One in 7 Xpert-positive retreatment cases are false positive. Patients with higher Xpert quantitative information, less time having passed since their previous tuberculosis, and a normal chest radiograph are more likely to have false-positive results. Xpert detects DNA in nonviable, nonintact cells.
Keywords: tuberculosis, diagnosis, Xpert, false positivity
Abstract
Background. Patients with previous tuberculosis may have residual DNA in sputum that confounds nucleic acid amplification tests such as Xpert MTB/RIF. Little is known about the frequency of Xpert-positive, culture-negative (“false positive”) results in retreatment patients, whether these are distinguishable from true positives, and whether Xpert's automated filter-based wash step reduces false positivity by removing residual DNA associated with nonintact cells.
Methods. Pretreatment patients (n = 2889) with symptoms of tuberculosis from Cape Town, South Africa, underwent a sputum-based liquid culture and Xpert. We also compared Xpert results from dilutions of intact or heat-lysed and mechanically lysed bacilli.
Results. Retreatment cases were more likely to be Xpert false-positive (45/321 Xpert-positive retreatment cases were false-positive) than new cases (40/461) (14% [95% confidence interval {CI}, 10%-18%] vs 8% [95% CI, 6%–12%]; P = .018). Fewer years since treatment completion (adjusted odds ratio [aOR], 0.85 [95% CI, .73–.99]), less mycobacterial DNA (aOR, 1.14 [95% CI, 1.03–1.27] per cycle threshold [CT]), and a chest radiograph not suggestive of active tuberculosis (aOR, 0.22 [95% CI, .06–.82]) were associated with false positivity. CT had suboptimal accuracy for false positivity: 46% of Xpert-positives with CT > 30 would be false positive, although 70% of false positives would be missed. CT's predictive ability (area under the curve, 0.83 [95% CI, .76–.90]) was not improved by additional variables. Xpert detected nonviable, nonintact bacilli without a change in CT vs controls.
Conclusions. One in 7 Xpert-positive retreatment patients were culture negative and potentially false positive. False positivity was associated with recent previous tuberculosis, high CT, and a chest radiograph not suggestive of active tuberculosis. Clinicians may consider awaiting confirmatory testing in retreatment patients with CT > 30; however, most false positives fall below this cut-point. Xpert can detect DNA from nonviable, nonintact bacilli.
Xpert MTB/RIF (Xpert; Cepheid) is an automated nucleic acid amplification test (NAAT) for Mycobacterium tuberculosis and rifampicin resistance [1–3], endorsed by the World Health Organization and the US Food and Drug Administration [4, 5]. Xpert is increasingly deployed in many countries as the initial diagnostic test for tuberculosis [6].
Xpert is used routinely in patients who have previously had tuberculosis [6, 7]. This is despite evidence that approximately 30% of patients who are microbiologically cured after 6 months of treatment are Xpert positive [8], a proven correlation between retreatment status and diminished specificity [9–11], and several case reports detailing false-positive (FP) Xpert results in retreatment cases [12–14]. Detectable mycobacterial DNA, which can be extracellular or associated with nonintact cells (and hence is not culturable), is a possible cause of this false positivity, which may trigger unwarranted treatment and unnecessarily expose patients to toxic drugs, delay establishing the correct underlying diagnosis and its appropriate treatment, and escalate healthcare costs. Although the manufacturer recommends that Xpert always be used in conjunction with culture [15], culture capacity is not mandatory for Xpert's use in the field [7] and, even in high-burden countries such as South Africa that do have culture capacity, most Xpert-positive patients do not receive culture, as per the national algorithm [16].
More than 700 000 patients with a history of tuberculosis were diagnosed in 2013 [17]; however, there are limited data about the frequency of Xpert false positivity in retreatment patients [18] and what factors, if any, may guide clinical practice [10]. We therefore examined the relationship between Xpert results (including M. tuberculosis complex–specific quantitative information), routinely collected clinical information, and culture results in a large cohort of patients evaluated for tuberculosis in the high-burden, high–human immunodeficiency virus (HIV) setting of Cape Town, South Africa. To interrogate claims that Xpert does not detect free DNA because of a preamplification wash step [19–22], which would potentially reduce the risk of an FP result in retreatment cases, we performed a laboratory-based substudy to ascertain whether Xpert can detect DNA from lysed nonviable cells.
METHODS
Patient Recruitment
We analyzed data from 3166 patients who had symptoms suggestive of tuberculosis. Patients were recruited from primary care clinics or hospitals in Cape Town, South Africa, as part of studies that evaluated the utility of Xpert. Patients included in the final analysis were Xpert positive and had cycle threshold (CT) data, were either culture positive or negative, had a known previous tuberculosis status, had not been on treatment for >48 hours, and had not taken antituberculosis treatment 60 days prior to testing. This study was approved by the University of Cape Town Faculty of Health Sciences Ethics Committee.
