Efavirenz-based combination antiretroviral therapy (cART) was associated with significantly lower atovaquone plasma concentrations in human immunodeficiency virus-infected subjects vs subjects not receiving cART. The currently recommended dose of atovaquone for mild-moderate Pneumocystis jiroveci pneumonia may not be adequate in patients receiving efavirenz.
Keywords: atovaquone, efavirenz, drug interaction, Pneumocystis jiroveci pneumonia, toxoplasma encephalitis
Abstract
Background. The current study was conducted to determine if efavirenz (EFV) or atazanavir/ritonavir (ATV/r)–based combination antiretroviral therapy (cART) impacted steady-state atovaquone plasma concentrations in human immunodeficiency virus (HIV)–infected patients receiving treatment doses of atovaquone.
Methods. Thirty HIV-infected volunteers were recruited, 10 taking no cART and 10 each taking cART that included EFV or ATV/r. Subjects were randomly assigned to atovaquone 750 mg twice daily (BID) for 14 days followed by atovaquone 1500 mg BID for 14 days, or vice-versa, with a washout period in between. On day 14 of each phase, blood was sampled for pharmacokinetic studies, and the area under the concentration-time curve (AUCτ) and average concentration (Cavg) were calculated and compared using an unpaired t test.
Results. Twenty-nine subjects completed both dosing cohorts. Subjects receiving EFV-based cART had 47% and 44% lower atovaquone AUCτ than subjects not receiving cART at atovaquone doses of 750 mg BID and 1500 mg BID, respectively (P ≤ .01). Only 5 of 10 subjects receiving EFV-based cART plus atovaquone 750 mg BID had an atovaquone Cavg >15 µg/mL, which has previously been associated with successful treatment of Pneumocystis jiroveci pneumonia. AUCτ and Cavg did not significantly differ for concurrent ATV/r for 750 mg BID or 1500 mg BID when compared to the group not receiving cART. Nine of 10 subjects not receiving cART, 8 of 10 subjects receiving ATV/r, and 2 of 10 subjects receiving EFV in combination with atovaquone 750 mg BID achieved an atovaquone Cavg >18.5 µg/mL, a concentration that has previously been associated with successful treatment of Toxoplasma encephalitis (TE).
Conclusions. These data suggest that the currently recommended dose of atovaquone 750 mg BID for treatment of mild to moderate PCP may not be adequate in patients receiving concurrent EFV. Furthermore, doses lower than the currently recommended dose of 1500 mg BID may achieve plasma concentrations adequate to treat TE in HIV-infected patients not receiving EFV.
Clinical Trials Registration. NTC01479361.
Pneumocystis jiroveci pneumonia (PCP) and Toxoplasma encephalitis (TE) are common opportunistic infections among patients with advanced human immunodeficiency virus (HIV) type 1 infection in the United States [1]. Even though trimethoprim-sulfamethoxazole (TMP-SMX) and the combination of pyrimethamine plus sulfadiazine are the preferred treatment regimens for PCP and TE, respectively, adverse effects such as bone marrow suppression and hypersensitivity reactions preclude the use of these agents in some patients [1]. Among alternatives for patients with intolerance to sulfa medications is atovaquone oral suspension [2–4].
Atovaquone is less effective than TMP-SMX in treating PCP; as such, it is used for the treatment of mild to moderate PCP, defined as an alveolar-arterial oxygen diffusion gradient [(A-a)DO2] ≤45 mm Hg, in adults and adolescents >13 years of age [1, 2]. Compared to other agents used as alternatives to TMP-SMX for the treatment and prophylaxis of PCP and/or TE such as pentamidine, dapsone plus pyrimethamine, clindamycin plus primaquine, and sulfadiazine, atovaquone is generally associated with fewer side effects [1, 2].
