Abstract
Activation of the maternal innate immune system, termed “maternal immune activation” (MIA), represents a common environmental risk factor for schizophrenia. Whereas evidence suggests dysregulation of GABA systems may underlie the pathophysiology of schizophrenia, a role for MIA in alteration of GABAergic systems is less clear. Here, pregnant rats received either the viral mimetic polyriboinosinic-polyribocytidilic acid or vehicle injection on gestational day 14. Glutamic acid decarboxylase-67 (GAD67) mRNA expression was examined in male offspring at postnatal day (P)14, P30 and P60. At P60, GAD67 mRNA was elevated in hippocampus and thalamus and decreased in prefrontal cortex of MIA offspring. MIA-induced alterations in GAD expression could contribute to the pathophysiology of schizophrenia.
Keywords: Schizophrenia, GAD, GABA, Poly I:C, mRNA, Inflammation, MIA
1. Introduction
Schizophrenia is a neurodevelopmental disorder affecting 1% of the population (Knapp et al., 2004). The etiology of schizophrenia requires a combination of genetic and environmental factors acting in concert. Epidemiological evidence demonstrates that maternal bacterial and viral infection during pregnancy is associated with increased risk of schizophrenia (Mednick et al., 1988; Brown, 2006; Clarke et al., 2009).
In animal models, prenatal exposure to maternal immune activation (MIA) is used to recapitulate this effect (Shi et al., 2003). One such model utilizes the synthetic double-stranded RNA polyriboinosinic-polyribocytidilic acid (Poly I:C). Injection of Poly I:C to pregnant dams activates the maternal innate immune response, stimulating pro-inflammatory cytokine systems (Fortier et al., 2004; Smith et al., 2007). This produces cellular, neurochemical, and behavioral alterations in the offspring attributable to the MIA rather than the virus itself (Zuckerman et al., 2003; Shi et al., 2005; Richtand et al., 2012; Missault et al., 2014; Vorhees et al., 2015) and of relevance to schizophrenia (Meyer et al., 2005, 2009; Brown and Derkits, 2010).
MIA offspring exhibit alterations in several neurotransmitter systems of relevance to schizophrenia including dopamine, glutamate, and γ-aminobutyric acid (GABA) systems (Samuelsson et al., 2006; Lanté et al., 2007; Meyer et al., 2008, 2009; Ibi et al., 2009; Bitanihirwe et al., 2010; Escobar et al., 2011; Roenker et al., 2011; Richetto et al., 2014). As the chief inhibitory neurotransmitter in the central nervous system, GABA is found in numerous locations including neocortical regions, the hippocampus and the thalamus. Imbalance between excitatory glutamate and inhibitory GABA function has been implicated in the pathophysiology of schizophrenia (Roberts, 1972; Huguenard and Prince, 1992; Hashimoto et al., 2003; Lewis et al., 2005; Woo et al., 2008; Chiang et al., 2012; Nakazawa et al., 2012). GABAergic systems are also critical in proper brain development and, therefore, a point of convergence and target in the study of schizophrenia (Wassef et al., 2003; Cellot and Cherubini, 2013; Schmidt and Mirnics, 2015). Modulation of GABA transmission in the brain is often monitored via glutamate decarboxylase isoform 67 kDa (GAD67), the rate-limiting enzyme in GABA synthesis. Here, we examined the consequences of MIA via Poly I:C exposure on GAD67 mRNA expression at multiple postnatal time points of the developing rat brain.
2. Methods
2.1 Poly I:C treatment
Female Sprague Dawley rats from Harlan Laboratories (Indianapolis, IN), aged 3–5 months, and males produced within the animal facility were paired for breeding. Animals were housed under standard conditions with access to food and water ad libitum. The Poly I:C treatment protocol was performed as previously described (Bronson et al., 2011; Hemmerle et al., 2015). Briefly, on gestational day 14 pregnant dams were injected with Poly I:C (8 mg/kg i.p.; Sigma, St. Louis, MO) dissolved in saline or with saline vehicle (1 ml/kg). On postnatal day (P) 14, P30 and P60 male offspring were sacrificed and their brains were subsequently processed for in situ hybridization. All experimental procedures were approved by the Institutional Animal Care and Use Committee.
