Abstract
Rationale: An asthma-like airway phenotype has been described in people with cystic fibrosis (CF). Whether these findings are directly caused by loss of CF transmembrane conductance regulator (CFTR) function or secondary to chronic airway infection and/or inflammation has been difficult to determine.
Objectives: Airway contractility is primarily determined by airway smooth muscle. We tested the hypothesis that CFTR is expressed in airway smooth muscle and directly affects airway smooth muscle contractility.
Methods: Newborn pigs, both wild type and with CF (before the onset of airway infection and inflammation), were used in this study. High-resolution immunofluorescence was used to identify the subcellular localization of CFTR in airway smooth muscle. Airway smooth muscle function was determined with tissue myography, intracellular calcium measurements, and regulatory myosin light chain phosphorylation status. Precision-cut lung slices were used to investigate the therapeutic potential of CFTR modulation on airway reactivity.
Measurements and Main Results: We found that CFTR localizes to the sarcoplasmic reticulum compartment of airway smooth muscle and regulates airway smooth muscle tone. Loss of CFTR function led to delayed calcium reuptake following cholinergic stimulation and increased myosin light chain phosphorylation. CFTR potentiation with ivacaftor decreased airway reactivity in precision-cut lung slices following cholinergic stimulation.
Conclusions: Loss of CFTR alters porcine airway smooth muscle function and may contribute to the airflow obstruction phenotype observed in human CF. Airway smooth muscle CFTR may represent a therapeutic target in CF and other diseases of airway narrowing.
Keywords: porcine, pig, cystic fibrosis, airways
At a Glance Commentary
Scientific Knowledge on the Subject
An asthma-like airway phenotype has been described in people with cystic fibrosis (CF), often referred to as “CF asthma.” Whether these findings are directly caused by loss of CF transmembrane conductance regulator (CFTR) function or secondary to chronic airway infection and/or inflammation is unknown.
What This Study Adds to the Field
This study highlights the direct role of CFTR in airway smooth muscle function and suggests that part of the airflow limitation seen in individuals with CF may be caused in part by airway smooth muscle dysfunction. Moreover, CFTR potentiation with ivacaftor decreased airway reactivity and may represent a therapeutic target in individuals with CF and airway narrowing diseases.
Cystic fibrosis (CF) is caused by loss of CF transmembrane conductance regulator (CFTR) function. CF pulmonary disease manifestations include bacterial infection, airway inflammation, mucus accumulation, and airflow obstruction. Although the causes of airflow obstruction are multifactorial, alterations in airway smooth muscle (ASM) physiology may contribute. Whether these changes reflect indirect effects of CF airway disease on ASM or direct/intrinsic defects in CF ASM is largely unknown.
Three lines of evidence support the hypothesis that CFTR plays a role in ASM function. First, people with CF commonly have symptoms typical of asthma and bronchial hyperresponsiveness, a condition sometimes referred to as “CF asthma” (1–4). More than 50% of people with CF show evidence of airway hyperresponsiveness and/or bronchodilator-induced reversible airway obstruction, a phenomena commonly seen in people with asthma (1, 5–7). Interestingly, one study found that people with asthma had a higher than expected carrier frequency of CFTR mutations, suggesting a potential role for CFTR in the asthma phenotype (8). Moreover, airway remodeling is present in both CF and asthma (9). Although host inflammation likely contributes to the altered ASM phenotype in the later stages of CF, the role of CFTR in ASM function in earlier stages of CF is not known.
Second, prior studies have demonstrated a potential role for CFTR in smooth muscle function (10–17). For example, CFTR activators cause vascular and ASM relaxation in mouse, rat, and human cells (10, 13, 14). However, CFTR−/− mice do not develop lung disease typical of human CF and human CF studies generally use tissues from individuals with long-standing airway infection and inflammation, thereby limiting conclusions for a direct role of CFTR in ASM function. Evidence from CFTR−/− pigs, which develop airway disease typical of human CF (18, 19), suggests that CFTR may be important for ASM function. On the day that CFTR−/− pigs are born, they have no airway inflammation or infection, yet newborn CFTR−/− pigs have abnormal-appearing trachealis smooth muscle and airflow obstruction (20, 21). These observations in the absence of airway inflammation suggest that CF ASM may have an intrinsic defect and that CFTR in ASM may play a role in underlying pathophysiology.
Finally, ivacaftor, a CFTR potentiator that increases the open probability of CFTR (22, 23), has been shown to improve pulmonary function demonstrated by increased FEV1 in people with CF by nearly 200 ml after only 3 days of treatment (24). Of note, ivacaftor blood levels reach steady state in 3–5 days. Given the rapid improvement in airflow obstruction, these effects might be mediated, in part, by improvements in ASM function.
In this study, we tested the hypothesis that CFTR is present in ASM and that loss of CFTR directly affects ASM function. By investigating ASM from newborn wild-type (WT; CFTR+/+ and CFTR+/−) and CFTR−/− pigs, we found that CFTR is intracellularly expressed in ASM and is relevant for ASM physiology. Data from CFTR−/− pigs suggest that impaired smooth muscle activity seen in CF ASM can occur before and independently of airway inflammation. Thus, CFTR could play an important role in regulating airway tone and may represent a potential therapeutic target for ASM dysfunction not only in the context of CF but also in other diseases, such as asthma. Some of the results of these studies have been previously reported in the form of abstracts (25, 26).
Methods
For additional information, see the Methods section in the online supplement.
Animals
We previously reported production of CFTR+/− and CFTR−/− pigs (27). Animal studies were reviewed and approved by the University of Iowa Animal Care and Use Committee.
ASM Tissue and Cells
Cells and tissues were isolated from newborn WT (CFTR+/+ and CFTR+/−) and CFTR−/− pigs using previously described protocols (28, 29).
Immunoblotting
For CFTR immunoblotting, cultured ASM cells or Calu-3 cells were lysed and supernatant was collected. CFTR was immunoprecipitated from the supernatant (1 mg for Calu-3 cells or 16 mg for ASM cells) using the CFTR antibodies, MM13–4 and M3A7 (Abcam, Cambridge, MA). The immunoprecipitant was run on a polyacrylamide gel, transferred to a polyvinylidene difluoride membrane, and immunoblotted using anti-CFTR mouse monoclonal primary antibody (1:100, clone 596, recognizes the NBD2 domain [aa 1204–1211]; University of North Carolina, Chapel Hill, NC) and with subsequent secondary antibody. Myosin light chain (MLC) blotting was performed as previously described (29) using trachealis muscle and cultured ASM cells from CFTR+/+ and CFTR−/− pigs.
Immunofluorescence
Cultured ASM cells were fixed and immunostained based on previously described methods (27, 30). Tyramide signal amplification kit #2 (Life Technologies, Foster City, CA) was used to label endogenous CFTR. Paired images were immunostained, visualized, and normalized to CFTR−/− controls.
