Abstract
In Parkinson's disease, cognitive deficits manifest as fronto-striatally-mediated executive dysfunction, with impaired attention, planning, judgment, and impulse control. We examined changes in executive function in mice lesioned with subchronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) using a 3-choice serial reaction-time (SRT) task, which included measures of sustained attention and impulse control. Each trial of the baseline SRT task comprised a pseudo-random pre-cue period ranging from 3 to 8 s, followed by a 1-s cue duration. MPTP impaired all measures of impulsive behavior acutely, but with additional training their performance normalized to saline control levels. When challenged with shorter cue durations, MPTP-lesioned mice had significantly slower reaction times than wild-type mice. When challenged with longer pre-cue times, the MPTP-lesioned mice exhibited a loss of impulse control at the longer durations. In lesioned mice, striatal dopamine was depleted by 54% and the number of tyrosine-hydroxylase-positive neurons in the substantia nigra pars compacta was reduced by 75%. Serotonin (5-HT) was unchanged in the striatum and prefrontal cortex (PFC), but the ratio of 5-hydroxyindolacetic acid (5-HIAA) to 5-HT was significantly reduced in the MPTP group in the PFC. In lesioned mice, prefrontal 5-HIAA/5-HT was significantly correlated with the executive impairments and striatal norepinephrine was associated with slower reaction times. None of the neurochemical measures was significantly associated with behavior in saline-treated controls. Taken together, these results show that prefrontal 5-HT turnover may play a pivotal role in MPTP-induced executive dysfunction.
Keywords: Parkinson's disease, Executive function, MPTP, Mice, Sustained attention, Impulse control
1. Introduction
Parkinson's disease is characterized neuropathologically by gradual degeneration of dopaminergic neurons in the substantia nigra and behaviorally by bradykinesia, tremor, and postural instability. In addition, approximately half of Parkinson patients exhibit age-related impairment of executive functions—a group of frontostriatally-mediated cognitive processes that includes sustained attention, short-term working memory, set-shifting, impaired decision-making, and loss of impulse control [1–3]. Although we have considerable knowledge about executive function and of the motor symptoms of Parkinson's disease, comparatively little is known about how executive dysfunction arises in Parkinson's disease.
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) has been used extensively to model Parkinson's disease in species as diverse as monkeys, mice, and worms, although with wide variability in sensitivity across and within species [4–9]. Injected systemically, MPTP destroys nigro-striatal dopaminergic neurons rapidly and reliably and induces motor deficits [10–12]. MPTP also recapitulates some of the non-motor symptoms that typically precede motor dysfunction in Parkinson's disease, such as anosmia/hyposmia, gastric slowing, and sleep disturbances [6,13–21]. In humans and monkeys MPTP induces robust executive deficits similar to those seen in Parkinson patients [22–24].
In contrast to the extensive work conducted in monkeys (reviewed in [13]), few studies have specifically examined executive function in rodent models of Parkinson's disease. In one study rats received intra-striatal injections of 6-OHDA and performed an attentional set-shifting task, which involves learning successively complex discriminations within and across sensory modalities (dimensions). Simple and complex discriminations and interdimensional shifts were unaffected by the 6-OHDA lesion, but extradimensional shifting (EDS) was significantly impaired relative to sham controls and unoperated rats [25]. In another study, Bissonette et al. [26] lesioned either the orbitofrontal cortex or medial prefrontal cortex (PFC) of mice using NMDA, and trained them on a set-shifting task. The mPFC, but not orbitofrontal or sham lesions, selectively impaired EDS. The deficits in these two studies are consistent with those in Parkinson's patients and demonstrate that lesion models can re-capitulate executive dysfunction in rodent models. However, despite being the most commonly-used method to induce parkinsonism, it is not known whether MPTP affects any measure of executive function in mice. The purpose of the present study was to determine whether MPTP would affect two aspects of executive function, and whether these processes are associated with the specific neurobiological features of MPTP-induced damage to the striatum or PFC.