2.2 In situ hybridization
Fresh-frozen brains (n=6/condition) were serially sectioned (at 10-μm thickness) throughout the forebrain using a cryostat, thaw-mounted onto Superfrost plus microslides (VWR, Batavia, IL), and stored at −20°C until hybridization. Semi-adjacent sections were processed for the in situ hybridization localization of GAD67 mRNA using a 35S-labeled cDNA probe, as previously described (Seroogy and Herman, 1997; Hemmerle et al., 2012, 2015; Makinson et al., 2015). Briefly, slides were pretreated, dehydrated and delipidated prior to hybridization. The hybridization probe was prepared from a linearized cDNA plasmid using T3 RNA polymerase and labeled with 35S-UTP (PerkinElmer, Boston, MA). The GAD67 plasmid (a generous gift from Dr. James Herman, University of Cincinnati) was contained in a Bluescript SK vector that consisted of 3086 bases (GenBank Gene ID: 24379). Sections were hybridized overnight, washed, treated with RNase, rinsed, air-dried, and exposed to BioMax MR film (Kodak, Rochester, NY) for 7 days. The films were developed with Kodak GPX developer and fixer.
2.3 Analysis
Analysis of the film autoradiograms was performed by taking densitometry measurements utilizing Scion Image software (NIH) as described previously (Numan et al., 2005; Hemmerle et al., 2012, 2015). At least six sections per area per animal were measured from the following regions: prefrontal cortex (PFC, including prelimbic, infralimbic and anterior cingulate cortex subdivisions), frontal, parietal and piriform cortices, striatum, hippocampus, and thalamus. Brain regions were selected for study based upon their participation in circuitry implicated in schizophrenia abnormalities modeled by MIA (Volk and Lewis, 2013). Boundaries of brain regions analyzed were determined using the Paxinos and Watson rat brain atlas (2007). Background measurements were taken from an unlabeled region of each section and subtracted from each optical density (OD) value to give a corrected OD value. The experimental data are shown as a percentage of the control group. Graph Pad Prism was used to determine group differences via t-test and results were considered significant when p < 0.05.
3. Results
3.1 Increased GAD67 mRNA expression in adult MIA offspring
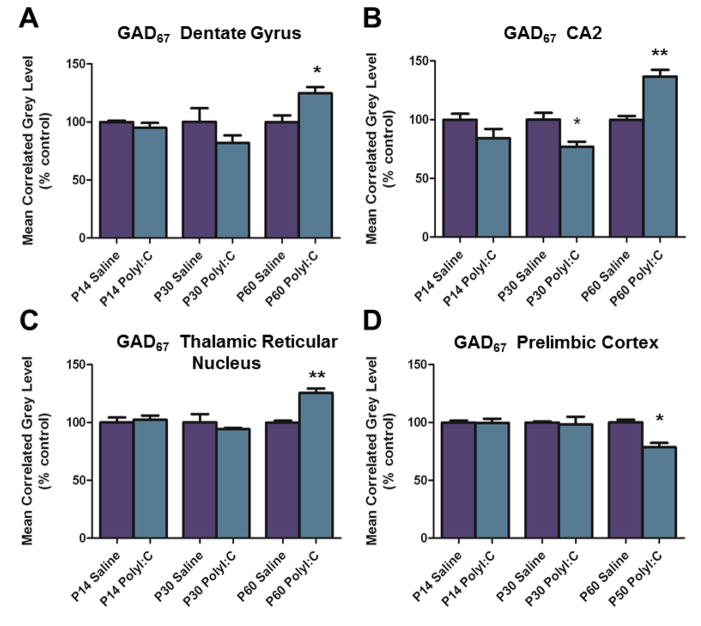
Hybridization for GAD67 mRNA was increased in multiple regions of adult (P60) MIA compared to control offspring, but not in young or adolescent offspring (P14 and P30, respectively). In the hippocampus, levels of GAD67 mRNA were elevated in the granule cell layer of the dentate gyrus (DG) (t(4) = 3.169, p < 0.05) and in region CA2 of the pyramidal cell layer (t(4) = 5.546, p < 0.01) (Figs. 1A–B, 2). A trend towards an increase in expression was detected in hippocampal region CA3 (t(4) = 2.606, p < 0.06). In the thalamic reticular nucleus, GAD67 mRNA levels were also upregulated in MIA compared to controls (t(4) = 0.415, p < 0.01) (Figs. 1C, 2). Increased expression of GAD67 was not seen in any other forebrain region evaluated, including region CA1 of the hippocampus.
Figure 1.