Measurement of ASM Force Generation
Tracheal rings or trachealis smooth muscle strips were mounted in individual muscle baths (Radnoti Glass, Monrovia, CA). The smooth muscle force was determined by obtaining concentration-response curves to acetylcholine (10−9 to 10−2 M) in the muscle bath. For GlyH-101 (CFTR inhibitor) studies, the muscle strips were hung in the muscle bath under 0.5 g tension and baseline force was measured before and after treating with GlyH-101 (100 µM).
Precision-Cut Lung Slice Preparation
Previously described techniques for precision-cut lung slices preparation were used (31). Lung tissue blocks were isolated from the caudal region of the porcine tracheal lobe or WT mice. For bronchodilator response measurements, lung slices were perfused with isoproterenol (10 mM). For CFTR potentiation studies, lung slices were pretreated with ivacaftor (10 μM; Selleck Chemical, Houston, TX) or dimethyl sulfoxide (0.1% final concentration) for 30 minutes and subsequently perfused with increasing concentrations of methacholine (10−7 to 10−4 M; Sigma-Aldrich, St. Louis, MO).
Calcium Measurements
To measure intracellular Ca2+ levels, freshly isolated ASM cells were loaded with Fura-2-AM (5 μM; Molecular Probes; Life Technologies) based on previously described protocols (28). Baseline fluorescence ratios (340/380) were measured before adding acetylcholine (1 μM). For analysis, the 340/380 response to acetylcholine was normalized to the baseline fluorescence ratio for an individual cell. A minimum of 15 cells per animal were analyzed.
Statistical Analysis
Data are expressed as mean ± SD or mean alone. For analyses that compared two groups, we used a paired or unpaired Student’s t test. For time course and drug dose response studies, the analysis used either a one-phase decay or a four-parameter logistic regression algorithm (sigmoidal curve fit), respectively, to fit and plotted the mean data values and the 95% confidence interval as a band. Datasets not significantly different from each other are plotted as a single curve fit, whereas datasets that are significantly different are plotted as two separate curves (GraphPad PRISM v6.0b; GraphPad Software, La Jolla, CA). P less than 0.05 was considered statistically significant.
Results
CFTR Is Present in Porcine ASM
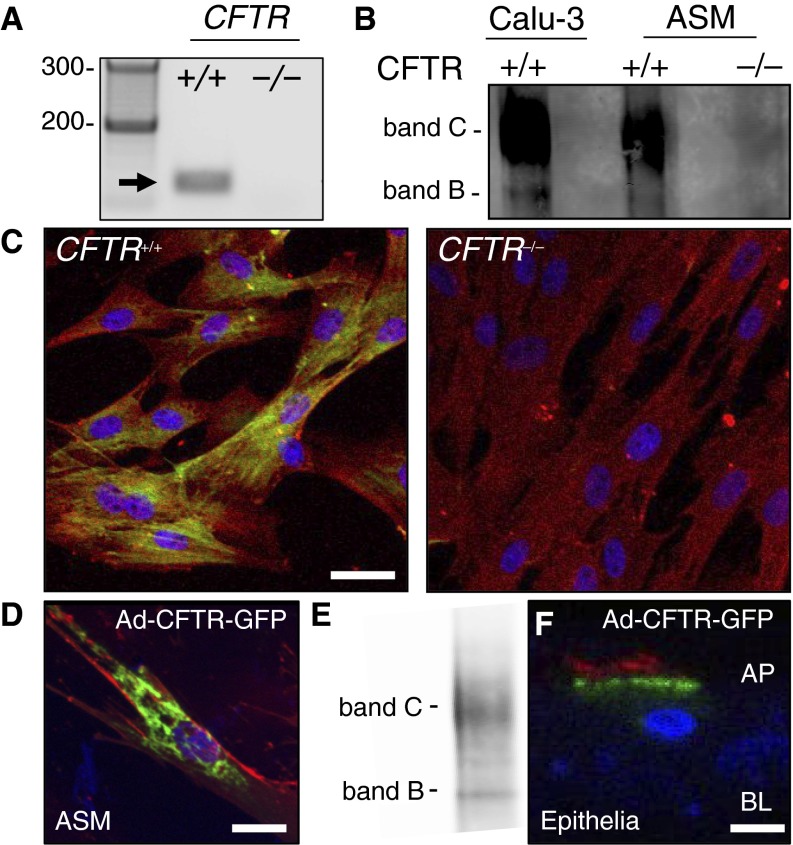
To investigate whether newborn porcine ASM expresses CFTR, we isolated RNA and performed reverse transcriptase polymerase chain reaction. CFTR mRNA was present in CFTR+/+ ASM cells, but absent in CFTR−/− ASM cells (Figure 1A). Consistent with these findings, we detected CFTR protein in ASM cells from CFTR+/+ pigs (Figure 1B). Similar to Calu-3 cells, an epithelial cell line that expresses high levels of CFTR (32), most CFTR protein in ASM cells was in the band C or the mature, glycosylated form (Figure 1B). We also tested for CFTR protein with immunocytochemistry using a tyramide signal amplification system (30). Immunostaining showed that CFTR primarily localized to an intracellular compartment in CFTR+/+ ASM. CFTR was not detected in ASM cells from CFTR−/− pigs (Figure 1C). Because CFTR typically localizes to the plasma membrane in epithelial cells (27, 33, 34), we were surprised to find the intracellular CFTR staining pattern in ASM cells. To confirm these findings, we expressed a functional CFTR, tagged at the N-terminal end with green fluorescent protein (GFP) (CFTR-GFP), in ASM cells (35). Similar to endogenous CFTR immunostaining, we found that recombinant CFTR was primarily localized intracellularly and expressed as band C (Figures 1D and 1E). In contrast, using the same CFTR-GFP construct, CFTR almost exclusively localized to the apical membrane of primary airway epithelial cells (Figure 1F). These findings demonstrate that CFTR is present in porcine ASM cells where it primarily localizes to an intracellular compartment.
Figure 1.
Cystic fibrosis transmembrane conductance regulator (CFTR) is present in porcine airway smooth muscle (ASM). (A) Reverse transcriptase polymerase chain reaction for CFTR mRNA (predicted size of 155 bp; arrow) in CFTR+/+ and CFTR−/− porcine ASM. Lane 1 is a 100-bp ladder. (B) CFTR protein in porcine ASM (CFTR+/+ and CFTR−/−) compared with Calu-3 epithelial cell control. For the immunoprecipitation step, differing amounts of total cell lysate were used (1 mg for Calu-3 and 16 mg for ASM cells). (C) Immunostaining of CFTR (yellow-green), α-smooth muscle actin (red), and nuclei (blue) in porcine ASM cells (CFTR+/+ and CFTR−/−). Scale bar = 40 μm. (D and E) CFTR localization (green) with WGA (red, membrane lectins) following Ad-CFTR-GFP transduction of ASM cells and associated CFTR immunoblot. (F) CFTR localization (green) and α-acetylated tubulin (red, apical membrane) following Ad-CFTR-GFP transduction of porcine airway epithelial cells. AP and BL = apical and basolateral membranes, respectively; GFP = green fluorescent protein. For D and F, scale bar = 20 μm.