Atovaquone is an antimicrobial agent with activity against P. jiroveci, Toxoplasma gondii, and Plasmodium species [1, 2]. It is highly lipophilic, highly protein bound, and metabolized via uridine diphosphate glucuronosyltransferase (UGT) enzymes. To date, few drugs have been shown to significantly interact with atovaquone [2]. However, it is possible that drugs that induce UGT activity, such as ritonavir (RTV) and efavirenz (EFV), may reduce atovaquone plasma concentrations, thereby compromising its efficacy [5–10].
A recent investigation compared atovaquone pharmacokinetics following a single dose of the antimalarial combination tablet Malarone (atovaquone 250 mg + proguanil 100 mg) in healthy HIV-uninfected volunteers receiving no antiretroviral therapy to HIV-infected patients receiving combination antiretroviral therapy (cART) containing EFV, lopinavir/ritonavir (LPV/r), or atazanavir/ritonavir (ATV/r). Area under the concentration-time curve (AUC) measurements for atovaquone were 75%, 74%, and 46% lower in patients who received EFV, LPV/r, and ATV/r, respectively, compared to HIV-uninfected volunteers [11]. Lower atovaquone exposure in patients taking these antiretroviral drugs may result in inadequate malaria prevention or treatment. It is unknown whether similar interactions occur when HIV-infected patients receiving these antiretroviral drugs are given atovaquone oral suspension for the treatment of PCP and TE.
Current guidelines recommend atovaquone 750 mg twice daily (BID) for PCP treatment, and 1500 mg BID for the treatment of TE [1]. Successful treatment of PCP and TE is predicted by average steady-state atovaquone plasma concentrations ≥15 µg/mL and ≥18.5 µg/mL, respectively [1–4]. Current product labeling for oral atovaquone suspension (Mepron) and drug interaction guidelines do not recommend adjustments in atovaquone dosing when it is coadministered with cART [1, 2]. To this end, the primary objective of this investigation was to assess the impact of EFV or ATV/r on the steady-state pharmacokinetics of atovaquone oral suspension in HIV-infected volunteers. A secondary objective was to compare the pharmacokinetics of the 2 dosing regimens currently recommended for the treatment of PCP and TE, given the poor and unpredictable absorption of atovaquone [12].
METHODS
Subjects
HIV-infected volunteers between the ages of 18 and 70 years, who were either not on cART or who were on cART containing either EFV plus 2 nucleoside reverse transcriptase inhibitors (NRTIs) or ATV/r plus 2 NRTIs were eligible for participation in this study. Each candidate underwent an evaluation that included medical history, physical examination, assessment of past medication compliance, and laboratory analysis to rule out any medical conditions that could place subjects at risk or potentially affect study results. Specific laboratory tests included a pregnancy test for females of childbearing potential (serum or urine), a basic chemistry panel, a lipid panel, complete blood count with differential, CD4 count, and HIV RNA measurement. HIV-infected participants taking cART were required to be on a stable regimen (≥90 days), be virologically suppressed (HIV RNA <200 copies/mL on at least 2 consecutive occasions, within 6 months prior to enrollment), and have a CD4+ count of ≥200 cells/µL. Participants not receiving cART had to have a CD4+ count ≥350 cells/µL. Concomitant routine therapy with any prescription, over-the-counter, herbal, or holistic medications known or suspected to alter atovaquone pharmacokinetics, including rifampin, rifabutin, and metoclopramide was not allowed during the 14-day period leading up to study participation. Additional exclusion criteria included receipt of, or an indication for, primary or secondary prophylaxis for PCP or TE, cART containing EFV in combination with a protease inhibitor, documented ongoing problems with medication adherence, and a high likelihood of switching cART regimens within 12 weeks of study initiation.
Informed consent was obtained from all study participants, and clinical research was conducted in accordance with guidelines for human experimentation as specified by the US Department of Health and Human Services. The study was approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board.