A. Quantification of GAD67 mRNA hybridization signal demonstrates increased levels in the dentate gyrus granule cell layer of Poly I:C (maternal immune activation) animals at P60. B. Quantification of GAD67 mRNA labeling in the CA2 region of the hippocampus revealed decreased expression at P30 and, in contrast, increased expression at P60 in Poly I:C offspring. C. Measurement of GAD67 mRNA expression in the thalamic reticular nucleus revealed an increase in hybridization signal at P60. D. Decreased cRNA-labeling for GAD67 mRNA was found in the prelimbic region of the medial prefrontal cortex of Poly I:C-treated animals at P60. Data are expressed as mean ± SEM. *p < 0.05, ** p < 0.01 compared to respective control values.
Figure 2.
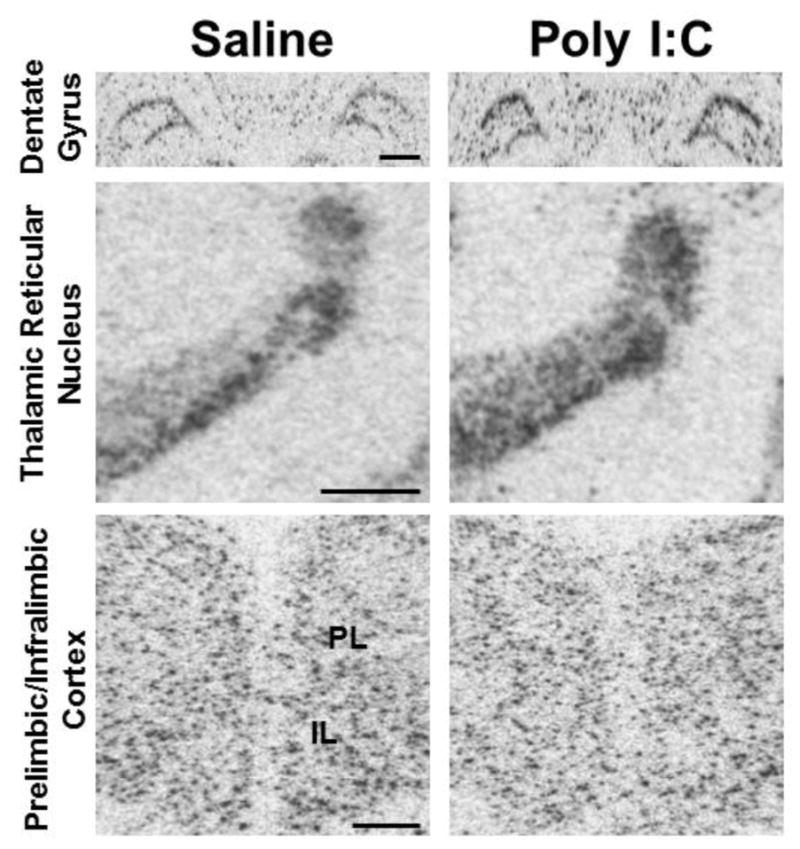
Representative autoradiograms of GAD67 mRNA labeling for several forebrain regions in saline (control) versus Poly I:C (maternal immune activation) conditions at P60. Top row: cRNA-labeling of GAD67 mRNA is increased in the dentate gyrus granule cell layer in Poly I:C animals compared to saline controls. Middle row: Increased hybridization signal for GAD67 mRNA is observed in the thalamic reticular nucleus under Poly I:C conditions. Bottom row: Decreased levels of GAD67 mRNA expression are present in the prelimbic (PL) region of the medial prefrontal cortex in Poly I:C animals. Note that levels in the infralimbic (IL) cortex also appear decreased, but this did not reach significance when quantified. Scale bar in top row and middle row = 1000 μm; scale bar in bottom row = 500 μm.
3.2 Decreased GAD67 mRNA expression in adult MIA offspring
Levels of GAD67 mRNA were decreased (t(4) = 3.156, p < 0.05) in the prelimbic region of the PFC in adult MIA offspring (P60), but again not at the earlier P14 and P30 time points (Figs. 1D, 2). Additionally, the infralimbic region at P60 exhibited a strong trend towards decreased expression (p = 0.0504). In hippocampal region CA2, there was a significant decrease in hybridization for GAD67 mRNA at the P30 time point (t(4) = 3.228, p < 0.05) in MIA offspring. Analysis of other forebrain regions revealed no significant alterations in mRNA expression in the anterior cingulate cortex, in the other cortical regions, or in the striatum.