Diagnostic Tests
Two paired sputum specimens were collected at recruitment; 1 was randomly selected for an Xpert test, and the other was used for a BACTEC MGIT 960 liquid culture (Becton, Dickinson, and Co). If patients were unable to expectorate sputum, sputum induction with hypertonic saline was performed. Tuberculosis morbidity score [23] data and chest radiograph (CXR) data were collected in a subset of patients, as determined by the parent protocol.
Xpert MTB/RIF Cell Lysis Experiment
To assess whether Xpert detected nonviable cells, 1 mL of M. tuberculosis H37Rv in phosphate-buffered saline and 0.25% Tween 80 (10 000, 1000, 500, and 0 colony-forming units [CFU] mL−1) was added to Xpert sample buffer (2:1 ratio) and, after 15 minutes of incubation with intermittent shaking, 2 mL was added to the Xpert cartridge (direct Xpert). In parallel, a 1.5-mL aliquot of each concentration underwent heat treatment (80°C, 1 hour), followed by mechanical disruption using Lysing Matrix B tubes (0.1 mm zirconium beads; MP Biochemicals) and a Fast Prep-24 machine (MP Biochemicals) (6.5 meters per second for three 30-second intervals with 1 minute resting on ice between intervals). After bead-beating, the lysate was allowed to settle for 2 minutes and 1 mL of supernatant was used for Xpert (lysed Xpert). Ten 10-µL aliquots of each dilution (direct and lysed) were plated on Middlebrook 7H10 agar supplement with oleic acid albumin dextrose complex and incubated for 6 weeks at 37°C to check for viability. This experiment was performed in triplicate.
Statistical Analysis
Xpert-positive, culture-positive patients were defined as true positive (TP) and Xpert-positive, culture-negative patients were defined as false positive (FP). The χ2 test was used for comparisons between proportions. The Mann–Whitney test was used to compare differences in nonparametric continuous data. Multivariable logistic regression was performed to adjust for potential confounding. A backward elimination strategy using the likelihood ratio test was used to finalize each model. Analyses were performed using GraphPad Prism version 6.0 (GraphPad Software) and Stata version 13 (StataCorp) software. All statistical tests are 2-sided at α = .05.
RESULTS
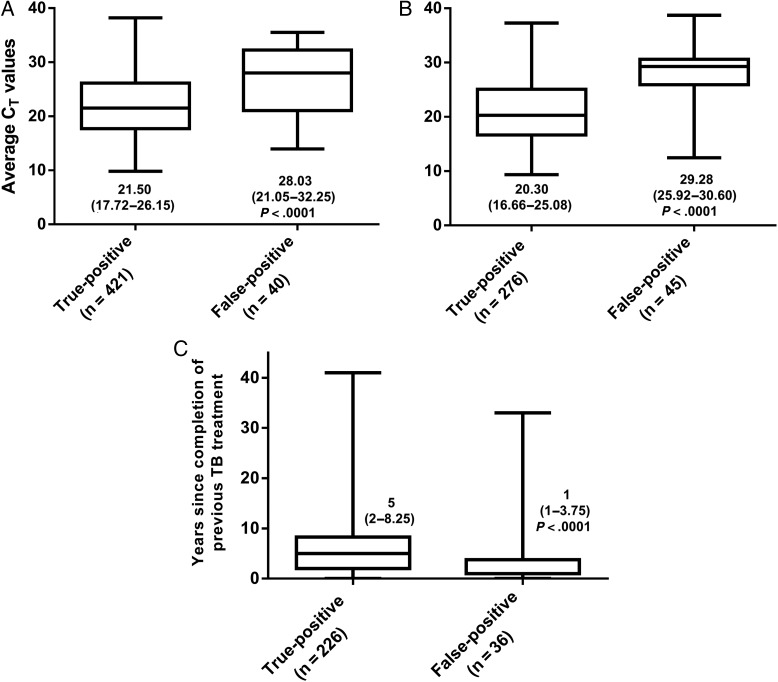
Of the 3166 patients, we excluded 263 (8%) patients (73 had did not have a positive- or negative-culture result, 86 were on treatment >48 hours, 104 Xpert-positive patients were missing CT data, and 14 were missing data on their previous tuberculosis history). Of the remaining 2889 patients with a known culture status, 837 (29%) were culture positive and 782 (27%) were Xpert positive. A total of 1220 (42%) patients were retreatment cases. A summary of the demographic and clinical characteristics of the cohort is shown according to previous tuberculosis status in Table 1. Retreatment patients were more likely to be older and HIV-infected. Differences in Xpert CT and years since completion of previous antituberculosis treatment in new and retreatment patients are shown in Figure 1.
Table 1.
