Abstract
Rationale: Septic shock is a common cause of acute kidney injury (AKI), and fluid resuscitation is a major part of therapy.
Objectives: To determine if structured resuscitation designed to alter fluid, blood, and vasopressor use affects the development or severity of AKI or outcomes.
Methods: Ancillary study to the ProCESS (Protocolized Care for Early Septic Shock) trial of alternative resuscitation strategies (two protocols vs. usual care) for septic shock.
Measurements and Main Results: We studied 1,243 patients and classified AKI using serum creatinine and urine output. We determined recovery status at hospital discharge, examined rates of renal replacement therapy and fluid overload, and measured biomarkers of kidney damage. Among patients without evidence of AKI at enrollment, 37.6% of protocolized care and 38.1% of usual care patients developed kidney injury (P = 0.90). AKI duration (P = 0.59) and rates of renal replacement therapy did not differ between study arms (6.9% for protocolized care and 4.3% for usual care; P = 0.08). Fluid overload occurred in 8.3% of protocolized care and 6.3% of usual care patients (P = 0.26). Among patients with severe AKI, complete and partial recovery was 50.7 and 13.2% for protocolized patients and 49.1 and 13.4% for usual care patients (P = 0.93). Sixty-day hospital mortality was 6.2% for patients without AKI, 16.8% for those with stage 1, and 27.7% for stages 2 to 3.
Conclusions: In patients with septic shock, AKI is common and associated with adverse outcomes, but it is not influenced by protocolized resuscitation compared with usual care.
Key words: sepsis, septic shock, resuscitation, early goal-directed therapy, acute kidney injury
At a Glance Commentary
Scientific Knowledge on the Subject
Protocolized resuscitation may reduce acute kidney injury and improve recovery and reduce need for renal replacement therapy.
What This Study Adds to the Field
In patients with septic shock, neither the development nor the course of acute kidney injury is influenced by protocolized resuscitation compared with current usual care. Because most sepsis-associated acute kidney injury is present at or soon after presentation, effective strategies should focus on improving resolution.
Acute kidney injury (AKI) is a common complication of critical illness, affecting nearly two-thirds of patients admitted to intensive care units (ICU) (1–3). Two of the most commonly identified etiologies for AKI are sepsis and shock (4); when these conditions occur together, the rate of AKI approaches 80% (1). Fluid resuscitation followed by vasopressor medications are the mainstays of initial treatment for shock. Protocolization of resuscitation aims to limit variation and improve deployment of these treatments. However, fluid overload may lead to congestion in the kidney and worsen injury (5, 6). Therefore, it is important to understand whether protocols that alter fluid therapy and pressor use during resuscitation provide benefit or harm to the kidney as manifested by differences in rates and severity of AKI, need for renal replacement therapy (RRT), or recovery of kidney function.
To address these questions, we conducted an ancillary study to the ProCESS (Protocolized Care for Early Septic Shock) study, a 31-site, randomized controlled trial of alternative resuscitation strategies (two experimental protocols vs. usual care) for septic shock (7). Specifically, we examined the occurrence of AKI on presentation, the development of new AKI, and the course of all AKI across treatment arms by clinical criteria and by novel kidney injury biomarkers. We also studied the use of RRT both during the hospitalization and out to 1 year. Our hypothesis was that protocolized resuscitation would reduce new AKI, improve recovery from new or existing AKI, and result in less short- and long-term dialysis use.
Methods
Study Design
Details of the ProCESS trial have been published previously (7). Briefly, ProCESS was a multicenter, randomized clinical trial that tested alternative resuscitation strategies for patients who presented to emergency departments with septic shock. Eligible patients had suspected infection plus hypotension, hyperlactemia, or both after an initial fluid bolus. One strategy, known as early goal-directed therapy (EGDT) (8), targeted fluids, vasoactive medication, and blood transfusions to central venous oxygen saturation (as a measure of oxygen delivery to the tissues.) Another strategy, termed protocol-based standard care (PSC), used a simpler structured approach based on blood pressure and heart rate, and the clinical assessment by the study team. The third arm of the trial was “usual care,” in which the clinical providers, not the study team, directed all care, with the study coordinator collecting data but not prompting any actions (Table 1). Lead investigators at a site could not serve as the bedside treating physician for patients in the usual care group.
Table 1.