2. Materials and methods
2.1. Subjects
Subjects were 40 male wild-type C57BL/6N mice (Charles River), 2 months old at the start of testing. Mice were housed 4–5 per cage in tub cages under standard conditions in an AALAC-approved vivarium, except for the 5 days during and 5 days following the MPTP injection regimen as described below. Mice had free access to food except for the duration of the SRT study. Water was freely available at all times. The week before SRT training began, mice were acclimated to food restriction by removing their food overnight. They had free access to food for 8 h on two consecutive days. This was reduced to 6 h on the next 2 days, and finally to 4 h per day. During the SRT study, mice received food immediately following the session and were allowed to eat undisturbed for 4 h. Under this food restriction regimen, mice tend to lose a little weight at the beginning, but gain it back and eventually weigh more than their free-feeding weights. The vivarium was maintained on a 12:12-h light/dark cycle, with lights on at 6 AM. All experiments were conducted during the light cycle and approved by the Institutional Animal Care and Use Committee.
2.2. Executive function
Mice were trained on a 3-choice serial reaction time (SRT) task that measured sustained attention (hits & reaction time) and impulse control (premature responding), as previously described [27–29]. SRT is particularly well- suited to assess deficits in parkinsonian subjects because it contains controls for motor impairments and slowness of movement (bradykinesia). For example, longer reaction times suggest impaired attention but may also result from bradykinesia. The SRT task includes several temporal response measures not directly related to sustained attention (e.g., latency to retrieve the reinforcer). If slowing occurs in all these measures it is more likely attributable to bradykinesia or motor deficits than inattention. The SRT task contains additional controls for motor impairment. Because it is a choice task, the sensorimotor requirements for correct and incorrect responses are identical. Although this will not eliminate the effects of all motor deficits, having subjects make a choice controls for many of the mild motor impairments mice exhibit following MPTP lesioning. The SRT task also tracks extraneous and adventitious responses (e.g., responding during the intertrial interval). These may not directly affect the measures of sustained attention or impulsivity but are suggestive of abnormal motor behavior.
SRT sessions were conducted in 15 commercially-available operant chambers (Med Associates, Georgia, VT). Mice were initially trained over four daily sessions to respond in one of three randomly-illuminated, equally-spaced nose-poke holes located on the back wall of the chambers, as previously described [30,31]. During response acquisition, the response hole remained illuminated until a response was made. Responses in the illuminated hole resulted in the delivery of a 14-mg food pellet reinforcer (#F05684, Bio-Serv, Flemington, NJ). Responses in non-illuminated response holes had no scheduled consequences. The house light remained lit for 7 s following reinforcer delivery, followed by a 10-s intertrial interval (ITI) in complete darkness and then re-illumination of the house light and another randomly-selected response hole. Four mice did not learn to respond on the response-acquisition task, so SRT data were generated for 18 mice per group. Initial SRT sessions were identical to response-acquisition sessions except that the illuminated response hole was extinguished after 10 s without a response. The cue duration was gradually reduced from 10 s (4 sessions) to 5 s (5 sessions) to 3 s (3 sessions) during training. Transitions from 5-s to 3-s cue sessions, and from 3-s to 1-s cue sessions were made after performance was stable, defined as the average accuracy and reaction times not varying more than 10% from either of the previous two sessions. Mice performed with an average accuracy of 90.1 ± 1.8% and had reaction times of 1.43 ± 0.04 s on the 3-s cue sessions before moving to sessions with a 1-s cue for the remainder of the study.
Daily SRT sessions with a 1-s cue duration consisted of 36 trials with a 10-s ITI. After a pseudo-random pre-cue period of 3–8 s signaled by turning on the house light, the cue was presented. A response in the correct nose-poke hole within 3 s of stimulus onset earned a reinforcer and was classified as a “hit”. A response in one of the other two holes was classified as a “commission error”. Response accuracy was calculated as hits/(hits + commission errors) × 100. Failure to respond on a trial was classified as a “miss” and resulted in immediate darkness and initiation of the ITI. Premature responses in the food well (hopper) or one of the nose-poke holes resulted in an immediate time-out, during which all lights were extinguished. The time-out duration was 4 s at the start of each session and increased in duration by 10% for each premature response. The log of the final time-out value was used as a measure of impulse control. Mice received MPTP or saline after performance reached asymptotic levels using the stability criteria described above. Following MPTP lesions, when responding was stable, mice were challenged in a 60-trial session with cue durations of 0.5, 0.25, or 0.125 s, presented pseudo-randomly. Regardless of the cue duration, mice had 3 s from cue onset in which to earn a reinforcer, just as in sessions with 1-s cues. This challenge session was followed by three additional training sessions with a 1-s cue duration, during which performance was stable using the same criteria as above, and then another 60-trial challenge session during which pre-cue durations were doubled, ranging from 6 to 16 s, presented pseudo-randomly.
