:3548–3553. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Ishiguro N, Higuchi K. Transmission of systemic AA amyloidosis in animals. Vet. Pathol. 2014;51:363–371. doi: 10.1177/0300985813511128. [DOI] [PubMed] [Google Scholar]
- Nath S, Agholme L, Kurudenkandy FR, Granseth B, Marcusson J, Hallbeck M. Spreading of neurodegenerative pathology via neuron-to-neuron transmission of β-amyloid. J. Neurosci. 2012;32:8767–8777. doi: 10.1523/JNEUROSCI.0615-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby GA, Lindquist S. Blessings in disguise: biological benefits of prion-like mechanisms. Trends Cell Biol. 2013;23:251–259. doi: 10.1016/j.tcb.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Nilsson KPR, Åslund A, Berg I, Nyström S, Konradsson P, et al. Imaging distinct conformational states of amyloid-βfibrils in Alzheimer’s disease using novel luminescent probes. ACS Chem. Biol. 2007;2:553–560. doi: 10.1021/cb700116u. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Masuda-Suzukake M, Arai T, Hasegawa Y, Akatsu H, et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 2013;4:124–134. doi: 10.1016/j.celrep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Oesch B, Westaway D, Wälchli M, McKinley MP, Kent SBH, et al. A cellular gene encodes scrapie PrP 27–30 protein. Cell. 1985;40:735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Paravastu AK, Leapman RD, Yau WM, Tycko R. Molecular structural basis for polymorphism in Alzheimer’s β-amyloid fibrils. PNAS. 2008;105:18349–18354. doi: 10.1073/pnas.0806270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paravastu AK, Qahwash I, Leapman RD, Meredith SC, Tycko R. Seeded growth of β-amyloid fibrils from Alzheimer’s brain-derived fibrils produces a distinct fibril structure. PNAS. 2009;106:7443–7448. doi: 10.1073/pnas.0812033106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann. Neurol. 1999;46:224–233. [PubMed] [Google Scholar]
- Pecho-Vrieseling E, Rieker C, Fuchs S, Bleckmann D, Esposito MS, et al. Transneuronal propagation of mutant huntingtin contributes to non-cell autonomous pathology in neurons. Nat. Neurosci. 2014;17:1064–1072. doi: 10.1038/nn.3761. [DOI] [PubMed] [Google Scholar]
- Peeraer E, Bottelbergs A, Van Kolen K, Stancu IC, Vasconcelos B, et al. Intracerebral injection of preformed synthetic tau fibrils initiates widespread tauopathy and neuronal loss in the brains of tau transgenic mice. Neurobiol. Dis. 2015;73:83–95. doi: 10.1016/j.nbd.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova AT, Leapman RD, Guo Z, Yau WM, Mattson MP, Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer’s β-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Some speculations about prions, amyloid, and Alzheimer’s disease. N. Engl. J. Med. 1984;310:661–663. doi: 10.1056/NEJM198403083101021. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Prions. PNAS. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. A unifying role for prions in neurodegenerative diseases. Science. 2012;336:1511–1513. doi: 10.1126/science.1222951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. Biology and genetics of prions causing neurodegeneration. Annu. Rev. Genet. 2013;47:601–623. doi: 10.1146/annurev-genet-110711-155524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nat. Rev. Neurol. 2012;8:423–434. doi: 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, LoCastro E, Kuceyeski A, Tosun D, Relkin N, et al. Network diffusion model of progression predicts longitudinal patterns of atrophy and metabolism in Alzheimer’s disease. Cell Rep. 2015;10:359–369. doi: 10.1016/j.celrep.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel LP, Costa DCF, Vieira TCRG, Silva JL. The aggregation of mutant p53 produces prion-like properties in cancer. Prion. 