Demographic and Clinical Characteristics of Patients Included in the Analysis Who Had Symptoms Suggestive of Tuberculosis
| Characteristic | No Previous TB (n = 1669) | Previous TB (n = 1220) | P Value |
|---|---|---|---|
| Demographic characteristics | |||
| Female sex, No. (%) | 773/1617 (48) | 548/1181 (46) | .463 |
| Age, y, median (IQR) | 36 (28–46) | 39 (32–49) | <.001 |
| Clinical characteristics | |||
| HIV-infected, No. (%) | 695/1617 (42) | 623/1198 (53) | <.001 |
| TB morbidity score, median (IQR) | 5 (3–6) | 5 (3–6) | .654 |
| Test characteristics | |||
| Culture-positive, No. (%) | 506/1669 (30) | 331/1220 (27) | .062 |
| Time-to-positivity, d, median (IQR) | 12 (8–17) | 13 (8–17) | .352 |
| Xpert MTB/RIF-positive, No. (%) | 461/1669 (28) | 321/1220 (26) | .434 |
| CT, median (IQR) | 21.20 (17.92–26.64) | 22.00 (17.23–27.06) | .309 |
| CXR compatible with active TB, No. (%) | 402/928 (43) | 329/708 (46) | .204 |
Abbreviations: CT, cycle threshold; CXR, chest radiograph; HIV, human immunodeficiency virus; IQR, interquartile range; TB, tuberculosis.
Figure 1.
Box-and-whisker plot comparison of Xpert MTB/RIF quantitative information (cycle threshold [CT] values) in true-positive (Xpert-positive, culture-positive) and false-positive (Xpert-positive, culture-negative) specimens for new (A) or retreatment (B) patients, and a comparison of the years since the completion of previous tuberculosis (TB) treatment in Xpert-positive retreatment patients according to culture status (C). Median values with interquartile ranges in parentheses are shown.
Xpert MTB/RIF False Positivity in Patients With Newly Diagnosed Tuberculosis
Xpert had a sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 83% (95% confidence interval [CI], 80%–86%), 97% (95% CI, 95%–98%), 91% (95% CI, 88%–94%), and 93% (95% CI, 91%–94%), respectively, in new patients. Forty of 461 (9%) Xpert-positive results were FP.
Correlates of Xpert MTB/RIF False Positivity
New tuberculosis patients with an FP Xpert were, compared to those who were TP, more likely to have higher median Xpert CT (28.03 [interquartile range {IQR}, 21.20–32.23] vs 21.50 [IQR, 17.72–26.10]; P < .001) and more likely to be female (25/40 [63%] of FP cases were women vs 187/414 [45%] of TP cases; odds ratio [OR], 2.02 [95% CI, 1.04–3.95]). In a multivariate logistic regression analysis (Table 2), each unit increase in CT was associated with a 14% increase in the relative risk (adjusted OR [aOR], 1.14 [95% CI, 1.08–1.21]) of Xpert false positivity, presuming the other variables held constant. Morbidity in patients with tuberculosis symptom score data (n = 168) was similar in those with an FP or TP Xpert result (median, 4 [IQR, 2–6] vs 4 [IQR, 4–5]; P = .389).
Table 2.
Factors Associated With Xpert False Positivity in New and Retreatment Cases
| New TB Patients (n = 461) | Univariate Analysis |
Multivariate Logistic Regression |
||||
|---|---|---|---|---|---|---|
| True-Positive Xpert (n = 421) | False-Positive Xpert (n = 40) | OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | |
| Demographic variables | ||||||
| Age, y, median (IQR) | 33 (27–43) | 32 (25–44) | 1.00 (.97–1.02) | .780 | … | … |
| Female, No. (%) | 187/414 (45) | 25/40 (63) | 2.02 (1.04–3.95) | .039 | … | … |
| Smoker, No. (%) | 125/347 (36) | 13/30 (43) | 1.36 (.64–2.89) | .427 | … | … |
| Clinical variables | ||||||
| HIV-infected, No. (%) | 162/404 (58) | 21/39 (50) | 1.74 (.90–3.37) | .099 | … | … |
| Xpert information | ||||||
| TB-specific CT values, median (IQR) | 21.50 (17.72–26.10) | 28.03 (21.20–32.23) | 1.14 (1.07–1.21) | <.001 | 1.14 (1.08–1.21) | <.001 |
| Retreatment Patients (n = 321) | True-Positive Xpert (n = 276) | False-Positive Xpert (n = 45) | OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
| Demographic variables | ||||||
| Age, y, median (IQR) | 37 (30–45) | 41 (21–48) | 1.03 (1–1.07) | .030 | … | … |
| Female, No. (%) | 108/274 (39) | 15/42 (36) | 1.17 (.60–2.30) | .647 | … | … |
| Smoker, No. (%) | 100/229 (44) | 18/32 (56) | 1.66 (.79–3.50) | .184 | … | … |
| Clinical variables | ||||||
| HIV-infected, No. (%) | 122/273 (45) | 20/43 (47) | 1.08 (.57–2.05) | .823 | … | … |
| Previous TB treatment not completed, No. (%) | 55/239 (23) | 26/39 (26) | 1.15 (.53–2.51) | .719 | … | … |
| Years since previous TB treatment stopped or completed, median (IQR) | 2 (0–5) | 1 (0–1) | 0.92 (.85–.99) | .033 | 0.91 (.84–.99) | .048 |
| Xpert information | ||||||
| TB-specific CT values, median (IQR) | 20.30 (16.71–25.05) | 29.28 (26.18–30.60) | 1.27 (1.18–1.37) | <.001 | 1.25 (1.15–1.35) | <.001 |
Versions of this table for all patients (Supplementary Table 1) or restricted to the subset of patients with chest radiographic data (Supplementary Table 2) are provided in the Supplementary Data.