4. Discussion
The present findings demonstrate differential, long-term alterations in GAD gene expression in multiple forebrain regions in MIA offspring, extending previous studies describing effects of maternal immune activation upon GABAergic systems (see Samuelsson et al., 2006; Meyer et al., 2008, 2009; Oskvig et al., 2012; Richetto et al., 2013, 2014; Tang et al., 2013; Volk and Lewis, 2013). The changes in GAD mRNA levels were not unidirectional; multiple forebrain regions displayed either decreases or increases in expression. Moreover, MIA offspring did not exhibit alterations in GAD67 expression until the later developmental time points, corresponding to the emergence of relevant behavioral alterations in the MIA model (Shi et al., 2003; Patterson, 2009). Though GABA levels were not directly measured, the current results may have important implications, as schizophrenia is a developmental disorder with initial overt symptom manifestation in late adolescence or early adulthood.
In studies of schizophrenia patients, changes in the GABAergic system are consistently found in the prefrontal cortex and hippocampus. Hippocampal abnormalities of the GABA system, such as reductions in parvalbumin-positive interneurons and increased GABA receptor expression, are observed in schizophrenia post-mortem tissue (Benes et al., 1998; Heckers and Konradi, 2010; Coyle et al., 2012). An imbalance between excitatory glutamatergic and inhibitory GABAergic neurotransmission is proposed to underlie hippocampal dysfunction in schizophrenia (Heckers and Konradi, 2010).
Similarly, in MIA models, GABAergic alterations have been consistently observed. These observations are perhaps not surprising given that cortical GABAergic neurons are born at the same time points the developing brains are exposed to MIA (Jakovcevski et al., 2011; Volk and Lewis, 2013). Studies have observed reduced GABAergic content in the hippocampus and PFC (Bitanihirwe et al., 2010), decreased GAD67 in the dorsal hippocampus and PFC (Dickerson et al., 2014; Richetto et al., 2014), reduction of parvalbumin-positive neurons in the PFC, hippocampus and entorhinal cortex (Meyer et al., 2008; Wischhof et al., 2015), and alterations in GABAA receptor subunits in select regions of the limbic system (Nyffeler et al., 2006; Samuelsson et al., 2006; Richetto et al., 2014, 2015). However, these GABAergic alternations are not universal in MIA models, emphasizing how variations in design paradigm can affect outcome (Winter et al., 2009; Jing et al., 2013). The data in these current experiments provide further evidence of the vulnerability of the GABAergic system to developmental disturbances.
Our findings of decreased levels of GAD67 mRNA in the prelimbic cortex of the offspring at P60 are in agreement with previous studies of the PFC in the MIA mouse model (Richetto et al., 2013, 2014; Labouesse et al., 2015). However, here we add regional specificity by localizing the decrease to the prelimbic subdivision of the PFC, as opposed to other PFC subregions. Inhibitory GABA signaling in the prelimbic cortex is believed to play a role in many cognitive functions known to be impaired in neuropsychiatric disorders, including the fear response and emotionality, acute stress response, aversive learning and working memory (Daviss and Lewis, 1995; Joshi et al., 2012; McKlveen et al., 2013; Piantoadosi and Floresco, 2014). Of note, our findings are also similar to observations in schizophrenia post-mortem studies in the PFC (Guidotti et al., 2000; Hashimoto et al., 2003; Lewis et al., 2005; Straub et al., 2007; Thompson Ray et al., 2011; Kimoto et al., 2014).
Consonant with previous findings suggesting decreases or no change in GAD67 mRNA expression in brains of schizophrenia patients and also in relevant animal models (Heckers et al., 2002; Thompson Ray et al., 2011), we found a significant reduction in expression of GAD67 mRNA at P30 in hippocampal region CA2, as well as no alterations of GAD67 levels in any other hippocampal (or extra-hippocampal) region at the earliest two time points (see Fig. 1). A reduction in GAD67 hippocampal mRNA expression was also observed in region CA2/3 of schizophrenia patients using laser-capture microdissection techniques (Benes et al., 2007). Other studies have found that two environmental insults, not simply one, were required to decrease the number of parvalbumin-positive GABA neurons in animals (Giovanoli et al., 2014). However, at the adult P60 time point and in contrast to the schizophrenia findings listed above, we observed increased GAD67 mRNA in the hippocampal formation, specifically in the DG and region CA2. The physiological implications for these increases are unknown. Other studies have found that GABAergic neuronal circuitry is vulnerable during adolescence, with effects persisting into adulthood (Guo et al., 2013).
