Arterolane is a rapid-acting, synthetic trioxolane with activity against all erythrocytic stages of Plasmodium falciparum. The advantages of arterolane maleate–piperaquine phosphate are once-daily dose, high clinical and parasitological response rates, and rapid parasite clearance.
Keywords: artemisinin combination therapy, arterolane maleate, malaria, fixed-dose combination, once-daily dose
Abstract
Background. Artemisinins, which are derived from plants, are subject to risk of supply interruption due to climatic changes. Consequently, an effort to identify a new synthetic antimalarial was initiated. A fixed-dose combination of arterolane maleate (AM), a new synthetic trioxolane, with piperaquine phosphate (PQP), a long half-life bisquinoline, was evaluated in patients with uncomplicated Plasmodium falciparum malaria.
Methods. In this multicenter, randomized, double-blind, comparative, parallel-group trial, 1072 patients aged 12–65 years with P. falciparum monoinfection received either AM–PQP (714 patients) once daily or artemether–lumefantrine (A–L; 358 patients) twice daily for 3 days. All patients were followed up until day 42.
Results. Of the 714 patients in the AM–PQP group, 638 (89.4%) completed the study; of the 358 patients in the A–L group, 301(84.1%) completed the study. In both groups, the polymerase chain reaction corrected adequate clinical and parasitological response (PCR–corrected ACPR) on day 28 in intent-to-treat (ITT) and per-protocol (PP) populations was 92.86% and 92.46% and 99.25% and 99.07%, respectively. The corresponding figures on day 42 in the ITT and PP populations were 90.48% and 91.34%, respectively. After adjusting for survival ITT, the PCR-corrected ACPR on day 42 was >98% in both groups. The overall incidence of adverse events was comparable.
Conclusions. AM–PQP showed comparable efficacy and safety to A–L in the treatment of uncomplicated P. falciparum malaria in adolescent and adult patients. AM–PQP demonstrated high clinical and parasitological response rates as well as rapid parasite clearance.
Clinical Trials Registration. India. CTRI/2009/091/000101.
Artemisinin combination therapies (ACTs) are recommended by the World Health Organization (WHO) as first-line treatment for uncomplicated Plasmodium falciparum malaria in all endemic regions [1, 2]. Artemisinin derivatives are advocated for use in antimalarial combination therapy because they quickly reduce the level of parasitemia [1]. Early effective treatment of malaria is the cornerstone of malaria control. The clinical effectiveness of the artemisinin derivatives in ACTs is due to their rapid onset of action and activity against all blood stages of the parasite. However, because artemisinins is derived from plants, the are subject to demand and supply problems [1, 3, 4].
Arterolane is a synthetic trioxolane that is an affordable, easy-to-synthesize, and rapid-acting oral antimalarial [5]. Arterolane exhibits a rapid onset of action and potent activity against all erythrocytic stages of P. falciparum and Plasmodium vivax. The tolerability, efficacy, pharmacokinetic profile, and low cost of piperaquine makes it a promising partner drug for use with short-acting and rapid-acting antimalarial agents. The advantages of arterolane maleate–piperaquine phosphate (AM–PQP) are its once-daily dose and low pill burden as well as a long duration of posttreatment prophylaxis.
Our primary aim in this phase 3 study was to assess the efficacy and safety of AM (150 mg)–PQP (750 mg) compared with artemether (A; 20 mg)–lumefantrine (L;120 mg) in adolescent and adult patients with uncomplicated P. falciparum malaria.
METHODS
Study Design
The study was a randomized, multicenter, comparative, double-blind, noninferiority trial conducted in Asia (India, Bangladesh, and Thailand) and Africa (Ivory Coast, Senegal, Mali, Democratic Republic of Congo, Mozambique, and Malawi).
Patients
Male and female patients aged 12–65 years with monoinfection with P. falciparum, asexual parasite densities of 1000–100 000/µL for patients in Asia (areas of low-to-moderate transmission) or 2000–200 000/µL for patients in Africa (areas of high transmission), fever (axillary temperature ≥37.5°C or oral temperature ≥38°C) or a history of fever within the preceding 24 hours, weight ≥35 kg, and hemoglobin ≥8 g/dL were enrolled in the study. Females of childbearing potential were enrolled if they were not lactating and agreed to practice contraception during the study period. All lactating and pregnant females at screening were excluded from the trial.
Randomization and Blinding
Eligible patients were randomly assigned in a 2:1 ratio to either the AM–PQP or A–L group, respectively. A randomization schedule was prepared for each site using SAS software. A double-dummy technique was followed to maintain blinding. An emergency code break scratch card was provided at each site for each participant in order to unblind the treatment in case of emergency.
Treatment
Hospitalized patients received AM–PQP 150 mg–750 mg tablets (714 patients) once daily or A–L 20 mg–120 mg tablets (358 patients) twice daily with food for 3 days. Patients who vomited within 1 hour of dosing were withdrawn and received rescue medication according to the country's Malaria Control Program guidelines. Patients were discharged after completion of 3 days of treatment and, thereafter, were followed up until day 42.