Abbreviations: CI, confidence interval; CT, cycle threshold; HIV, human immunodeficiency virus; IQR, interquartile range; OR, odds ratio; TB, tuberculosis.
Where CXR data (n = 193) were available, FP patients were less likely to have a CXR compatible with active tuberculosis than TP patients (9/19 [47%] vs 144/174 [83%]; P = .001) and, when included in a multivariable logistic regression model with previous tuberculosis and CT, a CXR compatible with active tuberculosis was associated with a 79% reduction in the relative odds of Xpert false positivity (aOR, 0.21 [95% CI, .08–.57]; Supplementary Table 2).
Receiver Operating Characteristic Curve Analyses
The area under the curve (AUC) for CT (0.70 [95% CI, .61–.80]) did not increase when CXR (0.78 [95% CI, .71–.86]) was included (Figure 2) (receiver operating characteristic [ROC] curves for CT only, and not those who also had CXR data, are shown in Supplementary Figure 1). At a rule-out cut-point (CT > 14.22) for Xpert false positivity (selected based on 95% sensitivity; ie, 95% of the 40 FP Xperts fell above this cut-point), CT alone had a specificity, NPV and negative likelihood ratio (LR) of 6%, 93%, and 0.78, respectively (Table 3). At a rule-in cut-point (selected based on 95% specificity) of >32.19, CT had a sensitivity, PPV, and positive LR of 3%, 33%, and 5.5, respectively. At a cut-point (CT > 27.08) corresponding to Youden index, CT had a sensitivity, specificity, NPV, PPV, positive LR, and negative LR of 55%, 80%, 21%, 95%, 2.79, and 0.56, respectively.
Figure 2.
Receiver operating characteristic curves of Xpert MTB/RIF cycle threshold values and clinical information for the prediction of Xpert MTB/RIF false positivity in new (A) or retreatment (B and C) patients. Abbreviations: AUC, area under the curve; CI, confidence interval; CT, cycle threshold; CXR, chest radiograph; TB, tuberculosis.
Table 3.
Accuracy of Cycle Threshold Values for Predicting Xpert MTB/RIF False Positivity in New and Retreatment Patients
| Test Use | Suggested CT
Cut-point |
Sensitivity, % (95% CI) |
Specificity, % (95% CI) |
PPV, % (95% CI) |
NPV, % (95% CI) |
Positive LR (95% CI) |
Negative LR (95% CI) |
|---|---|---|---|---|---|---|---|
| New patients (n = 461) | |||||||
| Rule-in | >32.19 | 3 (2–4) | 95 (92–97) | 34 (19–.54) | 93 (90–95) | 5.51 (2.87–10.60) | 0.76 (.63–.92) |
| Rule-out | >14.22 | 95 (82–99) | 6 (4–9) | 9 (6–12) | 93 (76–99) | 1.02 (.94–1.09) | 0.78 (.19–3.22) |
| Youden indexa | >27.08 | 55 (39–70) | 80 (76–84) | 21 (14–30) | 95 (92–97) | 2.79 (1.99–3.92) | 0.56 (.40–.79) |
| Retreatment patients (n = 321) | |||||||
| Rule-in | >30.56 | 27 (15–42) | 95 (91–97) | 46 (27–66) | 89 (85–92) | 5.26 (2.60–10.63) | 0.77 (.65–.92) |
| Rule-out | >26.80 | 95 (84–99) | 36 (30–42) | 20 (15–26) | 98 (92–100) | 1.39 (1.33–1.66) | 0.12 (.03–.49) |
| Youden indexa | >28.36 | 64 (49–78) | 90 (86–93) | 52 (38–65) | 94 (90–96) | 6.59 (4.33–10.01) | 0.39 (.27–.58) |
Abbreviations: CI, confidence interval; CT, cycle threshold; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
a Defined as the best compromise between sensitivity and specificity assuming equal weighting [24].
Figure 3.
