Abstract
Rationale: The goal of shared decision making is to match patient preferences, including evaluation of potential future outcomes, with available management options. Yet, it is unknown how patients with smoking-related thoracic diseases or their surrogates display future-oriented thinking.
Objectives: To document prevalent themes in patients’ and potential surrogate decision makers’ future-oriented thinking when facing preference-sensitive choices.
Methods: We conducted 44 scenario-based semistructured interviews among a diverse group of outpatients with smoking-associated thoracic diseases and potential surrogates for whom one of three preference-sensitive decisions would be medically relevant. Using content analysis, we documented prevalent themes to understand how these individuals display future-oriented thinking.
Measurements and Main Results: Patients and potential surrogates generally expressed expectations for future outcomes but also acknowledged their limitations in doing so. When thinking about potential outcomes, decision makers relied on past experiences, including those only loosely related; perceived familiarity with treatment options; and spirituality. The content of these expectations included effects on family, emotional predictions, and prognostication. For surrogates, a tension existed between hope-based and fact-based expectations.
Conclusions: Patients and surrogates may struggle to generate expectations, and these future-oriented thoughts may be based on loosely related past experiences or unrealistic optimism. These tendencies may lead to errors, preventing selection of treatments that promote true preferences. Clinicians should explore how decision makers engage in future-oriented thinking and what their expectations are as a component of the shared decision-making process. Future research should evaluate whether targeted guidance in future-oriented thinking may improve outcomes important to patients.
Keywords: shared decision making, patient preference, chronic pulmonary disease, solitary pulmonary nodule, non–small cell carcinoma
At a Glance Commentary
Scientific Knowledge on the Subject
The goal of shared decision making is to match patient preferences, including evaluation of potential future outcomes, with available management options. However, little is known about how patients with thoracic diseases or their surrogates generate predictions or the nature of such expectations.
What This Study Adds to the Field
Using qualitative interviews, this study identifies key aspects of patients’ and surrogates’ future predictions when faced with preference-sensitive treatment options in the management of smoking-associated thoracic diseases. These themes are integrated into a novel conceptual model of how expectations are formed and highlight ways in which clinicians and future interventions may help patients and surrogates make choices that better promote their future well-being.
Patients and their surrogate decision makers often must choose from multiple reasonable diagnostic and therapeutic options (1–7). These decisions are often complex given the potential tradeoffs between quality and duration of life. The advent of new technologies and a greater appreciation for patient-centered care expands these complexities, as in the case of recommendations for advance care planning in the routine management of chronic obstructive pulmonary disease (COPD) (8) and for low-dose computed tomography scanning of individuals with significant tobacco histories (9–13).
Patients and their surrogate decision makers ideally partner with clinicians to evaluate treatment choices in light of patient-specific values and goals (14). This process requires that clinicians not only communicate what the treatment entails but also that clinicians and patients have clear expectations for future outcomes resulting from the treatment (15). These elements are particularly important when evidence or clinical guidelines fail to unambiguously favor one management option.
Future-oriented thinking may lead patients to analyze the potential outcomes in the context of their own personal goals, thereby minimizing undesired effects (5, 16–22). However, individual patients may struggle to evaluate management choices with very different potential emotional and psychological outcomes. Current and former heavy smokers who have developed smoking-associated lung diseases are at particular risk of such difficulties in envisioning future outcomes. Individuals who smoke have demonstrated the inability to accept a short-term cost (e.g., loss of pleasure from smoking) to obtain the long-term benefits of better health (e.g., avoiding lung disease). Therefore, heavy smokers are likely to devalue future rewards or consequences in favor of immediate benefits (23–40).
Helping decision makers to focus on future outcomes may improve satisfaction with the decision-making process, the outcomes that follow from choices made, or both. Attention to these potential outcomes may also decrease negative emotional aspects of decision making, including regret (5, 18, 41–44). We, therefore, conducted a qualitative study of patients with smoking-associated thoracic diseases (or the potential for such disease) that included patients at risk for lung nodules, those with COPD, and potential surrogate decision makers of patients with advanced non–small cell lung cancer (NSCLC). Our goals were to explore whether patients with smoking-associated thoracic diseases and their surrogates display elements of future-oriented thinking when making decisions and to examine the content of the expectations they form. J.L.H. presented an earlier version of this work at the Society for Medical Decision Making Annual Meeting in October 2014 (45).
Methods
We conducted semistructured interviews with participants drawn from four outpatient clinics within a single health system between February 2014 and July 2014. These clinics included quaternary care subspecialty clinics and primary care offices serving a racially and economically diverse urban population.
Participants
Investigators screened electronic health records to identify individuals with smoking-associated thoracic conditions: (1) patients with a documented diagnosis of COPD, with FEV1 less than 50% predicted, or prescribed home oxygen therapy; (2) patients meeting United States Preventive Services Task Force preliminary lung cancer screening criteria, as defined as age 55–74 years with greater than 30 pack-years of smoking history who quit less than 15 years before enrollment (9); or (3) patients with stage IIIB or IV NSCLC. We sought consent from patients in the first two groups and surrogates of patients with NSCLC. We conducted stratified purposive sampling of participants to promote sociodemographic diversity (46). Additional details are provided in the online supplement.