CFTR Localizes to the Sarcoplasmic Reticulum in ASM
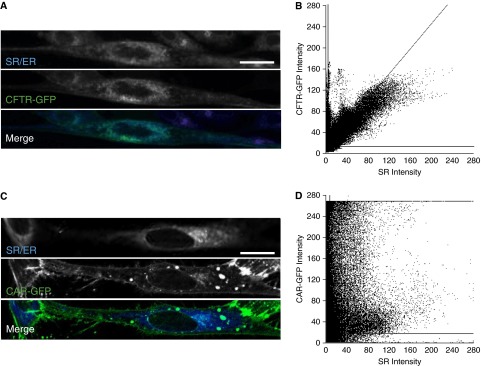
Given our finding of intracellular CFTR immunostaining pattern and the well-defined role of the sarcoplasmic reticulum (SR) in ASM contraction, we hypothesized that mature, glycosylated CFTR was localizing to the ASM SR compartment. Although stimulated emission depletion microscopy showed low levels of plasma membrane-associated CFTR-GFP, most CFTR-GFP had a high degree of colocalization with ER-Tracker (Invitrogen, Carlsbad, CA), which has previously been used to label the SR compartment (Figures 2A and 2B) (36, 37). As a control, we overexpressed the coxsackievirus and adenovirus receptor (GFP-labeled) (38). In ASM cells, coxsackievirus and adenovirus receptor–GFP predominantly localized to the plasma membrane and we found minimal colocalization with ER-Tracker dye (Figures 2C and 2D). These data suggest that CFTR predominately resides within the SR of ASM.
Figure 2.
Cystic fibrosis transmembrane conductance regulator (CFTR) localizes to the sarcoplasmic reticulum (SR) in airway smooth muscle (ASM) cells. (A) ER-Tracker (blue) and CFTR-GFP (green) staining in live porcine ASM cells. (B) Colocalization of CFTR-GFP to ER-Tracker as quantified by using JACoP (58, 59). Significant colocalizations were observed for CFTR-GFP and ER-Tracker with an average Pearson colocalization coefficient of 0.743. (C) ER-Tracker (blue) and coxsackievirus and adenovirus receptor (CAR)-GFP (green) staining in live ASM cells. (D) Localization of CAR-GFP and ER-Tracker as quantified by using JACoP. Localization plot for CAR-GFP and ER-Tracker showed a weak association with an average Pearson colocalization coefficient of 0.296. In B and D, horizontal and vertical lines indicate automated thresholding. Scale bar = 20 μm. ER = endoplasmic reticulum; GFP = green fluorescent protein.
CFTR Regulates ASM Basal Tone
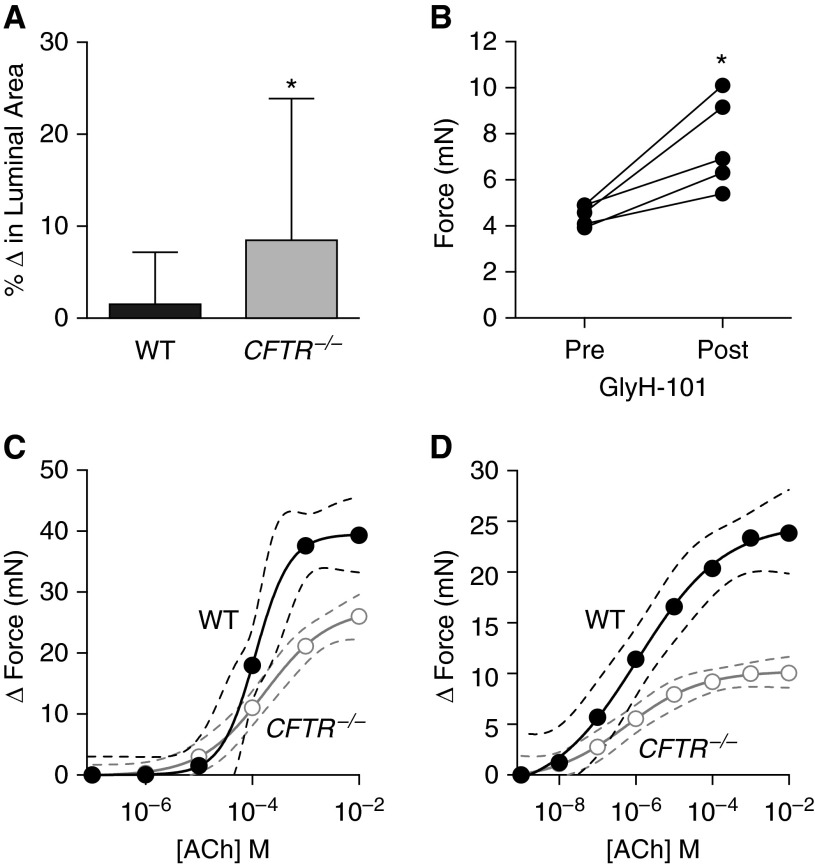
We previously reported that CFTR−/− pigs have narrowed airways and increased baseline airway resistance (20, 21), possibly suggesting increased ASM basal tone. To determine if CFTR regulates ASM contraction, we performed three experiments. First, we predicted that if CFTR−/− airways have increased basal tone then they would show greater dilation following bronchodilator treatment. We prepared precision-cut lung slices from newborn WT and CFTR−/− pig lungs and measured airway dilation following isoproterenol treatment. WT airways had minimal change in lumen area following isoproterenol treatment, but CFTR−/− airways increased their lumen area by nearly 10% under resting conditions (Figure 3A).
Figure 3.
Cystic fibrosis transmembrane conductance regulator (CFTR) regulates normal airway smooth muscle (ASM) force generation. (A) Percent change in airway lumen area of precision-cut lung slices in response to isoproterenol (wild type [WT], n = 34 slices from 11 animals; CFTR−/−, n = 38 slices from 15 animals were studied). (B) Basal isometric force measurements before and after GlyH-101 treatment in WT ASM strips (n = 5 animals). (C) WT (n = 9 animals) and CFTR−/− (n = 10 animals) tracheal ring isometric force generation following acetylcholine (ACh) treatment. (D) WT (n = 5 animals) and CFTR−/− (n = 5 animals) ASM trachealis muscle strip isometric force generation following ACh treatment. Solid circles denote WT and open circles denote CFTR−/−. In A, data are mean ± SD. *P < 0.05. In C and D, data are shown as mean values with the accompanying curve fit (solid line) and the 95% confidence interval displayed as a band (dashed lines).
Second, we hypothesized that CFTR inhibition would increase resting or basal tone in WT muscle. WT ASM tissue strips were calibrated to a resting tension of 0.5 g and GlyH-101 (a CFTR inhibitor) was added to the bath solution. Following GlyH-101 exposure, basal tone increased in the WT muscle strips (Figure 3B). These findings suggest that CFTR controlsvcontraction in ASM and loss of CFTR increases basal tone in CF airways.