Assessments
Physical examination, vital signs measurement, and clinical assessments were performed during the study. Body temperature (degrees Celsius) was recorded at screening, pre-dose on day 0, and at 6-hour intervals after the first dose of study medication until temperature normalized and it remained normal for 24 hours and on all follow-up days.
Parasitological assessments were performed according to WHO guidelines [6]. Asexual and sexual parasites were counted. Blood was also collected for polymerase chain reaction (PCR) analysis. Genotyping by PCR analysis was conducted at a central laboratory at National Institute of Malaria Research, New Delhi, for those patients whose parasitemia reappeared after initial clearance. Three polymorphic genetic markers, msp1, msp2, and glurp, were used to distinguish recrudescence from new infection. Two local microscopists who were blinded to treatment assignment examined the blood smear slides independently; the mean of the 2 parasite counts was used for efficacy assessments.
Laboratory evaluations for safety (hematology, biochemistry, and urinalysis [dipstick]) were performed during the study. A urine pregnancy test was performed at screening and on days 21 and 42 on all female patients of childbearing potential. A 12-lead electrocardiogram was performed at screening and between 2 and 4 hours after the last dose.
Blood samples were collected from each enrolled patient for pharmacokinetic analysis of arterolane and piperaquine or artemether, dihydroartemisinin, and lumefantrine. The compounds were measured using liquid chromatography tandem mass spectrometry methods validated as per the US Food and Drug Administration Guidance for Industry: Bioanalytical Method Validation, [7], in compliance with good laboratory practice regulations.
Statistical Analyses
Sample size calculation was based on primary study endpoint, that is, PCR–corrected adequate clinical and parasitological response (ACPR) at day 28. Nine hundred evaluable patients were required considering the expected response rate (primary endpoint) of 95%; power, 90%; noninferiority margin, 5%; alpha, 5%; and allocation ratio, 2:1. Two-sided 95% Wilson confidence interval (CI; without continuity corrected) of the difference between the groups was calculated for PCR-corrected and -uncorrected ACPR (cure rate) at day 42. The per-protocol (PP) population was selected for the primary efficacy analysis. A supporting efficacy analysis was done with the intent-to-treat (ITT) population. In addition, survival-adjusted PCR-corrected ACPR in the PP population at day 28 and ITT population at day 42 was estimated using Kaplan–Meier method and compared with log-rank test. Median times to parasite clearance time (PCT) and fever clearance time (FCT) were estimated and compared using survival analysis. Demographic and other baseline characteristics were summarized. Gametocyte prevalence was compared using Fisher exact test. The safety evaluations were performed using summary statistics. All statistical analyses were done using SAS, version 9.1.3, at the 2-sided 5% alpha level.
Ethics
The study was conducted in accordance with Good Clinical Practice, applicable regulatory requirements, and the Declaration of Helsinki. The respective site ethics committees as well as applicable regulatory authorities approved the protocol. Written informed consent was obtained from all participants or their guardians. Assent was obtained from patients aged <18 years.
RESULTS
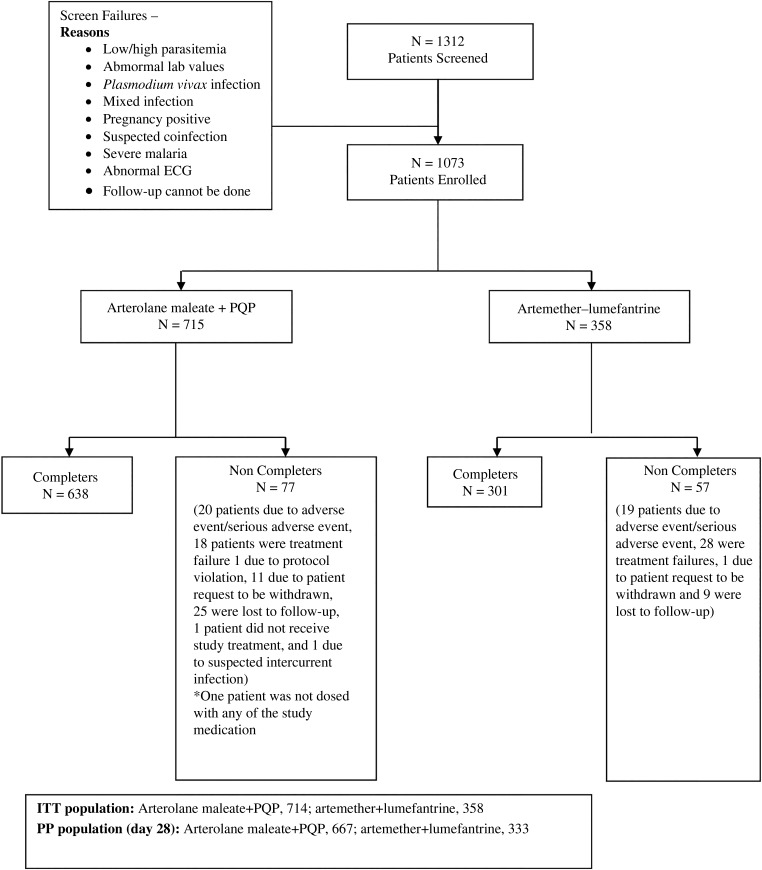
A total of 1073 patients were enrolled, of which 744 were of African origin (Democratic Republic of Congo, 157; Ivory Coast, 330; Malawi, 25; Mali, 32; Mozambique, 110; and Senegal, 90) and 329 were of Asian origin (Bangladesh, 19; India, 202; Thailand, 106; and origin unknown, 2). Of the 1073 patients enrolled, 1072 received at least 1 dose of the study treatment (714 received AM–PQP and 358 received A–L) and were included in the ITT analysis. A total of 667 of 714 patients and 333 of 358 patients were included in PP the population (Figure 1).