Clinicians were contacted to approve or decline recruitment of individual patients and physicians declined recruitment of 280 patients identified as potentially eligible (5.7%). Patients were excluded if they were non-English speaking, lacked decision-making capacity, or had previous exposure to the decision posed. All participants provided written consent; completed a demographic questionnaire; and participated in an audio-recorded, face-to-face interview.
Participants received $20 in exchange for their time and effort. The University of Pennsylvania Institutional Review Board and the Abramson Cancer Center Clinical Trials Scientific Review and Monitoring Committee approved all aspects of the study.
Semistructured Interviews
Data collection was performed by three interviewers trained by a medical anthropologist in semistructured interviewing techniques. Interviewers read aloud a standardized vignette describing a preference-sensitive medical decision tailored to the three participant groups: intubation or palliative care in the event of respiratory failure for patients with COPD, biopsy or surveillance for patients with a pulmonary nodule, and intensive care unit (ICU) admission or palliative care for patients with advanced NSCLC who become critically ill (Table 1; see online supplement for full text). The selection of these groups allowed us to explore a range of medically relevant decisions that the individual had not previously confronted but could be expected to encounter in the near future.
Table 1.
Participant and Scenario Types
| Type of Decision Maker | Participant Population | Scenario |
|---|---|---|
| Patient | Patients with severe or very severe chronic obstructive pulmonary disease* attending an outpatient appointment at the Penn Lung Center or Penn primary care clinics | Patient with severe chronic obstructive pulmonary disease completing an advance directive deciding between intubation with mechanical ventilation or palliative care in the event of respiratory failure |
| Patient | Patients meeting United States Preventive Services Task Force preliminary guidelines for low-dose computed tomography screening† attending an outpatient appointment at the Penn Lung Center or Penn primary care clinics | Patient with incidentally found pulmonary nodule deciding between immediate diagnostic biopsy or radiographic surveillance |
| Surrogate | Surrogate of patient with advanced lung cancer‡ attending an outpatient appointment at the Penn Infusion Center | Surrogate of patient with advanced lung cancer experiencing an acute illness in the emergency department deciding between admission to the intensive care unit or palliative care |
FEV1 <50% predicted or chronic oxygen dependence.
Age 55–74 years of age, >30 pack-year tobacco history, quit tobacco (if applicable) <15 years ago.
Stage IIIb or IV non–small cell lung cancer.
For each decision, the two options were described as being equally valid and therefore preference-sensitive. The participant was asked to describe his or her decision-making process and respond to an evolving set of prompts intended to elicit preferences regarding information seeking about outcomes of treatment decisions, consideration of possible futures, the proximity of the future outcomes considered, and future regret as a factor in decision making. We ceased interviewing after reaching thematic saturation, defined as the failure to identify new themes during three consecutive interviews.
Data Analysis
The audiotaped interviews were professionally transcribed verbatim. Using open coding techniques with an initial set of nine interviews (47), investigators identified themes and concepts regarding the participants’ perspectives of the future and expectation formation during the decision-making process. After independent identification of such themes, each subsequent interview was independently coded by two or three investigators using a codebook of these themes. Discrepancies in the application of codes were resolved by consensus. NVivo10 (QSR International, Melbourne, Australia) was used for database management. As prevalent themes emerged through ongoing concurrent data analysis, the interview guide and codebook were iteratively revised and past interviews were recoded. Additional details regarding our analytic methods are provided in the online supplement.
Results
Participant Characteristics
We approached 56 eligible patients with COPD and/or who met criteria for low-dose computed tomography screening for lung cancer. Of these, 27 (48.2%) completed an interview. Given the significant overlap between these two groups, we assigned patients who met criteria for both a priori to the group with the fewest completed interviews at the time of enrollment. We approached 31 potential surrogate decision makers, one for each of 31 patients with advanced NSCLC. Seventeen (54.8%) of these surrogates completed an interview. Fourteen subjects participated in the COPD scenario, 15 in the newly discovered lung nodule scenario, and 17 in the surrogate of a patient with NSCLC scenario. Participants’ demographic characteristics are described in Table 2.
Table 2.
Participant Demographics
| Characteristic | Patients with COPD (n = 14) | Patients Eligible for Lung Cancer Screening (n = 13) | Potential Surrogate Decision Makers of Patients with Lung Cancer (n = 17) |
|---|---|---|---|
| Age, yr, median (range) | 65.5 (48–83) | 67 (57–70) | 57.5 (29–80)* |
| Sex | |||
| Female | 8 | 6 | 10 |
| Male | 6 | 7 | 7 |
| Race | |||
| White | 7 | 4 | 10 |
| Black | 7 | 9 | 6 |
| Other | — | — | 1 |
| Religious affiliation | |||
| Baptist | 2 | 4 | 3 |
| Catholic | 4 | 2 | 5 |
| Christian, non-Catholic | 5 | 4 | 5 |
| Jewish | 1 | 0 | 2 |
| Muslim | 1 | 2 | 1 |
| Other† | 1 | 1 | 1 |
| Income, annual | |||
| <$30,000 | 4 | 5 | 1 |
| $30,000–69,000 | 6 | 2 | 4 |
| >$69,000 | 4 | 5 | 8 |
| Declined to answer | 2 | 1 | 3 |
| Highest level of education‡ | |||
| Less than high school | 3 | 3 | 1 |
| High school | 5 | 1 | 5 |
| Some college | 4 | 6 | 5 |
| College degree | 1 | 3 | 3 |
| More than college | — | — | 3 |
| Relationship to patient | |||
| Spouse/partner | — | — | 6 |
| Child | — | — | 6 |
| Parent | — | — | 2 |
| Other | — | — | 2 |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
One participant declined response.