Differences in Interventions by Treatment Arm
| Intervention* | EGDT (n = 439) | PSC (n = 446) | Usual Care (n = 456) | P Value† |
|---|---|---|---|---|
| Resuscitation | ||||
| Central venous catheterization | 411 (94%) | 252 (57%) | 264 (58%) | <0.0001 |
| Central venous oximeter catheterization | 409 (93%) | 18 (4%) | 16 (4%) | <0.0001 |
| Intravenous fluids, L, mean | 2.8 | 3.3 | 2.3 | <0.0001 |
| Vasopressor use | 241 (55%) | 233 (52%) | 201 (44%) | 0.003 |
| Dobutamine use | 35 (8%) | 5 (1%) | 4 (1%) | <0.0001 |
| Blood transfusion | 63 (14%) | 37 (8%) | 34 (8%) | 0.001 |
| Ancillary care | ||||
| Mechanical ventilation | 116 (26%) | 110 (25%) | 99 (22%) | 0.25 |
| Intravenous antibiotics | 428 (98%) | 433 (97%) | 442 (97%) | 0.90 |
| Corticosteroids | 54 (12%) | 48 (11%) | 37 (8%) | 0.16 |
| Activated protein C | 1 (0.2%) | 1 (0.2%) | 0 (0%) | 0.55 |
Definition of abbreviations: EGDT = early goal-directed therapy; PSC = protocol-based standard care.
Mechanical ventilation, central venous catheterization, and ancillary care (antibiotics, corticosteroids, and activated protein C) were counted from arrival at the emergency department to 6 hours. Resuscitation therapies (intravenous fluids, vasopressor, and dobutamine infusions, and blood product administration) were counted from randomization to 6 hours.
Values are n (%) unless otherwise indicated.
P values are shown for three-way comparison (Fisher’s exact test).
Patients and Study Procedures
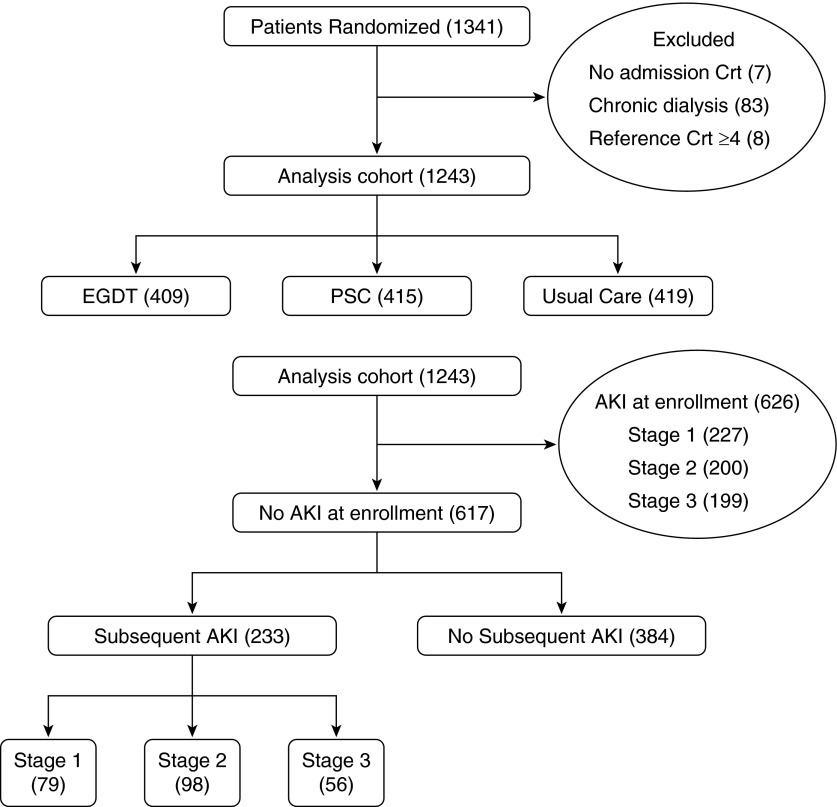
In ProCESS, we randomized 1,351 patients 1:1:1 to either EGDT, PSC, or usual care within 2 hours of meeting entry criteria. All patients or their legally authorized representatives provided written informed consent. We randomized subjects using a centralized Web-based program in variable block sizes of 3, 6, or 9, with stratification according to site and race. After excluding patients with end-stage renal disease (n = 83) or baseline serum creatinine values >4 mg/dl (n = 8), or with missing enrollment serum creatinine values (n = 7), 1,243 patients remained in the analysis (Figure 1).
Figure 1.
Study cohort. The top panel displays the analysis cohort by treatment arm. The bottom panel shows acute kidney injury (AKI) status at enrollment and subsequently, as well as AKI stages. Crt = creatinine; EGDT = early goal-directed therapy; PSC = protocol-based standard care.