2.3. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)
MPTP HCl (#M0896, lot #128K1549, Sigma) was dissolved in physiological (0.9%) saline at a concentration of 2.5 mg/ml free base (2.93 mg/ml salt). Injections were given subcutaneously at a volume of 10 ml/kg to achieve a dose of 25 mg/kg. Mice were given one injection of MPTP or saline per day for 5 consecutive days. Subchronic regimens such as this represent the most common method of modeling Parkinson's disease in mice. While receiving MPTP injections, mice were housed in disposable cages in a dedicated vivarium room under negative pressure; saline-injected mice were housed in their normal vivarium room. Injections were made under a fume hood to further protect personnel from aeration of the toxic MPP+ metabolite from the urine, feces, and bedding. The cages remained under the hood for 5 days after the last injection, when mice were transferred to clean tub cages and returned to the vivarium. MPTP and associated hazardous waste were handled and administered in strict accordance with the safety guidelines outlined by Przedborski et al. [32], and all procedures were approved and monitored by the Institutional Biosafety Committee. All behavioral procedures were conducted after the MPTP injections and the 5-day post-MPTP quarantine period.
All mice were sacrificed by cervical dislocation without anesthesia in the morning of the third or fourth day following the last SRT session, 5 weeks after the last MPTP injection. Brains were split at the midline and the left hemisphere dissected rapidly using a brain matrix. Punches (1-mm diam.) were taken from 1-mm thick coronal sections from the dorsolateral striatum and PFC, and flash-frozen in liquid nitrogen. Right hemispheres were fixed overnight in 4% paraformaldehyde in 0.1 M PBS (pH 7.4) and cryo-protected.
2.4. HPLC with electrochemical detection
Frozen striatal and prefrontal punches from 32 subjects (16 per group) were suspended in 400 μl aCSF and 600 μl 0.2 mM perchloric acid, homogenized for 30 s, and then centrifuged at 10,000 RPM for 15 min at 4 °C. The supernatant was filtered through a nylon syringe filter (0.2 μm) and frozen at −80 °C. Frozen samples (20 μl; 1:1 dilution with polished HPLC grade water) were automatically injected by an ESA 542 refrigerated autosampler (ESA, Inc.) onto a 150 ± 2 mm ODS C18 column connected to an ESA model 580HPLC pump. The mobile phase, containing 80 mM sodium dihydrogen phosphate monohydrate, 2.0 mM 1-octanesulfonic acid sodium salt, 100 μl/l triethylamine, 5 nM EDTA, and 10% acetonitrile, pH 3.0, was perfused at 0.25 ml/min. Dopamine levels were determined using an ESA 5041 high-sensitivity analytical cell, an ESA 5020 guard cell and an ESA Coulochem II 5200A electrochemical detector at a potential of 220 mV with the current gain at 10 nA and the guard cell at +350 mV. Under these conditions, the limit of detection for dopamine is 100 fg per injection. Dopamine (DA), norepinephrine (NE), and serotonin (5-HT) were measured, along with the dopamine metabolites 3, 4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 3-methoxytyramine (3-MT), and the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA). DOPAC/DA, HVA/DA, and 3-MT/DA were used as estimates of dopamine turnover, and 5-HIAA/5-HT as an estimate of serotonin turnover. DA/NE was used as an estimate of dopamine β-hydroxylase (DBH) activity, with lower ratios indicating greater DBH activity.
2.5. Histology and immunohistochemistry
Coronal sections (40 μm) were taken with a cryostat through the extent of the substantia nigra and striatum for histological analysis. Free-floating sections were treated first with phosphate buffered saline (PBS) containing 20% methanol and 3% hydrogen peroxide for 30 min., and rinsed thoroughly in PBS. Sections were then incubated in 10% normal horse serum in PBS containing 0.1% Triton X-100 for 30 min. and incubated overnight at 4 °C in the primary antibody (dilution 1:200) targeting tyrosine hydroxylase (TH; #AB152, Millipore). Following incubation sections were rinsed three times in PBS, incubated for an hour in a biotinylated anti-rabbit IgG (Vector Labs), washed thrice, and treated with avidin–biotin complex (Vectastain Elite ABC kit, Vector Labs) reagent for an hour and visualized using diaminobenzidine. Sections were then mounted on slides and air-dried. Optical density of TH-positive fibers in the dorsolateral striatum were quantified from 6 to 8 greyscale sections from 13 subjects (7 saline, 6 MPTP) using the public domain software ImageJ (http://rsb.info.nih.gov/ij).