Included one identifying as nondenominational, one atheist, and one participant who declined response.
One participant declined to respond.
The Process of Forming Expectations
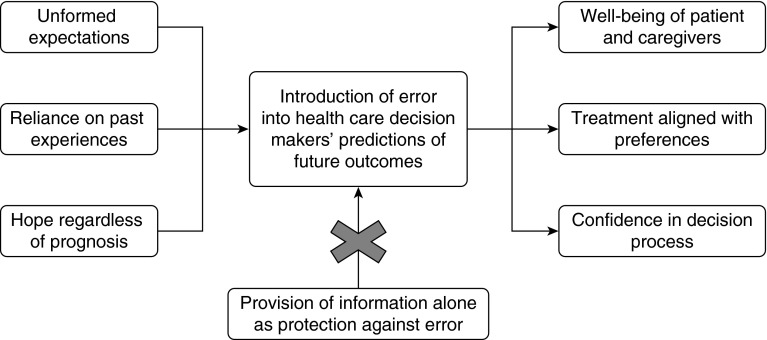
Participants engaged in future-oriented thinking during deliberation, but also discussed the limits of attempting to imagine an uncertain future (Table 3). These limits included participants feeling unprepared to fully engage in forecasting or unwilling to entertain notions of the future or of specifically negative outcomes (Figure 1). Those who self-identified as having a poor health state or limited future life expressed a relative ease of forecasting, including the need to plan for the dying process and focus on quality over quantity of life. This finding was predominant among those with severe COPD:
Table 3.
Key Themes among Participants and Key Patterns of Responses among Participant Groups
| Representative Quotations | |
|---|---|
| The process of forming
expectations |
|
| Limited ability to predict potential future states | It’s kind of hard to imagine . . . it’s just something that you have to be there and actually experience to know the feeling. |
| Avoidance of future-oriented thought | I don’t really imagine anything of the future I face . . . . We don’t know when we’re going to die. |
| Reliance on past experiences to formulate expectations | . . . with my dad they had him on a ventilator until all of us kids got there and we made the decision to take him off it. And he still lasted a week and just watching him, that was the longest week of my life, just watching him. So that really made me make sure that my husband and my sons all knew don’t put me on a machine and have me lie there like that. I don’t want that. |
| Reliance on spirituality to form expectations | You can’t picture the future or imagine the “what if’s.” It’s not there for you to choose the “what if’s.” As far as picturing it you don’t even picture it . . . . You’d be praying to the Lord up above to bring them out of this. But it’s always going to be His way, the way He wants it. |
| The content of expectations | |
| Potential impact of illness or treatment on family | I wouldn’t want to put anybody through what we went through with my brother . . . . I was with him every single day except two days from the time he was in the hospital . . . and it was exhausting and everybody starts getting cranky . . . and I wouldn’t want somebody to have to [go through] that for me. |
| Imagined intolerance of treatment itself | [Regarding repeated imaging for a lung nodule:] That would be aggravating having to keep coming back, coming back and doing the same thing over and over and over and over. |
| Loss of self, including of the caregiver role | [My mother’s] presence will be gone and it will be a void for a while. I can imagine a void. |
| Maintenance of positive emotional state regardless of outcome | It could always be worse than what it is and I’ve seen people in worse situations . . . so I still have to count my blessings. |
| Discussion of avoiding death as the dominant consideration | Am I going to live or die? That’s the most important thing. |
| Discussion of quality of life and suffering | To be on a ventilator, you’ve got to be confined to a bed, whether it’s in your home or in a nursing home, but you can’t get up, you can’t smell the flowers, you’re not outside, you’re not getting the sun. That’s not living. |
| Discussion of hope among potential surrogates of patients with advanced lung cancer | I always try to look at the positive and I try to have a lot of hope. I really probably wouldn’t see the [downsides to aggressive treatment]. |
Figure 1.
Conceptual model of the development of error in forecasting by health care decision makers.
“I’d say [I’m able to imagine] between 5 and 10 years [into the future]. I don’t go beyond that. I’m realistic. I have COPD, I had three stents put in my heart last year and I’m 63 years old and I was a smoker for 50 years. I don’t expect to live until I’m 100 by any means and I’m fine with that.”
Prior personal experiences heavily informed participants’ predictions of experiences and outcomes related to treatment options, despite clear differences between the past and current circumstances (Figure 1). A respondent discussing the management options of a newly found lung nodule relates this to other conceptually similar procedures with very different technical requirements and associated risks and benefits:
“I’ve had biopsies done before for . . . skin cancer and things like that. You automatically just get it done. So I wouldn’t be afraid of a biopsy.”
Past experiences, including those experienced by close contacts, sometimes dominated the deliberation process:
“What I’m thinking about is watching my little brother go through this 2 years ago . . . . That’s what I was thinking of while [the investigator was] reading this because it’s bringing back all the memories.”
Those who expressed feeling more familiar with the hypothetical situations engaged in more future thinking, although this perceived knowledge did not uniformly reflect greater factual knowledge about potential outcomes or experiences. When participants used past experiences to inform future predictions, they were often strongly positive or strongly negative:
“I really wouldn’t want to see [my loved one’s] face get blown up [by fluid] because I’ve been to a couple of funerals where people had cancer and died. You see the chemo and stuff made their skin complexion turn black.”