Study Design
This was a single-center, open-label, parallel-sequence, pharmacokinetic study. Thirty healthy HIV-infected participants were recruited into 3 groups based on their current cART regimen: either no cART (n = 10) or cART that included either ATV/r (300 mg/100 mg [n = 10]) once daily or EFV (600 mg [n = 10]) once daily. All participants received each of 2 atovaquone oral suspension (Mepron, GSK, Research Triangle Park, North Carolina) dosing regimens. All atovaquone doses were taken orally with food. Each subject received detailed counseling on adherence, the importance of taking atovaquone with food, information about potential side effects, and a diary card to record meals, missed doses, and side effects. Subjects brought diary cards and study medication bottles with them to each study visit where they were assessed by study personnel for adherence.
On study day 1, subjects were randomly assigned to receive either atovaquone 750 mg BID for 14 days (phase 1) followed by atovaquone 1500 mg BID for 14 days (phase 2), or vice-versa with a 2- to 6-week washout period between phases. On day 14 of each phase, blood samples were collected immediately before and 0.5, 1, 2, 3, 4, 5, 6, 8, and 12 hours after atovaquone administration. Adherence was assessed by self-report and examination of diary cards on day 14 of each phase. Adverse events were graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (version 1.0).
Analytical Methods
Atovaquone and deschloro atovaquone internal standard were separated and quantified using a newly developed ultraperformance liquid chromatography method.
Calibration curves for atovaquone were linear from 2.0 µg/mL to 120.0 µg/mL with R2 > 0.998. Percentage errors, as a measure of accuracy, were <15%, and the inter- and intra-assay coefficients of variation for atovaquone were 2.86%–6.39% and 4.61%–6.28%, respectively, at 3 different drug concentrations. The limit of quantitation for atovaquone was 0.50 µg/mL and the limit of detection was 0.10 µg/mL.
Pharmacokinetic Analysis
Plasma concentrations of atovaquone were analyzed by noncompartmental methods using Phoenix WinNonlin pharmacokinetic software version 6.4 (Certara, St Louis, Missouri). The maximum plasma concentration (Cmax) was obtained by direct inspection of the plasma concentration-time profiles, and area under the plasma concentration-time curve over the course of a 12-hour dosing interval at steady state (AUCτ) was determined using the log-linear trapezoidal rule. Average concentration (Cavg) was calculated by dividing AUCτ by τ.
Statistical Analysis
A difference in atovaquone (AUC) of at least 40% between the no-cART and cART groups was considered to be a clinically relevant effect size, arbitrarily chosen for the purpose of establishing sample size. A standard deviation of 40 was assumed for a mean of 100 based on previous data [11]. With α = .05, it was determined that a total of 30 subjects (10 per group) would provide >90% power to detect a 40% difference in atovaquone AUCτ with EFV or ATV/r compared with no cART. Results are reported as geometric mean and geometric mean ratio (GMR) and 90% confidence interval (CI). Atovaquone pharmacokinetic parameter values for the EFV and ATV/r arms were compared to the no-cART arm using a 2-tailed, unpaired t test. P < .05 was considered statistically significant for all analyses. SYSTAT software version 11 (Systat, Richmond, California) was used for sample size calculations.
Atovaquone Cavg values from both dosing cohorts in each of the 3 study groups were assessed in relation to those previously found to predict successful treatment of PCP and TE (≥15 µg/mL and ≥18.5 µg /mL, respectively) [3, 4]. Descriptive statistics were used to report these data (Microsoft Excel software version 14.0; Microsoft Corporation, Redmond, Washington).
RESULTS
Subjects
Thirty subjects (83% male; mean age, 42 ± 11 years) enrolled and completed 1 (n = 1) or both (n = 29) phases of the study. The single subject who only completed 1 phase of the study was in the no-cART group; this individual completed the 750-mg atovaquone course but not the 1500-mg course, as described in greater detail below. Fifty-seven percent of subjects were black, 33% white, 3% Asian, and 7% multiracial; 33% of subjects were Hispanic. The median CD4+ cell counts in the EFV, ATV/r, and no-cART groups were 602 (range, 212–1321), 616 (range, 357–916), and 585 (range, 412–912) cells/µL, respectively. HIV RNA was <50 copies/mL in all subjects in the EFV and ATV/r-based cART groups at study enrollment. Median HIV RNA in the no-cART group was 1224 (range, <40–26 743) copies/mL. Thirty-three subjects received at least 1 dose of study medication. Four participants withdrew due to side effects (n = 1), inability to comply with the study requirements (n = 1), or both (n = 2). One individual in the no-cART group completed the 750-mg atovaquone course but not the 1500-mg course due to dysgeusia and diarrhea; data from this subject are included for the 750-mg arm only. Of note, no subjects reported any missed doses of atovaquone throughout the course of the investigation.