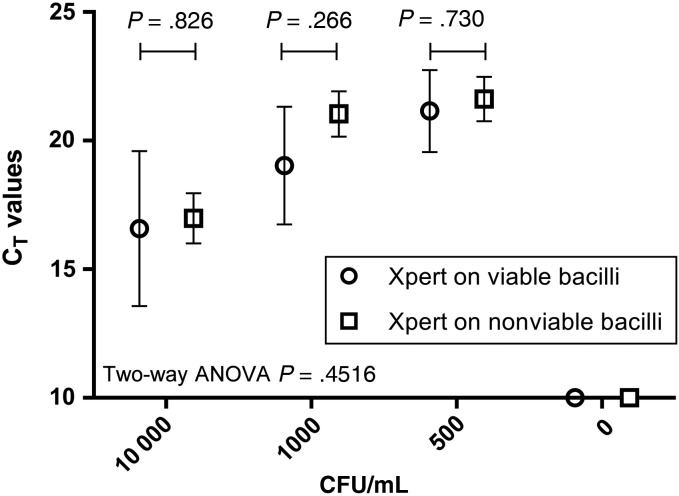
Comparison of Xpert MTB/RIF cycle threshold values (mean ± SEM) from a dilution series of bacilli, showing similar CT when Xpert MTB/RIF was performed on intact bacilli or nonviable, heat- and mechanically-lysed bacilli. Three experimental replicates were performed. Abbreviations: ANOVA, analysis of variance; CFU, colony-forming units; CT, cycle threshold; SEM, standard error of the mean.
Xpert MTB/RIF False Positivity in Retreatment Tuberculosis Patients
Xpert had a sensitivity, specificity, PPV, and NPV of 84% (95% CI, 79%–87%), 94% (95% CI, 93%–96%), 85% (95% CI, 82%–90%), and 94% (95% CI, 92%–95%) in retreatment patients. Forty-five of 321 (14%) Xpert-positive results were false positive (P = .018 compared to new cases).
Correlates of Xpert MTB/RIF False Positivity
Although retreatment patients with an FP Xpert were older than those with a TP Xpert in a univariate analysis (Median [IQR], 41 {21–48} vs 37 {30–45} years; P = .030), after multivariable adjustments were performed, only CT (aOR, 1.25 [95% CI, 1.15–1.35]; P < .001) and the number of years since stopping treatment for the previous episode of tuberculosis (aOR, 0.91 [95% CI, .84–.99]; P = .048) were independent predictors of Xpert FP (Table 2). There was no correlation between CT and years since stopping treatment for previous tuberculosis (P = .427; Supplementary Figure 2). When radiographic data were available, a CXR compatible with active tuberculosis was also an independent predictor of Xpert false positivity (aOR, 0.22 [95% CI, .06–.82]; Supplementary Table 2).
Receiver Operating Characteristic Curve Analyses
ROC curve AUCs of 0.83 (95% CI, .76–.90), 0.83 (95% CI, .75–.91), 0.78 (95% CI, .79–.86), and 0.84 (95% CI, .72–.95) were obtained for CT alone, a model incorporating CT and the number of years since stopping treatment, a model incorporating CT and CXR, and a model incorporating all 3 variables, respectively (Figure 2). CT had, at a cut-point for ruling out Xpert false positivity (selected based on 95% sensitivity; ie, 95% of the 45 FP Xperts in retreatment patients fell above this cut-point), a specificity, NPV, and negative LR of 36%, 98%, and 0.12, respectively (cut-point >18.28), whereas at a rule-in cut-point (selected based on 95% specificity), it had a sensitivity, PPV, and positive LR of 27%, 46%, and 5.26, respectively (cut-point >30.56) (Table 3). At a cut-point (>28.36) corresponding to Youden index, CT had a sensitivity, specificity, NPV, PPV, positive LR, and negative LR of 64%, 90%, 52%, 94%, 6.59, and 0.39, respectively.
Detection of DNA From Nonviable Bacilli
Each dilution of bacilli (10 000, 1000, and 500 CFU mL−1) was detected as positive when Xpert was done directly or on lysate. Similar CTs (SEM) were obtained (direct vs lysed): 16.58 (0.70) vs 16.98 (0.98; P = .826), 19.03 (0.53) vs 21.04 (0.88; P = .266), and 21.15 (0.37) vs 21.62 (0.86; P = .730) for the 10 000, 1000, and 500 CFU mL−1 dilutions, respectively (Figure 3). The 0 CFU mL−1 dilutions were undetected. After 6 weeks of incubation, each aliquot used for direct Xpert grew the expected number of CFUs, whereas no growth was observed from the aliquots of heat inactivated, bead-beaten bacilli.
DISCUSSION
Our key findings are as follows: (1) patients with an FP Xpert are more likely to have previous tuberculosis (and to have had this more recently), low mycobacterial DNA load (measured by CT), and a CXR not compatible with active tuberculosis; (2) about 1 in 7 Xpert-positive results in retreatment patients will be FP; (3) CT predicts false positivity, but has suboptimal discriminatory power (a specificity of 10% at a rule-out cut-point [95% sensitivity], and a sensitivity of 20% at a rule-in cut-point [95% specificity]) that is not enhanced by the incorporation of additional variables; (4) using a cut-point of CT > 30 in retreatment patients, 7 of 10 FP cases will be missed; however, about half of the patients falling above this cut-point will be FP; and (5) Xpert detects DNA from nonviable cells that are not intact, thereby suggesting that free DNA—and not just DNA from intact cells—is detected by Xpert.