“I have a friend who had an aneurysm several years ago . . . went through craniotomy, brain surgery, pneumonia, sepsis, ventilator, dopamine and everything. She was at my house yesterday for 5 hours . . . I have two friends who lived through the sepsis. It’s worth the chance because they all don’t die.”
The role of religion, God, or prayer in the future-focusing and decision-making processes was associated with whether and how individuals engaged in future-oriented thinking:
“I’m very faith based and . . . I always try to look at the positive and I try to have a lot of hope. I really probably wouldn’t see the cons [to aggressive treatment].”
Among those using spirituality to inform predictions or decision making, the content of participants’ forecasts varied in the evaluation of suffering and in their propensities to defer to clinician judgment.
Reliance on a specific clinician’s predictions and recommendations to form their own expectations varied among participants. Participants who described significant contact with certain providers expressed a high degree of dependence on the physician’s predictions and recommendations:
“I trust my doctors. My doctors that I had had through all my sickness when I started getting it, I just trust them. I figure they know more than I do, and they understand things more than I do.”
“I have a deep, deep faith in my husband’s oncologist and his heart doctor is also here in this hospital. I would base a lot of my decision on I think they would be very honest with me and what they would tell me the prognosis would be.”
In contrast, others expressed more skepticism regarding doctors’ input in the decision-making process, particularly in the absence of a longitudinal relationship:
“There are so, so many doctors that I just don’t trust . . . . When you went to see a doctor you went to see a doctor who knew you and you possibly knew him, certainly at least from the last time you saw them. Now you walk into a corporation. There are 10, 12, 15 names on the door. You don’t know who you’re going to talk to, usually, when you go in to see them.”
“This is what [doctors] do every day and they do it all day long. They come out and say things. I don’t think I would . . . want to hear from a doctor’s point of view because it’s just another number and they’re just going to tell you things that you don’t want to hear.”
The Content of Expectations
When participants’ described the future, they included potential effects of their decisions on family members, personal emotional expectations, and prognostic expectations. Potential surrogate decision makers also identified a conflict between predicted possible outcomes grounded in fact versus emotion or hope.
The potential impact of the described illness and treatment options on family members was a dominant consideration for patients and potential surrogates:
“Well, the most important things would be how my family feels about it and . . . what they feel about what is going to happen to me . . .”
Patients identified logistical concerns for their loved ones, as well as several emotional and coping considerations:
“Let’s say if I chose to be on a ventilator, it would be hard for [my family] to come visit whether it’s every day or every other day.”
“Probably the biggest thing [is to] provide as minimal discomfort to those that you love and not distressing them anymore than you would have to.”
Across all scenarios, participants engaged in affective forecasting or the prediction of emotions. This included anticipated intolerance of the treatment itself or predicted struggling with a loss of self. Similar expectations arose for potential surrogate decision makers, including losing the role of caregiver or companion with the death of their loved one. A conflicting sentiment also arose among participants who felt confident that they would maintain a positive emotional state even in the face of a negative outcome.
Patients and potential surrogates discussed potential health outcomes resulting from the choices presented over the course of the semistructured interview. Participants restated the provided information and reflected on the potential for mortality and loss of quality of life. Mortality was a central theme even among those asked to deliberate about lung nodule management, which did not include information about end-of-life considerations. Some participants in all scenarios viewed mortality to be the dominant future consideration, in contrast to others focused on quality of life.
Quality of life expectations were commonly constructed among those confronted with the option for mechanical ventilation in the event of respiratory failure in COPD or for ICU care for a loved one with advanced lung cancer (Table 3). The concept of suffering was prominent in these discussions and included impaired physical and mental functioning. Surrogate decision makers of patients with lung cancer in particular sought to make decisions that would prevent suffering:
“You want to do what is best, so you visualize, you are just visualizing this person that you love and care about lying here. What we consider treatment might be suffering to them or suffering in someone else’s eyes so either way you’ve got to live with the guilt. If you don’t treat it you have to live with the guilt of if I would have done this and then while you’re doing it you have to live with the guilt of maybe I should have just let go.”
For potential surrogate decision makers the concept of hope, or expectation of a desired outcome, was central to predictions for the future. However, a tension between personal hopes for the future and true expectations existed for surrogates:
“My reality is that my husband will probably pass sometime this year or maybe early [next year]. He shouldn’t be here now and he is. So if I’m using my intellect then I say I’m probably going to be a widow in [the next year]. But emotionally he and I both live with our heads in the clouds and we talk about 10 years from now.”
Participants also spontaneously acknowledged that hope may cause disruptions in outcome predictions, leading to inaccurate or incomplete evaluation of treatment options (Figure 1).