2014;8:75–84. doi: 10.4161/pri.27776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveendra BL, Siemer AB, Puthanveettil SV, Hendrickson WA, Kandel ER, McDermott AE. Characterization of prion-like conformational changes of the neuronal isoform of Aplysia CPEB. Nat. Struct. Mol. Biol. 2013;20:495–501. doi: 10.1038/nsmb.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravits JM, La Spada AR. ALS motor phenotype heterogeneity, focality, and spread: deconstructing motor neuron degeneration. Neurology. 2009;73:805–811. doi: 10.1212/WNL.0b013e3181b6bbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recasens A, Dehay B, Bové J, Carballo-Carbajal I, Dovero S, et al. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann. Neurol. 2014;75:351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat. Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Ciliax BJ, Wingo TS, Gearing M, Dooyema J, et al. Deficient high-affinity binding of Pittsburgh compound B in a case of Alzheimer’s disease. Acta Neuropathol. 2010;119:221–233. doi: 10.1007/s00401-009-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Fritz JJ, Dooyema J, Cintron AF, Hamaguchi T, et al. Exogenous seeding of cerebral β-amyloid deposition in βAPP-transgenic rats. J. Neurochem. 2012;120:660–666. doi: 10.1111/j.1471-4159.2011.07551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Walker LC, LeVine H., III PIB binding in aged primate brain: enrichment of high-affinity sites in humans with Alzheimer’s disease. Neurobiol. Aging. 2011;32:223–234. doi: 10.1016/j.neurobiolaging.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar J, Wille H, Itri V, Groth D, Serban H, et al. Eight prion strains have PrPSc molecules with different conformations. Nat. Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- Sanders DW, Kaufman SK, DeVos SL, Sharma AM, Mirbaha H, et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. 2014;82:1271–1288. doi: 10.1016/j.neuron.2014.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Wainer BH, German DC. Axonal and transneuronal transport in the transmission of neurological disease: potential role in system degenerations, including Alzheimer’s disease. Neuroscience. 1987;23:389–398. doi: 10.1016/0306-4522(87)90063-7. [DOI] [PubMed] [Google Scholar]
- Saunders SE, Bartelt-Hunt SL, Bartz JC. Occurrence, transmission, and zoonotic potential of chronic wasting disease. Emerg. Infect. Dis. 2012;18:369–376. doi: 10.3201/eid1803.110685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Preventing Alzheimer’s disease. Science. 2012;337:1488–1492. doi: 10.1126/science.1228541. [DOI] [PubMed] [Google Scholar]
- Si K, Lindquist S, Kandel ER. A neuronal isoform of the Aplysia CPEB has prion-like properties. Cell. 2003;115:879–891. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, et al. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, et al. Nomenclature 2014: amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid. 2014;21:221–224. doi: 10.3109/13506129.2014.964858. [DOI] [PubMed] [Google Scholar]
- Song HL, Shim S, Kim DH, Won SH, Joo S, et al. β-Amyloid is transmitted via neuronal connections along axonal membranes. Ann. Neurol. 2014;75:88–97. doi: 10.1002/ana.24029. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M. Tau pathology and neurodegeneration. Lancet Neurol. 2013;12:609–622. doi: 10.1016/S1474-4422(13)70090-5. [DOI] [PubMed] [Google Scholar]
- Stedman TL. Stedman’s Medical Dictionary for the Health Professions and Nursing. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2012. [Google Scholar]