Pharmacokinetics
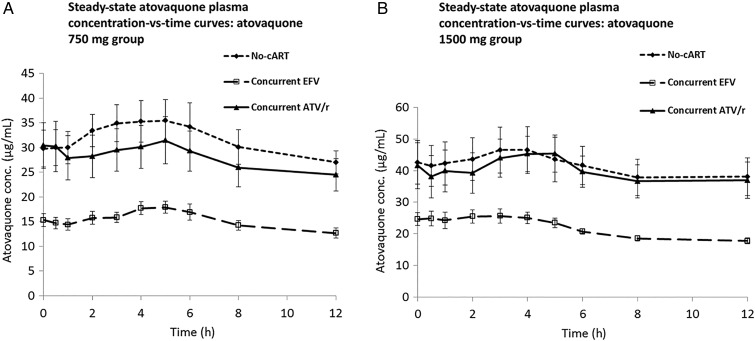
Geometric mean pharmacokinetic parameter values and GMRs for atovaquone are provided in Table 1. Concentration-time profiles for atovaquone at 750 mg BID and 1500 mg BID for all 3 study groups are shown in Figure 1 . In the EFV group, a significantly lower AUCτ was observed for both study doses of atovaquone compared to the no-cART group (750 mg BID: GMR, 0.53 [90% CI, .43–.64]; P = .001) and (1500 mg BID: GMR, 0.56 [90% CI, .43–.73]; P = .01). Conversely, pharmacokinetic parameter values did not significantly differ for the ATV/r group in either atovaquone cohort compared to the no-cART group.
Table 1.
Steady-State Atovaquone Pharmacokinetics in Human Immunodeficiency Virus-Infected Patients Receiving Efavirenz, Atazanavir/Ritonavir, or No Combination Antiretroviral Therapy
| Pharmacokinetics | Concurrent EFV | Concurrent ATV/r | No Concurrent cART | GMR (EFV: No cART) | GMR (ATV/r: No cART) |
|---|---|---|---|---|---|
| Atovaquone 750 mg BID × 14 d | |||||
| AUCτ, µg × h/mL, 90% CI | 178 (157–198) | 306 (231–380) | 364 (296–432) | 0.53 (.43–.64) | 0.9 (.67–1.21) |
| P value vs No cART | .001 | .4 | |||
| Cavg, µg/mL, 90% CI | 14.8 (13.1–16.5) | 25.5 (19.3–31.7) | 30.3 (24.6–36.0) | 0.53 (.43–.64) | 0.9 (.67–1.21) |
| P value vs No cART | .001 | .4 | |||
| Atovaquone 1500 mg BID × 14 d | |||||
| AUCτ, µg × h/mL, 90% CI | 256 (237–274) | 431 (327–535) | 455 (351–558) | 0.56 (.43–.73) | 0.95 (.65–1.38) |
| P value vs No cART | .01 | .8 | |||
| Cavg, µg/mL, 90% CI | 21.3 (19.7–22.9) | 35.9 (27.2–44.6) | 37.9 (29.2–36.5) | 0.56 (.43–.73) | 0.95 (.65–1.38) |
| P value vs No cART | .01 | .8 | |||
| GMR 1500 mg BID vs 750 mg BID | |||||
| AUCτ, µg × h/mL | 1.44 | 1.41 | 1.25 | ||
| Cavg, µg/mL | 1.44 | 1.41 | 1.25 | ||
Abbreviations: ATV/r, atazanavir/ritonavir; AUCτ, area under the plasma concentration-time curve over the course of a 12-hour dosing interval at steady state; BID, twice daily; Cavg, average concentration; cART, combination antiretroviral therapy; CI, confidence interval; EFV, efavirenz; GMR, geometric mean ratio.