Early evaluations of Xpert [25] contributed the majority of data to meta-analyses of test accuracy [26, 27]; however these studies excluded patients who were culture-negative and treated based on symptoms (including many Xpert-positive patients), despite the known poor specificity of empirical treatment [28, 29]. This led to calls that Xpert's specificity might be overestimated, especially in retreatment cases [12, 17]. A reanalysis of the pooled data found that, when these early evaluations were excluded, no significant change in specificity occurred; however, when patients' history of previous tuberculosis was included as a covariate, a trend between an increased prevalence of retreatment cases and diminished specificity existed [9].
The specificity of Xpert in retreatment cases in our study was 95% (95% CI, 93%–96%), indicating that 1 in 20 culture-negative patients will be FP by Xpert. This is less than the specificity reported in meta-analyses that included (99% [95% CI, 98%–99%]) [9] or excluded (98% [95% CI, 97%–99%]) data from the initial Xpert validation studies [25]. Our specificity is also less than that reported previously in retreatment cases in South Africa (99% [95% CI, 98%–100%]) [11], but higher than that seen among retreatment cases in Harare (87% [95% CI, 75%–94%]) [10]. As suggested by others [10, 11], our study indicates that about 1 in 7 Xpert-positive retreatment patients will be FP. In settings such as Cape Town, South Africa, where approximately 1 in 4 tuberculosis notifications are retreatment cases (approximately 7500 per annum) [30], this represents a potentially large public health problem.
We found CT to differentiate poorly between TP or FP Xpert results. For example, at an optimized rule-out cut-point (CT > 26.80; 95% sensitivity) in retreatment patients, only a third of true-positive patients would be correctly classified, and only 1 in 5 FP Xpert results would be correctly classified. Conversely, 70% of FP cases would be missed at an optimized rule-out cut-point (CT > 30.56; 95% specificity), and less than half of the Xpert-positive results with CT above this cut-point would be correctly classified as FP. Although suboptimal for use in routine clinical practice, this result suggests that clinicians should be cautious in interpreting Xpert-positive results in retreatment patients with CT > 30, and that they may wish to await the results of confirmatory culture-based testing before starting treatment. This study, as well as others that have demonstrated CT to be a useful proxy of bacterial load [31–33] and infectiousness [34–36], suggests that laboratories should consider routinely reporting these values.
Our study is the first to describe an inverse association between Xpert FP results and the time since previous treatment was stopped, and the utility of CXR in discriminating Xpert TP from FP patients. Although these tools reduced the odds of an FP result, they did not, unfortunately, improve upon the relatively poor discriminatory ability of CT alone. This is because several TP patients had recently been treated for active tuberculosis (which is reflective of our high transmission setting), or had a CXR not suggestive of active tuberculosis.
As Xpert does not detect DNA from nontuberculous mycobacteria [37], almost all positive results likely reflect the true detection of M. tuberculosis complex DNA [32]; however, this does not always correspond to the presence of active disease caused by viable, intact bacilli. Our research shows that the on-board sample processing system of Xpert is unable to remove genomic DNA from nonintact, nonviable cells, which may be present in retreatment cases. This is likely the mechanism by which Xpert FP occurs, and suggests that Xpert's automated mechanism to isolate intact bacilli prior to DNA extraction requires optimization if false positivity due to the detection of extracellular DNA or DNA in nonintact, nonviable cells is to be minimized. Notably, a study observed Xpert to effectively remove large numbers of amplicons in spiked sputum, preventing detection [37]; however, unlike our study, this earlier work used free DNA of low molecular weight.
Our study has limitations. Other causes of Xpert false positivity include variations in specimen quality and bacterial load in the different samples used for Xpert and culture, and the overlapping stochastic limits of detection of these 2 tests, which can cause false-negative reference standard results (and hence false-positive Xpert results, which may be minimized by repeated cultures). Culture itself is an imperfect reference standard with incomplete sensitivity, although it is used widely in both clinical practice and research. Several factors may underpin this incomplete sensitivity including sampling error, differential immune reactivity in retreatment cases, and technical reasons, among others. Furthermore, we lacked long-term systematic clinical outcome data to incorporate into a reference standard; however, this lacks specificity as empiric overtreatment is frequent in high-burden settings [28, 38, 39], patients without tuberculosis can still improve when on antituberculosis treatment, and patients with tuberculosis and a concomitant infection (eg, Pneumocystis) can still fail to improve. Laboratory error and sample cross-contamination are, as always, potential sources of error; however, Xpert is a closed system that generates few aerosols [40], and we performed Xpert in a quality-assured laboratory separate to that used for culture. Finally, it should also be noted that these findings, which are from a high-burden setting with a high intensity of transmission and where retreatment tuberculosis is relatively common, should undergo further validation, especially in different settings.