Outcome Measures
Our primary outcome was the development of new onset AKI (any stage) over the first 28 days after enrollment. Secondary outcomes included duration of AKI, recovery status at hospital discharge, development of fluid overload in the first 72 hours, and use of RRT. For the primary analysis, we restricted our sample to patients without AKI at enrollment, but for the secondary analyses, we included all patients. We also examined outcomes associated with AKI, including mortality (in-hospital to 60 d), 1-year survival, and death or use of dialysis at 60 days and 1 year.
We classified AKI according to Kidney Disease: Improving Global Outcomes criteria (9) using both serum creatinine and urine output. Patients with evidence of AKI based on serum creatinine at the time of enrollment were classified as having AKI at enrollment. We recorded hourly urine output for the first 72 hours or until ICU discharge if before 72 hours. We obtained daily serum creatinine values for 28 days for all subjects who remained hospitalized. We determined the stage of AKI each day based on maximum severity by either creatinine or urine output criteria (until ICU discharge or 72 h). We determined baseline (preadmission), admission, and reference (the lower of baseline and admission) serum creatinine as previously described (1, 10, 11). We only assigned an AKI stage by urine output criteria if the urine output values were recorded. Missing urine output was not imputed and did not contribute to staging for patients.
We determined duration of AKI and stage 2 to 3 AKI as previously described (1) using the first episode of AKI only and using a 72-hour criterion for sustained recovery off RRT and alive. We determined recovery status at hospital discharge truncated at 28 days, defining complete recovery as alive, free of RRT, and with a last known serum creatinine <1.5 times the reference creatinine. For recovery from stage 2 to 3 AKI, we considered partial recovery as alive, free of RRT, and improvement by at least one AKI stage but without return to <1.5 times reference creatinine as previously described (12, 13). We examined rates and duration of RRT across treatment arms. We considered inpatient RRT to end when the last treatment occurred as long as the patient was still in hospital for at least 96 hours. For patients who were discharged (alive or dead) before 96 hours, the last day of inpatient RRT was considered to be the date of discharge. We calculated fluid balance from all fluid in and out over the first 72 hours after enrollment, and expressed it as a percent based on body mass using 10% as the threshold that defined fluid overload (5). We determined postdischarge outcomes by linking to National Death Index and United States Renal Data System. An honest broker obtained the data from these sources and merged these results with the study data.
Biomarkers of Kidney Damage
In a subgroup of 270 patients, we measured urinary biomarkers at 0, 6, 24, and 72 hours after enrollment. Our panel included five urinary biomarkers: neutrophil gelatinase-associated lipocalin (NGAL); kidney injury molecule-1 (KIM-1); liver-fatty acid binding protein (L-FABP); pi glutathione S-transferase (πGST); and α glutathione S-transferase (αGST). Assays were performed using ELISA kits obtained from EKF diagnostics (Cardiff, UK) and performed according to the manufacturer’s specifications. Urine creatinine assessment used an enzymatic assay (EKF diagnostics) performed according to the manufacturer’s specifications.
Statistical Analysis
We analyzed data by intention to treat, testing the primary outcome for differences across all three arms using Fisher’s exact test. We also sequentially tested if protocolized resuscitation (EGDT or PSC) was superior to usual care, and if so, whether EGDT was superior to PSC. For this latter analysis, we detected a 12% absolute difference between protocolized treatment and usual care, assuming an α of 0.05 and a power of 80%. We used the Kruskal-Wallis test to determine whether duration of AKI varied by treatment arm due to a right skew in the distribution of duration of AKI. The distribution of values for percent positive fluid balance over 72 hours was normal, which allowed for use of analysis of variance. We performed sensitivity analyses by restricting to stage 2 to 3 AKI and including patients with stage 1 AKI at enrollment. We varied the criteria for AKI (serum creatinine, urine output, or both) and time for ascertainment of RRT. Subgroup analyses for patients who were enrolled on the basis of lactate only criterion fitted an interaction term between the treatment arm and entry method, and were assessed using the Wald test. We used Stata 13 (StataCorp LP, College Station, TX) for Kaplan-Meier curves and SAS 9.4 (SAS Institute Inc., Cary, NC) for all other statistical analyses.
Results
Baseline Patient Characteristics by AKI Status
At enrollment, 626 patients (50.4%) had AKI, with 399 (32.1%) having stage 2 to 3 AKI. Of the 617 patients without AKI at enrollment, 233 (37.8%) subsequently manifested AKI. Baseline characteristics for patients stratified by enrollment and subsequent AKI status are shown in Table 2.
Table 2.