Third, we hypothesized that if there is an increase in basal tone in CFTR−/− trachealis muscle, further activation of the muscle will result in production of less force (above the basal tone) because some of the myosin-actin cross-bridges will have already been recruited in tone generation. We measured isometric force development by tracheal rings from newborn WT and CFTR−/− pigs. Acetylcholine-induced force generation was decreased in CFTR−/− compared with WT tracheal rings (Figure 3C) (P < 0.0001 WT vs. CFTR−/− curves). To limit the effect of ring strain and cartilage and epithelial contributions to force production, and to further investigate for a smooth muscle–specific effect, we isolated and studied tracheal ASM tissue strips. Similar to the tracheal rings, the contractile response to acetylcholine was significantly reduced in CFTR−/−muscle strips, and the EC50 was similar between groups (Figure 3D) (P < 0.0001 WT vs. CFTR−/− curves). These data suggest that CFTR−/− ASM has an increased contractile tone before measurements and is thus nearer its maximal force response before cholinergic stimulation.
CFTR Regulates MLC Phosphorylation
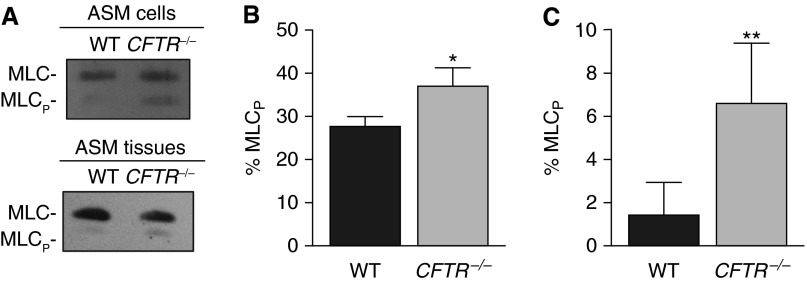
To investigate if CFTR activity is associated with molecular changes in the contractile machinery of ASM cells, we assayed regulatory MLC phosphorylation. Increased MLC phosphorylation is necessary for actin-myosin interactions, leading to contraction. Baseline MLC phosphorylation levels were increased in CFTR−/− trachealis muscle and in cultured CFTR−/− ASM cells, compared with WT samples (Figures 4A–4C). These results suggest that CFTR regulates MLC phosphorylation and loss of CFTR increases MLC phosphorylation, further supporting the conclusion that CFTR regulates ASM contractile tone.
Figure 4.
Cystic fibrosis transmembrane conductance regulator (CFTR) activity decreases regulatory myosin light chain (MLC) phosphorylation. (A) Immunoblot for total MLC and phosphorylated regulatory MLC (MLCp) in wild-type (WT) and CFTR−/− airway smooth muscle (ASM) cells and tissues. (B) Quantification of percent MLC phosphorylation in ASM cells from WT (n = 3) and CFTR−/− (n = 4) pigs. (C) Quantification of percent MLC phosphorylation in ASM tissue from WT (n = 7) and CFTR−/− (n = 5) pigs. Data are mean ± SD. *P < 0.05, **P < 0.01.
CFTR Regulates Ca2+ Reuptake in ASM
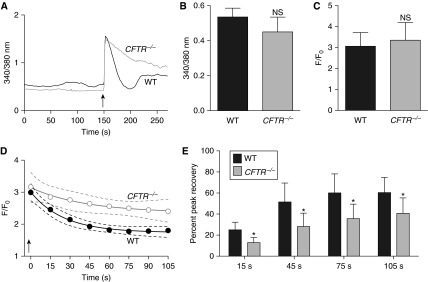
Identification of CFTR in the SR and alterations in MLC phosphorylation suggested that ASM Ca2+ handling might be regulated, in part, by CFTR. We measured Fura-2 fluorescence to quantify intracellular Ca2+ levels in freshly dissociated WT and CFTR−/− ASM cells (Figure 5A). Under basal conditions and following acute cholinergic treatment, intracellular Ca2+ levels were similar between WT and CFTR−/− ASM cells (Figures 5A–5C). However, after cholinergic stimulation, Ca2+ reuptake was delayed in CFTR−/− ASM cells (Figures 5A and 5D, P < 0.0001 WT vs. CFTR−/− curves; and Figure 5E). These data suggest that CFTR regulates proper ASM cell Ca2+ handling and that an SR-mediated mechanism may underlie the increased contractile tone observed in CFTR−/− ASM.
Figure 5.
Cystic fibrosis transmembrane conductance regulator (CFTR) regulates airway smooth muscle (ASM) cell Ca2+ handling. (A) The ratiometric indicator Fura-2 was used to quantify intracellular Ca2+ levels in freshly isolated ASM cells. A tracing from wild-type (WT) (black line) and CFTR−/− (gray line) cells after acetylcholine stimulation (arrow) is shown. (B) Baseline Fura-2 fluorescence values in CFTR−/− and WT ASM cells (n = 6 animals per genotype). (C) Maximal Fura-2 fluorescence normalized to baseline fluorescence in WT and CFTR−/− ASM cells (n = 6 animals per genotype). (D) Time course of Fura-2 fluorescence (normalized to baseline values) in WT (solid circles) and CFTR−/− (open circles) ASM cells in response to acetylcholine (arrow) (n = 6 animals per genotype). (E) Percent peak recovery of [Ca2+]i to baseline following cholinergic stimulation in WT and CFTR−/− ASM cells. Values at 15, 45, 75, and 105 seconds are shown. In B, C, and E, data are mean ± SD. *P < 0.05. NS = P > 0.05. In D, data are shown as mean values with the accompanying curve fit (solid line) and the 95% confidence interval displayed as a band (dashed lines).