Interestingly, region CA2 exhibited a transient decrease in GAD expression at the adolescent time point (P30), but a robust increase at the later adult time point (P60). It is possible the increase we observed in GAD67 mRNA is a compensatory response to the loss of inhibitory interneurons as mentioned above. Recent studies suggest that the CA2 region plays a role in social memory (Hitti and Siegelbaum, 2014). Specifically with respect to schizophrenia, imaging studies observed reduced non-pyramidal neurons, as well as decreased parvalbumin neuronal density in the CA2 region of schizophrenic patients (Benes et al., 1998; Knable et al., 2004). The GABA inhibitory neurons in the CA2 region and the hippocampus in general regulate the glutamatergic output of pyramidal neurons (Benes and Berretta, 2001) indicating that GAD67 expression changes could affect hippocampal excitatory output. It is also possible that the developmental alteration in hippocampal GAD67 expression observed in our study reflects a relative imbalance of hippocampal excitatory/inhibitory function. Previous studies have consistently identified altered indices of hippocampal function in the MIA model (Lanté et al., 2007; Lowe et al., 2008; Bitanihirwe et al., 2010; Oh-Nishi et al., 2010; Escobar et al., 2011; Ducharme et al., 2012; Dickerson and Bilkey, 2013; Patrich et al., 2016a,b).
Expression of GAD67 mRNA was also increased at P60 in the thalamic reticular nucleus, a GABAergic region implicated in schizophrenia and a major contributor to sensory gating, attentional processing and other reciprocal interactions between the thalamus and cortex (Ferrarelli and Tonoi, 2011; Pratt and Morris, 2015). Damage to the thalamic reticular nucleus results in atrophy of neurons in the PFC, hippocampus and nucleus accumbens and reduces exploratory behavior (Torres-García et al., 2012), suggesting a role for dysfunction of the thalamic reticular nucleus in alterations observed in the PFC of schizophrenia patients (Pratt and Morris, 2015).
The cellular mechanisms behind these differential long-term alterations in GAD expression in MIA offspring remain to be determined, though they could be related, for example, to neuroinflammatory-induced transcription factor modification, or changes in actual cell number. Determination of GAD67 protein levels in the present model is also necessary to further elucidate how MIA affects GABAergic gene regulation. Future investigations will explore whether the observed differential regulation of GAD/GABA homeostasis in MIA offspring has a role in the behavioral effects observed in MIA models, including fear conditioning and emotionality, as well as in attention and memory deficits (Meyer et al., 2008; Kranjac et al., 2012; Richtand et al., 2012; Vorhees et al., 2015).
Acknowledgments
Role of funding sources
Funding sources had no involvement in study design, in the writing of the report, or in the decision to submit the manuscript for publication.
This work was supported by the Morris Braun Foundation, Selma Schottenstein Harris Lab for Research in Parkinson’s, Gardner Family Center for Parkinson’s Disease and Movement Disorders, National Institute on Drug Abuse (R01 DA016778-01 and R21 DA031876-02), National Institute of Mental Health (R21 MH083192-01) and the Department of Veterans Affairs Medical Research Service. SNC and AMH were supported by National Institutes of Health grants T32 NS007453 and T32 DK059803, respectively.
Footnotes
Contributors
K.B. Seroogy and N.R. Richtand designed the study and methods and supervised the research. S.N. Cassella, A.M. Hemmerle, K.H. Lundgren, T.L. Kyser, R. Ahlbrand and S.L. Bronson carried out the experiments. S.N. Cassella and A.M. Hemmerle analyzed data, conducted the statistical analyses and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest
All authors declare that they have no conflicts of interest in relation to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44(2):88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA. 2007;104(24):10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology. 2010;35(12):2462–2478. doi: 10.1038/npp.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson SL, Ahlbrand R, Horn PS, Kern JR, Richtand NM. Individual differences in maternal response to immune challenge predict offspring behavior: contribution of environmental factors. Behav Brain Res. 2011;220(1):55–64. doi: 10.1016/j.bbr.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32(2):200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167(3):261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellot G, Cherubini E. Functinal role of ambient GABA in refining neuronal circuits early in postnatal development. Front Neural Circuits. 2013;7:136. doi: 10.3389/fncir.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang P, Wu P, Kuo T, Liu Y, Chan C, Chien T, Cheng J, Huang Y, Lien C. GABA is depolarizing in hippocampal dentate granule cells of the adolescent and adult rats. J Neurosci. 2012;32(1):62–67. doi: 10.1523/JNEUROSCI.3393-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166(9):1025–1030. doi: 10.1176/appi.ajp.2009.08010031. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb Exp Pharmacol. 2012;(213):267–295. doi: 10.1007/978-3-642-25758-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviss SR, Lewis DA. Local circuit neurons of the prefrontal cortex in schizophrenia: selective increase in the density of calbindin-immunoreactive neurons. Psychiatry Res. 1995;59(1–2):81–96. doi: 10.1016/0165-1781(95)02720-3. [DOI] [PubMed] [Google Scholar]