Baseline Characteristics of Patients According to Acute Kidney Injury Status
| Characteristic | AKI at Enrollment (n = 626) | AKI after Enrollment (n = 233) | No AKI (n = 384) | P Value* |
|---|---|---|---|---|
| Age, yr† | 60.7 ± 15.9 | 66.1 ± 15.8 | 59.2 ± 16.4 | <0.0001a |
| Male sex | 394 (62.9%) | 111 (47.6%) | 183 (47.7%) | <0.001 |
| Race | <0.001 | |||
| White | 395 (63.6%) | 183 (78.%) | 289 (75.3%) | |
| Black or African American | 184 (29.6%) | 41 (17.6%) | 62 (16.2%) | |
| Other | 42 (6.7%) | 9 (3.9%) | 33 (8.6%) | |
| Ethnicity‡ | 0.01 | |||
| Hispanic | 54 (8.6%) | 23 (9.9%) | 55 (14.4%) | |
| Non-Hispanic | 571 (91.4%) | 210 (90.1%) | 328 (85.6%) | |
| Comorbid conditions§ | ||||
| Charlson comorbidity score | 2 (1–4) | 2 (1–4) | 1 (1–3) | 0.03b |
| Hypertension | 369 (59.4%) | 142 (60.9%) | 204 (53.1%) | 0.08 |
| Diabetes mellitus | 221 (35.6%) | 80 (34.3%) | 103 (26.9%) | 0.02 |
| Chronic respiratory disease | 126 (20.3%) | 63 (27%) | 91 (23.7%) | 0.10 |
| Cancer | 103 (16.6%) | 46 (19.7%) | 78 (20.3%) | 0.28 |
| Renal impairment | 92 (14.8%) | 20 (8.6%) | 14 (3.7%) | <0.001 |
| Acute congestive heart failure | 58 (9.3%) | 40 (17.2%) | 41 (10.7%) | 0.005 |
| Prior myocardial infarction | 58 (9.3%) | 27 (11.6%) | 46 (12%) | 0.37 |
| Cerebral vascular disease | 68 (10.9%) | 31 (13.3%) | 20 (5.2%) | 0.001 |
| Peripheral vascular disease | 46 (7.4%) | 22 (9.4%) | 24 (6.3%) | 0.35 |
| Chronic dementia | 45 (7.3%) | 30 (12.9%) | 22 (5.7%) | 0.004 |
| Hepatic cirrhosis | 44 (7.1%) | 17 (7.3%) | 16 (4.2%) | 0.13 |
| Peptic ulcer disease | 38 (6.1%) | 9 (3.9%) | 21 (5.4%) | 0.45 |
| AIDS and related syndromes | 19 (3.1%) | 6 (2.6%) | 9 (2.3%) | 0.82 |
| Source of sepsis | 0.06 | |||
| After review judged not infected | 11 (1.8%) | 6 (2.6%) | 12 (3.1%) | 0.34 |
| Pneumonia | 188 (30.3%) | 91 (39.1%) | 136 (35.4%) | 0.03 |
| Intra-abdominal infection | 89 (14.3%) | 25 (10.7%) | 44 (11.5%) | 0.26 |
| Urosepsis | 144 (23.2%) | 44 (18.9%) | 84 (21.8%) | 0.40 |
| Skin and soft-tissue infections | 45 (7.3%) | 16 (6.9%) | 29 (7.6%) | 0.97 |
| Central nervous system | 3 (0.5%) | 3 (1.3%) | 4 (1%) | 0.43 |
| Endocarditis | 2 (0.3%) | 3 (1.3%) | 2 (0.5%) | 0.19 |
| Catheter-related infection | 10 (1.6%) | 7 (3%) | 8 (2.1%) | 0.42 |
| Unknown | 81 (13.0%) | 33 (14.2%) | 37 (9.7%) | 0.16 |
| Other | 48 (7.7%) | 5 (2.2%) | 28 (7.3%) | 0.005 |
| Blood culture positive | 211 (34%) | 66 (28.3%) | 93 (24.2%) | 0.006 |
| APACHE II score | 21 (17–27) | 20 (16–24) | 16 (13–20) | <0.001b |
| Entry criteria | ||||
| Refractory hypotension | 325 (52.3%) | 121 (51.9%) | 224 (58.3%) | 0.14 |
| Hyperlactatemia | 404 (65.1%) | 132 (56.7%) | 188 (49%) | <0.001 |
| Physiologic variables | ||||
| Systolic blood pressure, mm Hg | 95 ± 27 | 106 ± 28 | 107 ± 29 | <0.0001b |
| Serum lactate, mmol/L | 2.6 (1.3–4.5) | 2.0 (1.3–3.4) | 1.6 (0.9 – 3.0) | <0.001b |
| Anemia|| | 108 (17.6%) | 38 (16.5%) | 40 (10.6%) | 0.008 |
| Time to randomization | ||||
| From ED arrival,¶ min | 162 (118–210) | 162 (120–212) | 179 (131–243) | <0.001b |
| From meeting entry criteria, min | 69 (39–102) | 66 (38–91) | 60 (34–94) | 0.14b |
Definition of abbreviations: AKI = acute kidney injury; APACHE = Acute Physiology and Chronic Health Evaluation; ED = emergency department.