Discussion
Although reflecting on possible futures is central to medical decision making, prior quantitative evaluations of future-oriented thinking among health care decision makers have found that predictions are frequently flawed (48–52). Temporal discounting, or the tendency to undervalue future risks and rewards as compared with more proximal events or states, may contribute to these errors (53). Fortunately, emerging evidence suggests that this prevalent bias may be surmountable (54–56). This study demonstrates that patients or potential surrogate decision makers facing a preference-sensitive medical decision attempt to engage in future-oriented thinking, but that they often err in systematic ways. Specifically, we identified three potential sources of errors that are modifiable targets for improving deliberation and decision making among patients with smoking-associated thoracic diseases and their surrogates: (1) management of unformed expectations, (2) reliance on past experiences, and (3) the influence of hope on forecasting (Figure 1). Clinicians partnering with patients or their surrogates to make preference-sensitive decisions should be aware of these pitfalls and should specifically ask about the origins and content of patient-generated expectations as a component of shared decision making. This is particularly important for advance care planning, which may influence patients’ future quality of life and their family members’ bereavement outcomes (57–59), despite patients’ limited or absent lived experiences to guide such choices.
Management of Unformed Expectations
Health care decision makers rely on expectations to match preferences to management options, a central goal of shared decision making (14, 44, 60). The absence of expectations limits the ability to evaluate possible options. Although clinicians are encouraged to elicit preferences and goals from patients, and to provide the most accurate information possible, clinicians may not explore the content of patients’ or surrogates’ predicted outcomes. Although potential outcomes were explained in uniform fashion in this study’s vignettes, participants still varied considerably in their subsequent forecasting. This suggests that merely providing information may do little to promote patient-centered decision making. As a recent systematic review of decision aids concluded, although decision aids improve factual knowledge, there is little evidence that they better align choices with patients’ preferences and values (61). Rather, testing decision makers’ tendencies to generate expectations and the accuracy of those expectations may lead to interventions that direct and improve patients’ deliberation efforts. This may help patients match management selections to their true preferences (Figure 1).
Reliance on Past Experiences
Our finding that participants rely heavily on memories while engaging in future-oriented thinking is consistent with past work showing that specific positive or negative experiences directly informed cancer patients’ decisions (1, 62). Denberg and colleagues (62) found that among men with prostate cancer, even loosely related anecdotes strongly influenced patients’ treatment choices. Although previous anecdotes may help patients develop a framework for understanding potential outcomes of a novel decision, these “case studies” often overpower high-quality data on foreseeable outcomes. The familiarity heuristic suggests that individuals may rely heavily on situations that seem similar, leading to inaccurate assessments of risks and benefits, incomplete deliberation of all options, or a push or pull toward a particular option (63).
Our findings confirm that patients and potential surrogate decision makers are at high risk for familiarity bias, with many participants accepting or rejecting the most familiar option based on prior positive or negative experiences rather than considering both available options. Such reliance on subjective knowledge may be most problematic when there is a significant difference between previous experiences and likely future experiences. Thus, developing and testing interventions that reduce people’s tendencies to rely on memory and perceived familiarity may help patients and their families confront increasingly complex medical decisions. Alternatively, mimicking this familiarity with probabilistically accurate “experiences” may improve deliberation and decision making.
Additionally, these findings suggest that developing and testing interventions that reduce people’s tendencies to rely on memory and perceived familiarity may help patients and their families confront increasingly complex medical decisions. Alternatively, mimicking this familiarity with probabilistically accurate “experiences” may harness and redirect this bias to improve deliberation and decision making. Until further research provides clinicians with such tools, physicians should ask patients about past experiences when introducing multiple valid management choices to understand the framework an individual patient is using to make a choice. Clinician guidance that addresses the similarities and differences between past experiences and the present situation may enable patients to form more accurate expectations for potential outcomes and select an option better matched to their true preferences.
Role of Hope
Finally, this study among patients and potential surrogates of patients with smoking-associated thoracic diseases in the outpatient setting extends prior findings that patients with cancer (1) and surrogate decision makers in the ICU (64, 65) rely heavily on hope in deliberating among treatment options. Although hope and optimism may serve as protective coping tools (66, 67), expecting a desired outcome may introduce errors in judgment. Such optimism may cause patients to reject evidence- or expert judgment-based prognostic information. We found that patients with smoking-associated thoracic diseases and their surrogates were often aware of this cognitive contortion and were even able to articulate that they held conflicting expectations for the future. This is consistent with prior studies in patients with cancer (68, 69). Future exploratory studies are needed to develop and test theories of how health care decision makers integrate these conflicting “truths,” and to examine the impact of optimism on patients’ future well-being so as to determine the risks and benefits of hope.
Limitations
Decision-making processes under hypothetical conditions may differ from actual processes. We attempted to mitigate this by selecting participants for whom the clinical situation could occur given their condition at the time of enrollment. We also provided evidence-based details and information on the potential choices in an accessible manner that allowed patients to engage in a form of shared decision making. However, the decision makers were unable to benefit from the personal relationships patients may have with their clinicians during discussion of the potential options. Although potential surrogates of patients with NSCLC often asked for such guidance, the unfortunate reality is that the individual’s oncologist is unlikely to be present in the scenario provided. Additionally, although participants were recruited from four different clinics with representation of black and white respondents of varying educational attainment levels, the generalizability of our results beyond our health system or in patients with other illnesses is uncertain. We also did not vary the order of options presented in the clinical vignettes, which may have introduced bias into participants’ ability to form expectations for each.