Figure 1.
Steady-state atovaquone plasma concentration-time curves in human immunodeficiency virus-infected subjects receiving efavirenz (EFV), atazanavir/ritonavir (ATV/r), or no combination antiretroviral therapy (cART) in combination with atovaquone 750 mg twice daily for 14 days (A) or 1500 mg twice daily for 14 days (B). Solid diamonds and shorter dashed line: no cART; solid triangles and solid line: ATV/r; open squares and longer dashed line: EFV. Error bars represent standard error of the mean.
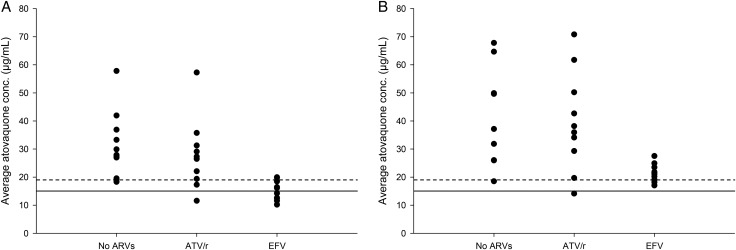
In subjects receiving atovaquone 750 mg BID, atovaquone Cavg was >15 µg/mL in 5 of 10 subjects taking EFV, 9 of 10 subjects taking ATV/r, and 10 of 10 subjects not receiving cART (Figure 2). In subjects receiving this same regimen, Cavg was >18.5 µg/mL in 2 of 10 subjects receiving EFV, 8 of 10 subjects receiving ATV/r, and 9 of 10 subjects not receiving cART (Figure 2). Of note, the 1 subject in the no-cART group who had a Cavg <18.5 µg/mL, had a Cavg of 18.3 µg/mL.
Figure 2.
Average atovaquone plasma concentrations (Cavg) in human immunodeficiency virus-infected patients receiving no antiretroviral therapy (no ARVs), atazanavir/ritonavir (ATV/r), or efavirenz (EFV) in combination with atovaquone 750 mg twice daily for 14 days (A) or 1500 mg twice daily for 14 days (B). Horizontal lines: solid line represents the Cavg previously associated with successful treatment of Pneumocystis jiroveci pneumonia (15 µg/mL); the dashed line represents the Cavg previously associated with successful treatment of toxoplasma encephalitis (18.5 µg/mL).
In subjects receiving atovaquone 1500 mg BID, Cavg was >18.5 µg/mL in 9 of 10 subjects taking EFV, 9 of 10 subjects taking ATV/r, and 9 of 9 subjects not receiving cART (Figure 2). In subjects receiving this same regimen, atovaquone Cavg was >15 µg/mL in subjects from all groups except for 1 individual in the ATV/r group who had a Cavg of 14.1 µg/mL (Figure 2).
Comparison of AUCτ values between the 750-mg BID and 1500-mg BID cohorts (ie, dose proportionality) is reported in Table 1. Comparison of Cavg between the 750-mg BID and 1500-mg BID cohorts is reported in Table 1 and Figure 3. If dose proportional, doubling the atovaquone dose would be expected to produce a doubling of the atovaquone Cavg and AUCτ; however, the increase observed in each group was 25%, 41%, and 44% for the no-cART, EFV, and ATV/r groups, respectively.
Figure 3.