Figure 1.
Patient disposition flow chart. Abbreviations: ECG, electrogram; ITT, intent to treat; PP, per protocol; PQP, piperaquine phosphate.
The baseline characteristics were comparable between the 2 treatment groups (Table 1).
Table 1.
Demographic and Baseline Clinical Characteristics of Study Participants
| Variable | Arterolane Maleate + Piperaquine Phosphate N = 715 | Artemether–Lumefantrine N = 358 |
|---|---|---|
| Sex, N (%) | ||
| Male | 427 (59.7) | 206 (57.5) |
| Female | 288 (40.3) | 152 (42.5) |
| Race, N (%) | ||
| African | 495 (69.2) | 249 (69.6) |
| Asiana | 220 (30.8) | 109 (30.4) |
| Age (y) | ||
| N | 715 | 358 |
| Mean ± SD | 26.6 ± 12.74 | 26.5 ± 12.17 |
| Median | 23.0 | 23.0 |
| Minimum, maximum | 12.0, 65.0 | 12.0, 64.3 |
| Weight (kg) | ||
| N | 715 | 358 |
| Mean ± SD | 54.3 ± 12.22 | 54.6 ± 12.27 |
| Median | 53.3 | 53.7 |
| Minimum, maximum | 35.0, 96.8 | 35.0, 112.4 |
| Plasmodium falciparum asexual parasites at screening (per microliter) | ||
| N | 715 | 358 |
| Mean ± SD | 28 463.2 ± 33003.50 | 26 288.6 ± 31 244.81 |
| Median | 16 900.0 | 16 031.0 |
| Minimum, maximum | 631.0, 198 400.0 | 1035.0, 272 470.0 |
| History of fever | ||
| Yes | 708 (99.0) | 353 (98.6) |
| No | 7 (1.0) | 5 (1.4%) |
| Body temperature, oral (°C) | ||
| N | 70 | 36 |
| Mean ± SD | 38.5 ± 0.54 | 38.4 ± 0.43 |
| Median | 38.3 | 38.2 |
| Minimum, maximum | 38.0, 40.2 | 38.0, 39.5 |
| Body temperature, axillary (°C) | ||
| N | 645 | 322 |
| Mean ± SD | 38.1 ± 1.06 | 38.1 ± 1.14 |
| Median | 38.0 | 38.0 |
| Minimum, maximum | 35.0, 42.0 | 35.7, 41.2 |
| P. falciparum gametocytes at screening (per microliter) | ||
| N | 715 | 358 |
| Mean ± SD | 36.8 ± 459.25 | 22.2 ± 280.88 |
| Median | 0.0 | 0.0 |
| Minimum, maximum | 0.0, 10 650.0 | 0.0, 5260.0 |
| Zero count, n (%) | 680 (95.1) | 341 (95.3) |
Abbreviation: SD, standard deviation.
a One Asian patient each was enrolled in the Democratic Republic of Congo and Mozambique.
Efficacy
The primary outcome was the PCR-corrected ACPR rate at day 28 (cure rate). In AM–PQP and A–L groups, the cure rates were 99.25% and 99.07% in the PP population (treatment difference, 1.8%; 95% CI, .99%–1.99%; Table 2) and 92.86% and 92.46 in the ITT population (treatment difference, 0.4%; 95% CI, 2.74%–4.02%; Table 3), respectively. In the ITT population, PCR-corrected ACPR on day 42 was 90.48% and 91.34% in the AM–PQP and A–L groups, respectively. The “survival-adjusted PCR-corrected ACPR” did not differ between treatment groups at day 28 in the PP population (Supplementary Figure 1; log-rank P value = .78) or at day 42 in the ITT population (Supplementary Figure 2; log-rank P value = .88) and it was more than 98% in both treatment groups.
Table 2.