In conclusion, this study suggests that when patients with smoking-associated thoracic diseases and their potential surrogates face health care decisions, they engage in future-oriented thinking when prompted, but nonetheless rely on past experiences with variable relevance to present decisions. Hope and familiarity also play large roles in determining the content of expectations for the future and may introduce biases as surrogates weigh possible health care choices. Future research that longitudinally follows patients to determine the frequency of errors in predictions, and the impact of such errors on outcomes important to patients and their families, is needed to determine the scope of this problem. Furthermore, rather than passively accepting these biases in how patients and surrogates engage in future-oriented thinking, future work should also explore whether interventions that harness and redirect these biases may improve the alignment of treatment choices with underlying goals for the future. Clinicians should consider exploring patients’ and surrogates’ expectations for potential future outcomes to assist them in making decisions that are most likely to promote their ultimate goals.
Acknowledgments
Acknowledgment
The authors thank Drs. Judy Shea and Fran Barg for guidance on qualitative methods and training in semistructured interviewing.
Footnotes
Supported by pilot grants from the Penn Roybal Center on Behavioral Economics and Health (P30AG034546); the Fostering Improvement in End-of-Life Decision Science Program, supported by the Otto Haas Charitable Trust; and National Institutes of Health/NHLBI grants T32 HL098054 and F32 HL124771-01 (J.L.H.). The content is solely the responsibility of the authors.
Author Contributions: Study conception and design: J.L.H., V.M., and S.D.H. Literature search: J.L.H. and E.P. Acquisition of data: J.L.H., E.P., and V.M. Data analysis: J.L.H., E.P., and V.M. Data interpretation: all authors. Drafting of manuscript: J.L.H. and E.P. Critical revision: all authors.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201505-0882OC on October 5, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Michael N, O'Callaghan C, Clayton J, Pollard A, Stepanov N, Spruyt O, Michael M, Ball D. Understanding how cancer patients actualise, relinquish, and reject advance care planning: implications for practice. Supportive Care Cancer. 2013;21:2195–2205. doi: 10.1007/s00520-013-1779-6. [DOI] [PubMed] [Google Scholar]
- 2.Pardon K, Deschepper R, Vander Stichele R, Bernheim J, Mortier F, Schallier D, Germonpré P, Galdermans D, Van Kerckhoven W, Deliens L. Are patients’ preferences for information and participation in medical decision-making being met? Interview study with lung cancer patients. Palliat Med. 2011;25:62–70. doi: 10.1177/0269216310373169. [DOI] [PubMed] [Google Scholar]
- 3.Slatore CG, Cecere LM, Letourneau JL, O’Neil ME, Duckart JP, Wiener RS, Farjah F, Cooke CR. Intensive care unit outcomes among patients with lung cancer in the surveillance, epidemiology, and end results: Medicare registry. J Clin Oncol. 2012;30:1686–1691. doi: 10.1200/JCO.2011.40.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakitas M, Kryworuchko J, Matlock DD, Volandes AE. Palliative medicine and decision science: the critical need for a shared agenda to foster informed patient choice in serious illness. J Palliat Med. 2011;14:1109–1116. doi: 10.1089/jpm.2011.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connolly T, Reb J. Regret in cancer-related decisions. Health Psychol. 2005;24:S29–S34. doi: 10.1037/0278-6133.24.4.S29. [DOI] [PubMed] [Google Scholar]
- 6.DuBenske LL, Wen KY, Gustafson DH, Guarnaccia CA, Cleary JF, Dinauer SK, McTavish FM. Caregivers’ differing needs across key experiences of the advanced cancer disease trajectory. Palliat Support Care. 2008;6:265–272. doi: 10.1017/S1478951508000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA. 2003;289:2387–2392. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 8.Lanken PN, Terry PB, Delisser HM, Fahy BF, Hansen-Flaschen J, Heffner JE, Levy M, Mularski RA, Osborne ML, Prendergast TJ, et al. ATS End-of-Life Care Task Force. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177:912–927. doi: 10.1164/rccm.200605-587ST. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, Zakher B, Fu R, Slatore CG. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2013;159:411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 10.Stefanek ME. Uninformed compliance or informed choice? A needed shift in our approach to cancer screening. J Natl Cancer Inst. 2011;103:1821–1826. doi: 10.1093/jnci/djr474. [DOI] [PubMed] [Google Scholar]
- 11.Tanner NT, Egede LE, Shamblin C, Gebregziabher M, Silvestri GA. Attitudes and beliefs toward lung cancer screening among US veterans. Chest. 2013;144:1783–1787. doi: 10.1378/chest.13-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med. 2012;185:363–372. doi: 10.1164/rccm.201104-0679CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA. 'The thing is not knowing': Patients' perspectives on surveillance of an indeterminate pulmonary nodule. Health Expect. 2012;18:355–365. doi: 10.1111/hex.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651–661. doi: 10.1016/s0277-9536(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 15.Rapley T. Distributed decision making: the anatomy of decisions-in-action. Sociol Health Illn. 2008;30:429–444. doi: 10.1111/j.1467-9566.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- 16.Martin LE, Stenmark CK, Thiel CE, Antes AL, Mumford MD, Connelly S, Devenport LD. The influence of temporal orientation and affective frame on use of ethical decision-making strategies. Ethics Behav. 2011;21:127–146. doi: 10.1080/10508422.2011.551470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman GB, Coups EJ. Emotions and preventive health behavior: worry, regret, and influenza vaccination. Health Psychol. 2006;25:82–90. doi: 10.1037/0278-6133.25.1.82. [DOI] [PubMed] [Google Scholar]