Values are number of subjects (%), median (95% confidence interval), or mean ± SD.
P values are shown for 3-way comparison (Fisher’s exact test unless otherwise noted: aanalysis of variance; bKruskal-Wallis).
Excludes one subject with missing age.
Excludes two subjects with missing ethnicity.
Chronic conditions defined as per Charlson comorbidity index (19).
Anemia was defined by hemoglobin <10 for males, <8 for females.
Not all subjects were eligible at time of ED arrival.
Treatments Received
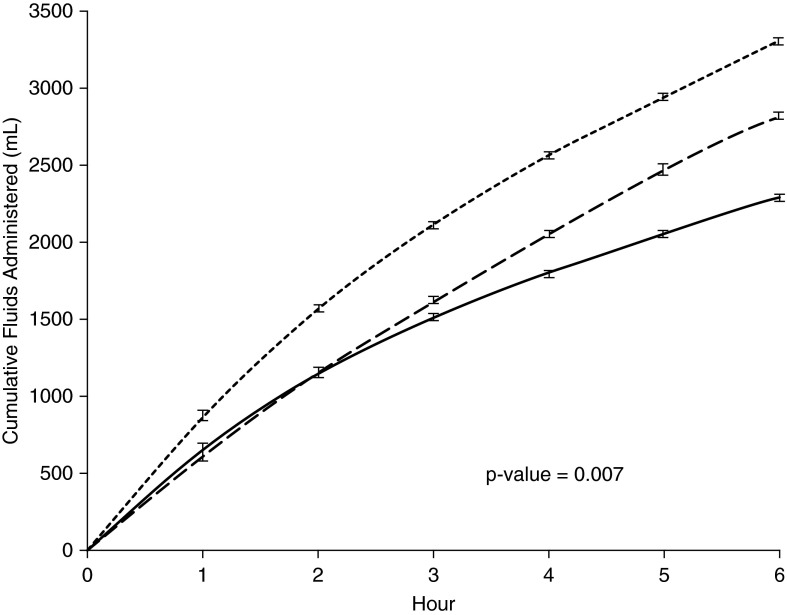
Treatments received by patients in each study arm are described in detail elsewhere (7) and are listed in Table 1. Total cumulative fluid balance by treatment arm over the 6-hour resuscitation period is shown in Figure 2, and was greatest with PSC and least with usual care (mean difference 1.0 L; P < 0.001). Ninety-four percent of the fluids given across all three arms was isotonic (0.9%) saline. Other differences in resuscitation can seen in Table 1, whereas ancillary care was similar in all three groups.
Figure 2.
Cumulative fluid use by study arm. Total fluid received over the first 6 hours. Solid line, usual care; long dashes, early goal-directed therapy; short dashes, protocol-based standard care.
Development of AKI by Treatment Arm
Development and duration of AKI, fluid overload, and use of RRT by treatment are shown in Table 3. There were no differences across treatment arms for presence of AKI on enrollment (P = 0.79), development of AKI after enrollment (P = 0.52), or development of stage 2 to 3 AKI after enrollment (P = 0.59). Analysis by EGDT+PSC versus usual care similarly showed no significant differences. In sensitivity analyses, when the criteria for AKI was restricted to only serum creatinine or only urine output criteria, there were no differences in AKI by treatment arm (see Table E1 in the online supplement). Similarly, the results were unchanged when we restricted analysis to patients enrolled on lactate criteria versus hypotension criteria versus both (see Figure E1). Additional sensitivity analyses are provided in Table E2. In the subgroup of 270 patients with urine biomarkers (KIM-1, NGAL, L-FABP), the three intervention groups did not differ in terms of biomarker trends over time (see Figure E2).
Table 3.