Adequate Clinical and Parasitological Response by Time Point: Per Protocol Population
| Parameter | Arterolane Maleate + Piperaquine Phosphate | Artemether-lumefantrine | Difference Wilson 95% Confidence Interval |
|---|---|---|---|
| Day 28 (N = 1000) | 667 | 333 | |
| PCR uncorrected ACPRa | 661 (99.10%; 98.05%–99.67%) | 320 (96.10%; 93.42%–97.91%) | 0.0300 (.0108, .0571) |
| Total failure$ | 6 (0.90%) | 13 (3.90%) | |
| Late clinical failure | 4 (0.60%) | 5 (1.50%) | |
| Late parasitological failure | 2 (0.30%) | 8 (2.40%) | |
| Day 28 (N = 987) | 664 | 323 | |
| PCR corrected ACPRa | 659 (99.25%; 98.25%–99.76%) | 320 (99.07%; 97.31%–99.81%) | 0.0018 (−.0099, .0199) |
| Total failure | 5 (0.75%) | 3 (0.93%) | |
| Late clinical failure | 4 (0.60%) | 1 (0.31%) | |
| Late parasitological failure | 1 (0.15%) | 2 (0.62%) | |
| $Recrudescence | 5 (0.75%) | 3 (0.93%) | |
| $Indeterminate PCR | 0 | 0 | |
| $Reinfection up to day 28b | 1 (0.15%) | 10 (3.10%) | |
| Day 42 (N = 983) | 655 | 328 | |
| PCR uncorrected ACPRa | 637 (97.25%; 95.69%–98.36%) | 301 (91.77%; 88.25%–94.51%) | 0.0012 (−.0321, .0390) |
| Total failures@ | 18 (2.75%) | 27 (8.23%) | |
| Late clinical failure | 10 (1.53%) | 9 (2.74%) | |
| Late parasitological failure | 8 (1.22%) | 18 (5.49%) | |
| Day 42 (N = 951) | 646 | 305 | |
| PCR corrected ACPRa | 637 (98.61%; 97.37%–99.36%) | 300 (98.36%; 96.22%–99.47%) | 0.0057 (−.0106, .0291) |
| Total failures | 9 (1.39%) | 5 (1.64%) | |
| Late clinical failure | 7 (1.08%) | 1 (0.33%) | |
| Late parasitological failure | 2 (0.31%) | 4 (1.31%) | |
| @Recrudescence | 9 (1.39%) | 5 (1.64%) | |
| @Indeterminate PCR | 2 (0.31%) | 0 | |
| @Reinfection up to day 42b | 7 (1.08%) | 22 (7.21%) |
$ signifies recrudescence, indeterminate PCR and Reinfection up to day 28 and @ signifies recrudescence, indeterminate PCR and Reinfection up to day 42.
Abbreviations: ACPR, adequate clinical and parasitological response; CI, confidence interval; PCR, polymerase chain reaction.
a Data presented includes (n: %, 95% CI). See “Methods” section for definition of outcomes.
b Patients with reinfection were not included in the PCR-corrected ACPR.
Table 3.
Adequate Clinical and Parasitological Response by Time Point: Intention-to-Treat Population
| Variable | Arterolane Maleate + Piperaquine Phosphate | Artemether–Lumefantrine | Difference: Wilson 95% Confidence Interval |
|---|---|---|---|
| Day 28 (N = 1072) | 714 | 358 | |
| PCR uncorrected ACPRa | 662 (92.72%; 90.56%–94.51%) | 321 (89.66%; 86.04%–92.62%) | 0.0305 (−.0043, .0701) |
| Total failure | 52 (7.28%) | 37 (10.34%) | |
| Missing (failure)b | 46 (6.44%) | 24 (6.70%) | |
| Late clinical failure | 4 (0.56%) | 5 (1.40%) | |
| Late parasitological failure | 2 (0.28%) | 8 (2.23%) | |
| Day 28 (N = 1072)$ | 714 | 358 | |
| PCR corrected ACPRa | 663 (92.86%; 90.72%–94.64%) | 331 (92.46%; 89.22%–94.97%) | 0.0040 (−.0274, .0402) |
| Total failure | 51 (7.14%) | 27 (7.54%) | |
| Missing (failure)b | 46 (6.44%) | 24 (6.70%) | |
| Late clinical failure | 4 (0.56%) | 1 (0.28%) | |
| Late parasitological failure | 1 (0.14%) | 2 (0.56%) | |
| $Recrudescence | 5 (0.70%) | 3 (0.84%) | |
| $Indeterminate PCR | 0 | 0 | |
| $Reinfection up to day 28c | 1 (0.14%) | 10 (2.79%) | |
| Day 42 (N = 1072) | 714 | 358 | |
| PCR uncorrected ACPRa | 638 (89.36%; 86.86%–91.52%) | 300 (83.80%; 79.57%–87.46%) | 0.0556 (.0131, .1021) |
| Total failure@ | 76 (10.64%) | 58 (16.20%) | |
| Missing (failure)b | 58 (8.12%) | 30 (8.37%) | |
| Late clinical failure | 10 (1.4%) | 9 (2.51%) | |
| Late parasitological failure | 8 (1.12%) | 19 (5.31%) | |
| Day 42 (N = 1072) | 714 | 358 | |
| PCR corrected ACPRa | 646 (90.48%; 88.08–92.53%) | 327 (91.34%; 87.93%–94.04%) | −0.0086 (−.0431, .0302) |
| Total failure | 68 (9.52%) | 31 (8.66%) | |
| Missing (failure)b | 57 (7.98%) | 4 (1.11%) | |
| Late clinical failure | 7 (0.98%) | 1 (0.28%) | |
| Late parasitological failure | 2 (0.28%) | 4 (1.12%) | |
| @Recrudescence | 9 (1.26%) | 5 (1.4%) | |
| @Indeterminate PCR | 2 (0.28%) | 0 | |
| @Reinfection up to day 42 | 7 (0.98%) | 22 (6.15%) |
$ signifies recrudescence, indeterminate PCR and Reinfection up to day 28 and @ signifies recrudescence, indeterminate PCR and Reinfection up to day 42.