In summary, patients with a history of tuberculosis, more recent previous tuberculosis, and a CXR incompatible with active tuberculosis are at a higher risk of Xpert false positivity; however, these do not add discriminatory power over and above CT alone. Although most FP cases would be missed, clinicians should treat CT > 30 in retreatment cases with caution. Further investigation is needed to discriminate NAAT FP patients from TP patients, including research into technologies that exclude DNA from nonintact cells (such as propidium monoazide or ethidium monoazide staining [41, 42]) or detect messenger RNA in live bacilli [43]. This is important as next-generation NAATs, such as Xpert Ultra, will purportedly have a sensitivity approaching that of culture [44], and hence be more likely to detect low quantities of residual tuberculosis DNA and have poor specificity in patients who have previously had tuberculosis.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. The authors are indebted to the patients who participated in this study. We thank Marietjie Pretorius, Ruth Wilson, and Raylene Titus for assistance.
Financial support. This work was supported by a Wellcome Trust Training Fellowship in Public Health and Tropical Medicine (G. T., 099854/Z/12/Z), and the South African Medical Research Council (Career Development Award to G. T. and a Collaborating Centre Award to K. D.).
Author contributions. Conception and design: G. T. and K. D. Collection of data: all authors. Analysis and first draft: G. T. and K. D. Interpretation and important intellectual input: all authors.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Boehme CC, Nabeta P, Hillemann D et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010; 363:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dheda K, Ruhwald M, Theron G, Peter J, Yam WC. Point-of-care diagnosis of tuberculosis: past, present and future. Respirology 2013; 18:217–32. [DOI] [PubMed] [Google Scholar]
- 3.Dheda K, Barry CE 3rd, Maartens G. Tuberculosis. Lancet 2015; doi:10.1016/S0140-6736(15)00151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. FDA permits marketing of first U.S. test labeled for simultaneous detection of tuberculosis bacteria and resistance to the antibiotic rifampin. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm362602.htm Accessed 13 January 2014. [PubMed]
- 5.World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system for the diagnosis of pulmonary and extrapulmonary TB in adults and children. WHO/HTM/TB/2013.14 Geneva, Switzerland: WHO, 2013. [PubMed] [Google Scholar]
- 6.World Health Organization. Tuberculosis diagnostics: Xpert MTB/RIF test roll-out update. Available at: http://www.who.int/tb/publications/Xpert_factsheet.pdf?ua=1 Accessed 17 April 2015.
- 7.World Health Organization. Updated: Xpert MTB/RIF implementation manual. Technical and operational ‘how-to’: practical considerations. WHO/HTM/TB/2014.1 Geneva, Switzerland: WHO, 2014. [PubMed] [Google Scholar]
- 8.Friedrich SO, Rachow A, Saathoff E et al. Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir Med 2013; 1:462–70. [DOI] [PubMed] [Google Scholar]
- 9.Steingart KR, Schiller I, Dendukuri N. In reply to ‘False-positive Xpert(R) MTB/RIF assays in previously treated patients’. Int J Tuberc Lung Dis 2015; 19:366–7. [DOI] [PubMed] [Google Scholar]
- 10.Metcalfe JZ, Makumbirofa S, Makamure B et al. Suboptimal specificity of Xpert MTB/RIF among treatment-experienced patients. Eur Respir J 2015; 45:1504–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorman SE, Chihota VN, Lewis JJ et al. Performance characteristics of the Cepheid Xpert MTB/RIF test in a tuberculosis prevalence survey. PLoS One 2012; 7:e43307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyles T, Hughes J, Cox V, Burton R, Meintjes G, Mendelson M. False-positive Xpert® MTB/RIF assays in previously treated patients: need for caution in interpreting results. Int J Tuberc Lung Dis 2014; 18:876–8. [DOI] [PubMed] [Google Scholar]
- 13.Kelly JD, Grace Lin SY, Barry PM, Keh C, Higashi J, Metcalfe JZ. Xpert MTB/RIF false detection of rifampin-resistant tuberculosis from prior infection. Am J Respir Crit Care Med 2014; 190:1316–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevenson DR, Biswas JR, Lievesley A et al. False-positive Xpert® MTB/RIF more than seven years after cure. Int J Tuberc Lung Dis 2015; 19:1264–5. [DOI] [PubMed] [Google Scholar]
- 15.Cepheid. Xpert MTB/RIF assay product insert. Available at: http://www.cepheid.com/manageddownloads/xpert-mtb-rif-english-package-insert-301-1404-rev-b-february-2015.pdf Accessed 17 December 2015.
- 16.South African Department of Health. National tuberculosis treatment guidelines. Available at: http://www.sahivsoc.org/upload/documents/NTCP_Adult_TB%20Guidelines%2027.5.2014.pdf. Accessed 17 April 2015.