- Dickerson DD, Bilkey DK. Aberrant neural synchrony in the maternal immune activation model: using translatable measures to explore targeted interventions. Front Behav Neurosci. 2013;7:217. doi: 10.3389/fnbeh.2013.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson DD, Overeem KA, Wolff AR, Williams JM, Abraham WC, Bilkey DK. Association of aberrant neural synchrony and altered GAD67 expression following exposure to maternal immune activation, a risk factor for schizophrenia. Transl Psychiatry. 2014;4:e418. doi: 10.1038/tp.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme G, Lowe GC, Goutagny R, Williams S. Early alterations in hippocampal circuitry and theta rhythm generation in a mouse model of prenatal infection: implications for schizophrenia. PLoS One. 2012;7:e29754. doi: 10.1371/journal.pone.0029754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar M, Crouzin N, Cavalier M, Quentin J, Roussel J, Lanté F, Batista-Novais AR, Cohen-Solal C, De Jesus Ferreira MC, Guiramand J, Barbanel G, Vignes M. Early, time-dependent disturbances of hippocampal synaptic transmission and plasticity after in utero immune challenge. Biol Psychiatry. 2011;70(10):992–999. doi: 10.1016/j.biopsych.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Tononi G. The thalamic reticular nucleus and schizophrenia. Schizophr Bull. 2011;37(2):306–315. doi: 10.1093/schbul/sbq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2004;287(4):R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- Giovanoli S, Weber L, Meyer U. Single and combined effects of prenatal immune activation and peripubertal stress on parvalbumin and reelin expression in the hippocampal formation. Brain Behav Immun. 2014;40:48–54. doi: 10.1016/j.bbi.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase 67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57(11):1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Guo N, Yoshizaki K, Kimura R, Suto F, Yanagawa Y, Osumi N. A sensitive period for GABAergic interneurons in the dentate gyrus in modulating sensorimotor gating. J Neurosci. 2013;33(15):6691–6704. doi: 10.1523/JNEUROSCI.0032-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk D, Eggan S, Mirnics K, Pierri J, Sun Z, Sampson A, Lewis D. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23(15):6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry. 2002;59(6):521–529. doi: 10.1001/archpsyc.59.6.521. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Curr Top Behav Neruosci. 2010;4:529–553. doi: 10.1007/7854_2010_43. [DOI] [PubMed] [Google Scholar]
- Hemmerle AM, Dickerson JW, Herring NR, Schaefer TL, Vorhees CV, Williams MT, Seroogy KB. (±)3,4-methylenedioxymethamphetamine (“ecstasy”) treatment modulates expression of neurotrophins and their receptors in multiple regions of adult rat brain. J Comp Neurol. 2012;520(11):2459–2474. doi: 10.1002/cne.23048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerle AM, Ahlbrand R, Bronson SL, Lundgren KH, Richtand NM, Seroogy KB. Modulation of schizophrenia-related susceptibility genes in the forebrain of adolescent and adult rats exposed to maternal immune activation. Schizophr Res. 2015;168(1-2):411–420. doi: 10.1016/j.schres.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508(7494):88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. A novel T-type current underlies prolonged Ca(2+)-dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. J Neurosci. 1992;12(10):3804–3817. doi: 10.1523/JNEUROSCI.12-10-03804.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D, Nagai T, Kitahara Y, Mizoguchi H, Koike H, Shiraki A, Takuma K, Kamei H, Noda Y, Nitta A, Nabeshima T, Yoneda Y, Yamada K. Neonatal polyI:C treatment in mice results in schizophrenia-like behavioral and neurochemical abnormalities in adulthood. Neurosci Res. 2009;64(3):297–305. doi: 10.1016/j.neures.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Mayer N, Zecevic N. Multiple origins of human neocortical interneurons are supported by distinct expression of transcription factors. Cereb Cortex. 2011;21:1771–1782. doi: 10.1093/cercor/bhq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Zhang H, Wolff AR, Bilkey DK, Liu P. Altered arginine metabolism in the hippocampus and prefrontal cortex of maternal immune activation rat offspring. Schizophr Res. 2013;148(1–3):151–156. doi: 10.1016/j.schres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Joshi D, Fung SJ, Rothwell A, Weickert CS. Higher gamma-aminobutyric acid neuron density in the white matter of orbital frontal cortex in schizophrenia. Biol Psychiatry. 