2.6. Stereological quantification of cells in SNc
For unbiased stereological quantification of TH-positive cells in 8–10 sections per mouse, every fourth section through the rostro-caudal extent of the SNc was measured using the optical fractionator counting method and Stereo Investigator software (MicroBrightField). The contour of the SNc was first delineated using Stereo Investigator's anatomical mapping tool at low power. TH-positive cells within the SNc were counted in from 10 to 20 frames measuring 25 × 25 μm in each of the selected sections, generated using Stereo Investigator's random sampling grid. The frames were selected using a systematic random sampling scheme, which provides an unbiased and efficient sampling technique. In every counting-frame location, the top of the section was identified, after which the plane of the focus was moved 4 μm deeper through the section (guard zone) to prevent counting inaccuracies due to uneven section surfaces. The resulting focal plane served as the first point of the counting process. All TH-positive cells that came into focus in the next 8-μm segment (dissector height) were counted if they were entirely within the counting frame or touching the upper or right side of the counting frame. Based on the these parameters and counts, the total number of TH-positive cells per selected region were counted using the optical fractionator formula N = 1/ssf × 1/asf × 1/hsf × Q, where ssf is the section sampling fraction, asf is the area sampling fraction, hsf is the height sampling fraction (dissector height divided by the section thickness after shrinkage), and Q denotes the total count of particles sampled for each region. After counting TH+ neurons, slides were counter-stained with cresyl violet (CV), and all SNc neurons positive for CV but negative for TH were counted stereologically, as previously described [12,33].
2.7. Statistical analysis
Most behavioral, histological, and neurochemical data were analyzed using 2-way analysis of variance (ANOVA) or repeated-measures ANOVA (RMANOVA), with lesion condition (saline or MPTP) as a between-subjects variable. Time-series data were analyzed using hierarchical linear modeling, with subject as a nominal random factor nested within lesion condition, and time as a balanced, continuous repeated measure. Degrees of freedom on repeated-measures analyses were corrected for variations in sphericity using Huyn-Feldt ε. HPLC data for striatal and PFC norepinephrine were significantly skewed (>8.7) and kurtotic (>3.0) and were reciprocal-transformed. The PFC values for dopamine and HVA were significantly kurtotic (>2.0) and were square-root transformed because reciprocal transformation made them more kurtotic. No other HPLC values were significantly skewed or kurtotic. Among the behavioral measures, only the distribution of hopper premature responses in the 14–16 s range was non-normal, and was square-root transformed because some subjects had zero values. Test statistics for skewness and kurtosis were calculated as skewness and excess kurtosis divided by their respective standard errors. Critical values for both tests statistics is 2.0 [34]. Conceptually-similar measures were analyzed first using multivariate ANOVA (MANOVA) followed by individual tests when appropriate. Follow-up comparisons were made using orthogonal paired t-tests. To further protect against spurious Type I errors, follow-up analyses were conducted only after a significant omnibus effect, except for comparisons having specific a priori hypotheses. All statistical tests were two-tailed with α = .05.
3. Results
Mice were trained to stable baseline performance on the SRT task, at which time MPTP or saline was administered. Baseline performance before and after injections were compared to determine the short-term effects of MPTP intoxication. There were no significant main effects of lesion, but there were significant Lesion × Time (pre-post MPTP) interactions on measures associated with impulse control. There were no main or interaction effects on hits, reaction time, hopper latency, or commission errors [F's < 2.7, p's > .111]. Follow-up analyses show that there were no differences between the two groups before MPTP treatment [t's < 0.7, p's > .429], but significant group differences emerged following MPTP (Table 1). Specifically, MPTP-lesioned mice exhibited a loss of impulse control compared with saline-treated mice, as measured by significantly increased hopper errors, premature responses, and a longer final time-out value. However, performance improved with extended training and by the time of the challenge sessions the MPTP-lesioned mice were performing as well as saline control mice.
Table 1.