CFTR Potentiation Inhibits Cholinergic-induced Airway Narrowing
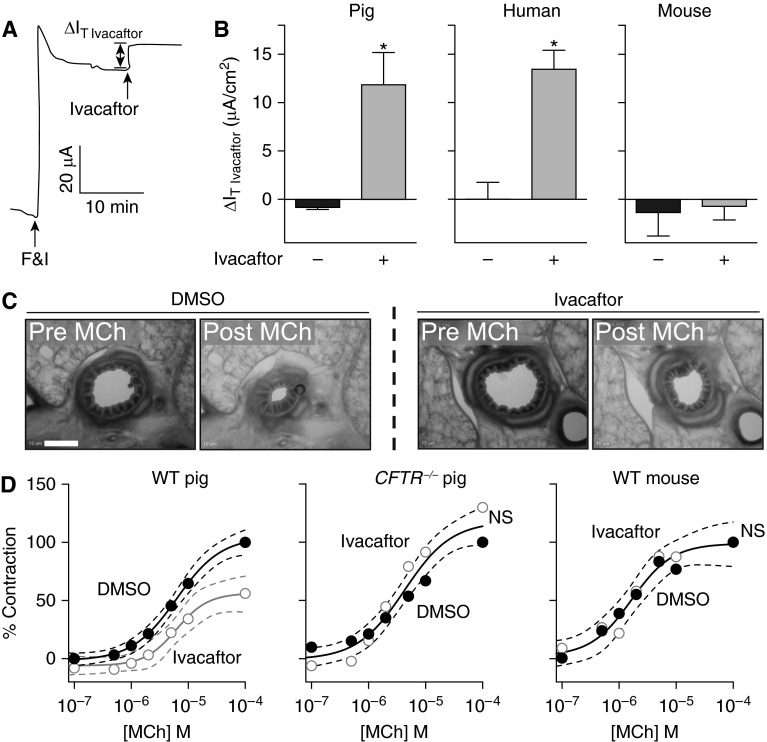
In contrast to the studies described previously in which we eliminated or inhibited CFTR, we predicted that increasing CFTR activity would have the opposite effect, attenuating contraction. To test this, we used the CFTR potentiator ivacaftor, which increases the open-state probability of human WT CFTR and some mutant CFTR channels (22, 24, 39, 40). We first confirmed that ivacaftor potentiates porcine CFTR by studying airway epithelial cell cultures from WT pigs and comparing the response to human and murine airway epithelial cell cultures. After forskolin and IBMX treatment, airway epithelial cell cultures were treated with ivacaftor and CFTR-mediated transport was determined (Figure 6A). Ivacaftor increased the transepithelial current in both porcine and human airway epithelial cells, but not murine (Figure 6B). These data show that ivacaftor potentiates porcine CFTR to levels observed in human airway epithelial cell cultures.
Figure 6.
Cystic fibrosis transmembrane conductance regulator (CFTR) potentiation decreases airway contraction in porcine precision-cut lung slices. (A) Current trace in response to indicated agents in porcine wild-type (WT) airway epithelial cell cultures. F&I = forskolin and IBMX. ∆IT Ivacaftor represents the change in current following ivacaftor treatment. Studies were performed in the presence of a Cl− gradient and following basalolateral nystatin permeabilization. (B) Data show change in current in response to ivacaftor in primary porcine (left), human (middle), and murine (right) differentiated airway epithelial cell cultures. Epithelia were pretreated with amiloride and DIDS. Cells were treated with forskolin/IBMX followed by ivacaftor. *P < 0.05 for dimethyl sulfoxide (DMSO) (−) versus ivacaftor (+). (C and D) Precision-cut lung slices were prepared from porcine (WT and CFTR−/−) and murine (WT) lungs. (C) Representative images of porcine WT precision-cut lung slices with control DMSO or ivacaftor pretreatment before and after methacholine (MCh) treatment. Scale bar = 300 μm. (D) Percent airway lumen area contraction in response to MCh in precision-cut lung slices pretreated with control vehicle (DMSO, solid circles) or ivacaftor (open circles) in WT (left; n = 6–8 animals per group) and CFTR−/− (middle; n = 4 animals per group) porcine and WT murine (right; n = 4 animals per group) normalized to maximal untreated contractile response. In B, data are mean ± SD. *P < 0.0001. In D, data are shown as mean values with the accompanying curve fit (solid line) and the 95% confidence interval displayed as a band (dashed lines). NS = P > 0.05.
Adding methacholine to WT porcine lung slices quickly reduced airway lumen area. Pretreating lung slices with ivacaftor attenuated airway narrowing by nearly 50% (Figures 6C and 6D; see Movies E1 and E2 in the online supplement) (P < 0.0001 dimethyl sulfoxide control vs. ivacaftor curves in WT pig). To test if the effect of ivacaftor was CFTR-dependent, we repeated the studies on CFTR−/− porcine lung slices. As an additional control, we performed the studies on WT murine lung slices because ivacaftor does not affect murine CFTR (Figure 6B) (22). Methacholine caused a similar degree of airway narrowing in the CFTR−/− pig and WT mouse tissues, but ivacaftor did not inhibit the response (Figure 6D). These data indicate that CFTR potentiation attenuated cholinergic-induced airway narrowing, and the effect was CFTR-dependent.
Discussion
Prior studies have shown that more than 50% of people with CF have some form of airway hyperresponsiveness (5, 6). Despite ASM being the primary cell type contributing to airway narrowing, the asthmatic-like abnormalities in ASM of people with CF are not well characterized. The largest confounding factor in assessing the role of CFTR in human CF ASM is the presence of chronic airway inflammation and infection. Here, newborn CFTR−/− pigs, which lack airway inflammation and infection at birth, provide a unique opportunity to study the intrinsic role of CFTR in ASM function in the absence of those secondary manifestations. Accordingly, our findings support a primary role for CFTR in ASM function. We demonstrated that CFTR regulates ASM Ca2+ reuptake and loss of CFTR caused changes in basal tone and contractility. In this regard, unlike epithelial cells where CFTR is normally expressed in the apical membrane, immunostaining studies surprisingly showed that CFTR is predominately localized to an intracellular compartment in ASM, likely the SR. Consistently, Ca2+ handling defects were also found in CFTR−/− ASM cells, highlighting an underlying mechanism for CF-associated ASM dysfunction and a possible role for CFTR potentiation in modulating airway narrowing.
We were initially surprised to find that in ASM cells both endogenous and recombinant CFTR were fully glycosylated, but predominately localized to an intracellular compartment. Precise CFTR localization within smooth muscle has been difficult because of the low levels of endogenous protein and lack of gene inactivated controls needed to confirm specificity of CFTR antibody binding. Immunostaining for CFTR in rat tracheal smooth muscle cells and human bronchial ASM cells showed a diffuse cytoplasmic distribution of CFTR, with high perinuclear staining (12, 13). These findings and our current data contrast with the typical localization of CFTR to the plasma membrane of polarized epithelial and serous cells (27, 33, 34). We further localized CFTR to the SR in ASM cells, a finding consistent with earlier reports of CFTR localization to the SR in skeletal muscle cells (41, 42). CF disease manifestations are typically related to loss of CFTR function at the plasma membrane in epithelial cells. However, differing mechanisms of CFTR function in nonepithelial cells have been proposed, including intracellular vesicle acidification and SR calcium homeostasis in skeletal muscle (41–44). The lack of localized plasma membrane CFTR staining in ASM is similar to that in other nonepithelial cells and suggests an intracellular function of CFTR in ASM. Moreover, CFTR may be similar to other Cl− channels, such as Bestrophins, CLCs, and TMEM16a, which have been reported to have dual plasma membrane and intracellular functions (45–47).