Atovaquone plasma concentration-time curves in human immunodeficiency virus-infected subjects receiving atovaquone 750 mg and 1500 mg twice daily for 14 days without concurrent antiretroviral therapy: dose proportionality comparison under steady-state conditions. Solid diamonds and solid line: atovaquone 750-mg regimen; solid squares and solid line: 1500-mg atovaquone regimen; open triangles and dashed line: the concentration-time curve that would be expected for the 1500-mg atovaquone regimen if it were proportional to the 750-mg atovaquone regimen (ie, plasma concentrations for the 750-mg atovaquone regimen multiplied by 2).
Safety and Tolerability
Overall, atovaquone oral suspension was well tolerated. No serious adverse events were reported. A total of 32 adverse events (90 episodes) were observed, 11 of which were grade 2 in severity and the remainder were grade 1. The most common adverse events were diarrhea (9 episodes) and nausea (8 episodes), as expected, based on known adverse effects of atovaquone.
DISCUSSION
In this study, we found that atovaquone exposure was significantly lower (44%–47%) in HIV-infected subjects receiving an EFV-containing cART regimen compared with those not receiving cART. These results show a similar trend to those reported by Van Luin et al, who found that atovaquone exposure was 75% lower in HIV-infected EFV recipients taking atovaquone 250-mg (plus proguanil 100 mg) tablets, compared to HIV-uninfected volunteers [11]. In contrast to their study, however, we found no significant effect of ATV/r on atovaquone exposure. The results from our investigation are likely more applicable to HIV-infected individuals, as we utilized atovaquone oral suspension (without proguanil) at doses used for the treatment of PCP and TE, and included HIV-infected patients not on cART as a comparator group.
As a result of the altered pharmacokinetics in the EFV group, Cavg values were <15 µg/mL in 50% of participants taking atovaquone 750 mg BID. In a prospective, randomized study using atovaquone for treatment of PCP, average plasma atovaquone concentrations ≥15 µg/mL were associated with a 98% response rate (42/43 patients), whereas concentrations of 10 to <15 µg/mL were associated with a response rate of 79% (30/38 patients) and concentrations <10 µg/mL were associated with a response rate of only 50% (16/32 patients) [4]. Thus, the dose of atovaquone may need to be increased in patients receiving EFV to maximize therapeutic response. Indeed, in the current study, all subjects receiving atovaquone 1500 mg BID in the EFV group achieved Cavg >15 µg/mL (Figure 2).
In the 1500-mg BID dosing group, all but 2 of the 29 subjects (1 each in the ATV/r and EFV groups) achieved Cavg of >18.5 µg/mL. In the 750-mg BID dosing group, Cavg was >18.5 µg/mL in 9 of 10 subjects in the no-cART group and 8 of 10 in the ATV/r group, but only in 2 of 10 in the EFV group. Because this concentration was associated with the best response to TE in a salvage study of atovaquone when used as a single agent [3], these data suggest that the lower dose may be adequate to treat TE in patients not receiving EFV-based cART, or at the very least, that doses lower than the currently recommended 1500 mg BID may be sufficient in such patients. In a randomized study of 1500 mg BID atovaquone given with either sulfadiazine or pyrimethamine for treating toxoplasmosis, 28% of patients discontinued treatment because of intolerance (primarily nausea and vomiting or unacceptable taste), and another 31% temporarily interrupted treatment because of various adverse events [13]. Based on our pharmacokinetic data, dose reduction in patients intolerant of higher atovaquone doses may be considered in patients receiving cART regimens not containing EFV. However, it is important to note that only ATV/r was evaluated in this trial; any atovaquone dose reduction, irrespective of concurrent ARV regimen, should be undertaken with caution.
The mechanism of the apparent interaction between EFV and atovaquone is unknown but may be related to UGT induction of atovaquone metabolism by EFV. However, this is not possible to determine in our study as we did not characterize the terminal elimination of the drug, but instead designed the study to assess steady-state AUCτ and Cavg, the latter of which has been shown to be predictive of clinical efficacy.