Abbreviations: ACPR, Adequate Clinical and Parasitological Response; CI, confidence interval; PCR, polymerase chain reaction.
a Data presented includes (n: %, 95% CI). See “Methods” section for definition of outcomes.
b Patients with missing data were regarded as having treatment failure.
c Patients with reinfection were not included in the PCR-corrected ACPR.
The rate of reinfection in the AM–PQP and A–L groups was 0.14% and 2.79%, respectively, at day 28 and 0.98% and 6.15%, respectively, at day 42 in the ITT population.
No difference was observed in the median PCT (24 hours) in both treatment groups. Median FCT was 6 hours in the AM–PQP group and 12 hours in the A–L group.
On day 7 and day 14, gametocyte prevalence was significantly higher in the AM–PQP group. However, at day 28 and day 42, the gametocytes were cleared and comparable in both treatment groups (Table 4).
Table 4.
Proportion of Patients Having Zero Gametocyte Counts
| Time | Arterolane Maleate + Piperaquine Phosphate | Artemether–Lumefantrine | P Value (Fisher Exact) |
|---|---|---|---|
| n/N (%) | n/N (%) | ||
| Day 7 | 636/688 (92.4) | 339/349 (97.1) | .0021 |
| Day 14 | 645/679 (95.0) | 338/341 (99.1) | .0005 |
| Day 28 | 661/665 (99.4) | 335/335 (100.0) | .3071 |
| Day 42 | 651/651 (100.0) | 316/317 (99.7) | .3275 |
Safety
The overall incidence of treatment-emergent adverse events (AEs) was comparable in the 2 treatment groups (AM–PQP, 98.2%; A–L, 99.7%). The most commonly observed AEs were headache, abdominal pain, cough, vomiting, nausea, and diarrhea (Table 5). Serious adverse events (SAEs) occurred in 4 patients each in the AM–PQP group (abdominal pain, spontaneous abortion, sepsis, and suspected meningeal syndrome) and the A–L group (Wenkebach's phenomenon on electrocardiogram, pneumonia, cellulitis, and preterm labor). All SAEs were considered “not related” to the study medication except Wenkebach's phenomenon seen in the A–L group, which was considered “probably related” by the investigator. Overall, 2.8% of patients in the AM–PQP group and 5.3% in the A–L group were discontinued from the study. No death was reported.
Table 5.
Clinical Adverse Events
| Preferred Term | Arterolane Maleate + Piperaquine Phosphate N = 714 | Artemether + Lumefantrine N = 358 |
|---|---|---|
| Patients discontinued from the study | ||
| Total number of patient discontinued (%) | 20 (2.8) | 19 (5.3) |
| Vomiting | 11 (1.54) | 4 (1.12) |
| Malaria (Plasmodium vivax, Plasmodium malariae, Plasmodium ovale) | 8 (1.12) | 13 (3.63) |
| Others | 1 (0.14) | 2 (0.56) |
| Number of patients with at least 1 AE | 701 (98.2) | 357 (99.7) |
| Total unique AEs | 4276 | 2213 |
| Total number of AEs | 4620 | 2371 |
| AEs reported (intention-to-treat population; %) | ||
| Anemia | 65 (9.1) | 33 (9.2) |
| Abdominal pain | 48 (6.7) | 32(8.9) |
| Vomiting | 41 (5.7) | 16 (4.5) |
| Headache | 92 (12.9) | 38 (10.6) |
| Cough | 45 (6.3) | 21 (5.9) |
| Diarrhea | 16 (2.2) | 4 (1.1) |
| Nausea | 24 (3.4) | 6 (1.7) |
Abbreviation: AE, adverse event.
An initial decrease in hemoglobin (<12 g/dL) that returned to baseline levels by day 28 in both treatment groups was observed. There were 15.7% of patients in the AM–PQP group and 18.7% in the A–L group who had at least 1 incidence of reduced platelet count during the trial. Severe thrombocytopenia (<50 000/µL) was reported in 5 (0.70%) patients in the AM–PQP group and 3 (0.84%) patients in the A–L group. All cases were resolved without sequelae. There were no differences in laboratory measurements between groups. The mean increases in alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase values were similar in both groups. Most of the increase in hepatic enzymes levels was mild to moderate in severity (Supplementary Table 2).
The mean change in corrected QT (QTc) interval from baseline until day 2 in patients treated with AM–PQP and A–L was recorded as 21.8 msec (95% CI, 19.77–23.91) and 11 msec (95% CI, 8.55–13.35), respectively. In the AM–PQP group, 4 patients had Fridericia's corrected QT interval (QTc(F)) >500 msec on day 2; 49 patients (6.9%) in the AM–PQP group and 6 patients (1.7%) in the A–L group had an increase in QTc(F) >60 msec over baseline on day 2. However, no cardiovascular events were reported in any of those patients.