- 18.Connolly T, Zeelenberg M. Regret in decision making. Curr Dir Psychol Sci. 2002;11:212–216. [Google Scholar]
- 19.Joseph-Williams N, Edwards A, Elwyn G. The importance and complexity of regret in the measurement of ‘good’ decisions: a systematic review and a content analysis of existing assessment instruments. Health Expect. 2011;14:59–83. doi: 10.1111/j.1369-7625.2010.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reb J. Regret aversion and decision process quality: effects of regret salience on decision process carefulness. Organ Behav Hum Decis Process. 2008;105:169–182. [Google Scholar]
- 21.Summerville A. Counterfactual seeking: the scenic overlook of the road not taken. Pers Soc Psychol Bull. 2011;37:1522–1533. doi: 10.1177/0146167211413295. [DOI] [PubMed] [Google Scholar]
- 22.Zeelenberg M, Beattie JM, Van der Pligt J, de Vries NK. Consequences of regret aversion: effects of expected feedback on risky decision making. Organ Behav Hum Decis Process. 1996;65:148–158. [Google Scholar]
- 23.Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, Cavallo DA, Carroll KM, Potenza MN. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheffer CE, Christensen DR, Landes R, Carter LP, Jackson L, Bickel WK. Delay discounting rates: a strong prognostic indicator of smoking relapse. Addict Behav. 2014;39:1682–1689. doi: 10.1016/j.addbeh.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheffer C, Mackillop J, McGeary J, Landes R, Carter L, Yi R, Jones B, Christensen D, Stitzer M, Jackson L, et al. Delay discounting, locus of control, and cognitive impulsiveness independently predict tobacco dependence treatment outcomes in a highly dependent, lower socioeconomic group of smokers. Am J Addict. 2012;21:221–232. doi: 10.1111/j.1521-0391.2012.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Exp Clin Psychopharmacol. 2007;15:176–186. doi: 10.1037/1064-1297.15.2.186. [DOI] [PubMed] [Google Scholar]
- 27.MacKillop J, Amlung M, Few L, Ray L, Sweet L, Munafò M. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Story GW, Vlaev I, Seymour B, Darzi A, Dolan RJ. Does temporal discounting explain unhealthy behavior? A systematic review and reinforcement learning perspective. Front Behav Neurosci. 2014;8:76. doi: 10.3389/fnbeh.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- 30.Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- 31.Epstein LH, Richards JB, Saad FG, Paluch RA, Roemmich JN, Lerman C. Comparison between two measures of delay discounting in smokers. Exp Clin Psychopharmacol. 2003;11:131–138. doi: 10.1037/1064-1297.11.2.131. [DOI] [PubMed] [Google Scholar]
- 32.Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology (Berl) 2005;182:508–515. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds B. Do high rates of cigarette consumption increase delay discounting? A cross-sectional comparison of adolescent smokers and young-adult smokers and nonsmokers. Behav Processes. 2004;67:545–549. doi: 10.1016/j.beproc.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav Processes. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 36.Litvin EB, Brandon TH. Testing the influence of external and internal cues on smoking motivation using a community sample. Exp Clin Psychopharmacol. 2010;18:61–70. doi: 10.1037/a0017414. [DOI] [PubMed] [Google Scholar]
- 37.Friedel JE, DeHart WB, Madden GJ, Odum AL. Impulsivity and cigarette smoking: discounting of monetary and consumable outcomes in current and non-smokers. Psychopharmacology (Berl) 2014;231:4517–4526. doi: 10.1007/s00213-014-3597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sweitzer MM, Donny EC, Dierker LC, Flory JD, Manuck SB. Delay discounting and smoking: association with the Fagerström Test for Nicotine Dependence but not cigarettes smoked per day. Nicotine Tob Res. 2008;10:1571–1575. doi: 10.1080/14622200802323274. [DOI] [PubMed] [Google Scholar]
- 39.Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- 40.MacKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug Alcohol Depend. 2009;104:197–203. doi: 10.1016/j.drugalcdep.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hickman RL, Jr, Daly BJ, Lee E. Decisional conflict and regret: consequences of surrogate decision making for the chronically critically ill. Appl Nurs Res. 2012;25:271–275. doi: 10.1016/j.apnr.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam WW, Chan M, Or A, Kwong A, Suen D, Fielding R. Reducing treatment decision conflict difficulties in breast cancer surgery: a randomized controlled trial. J Clin Oncol. 2013;31:2879–2885. doi: 10.1200/JCO.2012.45.1856. [DOI] [PubMed] [Google Scholar]
- 43.Aning JJ, Wassersug RJ, Goldenberg SL. Patient preference and the impact of decision-making aids on prostate cancer treatment choices and post-intervention regret. Curr Oncol. 2012;19:S37–S44. doi: 10.3747/co.19.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan RM, Frosch DL. Decision making in medicine and health care. Annu Rev Clin Psychol. 2005;1:525–556. doi: 10.1146/annurev.clinpsy.1.102803.144118. [DOI] [PubMed] [Google Scholar]