In contrast to the influence of EFV on atovaquone pharmacokinetics, ATV/r pharmacokinetic parameter values did not differ significantly from the no-cART group at either of the studied doses. This may have resulted due to the opposing effects of ATV and RTV on UGT activity. ATV has been shown to inhibit a variety of UGT isoforms in vitro and is a notable inhibitor of UGT1A1 in vivo [6, 14, 15]. Conversely, RTV is known to induce a variety of UGT enzymes [5–9]. However, as the specific UGT isoform(s) involved in atovaquone metabolism is (are) unknown, it is difficult to speculate on the exact mechanism behind the lack of a significant interaction between atovaquone and ATV/r in this study.
Atovaquone was previously available in a tablet formulation that had poor bioavailability as a result of slow, irregular, and highly variable absorption [16]. In an effort to improve the bioavailability of atovaquone, an oral suspension was developed [17]. The absolute bioavailability of the oral suspension is twice that of the tablet formulation; however, it is still suboptimal at 46% [16, 17]. Indeed, low bioavailability and saturable absorption likely explain the lack of dose proportionality we observed when doubling the atovaquone dose from 750 mg to 1500 mg. When the atovaquone dose was doubled, instead of a doubling of the atovaquone AUC, increases ranged from 25% to 44% among the 3 study groups. To this end, data from our study further support the need for the development of new atovaquone formulations with improved bioavailability and a more predictable pharmacokinetic profile [12].
Atovaquone (1500 mg once daily) is also used for prophylaxis to prevent the occurrence of PCP and TE. Therapeutic plasma concentrations needed for prophylaxis have not been defined for either of these indications; thus, it is unknown if the interaction between EFV and atovaquone will impact efficacy in this setting. With TMP-SMX, doses that provide effective prophylaxis against PCP and TE are less than those needed to treat these diseases. Therefore, it is likely that atovaquone doses required for effective prophylaxis against PCP and TE will also be less than those needed to treat these opportunistic infections.
In summary, HIV-infected subjects receiving EFV had significantly lower atovaquone exposure compared with individuals not receiving cART. Moreover, EFV recipients receiving atovaquone 750 mg BID were more likely to have atovaquone Cavg values that were <15 µg/mL compared with subjects not receiving cART (5/10 vs 0/10, respectively). In addition, among subjects taking atovaquone 750 mg BID, atovaquone Cavg values >18.5 µg/mL were achieved in 17 of 20 subjects not receiving an EFV-based ARV regimen, suggesting that this dosing regimen may be adequate for the treatment of TE in subjects not receiving EFV-based cART.
As the current dose of atovaquone oral suspension 750 mg BID for mild to moderate PCP treatment may not be adequate for patients receiving concurrent EFV, clinicians may wish to choose a cART regimen that is less likely to interact with atovaquone, such as an ATV/r-based regimen. Other options that may be considered include increasing the atovaquone daily dose or increasing the dosing frequency; however, both of these approaches may lead to problems with adherence, given the poor tolerability of the currently available atovaquone oral suspension. These results further highlight the need for new oral formulations of atovaquone with improved bioavailability and more predictable pharmacokinetic profiles.
Supplementary Material
Notes
Acknowledgments. The investigators express their sincere gratitude to the study participants, who made this research possible.
Financial support. This research was supported by the Intramural Research Program of the National Institutes of Health (NIH) Clinical Center and the National Institute of Allergy and Infectious Diseases, NIH. This project was also funded in part with federal funds from the National Cancer Institute, NIH (contract number HHSN2612008000).
Potential conflicts of interest. J. A. K. and P. K. are collaborating with Matinas Biopharma to develop a nanoformulation of atovaquone. The NIH is in the process of establishing a cooperative research and development agreement with Matinas to support clinical trials of nanoformulated drugs. This study was completed while S. R. P. was employed by the Clinical Research Center, Clinical Center Pharmacy Department, NIH, and while M. M. C. was employed by Leidos Biomedical Research, Inc, NCI-Frederick. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf Accessed 1 February 2016.