How might CFTR regulate ASM contractile tone? It has been proposed that Cl− is the principal anion that contributes to charge neutralization during Ca2+ reuptake in smooth muscle SR (48, 49). Hirota and colleagues (50) have shown that contractile agonists are able to contribute to Ca2+ flux via Cl− channels in the smooth muscle SR, indicating a potential function for Cl− channels, such as CFTR in SR Ca2+ handling. We found that newborn CFTR−/− porcine ASM cells had delayed restoration of intracellular Ca2+ levels following cholinergic stimulation. This finding could be consistent with decreased CFTR-mediated Cl− transport from the cytoplasmic space to the SR leading to a positive charge accumulation on the inner SR membrane (from Ca2+ movement into the SR) thereby impairing or delaying Ca2+ reuptake. Although we found that the peak Ca2+ response following cholinergic stimulation was similar between WT and CFTR−/− porcine ASM cells, others have found that the Ca2+ response to histamine and IL-8 was reduced in human CF ASM cells (51). Potential factors that might account for the divergent Ca2+ responses could include the presence of long-standing disease and/or remodeling in the human CF tissues, the choice of contractile agonists, or species-dependent differences.
Earlier studies in people with CF have found variable bronchodilator and methacholine responses (1, 5–7). This incomplete penetrance could reflect the varying severity and class of CFTR mutations on smooth muscle function. For example, CFTR mutations, such as ∆F508, which lead to aberrant trafficking still retain some function in the endoplasmic reticulum of epithelia (52) and might be functionally active in the SR of ASM. In contrast, other CFTR mutations that lead to a nonfunctional protein may more closely resemble the ASM alterations observed in the CFTR−/− pig. Additional studies investigating the effects of different CFTR mutations on smooth muscle function will likely be very informative.
Our studies have both advantages and limitations. Advantages include (1) the CFTR−/− pig recapitulates many of the disease phenotypes seen in human CF (18, 19); (2) the newborn porcine airway more closely resembles human airways than other species (53); (3) by using newborn CFTR−/− pigs, we were able to limit confounding factors, such as airway infection and inflammation, thereby studying the primary effects of CFTR; and (4) we used multiple approaches and length-scales to determine the importance of CFTR on ASM contraction. Limitations include first that we cannot exclude the possibility that developmental changes might also contribute to the ASM defects in CF pigs. However, data from acute CFTR inhibition experiments, CFTR−/− pigs, and ivacaftor studies indicate that the phenotype is, in part, an intrinsic defect due to loss of CFTR. For example, we found increased MLC phosphorylation in both freshly isolated trachealis muscle samples and cultured ASM cells, suggesting a primary defect. Second, although we observed a cell-specific effect of CFTR activity in ASM function, we cannot rule out contributing effects of CFTR in other cell types, such as epithelia or neurons. Finally, although the CFTR potentiator used promotes airway relaxation presumably through CFTR’s ion channel activity, we cannot exclude other effects by CFTR, such as its role as a scaffold protein for other protein-binding partners (43).
What are the implications of our findings? In total, our data suggest that CFTR regulates ASM function, whereas loss of CFTR in ASM leads to an altered contractile phenotype represented by increased basal tone. Moreover, these alterations are likely caused in part by a role for CFTR in SR Ca2+ handling. These findings could have important implications for both CF and non-CF diseases involving excessive airway narrowing. Although some of the “asthma-like” symptoms in CF are probably secondary to chronic airway infection and inflammation, our data provide further support for a direct role of CFTR in ASM and that loss of CFTR causes a primary defect in CF ASM cells. Whether similar smooth muscle defects are present in other organs of the porcine CFTR−/− model is unknown, but intestinal dysmotility has been linked to CF intestinal disease, consistent with a role for CFTR in intestinal smooth muscle function (54–57).
These data also have important implications for treating both CF and non-CF airway diseases. Ivacaftor is a CFTR potentiator for both WT and G551D-CFTR. Ivacaftor improves FEV1 in people with CF and the G551D-CFTR mutation by nearly 200 ml after only 3 days of treatment (24), suggesting that improvements in airflow obstruction could be, in part, mediated by relaxation of ASM. We found that ivacaftor inhibited cholinergic-induced airway narrowing in WT porcine lung slices, but had no effect in murine WT or porcine CFTR−/− tissues, demonstrating a CFTR-dependent effect. These findings suggest that CFTR potentiators may lessen airway narrowing in individuals with select CFTR mutations for which ivacaftor is effective. Moreover, CFTR modulation in ASM might represent a novel therapeutic target for those individuals with asthma or COPD refractory to conventional treatment, such as in the case of tachyphylaxis or select β-adrenergic polymorphisms.
In summary, using a CFTR knockout porcine model, absent the chronic airway infection and inflammation characteristic of CF, we found CFTR plays a primary role in ASM function. The contribution of ASM dysfunction to the pathogenesis of CF airway disease remains to be determined, but could play an important role. CFTR might represent a novel target in airway-narrowing diseases associated with abnormal smooth muscle function.
Acknowledgments
Acknowledgment
The authors thank P. Karp, T. Mayhew, T. Moninger, A. Pezzulo, T. Rokhlina, J. Rodgers, and P. Taft for excellent assistance and advice. They also thank A. Comellas, P. McCray, M. Welsh, and J. Zabner for insightful discussions and guidance. GlyH-101 was a gift from Cystic Fibrosis Foundation Therapeutics and Robert Bridges, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois.
Footnotes
Funded by an Early Excellence Award from the American Asthma Foundation (D.A.S.), the National Institutes of Health (HL091842, HL51670, HL117744, T32 GM007737, T32 HL007638), and the Cystic Fibrosis Foundation (Research Development Program). D.A.S. was supported by the Gilead Sciences Research Scholars Program in Cystic Fibrosis.