Pharmacokinetics
The mean maximum (or Peak) plasma concentration (Cmax) of arterolane ranged from 12.66 to 101.28 ng/mL across all sites. It was also observed that the mean exposure of arterolane (AUClast; as estimated based on 3 time points available) in patients ranged from 144.57 to 2693.98 h × ng/mL. The mean Cmax of piperaquine was observed to be consistent across all sites examined (71.19–464.64 ng/mL). Piperaquine, known to be a highly variable drug, showed a mean exposure of 66 843.90 h × ng/mL (Supplementary Table 1).
DISCUSSION
Early effective treatment of malaria is the cornerstone of malaria control. ACTs are the recommended first-line treatment for uncomplicated falciparum malaria. AM, a new synthetic trioxolane, was developed as an alternative to artemisinins. The tolerability, efficacy, pharmacokinetic profile, and low cost of piperaquine makes it a promising partner drug for use with short- and rapid-acting antimalarial agents.
The present study has demonstrated that the efficacy of AM–PQP is comparable to that of A–L in the treatment of uncomplicated P. falciparum malaria in adolescent and adult patients. A 6-dose regimen for Coartem is the standard antimalarial treatment for uncomplicated P. falciparum and mixed infections and has been globally adopted as first-line antimalarial treatment.
Overall, PCR-corrected ACPR at day 28 was >95% in both treatment groups. These results confirm the reported results of a phase 2 study in which 100% cure rates were reported in a PP population [8]. There was no early treatment failure in this study. Since all patients who did not complete the study were considered as failures in statistical analysis, a slightly lesser PCR-corrected ACPR of about 93% at day 28 was observed in the ITT population in both arms. Similar ACPR rates have been reported in Asia and Africa for A–L in various studies [5, 9]. The difference in PCR-corrected and uncorrected evaluations results in varying estimates of antimalarial drug efficacy from which they are derived [10]. Tshefu et al reported a high rate of reinfection with A–L [9], and the same was observed in our study. However, there was a difference in the rate of reinfection between groups at days 28 and 42. The rate of reinfection was lower and time to reinfection was longer in the AM–PQP group than in the A–L group. This suggests that AM–PQP has a longer duration of antimalarial activity and may provide a longer post-treatment prophylactic effect due to the long half-life of PQP. Similar observations in the current trial could be partially attributed to the long elimination half-life of piperaquine, resulting in longer protection for the patient.
The median time to parasite clearance was identical in both treatments. Day 3 parasitemia after treatment with a full dose of ACT is considered a good indicator of sensitivity of P. falciparum to artemisinins and efficacy of ACTs [11]. Like the artemisinin component, arterolane is mostly responsible for the rapid parasite clearance. AM has similar action to clear parasites rapidly and achieve PCT within 3 days. It has been proposed that AM, the ozonide, reacts with Fe2+ in the parasite to produce free radicals, leading to alkylation of key parasitic proteins [12]. It was demonstrated in early-phase studies that arterolane concentrations were less in parasitemic patients than in aparasitemic patients as the drug is more concentrated (approximately 200 times) in parasitized red blood cells than in plasma [13]. Median FCT was 6 hours in the AM–PQP group and 12 hours in the A–L group. In both treatment groups, gametocytes were completely cleared by day 42. The high gametocidal action of AM–PQP may help in the reducing transmission of parasites and in preventing the relapse of malaria.
The pharmacokinetic analysis for AM and PQP in phase 1, 2, and 3 studies demonstrated longer elimination half-life of PQP [14, 15], which corroborates the finding in the previous study [8].
The overall incidences of treatment-emergent AEs were comparable in both treatment arms. The safety profile of AM–PQP was consistent with results obtained in earlier studies in India, Thailand, and Africa [5, 8]. Most AEs were possibly/probably related to the study medication. Also, the incidence of all AEs was similar to incidences reported in patients treated with PQP combinations with dihydroartemisinin in other studies [16–18]. Wenkebach's phenomenon was seen in 1 patient in the A–L group. Though A–L may cause a condition that affects heart rhythm, specifically QT prolongation, the cause of conduction disturbance in this patient could not be ascertained and was categorized as “probably related” to the study medication. Two patients, one with spontaneous abortion and another with preterm labor, were not pregnant at the time of enrollment in the study. The cause of preterm labor in 1 patient on A–L was identified as urinary infection. Safety data from an observational study of approximately 500 pregnant women who were exposed to A–L (including a third exposed during the first trimester) and published data of more than 1000 pregnant patients who were exposed to artemisinin derivatives did not show an increase in adverse pregnancy outcomes or teratogenic effects over the background rate. However, A–L is only recommended for use during pregnancy when the benefit outweighs the risk. One patient on AM–PQP, who became pregnant during the course of the study, had spontaneous abortion in the first trimester. The efficacy of AM–PQP in the treatment of acute uncomplicated malaria in pregnant women has not been established.
Some patients had an increased QT interval after receiving either AM–PQP or A–L. Evaluation of QT interval changes is difficult in malaria because of systematic differences between acute febrile admission before antimalarial drugs are given and early convalescence. At presentation, patients are usually anxious, fasting, and febrile, with increased autonomic tone and a raised heart rate. This contrasts with the relaxed, fed, supine, and afebrile state 3 days later when most antimalarial treatments finish and antimalarial concentrations are at their highest. A reduction in sympathetic activity with recovery leads to a consistent increase in the QT interval, which has been mistakenly ascribed to antimalarial drug effects [19]. The mean increase in QTc interval in patients treated with AM–PQP and A–L is consistent with data reported in the literature in various clinical trials with dihydroartemisinin–PQP and A–L, respectively [5, 19–22].