Baseline behavioral measures before and after MPTP injections.
| Pre-lesion |
Post-lesion |
|||
|---|---|---|---|---|
| Measure | Saline | MPTP | Saline | MPTP |
| Accuracy (%) | 83.54 ± 2.27 | 84.33 ± 2.52 | 88.54 ± 2.67 | 85.01 ± 2.31 |
| Hopper errors (%) | 5.82 ± 0.82 | 6.92 ± 0.84 | 8.83 ± 0.96 | 11.64 ± 1.21* |
| Hit reaction time (s) | 0.77 ± 0.03 | 0.76 ± 0.03 | 0.76 ± 0.02 | 0.81 ± 0.02 |
| Comm. error reaction time (s) | 1.24 ± 0.09 | 1.36 ± 0.12 | 1.22 ± 0.10 | 1.38 ± 0.08 |
| Hopper latency (s) | 1.90 ± 0.08 | 1.93 ± 0.10 | 2.08 ± 0.10 | 2.14 ± 0.07 |
| ITI responses | 2.16 ± 0.52 | 2.50 ± 0.62 | 2.23 ± 0.34 | 4.24 ± 0.92 |
| Total premature responses | 11.94 ± 1.89 | 11.27 ± 1.77 | 10.57 ± 1.05 | 18.97 ± 1.84*** |
| Cue premature responses | 8.44 ± 1.54 | 7.28 ± 1.48 | 5.46 ± 0.82 | 11.21 ± 1.46** |
| Hopper premature responses | 3.51 ± 0.49 | 4.03 ± 0.47 | 5.13 ± 0.66 | 7.79 ± 1.20* |
| Final timeout value [log(s)] | 0.76 ± 0.11 | 0.68 ± 0.11 | 0.79 ± 0.08 | 1.26 ± 0.11* |
| Premature response trials | 6.76 ± 0.64 | 6.36 ± 0.76 | 6.83 ± 0.50 | 9.07 ± 0.52* |
Mice were trained to a stable baseline on the 3-choice SRT task with a 1-s stimulus duration, then given five daily injections of MPTP or saline. Five days following the last injection, additional SRT sessions were conducted. MPTP-treated mice exhibited a transient loss of impulse control immediately after the lesion, as indicated by increased premature responding and trials with at least one premature response, longer final time-out values, and a greater proportion of trials with at least one premature response. The means (±SEM) of the last four pre-lesion and first four post-lesion sessions were used as baseline values.
p < .05.
p < .01.
p < .001.
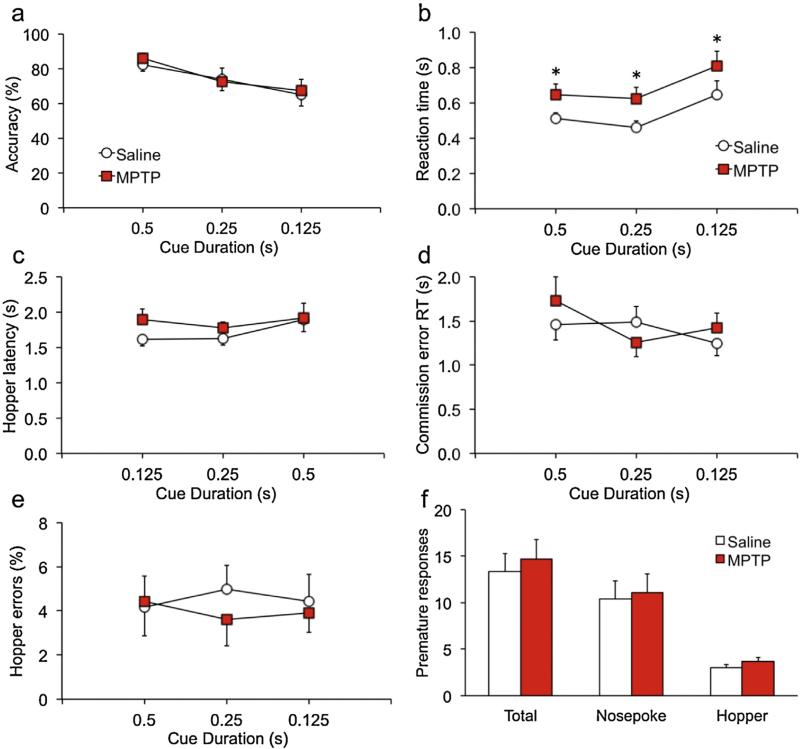
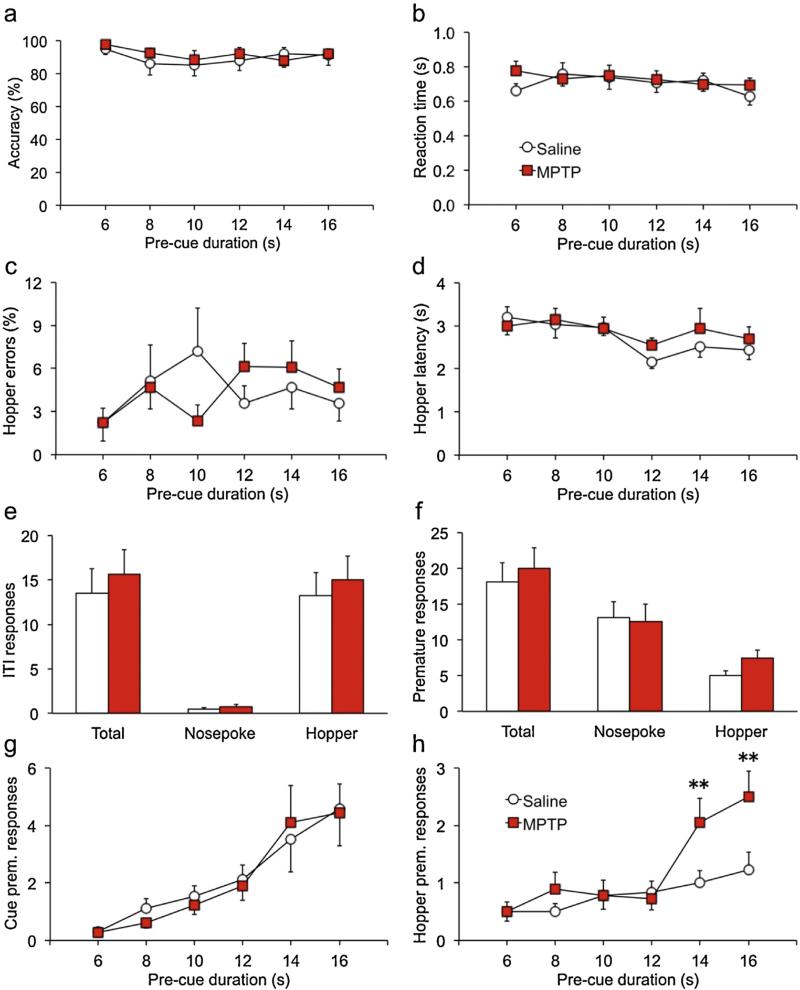