Author Contributions: D.P.C., M.V.R., D.C.B., A.S.M., M.A.T., Y.S.P., D.K.M., C.Y.S., and D.A.S., conceived of and designed research. D.P.C., M.V.R., D.C.B., A.S.M., N.D.G., L.R.R., X.L., M.R.S., L.S.O., and D.A.S., performed experiments and data acquisition. D.P.C., M.V.R., D.C.B., A.S.M., N.D.G., X.L., M.R.S., M.H.A.A., C.Y.S., and D.A.S., analyzed data. D.P.C., M.V.R., D.C.B., A.S.M., L.R.R., X.L., L.S.O., M.H.A.A., R.K., C.Y.S., and D.A.S., interpreted results of experiments. D.P.C., M.V.R., D.C.B., A.S.M., N.D.G., X.L., M.R.S., and D.A.S., prepared figures. D.P.C., M.V.R., D.C.B., A.S.M., D.K.M., C.Y.S., and D.A.S., drafted manuscript. D.P.C., M.V.R., D.C.B., A.S.M., N.D.G., L.R.R., X.L., M.R.S., L.S.O., M.H.A.A., M.A.T., Y.S.P., R.K., D.K.M., C.Y.S., and D.A.S., edited, reviewed, and approved final version of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201508-1562OC on October 21, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Weinberger M. Airways reactivity in patients with CF. Clin Rev Allergy Immunol. 2002;23:77–85. doi: 10.1385/CRIAI:23:1:077. [DOI] [PubMed] [Google Scholar]
- 2.Kent BD, Lane SJ, van Beek EJ, Dodd JD, Costello RW, Tiddens HA. Asthma and cystic fibrosis: a tangled web. Pediatr Pulmonol. 2014;49:205–213. doi: 10.1002/ppul.22934. [DOI] [PubMed] [Google Scholar]
- 3.Balfour-Lynn IM, Elborn JS. “CF asthma”: what is it and what do we do about it? Thorax. 2002;57:742–748. doi: 10.1136/thorax.57.8.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balfour-Lynn IM. Asthma in cystic fibrosis. J R Soc Med. 2003;96:30–34. [PMC free article] [PubMed] [Google Scholar]
- 5.van Haren EH, Lammers JW, Festen J, van Herwaarden CL. Bronchial vagal tone and responsiveness to histamine, exercise and bronchodilators in adult patients with cystic fibrosis. Eur Respir J. 1992;5:1083–1088. [PubMed] [Google Scholar]
- 6.Sanchez I, Powell RE, Pasterkamp H. Wheezing and airflow obstruction during methacholine challenge in children with cystic fibrosis and in normal children. Am Rev Respir Dis. 1993;147:705–709. doi: 10.1164/ajrccm/147.3.705. [DOI] [PubMed] [Google Scholar]
- 7.Colombo JL. Long-acting bronchodilators in cystic fibrosis. Curr Opin Pulm Med. 2003;9:504–508. doi: 10.1097/00063198-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Tzetis M, Efthymiadou A, Strofalis S, Psychou P, Dimakou A, Pouliou E, Doudounakis S, Kanavakis E. CFTR gene mutations--including three novel nucleotide substitutions--and haplotype background in patients with asthma, disseminated bronchiectasis and chronic obstructive pulmonary disease. Hum Genet. 2001;108:216–221. doi: 10.1007/s004390100467. [DOI] [PubMed] [Google Scholar]
- 9.Regamey N, Ochs M, Hilliard TN, Mühlfeld C, Cornish N, Fleming L, Saglani S, Alton EW, Bush A, Jeffery PK, et al. Increased airway smooth muscle mass in children with asthma, cystic fibrosis, and non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2008;177:837–843. doi: 10.1164/rccm.200707-977OC. [DOI] [PubMed] [Google Scholar]
- 10.Robert R, Norez C, Becq F. Disruption of CFTR chloride channel alters mechanical properties and cAMP-dependent Cl- transport of mouse aortic smooth muscle cells. J Physiol. 2005;568:483–495. doi: 10.1113/jphysiol.2005.085019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert R, Savineau JP, Norez C, Becq F, Guibert C. Expression and function of cystic fibrosis transmembrane conductance regulator in rat intrapulmonary arteries. Eur Respir J. 2007;30:857–864. doi: 10.1183/09031936.00060007. [DOI] [PubMed] [Google Scholar]
- 12.Michoud MC, Robert R, Hassan M, Moynihan B, Haston C, Govindaraju V, Ferraro P, Hanrahan JW, Martin JG. Role of the cystic fibrosis transmembrane conductance channel in human airway smooth muscle. Am J Respir Cell Mol Biol. 2009;40:217–222. doi: 10.1165/rcmb.2006-0444OC. [DOI] [PubMed] [Google Scholar]
- 13.Vandebrouck C, Melin P, Norez C, Robert R, Guibert C, Mettey Y, Becq F. Evidence that CFTR is expressed in rat tracheal smooth muscle cells and contributes to bronchodilation. Respir Res. 2006;7:113. doi: 10.1186/1465-9921-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norez C, Jayle C, Becq F, Vandebrouck C. Bronchorelaxation of the human bronchi by CFTR activators. Pulm Pharmacol Ther. 2014;27:38–43. doi: 10.1016/j.pupt.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Bazett M, Haston CK. Airway hyperresponsiveness in FVB/N delta F508 cystic fibrosis transmembrane conductance regulator mice. J Cyst Fibros. 2014;13:378–383. doi: 10.1016/j.jcf.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Wallace HL, Southern KW, Connell MG, Wray S, Burdyga T. Abnormal tracheal smooth muscle function in the CF mouse. Physiol Rep. 2013;1:e00138. doi: 10.1002/phy2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonvin E, Le Rouzic P, Bernaudin JF, Cottart CH, Vandebrouck C, Crié A, Leal T, Clement A, Bonora M. Congenital tracheal malformation in cystic fibrosis transmembrane conductance regulator-deficient mice. J Physiol. 2008;586:3231–3243. doi: 10.1113/jphysiol.2008.150763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyerholz DK, Stoltz DA, Namati E, Ramachandran S, Pezzulo AA, Smith AR, Rector MV, Suter MJ, Kao S, McLennan G, et al. Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Respir Crit Care Med. 2010;182:1251–1261. doi: 10.1164/rccm.201004-0643OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adam RJ, Michalski AS, Bauer C, Abou Alaiwa MH, Gross TJ, Awadalla MS, Bouzek DC, Gansemer ND, Taft PJ, Hoegger MJ, et al. Air trapping and airflow obstruction in newborn cystic fibrosis piglets. Am J Respir Crit Care Med. 2013;188:1434–1441. doi: 10.1164/rccm.201307-1268OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H, Burton B, Huang CJ, Worley J, Cao D, Johnson JP, Jr, Urrutia A, Joubran J, Seepersaud S, Sussky K, et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros. 2012;11:237–245. doi: 10.1016/j.jcf.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook DP, Bouzek DC, Stroik M, Reznikov LR, Gansemer ND, Meyerholz DK, Stoltz DA. Loss of CFTR leads to altered calcium handling in airway smooth muscle. B59 asthma-like phenotype: emergence of (Epi)genetics and targeted transgenesis [abstract] Am J Respir Crit Care Med. 2015;191:A3528. [Google Scholar]
- 26.Rector M, Gansemer N, Xiaopeng L, Ryan A, Meyerholz D, Stoltz D. Abnormal regulation of airway smooth muscle function in newborn cystic fibrosis pigs. Pediatr Pulmonol. 2012;47:1–463. [Google Scholar]
- 27.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sathish V, Delmotte PF, Thompson MA, Pabelick CM, Sieck GC, Prakash YS. Sodium-calcium exchange in intracellular calcium handling of human airway smooth muscle. PLoS One. 2011;6:e23662. doi: 10.1371/journal.pone.0023662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lan B, Wang L, Zhang J, Pascoe CD, Norris BA, Liu JC, Solomon D, Paré PD, Deng L, Seow CY. Rho-kinase mediated cytoskeletal stiffness in skinned smooth muscle. J Appl Physiol (1985) 2013;115:1540–1552. doi: 10.1152/japplphysiol.00654.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clay H, Ramakrishnan L. Multiplex fluorescent in situ hybridization in zebrafish embryos using tyramide signal amplification. Zebrafish. 2005;2:105–111. doi: 10.1089/zeb.2005.2.105. [DOI] [PubMed] [Google Scholar]