2012;72(9):725–733. doi: 10.1016/j.biopsych.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Kimoto S, Bazmi H, Lewis DA. Lower expression of glutamic acid decarboxylase 67 in the prefrontal cortex in schizophrnia: contribution of altered regulation by zif268. Am J Psychiatry. 2014;171(9):969–978. doi: 10.1176/appi.ajp.2014.14010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knable KB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psych. 2004;9(6):609–620. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- Knapp M, Mangalore R, Simon J. The global costs of schizophrenia. Schizophr Bull. 2004;30(2):279–293. doi: 10.1093/oxfordjournals.schbul.a007078. [DOI] [PubMed] [Google Scholar]
- Kranjac D, McLinden KA, Koster KM, Kaldenbach DL, Chumley MJ, Boehm GW. Peripheral administration of poly I:C disrupts contextual fear memory consolidation and BDNF expression in mice. Behav Brain Res. 2012;228(2):452–457. doi: 10.1016/j.bbr.2011.12.031. [DOI] [PubMed] [Google Scholar]
- Labouesse MA, Dong E, Grayson D, Guidotti A, Meyer U. Maternal immune activation induces GAD1 and GAD2 promoter remodeling in the offspring prefrontal cortex. Epigenetics. 2015 doi: 10.1080/15592294.2015.1114202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanté F, Meunier J, Guiramand J, Maurice T, Cavalier M, de Jesus Ferreira MC, Aimar R, Cohen-Solal C, Vignes M, Barbanel G. Neurodevelopmental damage after prenatal infection: role of oxidative stress in the fetal brain. Free Radic Biol Med. 2007;42(8):1231–1245. doi: 10.1016/j.freeradbiomed.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lowe GC, Luheshi GN, Williams S. Maternal infection and fever during late gestation are associated with altered synaptic transmission in the hippocampus of juvenile offspring rats. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1563–R1571. doi: 10.1152/ajpregu.90350.2008. [DOI] [PubMed] [Google Scholar]
- Makinson R, Lundgren KH, Seroogy KB, Herman JP. Chronic social subordination stress modulates glutamic acid decarboxylase (GAD) 67 mRNA expression in central stress circuits. Physiol Behav. 2015;146:7–15. doi: 10.1016/j.physbeh.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB, Herman JP. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry. 2013;74(9):672–679. doi: 10.1016/j.biopsych.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45(2):189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29(6):913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22(4):469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35(5):959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missault S, Van den Eynde K, Vanden Berghe W, Fransen E, Weeren A, Timmermans JP, Kumar-Singh S, Dedeurwaerdere S. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav Immun. 2014;42:138–146. doi: 10.1016/j.bbi.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62(3):1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan S, Gall CM, Seroogy KB. Developmental expression of neurotrophins and their receptors in postnatal rat ventral midbrain. J Mol Neurosci. 2005;27(2):245–260. doi: 10.1385/JMN:27:2:245. [DOI] [PubMed] [Google Scholar]
- Nyffeler M, Meyer U, Yee BK, Feldon J, Knuesel I. Maternal immune activation during pregnancy increases limbic GABAA receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience. 2006;143(1):51–62. doi: 10.1016/j.neuroscience.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Oh-Nishi A, Obayashi S, Sugihara I, Minamimoto T, Suhara T. Maternal immune activation by polyriboinosinic-polyribocytidilic acid injection produces synaptic dysfunction but not neuronal loss in the hippocampus of juvenile rat offspring. Brain Res. 2010;1363:170–179. doi: 10.1016/j.brainres.2010.09.054. [DOI] [PubMed] [Google Scholar]
- Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun. 2012;26(4):623–634. doi: 10.1016/j.bbi.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrich E, Piontkewitz Y, Peretz A, Weiner I, Attali B. Maternal immune activation produces neonatal excitability defects in offspring hippocampal neurons from pregnant rats treated with poly I:C. Sci Rep. 2016a;6:19106. doi: 10.1038/srep19106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrich E, Piontkewitz Y, Peretz A, Weiner I, Attali B. Maturation- and sex-sensitive depression of hippocampal excitatory transmission in a rat schizophrenia model. Brain Behav Immun. 2016b;51:240–251. doi: 10.1016/j.bbi.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204(2):313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press; Sydney: 2007. [DOI] [PubMed] [Google Scholar]