- 45.Hart JL.Thinking forward: the time orientation of patients making treatment decisionsPresented at the Society for Medical Decision Making Annual Meeting. October 20, 2014, Miami, FL. Abstract 8442 [Google Scholar]
- 46.Ritchie J, Lewis J, McNaughton Nicholls C, Ormston R.Qualitative research practice: a guide for social science students and researchers.Thousand Oaks, CA: Sage Publications Ltd; 2014 [Google Scholar]
- 47.Walker D, Myrick F. Grounded theory: an exploration of process and procedure. Qual Health Res. 2006;16:547–559. doi: 10.1177/1049732305285972. [DOI] [PubMed] [Google Scholar]
- 48.Smith D, Loewenstein G, Jepson C, Jankovich A, Feldman H, Ubel P. Mispredicting and misremembering: patients with renal failure overestimate improvements in quality of life after a kidney transplant. Health Psychol. 2008;27:653–658. doi: 10.1037/a0012647. [DOI] [PubMed] [Google Scholar]
- 49.Smith DM, Loewenstein G, Jankovic A, Ubel PA. Happily hopeless: adaptation to a permanent, but not to a temporary, disability. Health Psychol. 2009;28:787–791. doi: 10.1037/a0016624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ubel PA, Loewenstein G, Hershey J, Baron J, Mohr T, Asch DA, Jepson C. Do nonpatients underestimate the quality of life associated with chronic health conditions because of a focusing illusion? Med Decis Making. 2001;21:190–199. doi: 10.1177/0272989X0102100304. [DOI] [PubMed] [Google Scholar]
- 51.Ubel PA, Loewenstein G, Jepson C. Disability and sunshine: can hedonic predictions be improved by drawing attention to focusing illusions or emotional adaptation? J Exp Psychol Appl. 2005;11:111–123. doi: 10.1037/1076-898X.11.2.111. [DOI] [PubMed] [Google Scholar]
- 52.Ubel PA, Loewenstein G, Schwarz N, Smith D. Misimagining the unimaginable: the disability paradox and health care decision making. Health Psychol. 2005;24:S57–S62. doi: 10.1037/0278-6133.24.4.S57. [DOI] [PubMed] [Google Scholar]
- 53.Odum AL. Delay discounting: I’m a k, you’re a k. J Exp Anal Behav. 2011;96:427–439. doi: 10.1901/jeab.2011.96-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Senecal N, Wang T, Thompson E, Kable JW. Normative arguments from experts and peers reduce delay discounting. Judgm Decis Mak. 2012;7:568–589. [PMC free article] [PubMed] [Google Scholar]
- 55.Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 56.Hershfield HE, Goldstein DG, Sharpe WF, Fox J, Yeykelis L, Carstensen LL, Bailenson JN. Increasing saving behavior through age-progressed renderings of the future self. J Mark Res. 2011;48:S23–S37. doi: 10.1509/jmkr.48.SPL.S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silveira MJ, Kim SY, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362:1211–1218. doi: 10.1056/NEJMsa0907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teno JM, Gruneir A, Schwartz Z, Nanda A, Wetle T. Association between advance directives and quality of end-of-life care: a national study. J Am Geriatr Soc. 2007;55:189–194. doi: 10.1111/j.1532-5415.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- 59.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. doi: 10.1136/bmj.c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Politi MC, Lewis CL, Frosch DL. Supporting shared decisions when clinical evidence is low. Med Care Res Rev. 2013;70(Suppl. 1:):113S–128S. doi: 10.1177/1077558712458456. [DOI] [PubMed] [Google Scholar]
- 61.Stacey D, Légaré F, Col NF, Bennett CL, Barry MJ, Eden KB, Holmes-Rovner M, Llewellyn-Thomas H, Lyddiatt A, Thomson R, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2014:CD001431 [DOI] [PubMed]
- 62.Denberg TD, Melhado TV, Steiner JF. Patient treatment preferences in localized prostate carcinoma: The influence of emotion, misconception, and anecdote. Cancer. 2006;107:620–630. doi: 10.1002/cncr.22033. [DOI] [PubMed] [Google Scholar]
- 63.Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- 64.Schenker Y, White DB, Crowley-Matoka M, Dohan D, Tiver GA, Arnold RM. “It hurts to know... and it helps”: exploring how surrogates in the ICU cope with prognostic information. J Palliat Med. 2013;16:243–249. doi: 10.1089/jpm.2012.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Apatira L, Boyd EA, Malvar G, Evans LR, Luce JM, Lo B, White DB. Hope, truth, and preparing for death: perspectives of surrogate decision makers. Ann Intern Med. 2008;149:861–868. doi: 10.7326/0003-4819-149-12-200812160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Applebaum AJ, Stein EM, Lord-Bessen J, Pessin H, Rosenfeld B, Breitbart W. Optimism, social support, and mental health outcomes in patients with advanced cancer. Psychooncology. 2014;23:299–306. doi: 10.1002/pon.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horney DJ, Smith HE, McGurk M, Weinman J, Herold J, Altman K, Llewellyn CD. Associations between quality of life, coping styles, optimism, and anxiety and depression in pretreatment patients with head and neck cancer. Head Neck. 2011;33:65–71. doi: 10.1002/hed.21407. [DOI] [PubMed] [Google Scholar]
- 68.Weeks JC, Catalano PJ, Cronin A, Finkelman MD, Mack JW, Keating NL, Schrag D. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367:1616–1625. doi: 10.1056/NEJMoa1204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen AB, Cronin A, Weeks JC, Chrischilles EA, Malin J, Hayman JA, Schrag D. Expectations about the effectiveness of radiation therapy among patients with incurable lung cancer. J Clin Oncol. 2013;31:2730–2735. doi: 10.1200/JCO.2012.48.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]