- 31.Sanderson MJ. Exploring lung physiology in health and disease with lung slices. Pulm Pharmacol Ther. 2011;24:452–465. doi: 10.1016/j.pupt.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illek B, Tam AW, Fischer H, Machen TE. Anion selectivity of apical membrane conductance of Calu 3 human airway epithelium. Pflugers Arch. 1999;437:812–822. doi: 10.1007/s004240050850. [DOI] [PubMed] [Google Scholar]
- 33.Denning GM, Ostedgaard LS, Cheng SH, Smith AE, Welsh MJ. Localization of cystic fibrosis transmembrane conductance regulator in chloride secretory epithelia. J Clin Invest. 1992;89:339–349. doi: 10.1172/JCI115582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford I, Maloney PC, Zeitlin PL, Guggino WB, Hyde SC, Turley H, Gatter KC, Harris A, Higgins CF. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proc Natl Acad Sci USA. 1991;88:9262–9266. doi: 10.1073/pnas.88.20.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granio O, Norez C, Ashbourne Excoffon KJ, Karp PH, Lusky M, Becq F, Boulanger P, Zabner J, Hong SS. Cellular localization and activity of Ad-delivered GFP-CFTR in airway epithelial and tracheal cells. Am J Respir Cell Mol Biol. 2007;37:631–639. doi: 10.1165/rcmb.2007-0026TE. [DOI] [PubMed] [Google Scholar]
- 36.Esfandiarei M, Fameli N, Choi YY, Tehrani AY, Hoskins JG, van Breemen C. Waves of calcium depletion in the sarcoplasmic reticulum of vascular smooth muscle cells: an inside view of spatiotemporal Ca2+ regulation. PLoS One. 2013;8:e55333. doi: 10.1371/journal.pone.0055333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fedoryak OD, Searls Y, Smirnova IV, Burns DM, Stehno-Bittel L. Spontaneous Ca2+ oscillations in subcellular compartments of vascular smooth muscle cells rely on different Ca2+ pools. Cell Res. 2004;14:379–388. doi: 10.1038/sj.cr.7290238. [DOI] [PubMed] [Google Scholar]
- 38.Excoffon KJ, Traver GL, Zabner J. The role of the extracellular domain in the biology of the coxsackievirus and adenovirus receptor. Am J Respir Cell Mol Biol. 2005;32:498–503. doi: 10.1165/rcmb.2005-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clancy JP, Jain M. Personalized medicine in cystic fibrosis: dawning of a new era. Am J Respir Crit Care Med. 2012;186:593–597. doi: 10.1164/rccm.201204-0785PP. [DOI] [PubMed] [Google Scholar]
- 41.Divangahi M, Balghi H, Danialou G, Comtois AS, Demoule A, Ernest S, Haston C, Robert R, Hanrahan JW, Radzioch D, et al. Lack of CFTR in skeletal muscle predisposes to muscle wasting and diaphragm muscle pump failure in cystic fibrosis mice. PLoS Genet. 2009;5:e1000586. doi: 10.1371/journal.pgen.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamhonwah AM, Bear CE, Huan LJ, Kim Chiaw P, Ackerley CA, Tein I. Cystic fibrosis transmembrane conductance regulator in human muscle: dysfunction causes abnormal metabolic recovery in exercise. Ann Neurol. 2010;67:802–808. doi: 10.1002/ana.21982. [DOI] [PubMed] [Google Scholar]
- 43.Bronckers A, Kalogeraki L, Jorna HJ, Wilke M, Bervoets TJ, Lyaruu DM, Zandieh-Doulabi B, Denbesten P, de Jonge H. The cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in maturation stage ameloblasts, odontoblasts and bone cells. Bone. 2010;46:1188–1196. doi: 10.1016/j.bone.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang H, Yang L, Ma T, Zhao Y. Functional expression of cystic fibrosis transmembrane conductance regulator in mouse chondrocytes. Clin Exp Pharmacol Physiol. 2010;37:506–508. doi: 10.1111/j.1440-1681.2009.05319.x. [DOI] [PubMed] [Google Scholar]
- 45.Gomez NM, Tamm ER, Straubeta O. Role of bestrophin-1 in store-operated calcium entry in retinal pigment epithelium. Pflugers Arch. 2013;465:481–495. doi: 10.1007/s00424-012-1181-0. [DOI] [PubMed] [Google Scholar]
- 46.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 47.Danielsson J, Perez-Zoghbi J, Bernstein K, Barajas MB, Zhang Y, Kumar S, Sharma PK, Gallos G, Emala CW. Antagonists of the TMEM16A calcium-activated chloride channel modulate airway smooth muscle tone and intracellular calcium. Anesthesiology. 2015;123:569–581. doi: 10.1097/ALN.0000000000000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pollock NS, Kargacin ME, Kargacin GJ. Chloride channel blockers inhibit Ca2+ uptake by the smooth muscle sarcoplasmic reticulum. Biophys J. 1998;75:1759–1766. doi: 10.1016/S0006-3495(98)77617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirota S, Trimble N, Pertens E, Janssen LJ. Intracellular Cl- fluxes play a novel role in Ca2+ handling in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1146–L1153. doi: 10.1152/ajplung.00393.2005. [DOI] [PubMed] [Google Scholar]
- 50.Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J. 2007;30:114–133. doi: 10.1183/09031936.00147706. [DOI] [PubMed] [Google Scholar]
- 51.Govindaraju V, Michoud MC, Ferraro P, Arkinson J, Safka K, Valderrama-Carvajal H, Martin JG. The effects of interleukin-8 on airway smooth muscle contraction in cystic fibrosis. Respir Res. 2008;9:76. doi: 10.1186/1465-9921-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pasyk EA, Foskett JK. Mutant (ΔF508) cystic fibrosis transmembrane conductance regulator Cl- channel is functional when retained in endoplasmic reticulum of mammalian cells. J Biol Chem. 1995;270:12347–12350. doi: 10.1074/jbc.270.21.12347. [DOI] [PubMed] [Google Scholar]
- 53.Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT, McCray PB, Jr, McLennan G, Meyerholz DK, Namati E, Ostedgaard LS, et al. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L240–L263. doi: 10.1152/ajplung.90203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Risse PA, Kachmar L, Matusovsky OS, Novali M, Gil FR, Javeshghani S, Keary R, Haston CK, Michoud MC, Martin JG, et al. Ileal smooth muscle dysfunction and remodeling in cystic fibrosis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1–G8. doi: 10.1152/ajpgi.00356.2011. [DOI] [PubMed] [Google Scholar]
- 55.De Lisle RC, Meldi L, Mueller R. Intestinal smooth muscle dysfunction develops postnatally in cystic fibrosis mice. J Pediatr Gastroenterol Nutr. 2012;55:689–694. doi: 10.1097/MPG.0b013e3182638bf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol. 2010;176:1377–1389. doi: 10.2353/ajpath.2010.090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Lisle RC, Borowitz D. The cystic fibrosis intestine. Cold Spring Harb Perspect Med. 2013;3:a009753. doi: 10.1101/cshperspect.a009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86:3993–4003. doi: 10.1529/biophysj.103.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bolte S, Cordelières FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]