Initially, the dose of AM was selected as 200 mg based on the results of a phase 2 dose–range finding study on arterolane alone. Subsequently, in the phase 1 study of AM − PQP, the AM exposure almost doubled upon coadministration with PQP (1.57- to 1.97-fold) while the increase in Cmax ranged from 1.88- to 2.27-fold. Based on these results, the final dose of AM in AM − PQP was selected as 150 mg.
It has been reported that the plasma concentrations of piperaquine on day 7 should be >30 ng/mL as it could be an indicator of low probability of recrudescence [23]. The dose of PQP in AM–PQP was selected based on the results of a phase 1 study with PQP. The PQP concentration was maintained at >30 ng/mL for 7 days with PQP 750 mg administered for 3 days. In another phase 1 study with AM − PQP, 2 of 6 patients experienced vomiting at a dose of PQP 1000 mg + AM 200 mg. Hence, the dose of PQP was determined to be 750 mg for combination therapy. These findings were corroborated in the present study wherein the day 7 PQP concentrations were >30 ng/mL.
The advantages of AM–PQP are once-daily dosing and low pill burden as well as a long duration of post-treatment prophylaxis. Availability of this synthetic, rapid-acting medicine would have beneficial impact on patient access, treatment, and adherence.
AM–PQP showed efficacy and safety that were to those of A–L in the treatment of uncomplicated P. falciparum malaria in adolescent and adult patients. AM–PQP demonstrated high clinical and parasitological response rates as well as rapid parasite clearance.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. The authors thank Sanjay K. Sharma, Amit Nasa, Monika Obrah, Sarfaraz Ahmed, Anita Zutshi, Gaurav Kumar Nigam, Kanchan Tyagi, and Sanjukta Bhattacharrya who were in charge of study monitoring; Bina Srivastava, National Institute of Malaria Research (NIMR), who undertook reading of the slides. The authors express their deep gratitude to Dr B. Shahi (deceased), who contributed significantly to the present study. This article bears the NIMR publication screening committee approval no. 02/2014.
The Arterolane maleate - piperaquine phosphate (AM–PQP) study team included the following (in addition to the named authors): Coulibaly M'lanhoro Ange Aristide, Ayame General Hospital, Ayame, Ivory Coast; Landry Tiacoh, North Abobo General Hospital, Abidjan, Ivory Coast; Sonia Enosse, Chókwè Health Research and Training Centre, National Institute of Health, Chokwé, Mozambique; Noppadon Tangpukdee, Mahidol University, Bangkok, Thailand; Jack Kokolomami, Centre hospitalier de Kingasani, Democratic Republic of Congo; Jean-Louis Ndiaye, Service de Parasitologie, Faculté de Médecine, Université Cheikh Anta Diop, Dakar, Senegal; Deepak Rao, Kartuba Medical College, Wenlock District Hospital, Mangalore, India; Ntamabyaliro Nsengi Yumva, Kinshasa School of Public Health, University of Kinshasa, Democratic Republic of the Congo; Bouran Sidibe, Kolle Health Centre, Malaria Research and Training Center, Bamako, Mali; Rajesh Mohanty and AC Jha, Tata Main Hospital, Jamshedpur, India; Mulinda Nyirenda, Queen Elizabeth Central Hospital, Blantyre, Malawi; and Peter Starzengruber and Paul Swoboda, Malaria Research Initiative Bandarban, Bandarban, Bangladesh.
Authors’ Contributions. Literature search: O. A. T., B. S. R., O. G., and V. M.; creation of figures and tables: A. R., A. R. A., O. A. T., A. K. T., R. T., and P. S.; study design: N. V., S. K., B. H. K. R., T. K. B., S. M., H. N., R. K. J., S. A., and N. S.; data collection: O. A. T., A. K. T., R. T., N. V., S. K., O. G., B. H. K. R., I. S., T. K. B., S. M., B. S. R., A. R. A., V. M., H. N., A. R., S. S. I., P. S., N. S., and the AM–PQP study team; data analysis: A. R., S. S. I., N. V., O. A. T., I. S., and B. S. R.; data interpretation: N. V., N. S., and O. A. T.; and manuscript writing: N. V., N. S., A. R. A., A. N., and S. K. S.
Financial support. Sun Pharmaceutical Industries Limited (erstwhile Ranbaxy Laboratories Ltd), with funding from a partial financial grant from the Department of Science and Technology, Government of India, sponsored this trial as part of the clinical development program of AM–PQP.