- Piantoadosi PT, Floresco SB. Prefrontal cortical GABA transmission discrimination and latent inhibition of conditioned fear: relevance for schizophrenia. Neuropsychopharmacology. 2014;39(10):2473–2484. doi: 10.1038/npp.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt JA, Morris BJ. The thalamic reticular nucleus: a functional hub for thalamocortical network dysfunction in schizophrenia and a target for drug discovery. J Psychopharmacol. 2015;29(2):127–137. doi: 10.1177/0269881114565805. [DOI] [PubMed] [Google Scholar]
- Richetto J, Calabrese F, Meyer U, Riva MA. Prenatal versus postnatal maternal factors in the development of infection-induced working memory impairments in mice. Brain Behav Immun. 2013;33:190–200. doi: 10.1016/j.bbi.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Richetto J, Calabrese F, Riva MA, Meyer U. Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. Schizophr Bull. 2014;40(2):351–361. doi: 10.1093/schbul/sbs195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richetto J, Labouesse MA, Poe MM, Cook JM, Grace AA, Riva MA, Meyer U. Behavioral effects of the benzodiazepine-positive allosteric modulator SH-053–2′F-S-CH(3) in an immune-mediated neurodevelopmental disruption model. Int J Neuropsychopharmacol. 2015;18(4):pyu055. doi: 10.1093/ijnp/pyu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtand NM, Ahlbrand R, Horn PS, Chambers B, Davis J, Benoit S. Effects of prenatal immune activation and peri-adolescent stress on amphetamine-induced conditioned place preference in the rat. Psychopharmacology (Berl) 2012;222(2):313–324. doi: 10.1007/s00213-012-2646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E. Prospects for research on schizophrenia. An hypotheses suggesting that there is a defect in the GABA system in schizophrenia. Neurosci Res Program Bull. 1972;10(4):468–482. [PubMed] [Google Scholar]
- Roenker N, Gudelsky GA, Ahlbrand RL, Bronson SL, Kern JR, Waterman H, Richtand NM. Effect of paliperidone and risperidone on extracellular glutamate in the prefrontal cortex of rats exposed to prenatal immune activation or MK-801. Neurosci Lett. 2011;500(3):167–171. doi: 10.1016/j.neulet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1345–R1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190–206. doi: 10.1038/npp.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seroogy KB, Herman JP. In situ hybridization approaches to the study of the nervous system. In: Turner A, Bachelard H, editors. Neurochemistry - A Practical Approach. Oxford University Press; Oxford: 1997. pp. 121–150. [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23(1):297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int J Dev Neurosci. 2005;23(2–3):299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12(9):854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Tang B, Jia H, Kast RJ, Thomas EA. Epigenetic changes at gene promoters in response to immune activation in utero. Brain Behav Immun. 2013;30:168–175. doi: 10.1016/j.bbi.2013.01.086. [DOI] [PubMed] [Google Scholar]
- Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36(3):195–203. doi: 10.1503/jpn.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-García ME, Solis O, Patricio A, Rodríguez-Moreno A, Camacho-Abrego I, Limón ID, Flores G. Dendritic morphology changes in neurons from the prefrontal cortex, hippocampus and nucleus accumbens in rats after lesion of the thalamic reticular nucleus. Neuroscience. 2012;223:429–438. doi: 10.1016/j.neuroscience.2012.07.042. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Prenatal ontogeny as a susceptibility period for cortical GABA neuron disturbances in schizophrenia. Neuroscience. 2013;248:154–164. doi: 10.1016/j.neuroscience.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Graham DL, Braun AA, Schaefer TL, Skelton MR, Richtand NM, Williams MT. Prenatal immune challenge in rats: Effects of polyinosinic-polycytidylic acid on spatial learning, prepulse inhibition, conditioned fear, and responses to MK-801 and amphetamine. Neurotoxicol Teratol. 2015;47:54–65. doi: 10.1016/j.ntt.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol. 2003;23(6):601–640. doi: 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- Winter C, Djodari-Irani A, Sohr R, Morgenstern R, Feldon J, Juckel G, Meyer U. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int J Neuropsychopharmacol. 2009;12(4):513–524. doi: 10.1017/S1461145708009206. [DOI] [PubMed] [Google Scholar]
- Wischhof L, Irrsack E, Osorio C, Koch M. Prenatal LPS-exposure--a neurodevelopmental rat model of schizophrenia--differentially affects cognitive functions, myelination and parvalbumin expression in male and female offspring. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:17–30. doi: 10.1016/j.pnpbp.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Woo TU, Kim AM, Viscidi E. Disease-specific alterations in glutamatergic neurotransmission on inhibitory interneurons in the prefrontal cortex in schizophrenia. Brain Res. 2008;1218:267–277. doi: 10.1016/j.brainres.2008.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropharmacology. 2003;28(10):1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]