Acute Kidney Injury by Treatment Arm
| Outcome | EGDT (n = 409) | PSC (n = 415) | Usual Care (n = 419) | P Value* |
|---|---|---|---|---|
| AKI | ||||
| AKI at enrollment | 203/409 (49.6%) | 206/415 (49.6%) | 217/419 (51.9%) | 0.79 |
| Stage 2–3 AKI at enrollment | 134/409 (32.8%) | 130/415 (31.3%) | 135/419 (32.2%) | 0.91 |
| AKI after enrollment | 83/206 (40.3%) | 73/209 (34.9%) | 77/202 (38.1%) | 0.52 |
| Stage 2–3 AKI after enrollment | 58/206 (28.2%) | 47/209 (22.5%) | 49/202 (24.3%) | 0.39 |
| Development of stage 2–3 AKI† | 90/275 (32.7%) | 86/285 (30.2%) | 97/284 (34.2%) | 0.59 |
| Median (95% CI) duration of AKI,‡ d | ||||
| Any AKI | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.45 |
| Stage 2–3 | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.79 |
| Recovery from AKI‡ | 0.89§ | |||
| Complete | 164/286 (57.3%) | 154/279 (55.2%) | 159/294 (54.1%) | |
| Partial | 31/286 (10.8%) | 27/279 (9.7%) | 31/294 (10.5%) | |
| None | 91/286 (31.8%) | 98/279 (35.1%) | 104/294 (35.4%) | |
| Renal replacement therapy use‡ | ||||
| Any time during hospitalization | 25/407 (6.1%) | 32/414 (7.7%) | 18/419 (4.3%) | 0.11 |
| At 1 wk | 12/382 (3.1%) | 24/399 (6.0%) | 11/397 (2.8%) | 0.04 |
| At 48 h | 13/405 (3.2%) | 16/411 (3.9%) | 10/417 (2.4%) | 0.48 |
| Fluid overload | ||||
| >10% over first 72 h | 40/408 (9.8%) | 28/414 (6.8%) | 26/415 (6.3%) | 0.13 |
| % positive fluid balance over 72 h, mean (SD) | 1.6% (6.4%) | 1.7% (5.3%) | 1.2% (5.6%) | 0.40‖ |
Definition of abbreviations: AKI = acute kidney injury; CI = confidence interval; EGDT= early goal-directed therapy; PSC = protocol-based standard care.
P values are shown for three-way comparison (Fisher’s exact test unless otherwise specified), analysis by EGDT+PSC versus usual care showed no significant differences.
Includes patients with stage 1 AKI on enrollment.
Includes patients with AKI (any stage) on enrollment.
P value is shown for comparison across all categories and groups (Fisher’s exact).
Analysis of variance.
Use of RRT and presence of fluid overload are also shown by treatment arm in Table 3. Receipt of RRT on day 7 was greater with PSC (P = 0.04) compared with the other arms as previously reported (7). However, we found no differences for RRT at 48 hours after enrollment or over the entire course of hospitalization. Neither did we find any differences for RRT when comparing analysis by EGDT+PSC versus usual care at any time point.
Survival and Renal Replacement Therapy by AKI Status
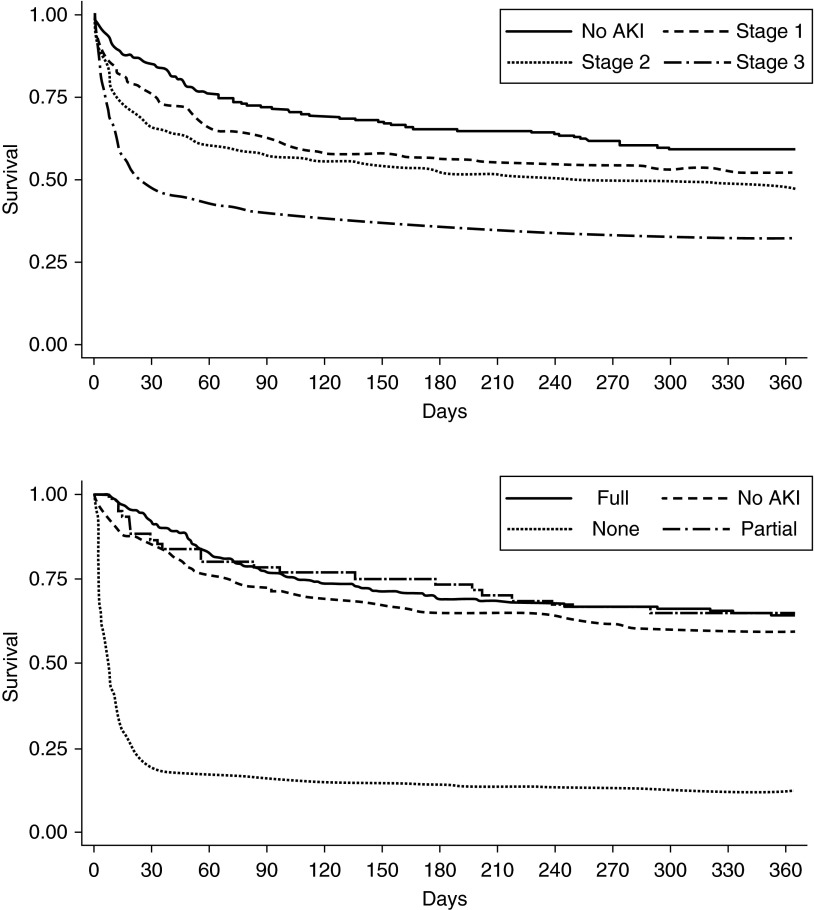
Hospital mortality truncated at 60 days was 6.2% for patients without AKI, 16.8% for patients with maximum AKI stage 1, and 27.7% for stage 2 to 3 AKI (P < 0.0001). Outcomes were not different for patients who manifested AKI at enrollment versus afterwards: hospital mortality (to 60 d) 23.5% versus 28.3% (P = 0.16). One-year survival for patients with and without AKI, and with AKI stratified by degree of recovery of kidney function is shown in Figure 3. Dialysis at day 60 or in-hospital death by 60 days occurred in 22.1% for EGDT, 18.0% for PSC, and 20.7% for usual care groups (P = 0.35, three-way; P = 0.85, two-way). Death or dialysis at 1 year occurred in 38.9% for EGDT, 37.0% for PSC, and 38.6% for usual care groups (P = 0.84, three-way; P = 0.90 two-way).