- Stöhr J, Condello C, Watts JC, Bloch L, Oehler A, et al. Distinct synthetic Aβ prion strains producing different amyloid deposits in bigenic mice. PNAS. 2014;111:10329–10334. doi: 10.1073/pnas.1408968111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr J, Watts JC, Mensinger ZL, Oehler A, Grillo SK, et al. Purified and synthetic Alzheimer’s amyloid beta (Aβ) prions. PNAS. 2012;109:11025–11030. doi: 10.1073/pnas.1206555109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S, Tanaka M. Self-propagating amyloid as a critical regulator for diverse cellular functions. J. Biochem. 2014;155:345–351. doi: 10.1093/jb/mvu026. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Collins SR, Toyama BH, Weissman JS. The physical basis of how prion conformations determine strain phenotypes. Nature. 2006;442:585–589. doi: 10.1038/nature04922. [DOI] [PubMed] [Google Scholar]
- Tuite MF. The natural history of yeast prions. Adv. Appl. Microbiol. 2013;84:85–137. doi: 10.1016/B978-0-12-407673-0.00003-5. [DOI] [PubMed] [Google Scholar]
- Udan M, Baloh RH. Implications of the prion-related Q/N domains in TDP-43 and FUS. Prion. 2011;5:1–5. doi: 10.4161/pri.5.1.14265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Langenhove T, van der Zee J, Van Broeckhoven C. The molecular basis of the frontotemporal lobar degeneration–amyotrophic lateral sclerosis spectrum. Ann. Med. 2012;44:817–828. doi: 10.3109/07853890.2012.665471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Callahan MJ, Bian F, Durham RA, Roher AE, Lipinski WJ. Exogenous induction of cerebral β-amyloidosis in βAPP-transgenic mice. Peptides. 2002;23:1241–1247. doi: 10.1016/s0196-9781(02)00059-1. [DOI] [PubMed] [Google Scholar]
- Walker LC, LeVine H., III Corruption and spread of pathogenic proteins in neurodegenerative diseases. J. Biol. Chem. 2012;287:33109–33115. doi: 10.1074/jbc.R112.399378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wang X, Yuan CG, Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Condello C, Stöhr J, Oehler A, Lee J, et al. Serial propagation of distinct strains of Aβ prions from Alzheimer’s disease patients. PNAS. 2014;111:10323–10328. doi: 10.1073/pnas.1408900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Giles K, Grillo SK, Lemus A, DeArmond SJ, Prusiner SB. Bioluminescence imaging of Aβ deposition in bigenic mouse models of Alzheimer’s disease. PNAS. 2011;108:2528–2533. doi: 10.1073/pnas.1019034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, et al. Transmission of multiple system atrophy prions to transgenic mice. PNAS. 2013;110:19555–19560. doi: 10.1073/pnas.1318268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark GT, Westermark P. Prion-like aggregates: infectious agents in human disease. Trends Mol. Med. 2010;16:501–507. doi: 10.1016/j.molmed.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Wickner RB, Edskes HK, Bateman DA, Kelly AC, Gorkovskiy A, et al. Amyloids and yeast prion biology. Biochemistry. 2013;52:1514–1527. doi: 10.1021/bi301686a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ES. Chronic wasting disease. Vet. Pathol. 2005;42:530–549. doi: 10.1354/vp.42-5-530. [DOI] [PubMed] [Google Scholar]
- Wu JW, Herman M, Liu L, Simoes S, Acker CM, et al. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J. Biol. Chem. 2013;288:1856–1870. doi: 10.1074/jbc.M112.394528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Nakamura A, Chiba T, Kogishi K, Matsushita T, et al. Transmission of mouse senile amyloidosis. Lab. Investig. 2001;81:493–499. doi: 10.1038/labinvest.3780257. [DOI] [PubMed] [Google Scholar]
- Xu J, Reumers J, Couceiro JR, De Smet F, Gallardo R, et al. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat. Chem. Biol. 2011;7:285–295. doi: 10.1038/nchembio.546. [DOI] [PubMed] [Google Scholar]
- Ye L, Hamaguchi T, Fritschi SK, Eisele YS, Obermüller U, et al. Progression of seed-induced Aβ deposition within the limbic connectome. Brain Pathol. 2015 doi: 10.1111/bpa.12252. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang F, Wang X, Zhang Z, Xu Y, et al. Comparison of 2 synthetically generated recombinant prions. Prion. 2014;8:215–220. doi: 10.4161/pri.28669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73:1216–1227. doi: 10.1016/j.neuron.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]