- 2.GlaxoSmithKline. Mepron [package insert]. Research Triangle Park, NC: GlaxoSmithKline, 2008. [Google Scholar]
- 3.Torres RA, Weinberg W, Stansell J et al. Atovaquone for salvage treatment and suppression of toxoplasmic encephalitis in patients with AIDS. Clin Infect Dis 1997; 24:422–9. [DOI] [PubMed] [Google Scholar]
- 4.Hughes W, Leoung G, Kramer F et al. Comparison of atovaquone (566C80) with trimethoprim-sulfamethoxazole to treat Pneumocystis carinii pneumonia in patients with AIDS. N Engl J Med 1993; 328:1521–7. [DOI] [PubMed] [Google Scholar]
- 5.van der Lee MJ, Dawood L, ter Hofstede HJ et al. Lopinavir/ritonavir reduces lamotrigine plasma concentrations in healthy subjects. Clin Pharmacol Ther 2006; 80:159–68. [DOI] [PubMed] [Google Scholar]
- 6.Burger DM, Huisman A, Van Ewijk N et al. The effect of atazanavir and atazanavir/ritonavir on UDP-glucuronosyltransferase using lamotrigine as a phenotypic probe. Clin Pharmacol Ther 2008; 84:698–703. [DOI] [PubMed] [Google Scholar]
- 7.Sheehan NL, Brouillette MJ, Delisle MS, Allan J. Possible interaction between lopinavir/ritonavir and valproic acid exacerbates bipolar disorder. Ann Pharmacother 2006; 40:147–50. [DOI] [PubMed] [Google Scholar]
- 8.Abbott Laboratories. Norvir [package insert] North Chicago, IL: Abbott Laboratories, 2010. [Google Scholar]
- 9.Ouellet D, Hsu A, Qian J et al. Effect of ritonavir on the pharmacokinetics of ethinyl oestradiol in healthy female volunteers. Br J Clin Pharmacol 1998; 46:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishna G, Moton A, Ma L, Martinho M, Seiberling M, McLeod J. Effects of oral posaconazole on the pharmacokinetics of atazanavir alone and with ritonavir or with efavirenz in healthy adult volunteers. J Acquir Immune Defic Syndr 2009; 51:437–44. [DOI] [PubMed] [Google Scholar]
- 11.Van Luin M, Van der Ende ME, Richter C et al. Lower atovaquone/proguanil concentrations in patients taking efavirenz, lopinavir/ritonavir or atazanavir/ritonavir. AIDS 2010; 24:1223–6. [DOI] [PubMed] [Google Scholar]
- 12.Borhade V, Pathak S, Sharma S, Patravale V. Formulation and characterization of atovaquone nanosuspension for improved oral delivery in the treatment of malaria. Nanomedicine (Lond) 2014; 9:649–66. [DOI] [PubMed] [Google Scholar]
- 13.Chirgwin K, Hafner R, Leport C et al. Randomized phase II trial of atovaquone with pyrimethamine or sulfadiazine for treatment of toxoplasmic encephalitis in patients with acquired immunodeficiency syndrome: ACTG 237/ANRS 039 study. Clin Infect Dis 2002; 34:1243–50. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, Chando TJ, Everett DW, Patten CJ, Dehal SS, Humphreys WG. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationships of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos 2005; 33:1729–39. [DOI] [PubMed] [Google Scholar]
- 15.Bristol-Myers Squibb. Atazanavir [package insert] Princeton, NJ: Bristol-Myers Squibb, 2010. [Google Scholar]
- 16.Falloon J, Sargent S, Piscitelli S et al. Atovaquone suspension in HIV-infected volunteers: pharmacokinetics, pharmacodynamics, and TMP-SMX interaction study. Pharmacother 1999; 19:1050–6. [DOI] [PubMed] [Google Scholar]
- 17.Dixon R, Pozniak AL, Watt HM, Rolan P, Posner J. Single-dose and steady-state pharmacokinetics of a novel microfluidized suspension of atovaquone in human immunodeficiency virus-seropositive patients. Antimicrob Agents Chemother 1996; 40:556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.