Figure 3.
Survival by acute kidney injury (AKI) and recovery status. (Top panel) One-year survival by AKI status (no AKI, stage 1, 2, or 3). (Bottom panel) One-year survival for stage 2 to 3 AKI by recovery status (complete, partial, none).
Discussion
In this preplanned ancillary analysis of a large randomized trial of protocol-based fluid resuscitation for septic shock, we found no benefit for protocolized management in terms of development or severity of AKI or for renal outcomes, including severity of fluid overload, use of RRT, and recovery of kidney function. Our results were consistent across subgroups defined by patient characteristics (lactate vs. hypotension criteria), or AKI criteria (creatinine, urine output, novel biomarkers). Our results are important clinically because fluid resuscitation is a mainstay of treatment for septic shock, and protocolized fluid resuscitation is often recommended to prevent kidney damage. Protocolized hemodynamic management for patients with septic shock is recommend by the Kidney Disease: Improving Global Outcomes clinical practice guideline (9) based on a previous metaanalysis (14). Our results do not support the adoption of either of the protocols studied to prevent new AKI or alter the course of existing AKI in patients presenting with septic shock.
Our results should not be interpreted to imply that fluid therapy is unimportant in the management of septic shock or that alternative protocols for resuscitation might be better than current practice. Although volume overload can result in respiratory compromise and venous congestion in the kidney and other organs (5, 6, 15), all our patients received aggressive fluid therapy, and rates of fluid overload and RRT were rather low (both <10%). Nevertheless, the lack of benefit with protocolized resuscitation for AKI despite more mean fluid use should temper enthusiasm for systematic approaches that simply increase fluid use in resuscitation in the setting of septic shock. Similarly, the use of central venous oxygen saturation to titrate vasoactive medication and blood product use (as per the EGDT arm) was not effective in altering the course of AKI.
These results also shed light on the epidemiology of AKI in patients with septic shock by demonstrating several key aspects. First, AKI is extremely common with more than two-thirds of patients developing AKI within the first 7 days. Almost three-quarters of episodes of AKI manifested at or soon after presentation to the emergency department. Although progression of AKI was still common after admission, the substantial burden of kidney injury evident on presentation combined with the well-known delay in clinical manifestations of AKI using functional criteria (creatinine and urine output) (16) means that for sepsis, primary AKI prevention will be nearly impossible. Second, AKI is often transient (median duration was only 2 days, and RRT was used in only 4–8%), but is nevertheless severe (25% of all patients have stage 2 to 3 AKI within the first week) and was strongly associated with increased mortality at 60 days. Third, patients who fail to recover renal function have a dismal survival, and the hazard for this association manifests in the first 30 days (Figure 3). Conversely, when patients with sepsis-associated AKI recover renal function, even incompletely, their survival seems to be similar to patients without AKI. To our knowledge, this relationship has not been reported previously. Together these findings imply that for treatments for AKI in the setting of sepsis to be successful, they will need to be effective after initiation of kidney injury. However, should they result in increased recovery, the prognosis, out to a year at least, may be considerably improved.