Potential conflicts of interest. R. K. J., S. A., A. R., N. S., S. S. I., P. S., A. N., S. K. S., M. O., S. A., A. Z., G. K. N., K. T., and S. B. are or were employed in Sun Pharmaceutical Industries Limited (erstwhile Ranbaxy Laboratories Ltd) while developing the product. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the AM–PQP Study Team, Landry Tiacoh, Sonia Enosse, Noppadon Tangpukdee, Jack Kokolomami, Jean-Louis Ndiaye, Deepak Rao, Ntamabyaliro Nsengi Yumva, Bouran Sidibe, Rajesh Mohanty, A.C. Jha, Mulinda Nyirenda, Peter Starzengruber, and Paul Swoboda
References
- 1.WHO. Global Report on Antimalarial Drug Efficacy and Drug Resistance: 2000–2010. Geneva, Switzerland: World Health Organization, 2010. [Google Scholar]
- 2.WHO. Guidelines for the Treatment of Malaria. Geneva, Switzerland: World Health Organization, 2010. [Google Scholar]
- 3.Newman RD. Malaria control beyond 2010. BMJ 2010; 340:c2714. [DOI] [PubMed] [Google Scholar]
- 4.Noorden RV. Demand for malaria drug soars. Nature 2010; 466:672–3. [DOI] [PubMed] [Google Scholar]
- 5.Valecha N, Looareesuwan S, Martensson A et al. Arterolane, a new synthetic trioxolane for treatment of uncomplicated Plasmodium falciparum malaria: a phase II, multicenter, randomized, dose-finding clinical trial. Clin Infect Dis 2010; 51:684–91. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria 2003. Geneva, Switzerland: World Health Organization, 2003. Available at: http://whqlibdoc.who.int/hq/2003/WHO_HTM_RBM_2003.50.pdf. Accessed 9 February 2016. [Google Scholar]
- 7.Guidance for Industry, Bioanalytical Method Validation, U.S. Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER), Centre for Veterinary Medicine (CVM), 2001.
- 8.Valecha N, Krudsood S, Tangpukdee N et al. Arterolane maleate plus piperaquine phosphate for treatment of uncomplicated Plasmodium falciparum malaria: a comparative, multicenter, randomized clinical trial. Clin Infect Dis 2012; 55:663–71. [DOI] [PubMed] [Google Scholar]
- 9.Tshefu AK, Gaye O, Kayentao K et al. Efficacy and safety of a fixed-dose oral combination of pyronaridine-artesunate compared with artemether-lumefantrine in children and adults with uncomplicated Plasmodium falciparum malaria: a randomised non-inferiority trial. Lancet 2010; 375:1457–67. [DOI] [PubMed] [Google Scholar]
- 10.Verret WJ, Dorsey G, Nosten F, Price RN. The effect of varying analytical methods on estimates of anti-malarial clinical efficacy. Malar J 2009; 8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Global Plan for Artemisinin Resistance Containment (GPARC). Geneva, Switzerland: World Health Organization, 2011. Available at: http://www.who.int/malaria/publications/atoz/artemisinin_resistance_containment_2011.pdf. Accessed 9 February 2016. [Google Scholar]
- 12.Uhlemann AC, Wittlin S, Matile H, Bustamante LY, Krishna S. Mechanism of antimalarial action of the synthetic trioxolane RBX11160 (OZ277). Antimicrob Agents Chemother 2007; 51:667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maerki S, Brun R, Charman SA, Dorn A, Matile H, Wittlin S. In vitro assessment of the pharmacodynamic properties and the partitioning of OZ277/RBx-11160 in cultures of Plasmodium falciparum. J Antimicrob Chemother 2006; 58:52–8. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed T, Sharma P, Gautam A et al. Safety, tolerability, and single- and multiple-dose pharmacokinetics of piperaquine phosphate in healthy subjects. J Clin Pharmacol 2008; 48:166–75. [DOI] [PubMed] [Google Scholar]
- 15.Gautam A, Ahmed T, Sharma P et al. Pharmacokinetics and pharmacodynamics of arterolane maleate following multiple oral doses in adult patients with P. falciparum malaria. J Clin Pharmacol 2011; 51:1519–28. [DOI] [PubMed] [Google Scholar]
- 16.Valecha N, Phyo AP, Mayxay M et al. An open-label, randomised study of dihydroartemisinin-piperaquine versus artesunate-mefloquine for falciparum malaria in Asia. PLoS One 2010; 5:e11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwang J, Ashley EA, Karema C et al. Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One 2009; 4:e6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashley EA, McGready R, Hutagalung R et al. A randomized, controlled study of a simple, once-daily regimen of dihydroartemisinin-piperaquine for the treatment of uncomplicated, multidrug-resistant falciparum malaria. Clin Infect Dis 2005; 41:425–32. [DOI] [PubMed] [Google Scholar]
- 19.Mytton OT, Ashley EA, Peto L et al. Electrocardiographic safety evaluation of dihydroartemisinin piperaquine in the treatment of uncomplicated falciparum malaria. Am J Trop Med Hyg 2007; 77:447–50. [PubMed] [Google Scholar]
- 20.Kamya MR, Yeka A, Bukirwa H et al. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of malaria: a randomized trial. PLoS Clin Trials 2007; 2:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karunajeewa H, Lim C, Hung TY et al. Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin) in Cambodian children and adults with malaria. Br J Clin Pharmacol 2004; 57:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart 2003; 89:1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price RN, Hasugian AR, Ratcliff A et al. Clinical and pharmacological determinants of the therapeutic response to dihydroartemisinin-piperaquine for drug-resistant malaria. Antimicrob Agents Chemother 2007; 51:4090–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.