- 17.World Health Organization. Global tuberculosis control 2014. WHO/HTM/TB/2014.08 Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 18.Boyles TH, Hughes J, Cox V, Burton R, Meintjes G, Mendelson M. False-positive Xpert (®) MTB/RIF assays and previous treatment. Int J Tuberc Lung Dis 2015; 19:495. [DOI] [PubMed] [Google Scholar]
- 19.Gous N, Cunningham B, Kana B, Stevens W, Scott L. Performance monitoring of Mycobacterium tuberculosis dried culture spots for use with the GeneXpert system within a national program in South Africa. J Clin Microbiol 2013; 51:4018–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawn SD, Kerkhoff AD, Vogt M, Wood R. High diagnostic yield of tuberculosis from screening urine samples from HIV-infected patients with advanced immunodeficiency using the Xpert MTB/RIF assay. J Acquir Immune Defic Syndr 2012; 60:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shenai S, Amisano D, Ronacher K et al. Exploring alternative biomaterials for diagnosis of pulmonary tuberculosis in HIV-negative patients by use of the GeneXpert MTB/RIF assay. J Clin Microbiol 2013; 51:4161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cepheid. GeneXpert Xpert cartridge, a look inside. Available at: https://www.youtube.com/watch?v=mIsBLmjus6Q Accessed 17 June 2015.
- 23.Wejse C, Gustafson P, Nielsen J et al. TBscore: signs and symptoms from tuberculosis patients in a low-resource setting have predictive value and may be used to assess clinical course. Scand J Infect Dis 2008; 40:111–20. [DOI] [PubMed] [Google Scholar]
- 24.Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3:32–5. [DOI] [PubMed] [Google Scholar]
- 25.Boehme CC, Nicol MP, Nabeta P et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011; 377:1495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steingart K, Sohn H, Schiller I et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. WHO/HTM/TB/2011.4 Geneva, Switzerland: WHO, 2011. [PubMed] [Google Scholar]
- 28.Theron G, Peter J, Dowdy D, Langley I, Squire SB, Dheda K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis 2014; 14:527–32. [DOI] [PubMed] [Google Scholar]
- 29.Walusimbi S, Bwanga F, De Costa A, Haile M, Joloba M, Hoffner S. Meta-analysis to compare the accuracy of GeneXpert, MODS and the WHO 2007 algorithm for diagnosis of smear-negative pulmonary tuberculosis. BMC Infect Dis 2013; 13:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood R, Lawn SD, Caldwell J, Kaplan R, Middelkoop K, Bekker L-G. Burden of new and recurrent tuberculosis in a major South African city stratified by age and HIV-status. PLoS One 2011; 6:e25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theron G, Peter J, Calligaro G et al. Determinants of PCR performance (Xpert MTB/RIF), including bacterial load and inhibition, for TB diagnosis using specimens from different body compartments. Sci Rep 2014; 4: 4:5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theron G, Peter J, van Zyl-Smit R et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med 2011; 184:132–40. [DOI] [PubMed] [Google Scholar]
- 33.Rachow A, Zumla A, Heinrich N et al. Rapid and accurate detection of Mycobacterium tuberculosis in sputum samples by Cepheid Xpert MTB/RIF assay—a clinical validation study. PLoS One 2011; 6:e20458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanrahan CF, Theron G, Bassett J et al. Xpert MTB/RIF as a measure of sputum bacillary burden: variation by HIV status and immunosuppression. Am J Respir Crit Care Med 2014; 189:1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theron G, Pinto L, Peter J et al. The use of an automated quantitative polymerase chain reaction (Xpert MTB/RIF) to predict the sputum smear status of tuberculosis patients. Clin Infect Dis 2012; 54:384–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blakemore R, Nabeta P, Davidow AL et al. A multisite assessment of the quantitative capabilities of the Xpert MTB/RIF assay. Am J Respir Crit Care Med 2011; 184:1076–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blakemore R, Story E, Helb D et al. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol 2010; 48:2495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theron G, Zijenah L, Chanda D et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 2013; 383:424–35. [DOI] [PubMed] [Google Scholar]
- 39.Churchyard GJ, Stevens WS, Mametja LD et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Global Health 2015; 3:e450–7. [DOI] [PubMed] [Google Scholar]
- 40.Banada P, Sivasubramani SK, Blakemore R et al. Containment of bioaerosol infection risk by the Xpert(R) MTB/RIF assay and its applicability to point-of-care settings. J Clin Microbiol 2010; 48:3551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikolayevskyy V, Miotto P, Pimkina E et al. Utility of propidium monoazide viability assay as a biomarker for a tuberculosis disease. Tuberculosis 2015; 95:179–85. [DOI] [PubMed] [Google Scholar]
- 42.Miotto P, Bigoni S, Migliori GB, Matteelli A, Cirillo DM. Early tuberculosis treatment monitoring by Xpert MTB/RIF. Eur Respir J 2012; 39:1269–71. [DOI] [PubMed] [Google Scholar]
- 43.Honeyborne I, McHugh TD, Phillips PPJ et al. Molecular bacterial load assay, a culture-free biomarker for rapid and accurate quantification of sputum Mycobacterium tuberculosis bacillary load during treatment. J Clin Microbiol 2011; 49:3905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alland D, Rowneki M, Smith L et al. Xpert MTB/RIF Ultra: a new near-patient TB test with sensitivity equal to culture [abstract 91]. In: Conference of Retroviruses and Opportunistic Infections, 2015. Available at: http://www.croiconference.org/sessions/xpert-mtbrif-ultra-new-near-patient-tb-test-sensitivity-equal-culture. Accessed 20 December 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.