Among the strengths of this study are a large protocolized cohort conducted in 31 sites (7), prompt enrollment (patients were enrolled with 2 h of meeting criteria), availability of baseline and postintervention fluid and renal specific endpoints, assessment of long-term outcomes, and AKI biomarkers. Nonetheless, there are important limitations. First, we did not examine fluid type because 0.9% saline was the predominant fluid used. Second, our study did not address the initial fluid resuscitation that was given to qualify for entry into ProCESS; typically, this was 2 L before enrollment. It is possible that even earlier or greater volumes of fluid at this stage could have been beneficial. However, because additional fluids given to patients with evidence of ongoing shock (hypotension and/or hyperlactatemia) did not improve outcome, it is reasonable to hypothesize that less fluids might have been better in the initial resuscitation. There is some evidence that this initial bolus therapy could be deleterious in some situations. For example, in the FEAST (Fluid Expansion as Supportive Therapy) trial, bolus fluids resulted in worse outcome in children with sepsis in Africa (17). However, we did not observe harm with protocolized resuscitation, and although not significantly different, rates of stage 2 to 3 AKI developing at anytime after enrollment were actually highest with usual care. Meanwhile, although the rate of RRT use did not differ over the entire course of hospitalization or at 48 hours after enrollment across treatment groups, at 7 days, RRT use was highest in the PSC arm, the arm receiving the most fluid. This contradictory signal, in which AKI rates and RRT rates move in opposite directions, has been seen with other fluid trials—most notably, the CHEST trial (Crystalloid versus Hydroxyethyl Starch Trial) (18), in which AKI rates were lowest with starch, but dialysis rates were increased. We speculate that apart from any direct toxicity of fluids, early reversal of shock is beneficial, but excess fluids are harmful and strategies that provide more rapid resolution of shock might pay a price in greater fluid overload and use of RRT. Third, although we used the latest consensus criteria for AKI and recovery, and examined biomarkers of kidney damage, we cannot exclude that some patients may have had “subclinical AKI” or may have been missed, particularly if AKI occurred late. Finally, our protocols dictated a “pattern of management” rather than specific amounts of fluid, blood, or vasopressor; we did not study the relationship between individual fluid volumes or vasopressor doses and outcomes. Some subbjects in each arm may have received far more or less than the mean amounts. Therefore, we are best able to comment on the effect of systematic resuscitation targets, not specific amounts of therapy per se.
In conclusion, in patients with septic shock, AKI is common and associated with adverse outcomes, but it was not influenced by the use of either EGDT or an alternative resuscitation protocol compared with usual care.
Footnotes
Supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK083961 and NIH/National Institute of General Medical Sciences grant P50 GM076659.
Author Contributions: Conception and design: J.A.K., L.S.C., K.S., P.M.P., D.M.Y., and D.C.A. Analysis and interpretation: all authors. Drafting the manuscript for important intellectual content: all authors.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201505-0995OC on September 23, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26:2231–2238. doi: 10.1681/ASN.2014070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandelbaum T, Scott DJ, Lee J, Mark RG, Malhotra A, Waikar SS, Howell MD, Talmor D. Outcome of critically ill patients with acute kidney injury using the Acute Kidney Injury Network criteria. Crit Care Med. 2011;39:2659–2664. doi: 10.1097/CCM.0b013e3182281f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murugan R, Kellum JA. Acute kidney injury: what’s the prognosis? Nat Rev Nephrol. 2011;7:209–217. doi: 10.1038/nrneph.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 5.Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, Hackbarth R, Somers MJG, Baum M, Symons JM, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55:316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 6.Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6:107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- 7.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, et al. ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 9.KDIGO AKI Work Group. Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 10.Hoste EAJ, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Závada J, Hoste E, Cartin-Ceba R, Calzavacca P, Gajic O, Clermont G, Bellomo R, Kellum JA AKI6 investigators. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant. 2010;25:3911–3918. doi: 10.1093/ndt/gfp766. [DOI] [PubMed] [Google Scholar]
- 12.Srisawat N, Murugan R, Lee M, Kong L, Carter M, Angus DC, Kellum JA Genetic and Inflammatory Markers of Sepsis (GenIMS) Study Investigators. Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int. 2011;80:545–552. doi: 10.1038/ki.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellum JA. How can we define recovery after acute kidney injury? Considerations from epidemiology and clinical trial design. Nephron Clin Pract. 2014;127:81–88. doi: 10.1159/000363681. [DOI] [PubMed] [Google Scholar]
- 14.Brienza N, Giglio MT, Marucci M, Fiore T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med. 2009;37:2079–2090. doi: 10.1097/CCM.0b013e3181a00a43. [DOI] [PubMed] [Google Scholar]
- 15.Boyd JH, Forbes J, Nakada T-A, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 16.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20:672–679. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, et al. FEAST Trial Group. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 18.Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, Glass P, Lipman J, Liu B, McArthur C, et al. CHEST Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 19.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]