Once performance was stable in sessions with a 1-s cue duration, a challenge session was conducted in which shorter cue durations were presented pseudo-randomly. Under these conditions, accuracy did not differ between groups [Fig. 1a; F(2,33) = .03, p = .875]. However, accuracy diminished significantly with shorter cue durations, reflecting an increased proportion of commission errors in both groups when cue durations were short [ε = .741, F(1,48) = 5.6, p = .0117]. MPTP-treated mice had significantly slower reaction times on hit trials across all three cue durations [Fig. 1b; main F(1,30) = 5.0, p = .0336; interaction ε = .937, F(1,56) = 0.002, p = .997]. The other measures of response speed—hopper latency and reaction time after commission errors—did not differ between groups [Fig. 1c,d; F's < 2.4; p's > .138]. There was also no effect of MPTP on any measure of impulsive or adventitious responding [Fig. 1e,f; F's < 1.4, p's > .260]. Following the first challenge session, mice were restabilized on baseline sessions with a 1-s cue duration. At that time a second challenge session was conducted in which pre-cue durations were doubled. Under these conditions MPTP-lesioned mice performed normally on nearly every measure of sustained attention and impulsivity [Fig. 2a–g; F's < 2.6; p's > .120]. However, there was a significant overall effect of MPTP on premature hopper responses [Fig. 2 h; F(1,34) = 4.1, p = .04996] that differed across pre-cue duration [ε = .927, F(4157) = 7.8, p = .0176]. Follow-up tests showed that groups did not differ when pre-cue durations were less than 14 s [t's < 1.1, p's > .312], but at the two longer durations MPTP-lesioned mice emitted more premature responses than saline controls [t's > 2.7, p's < .007].
Fig. 1.
MPTP-lesioned mice have slower reaction times when challenged with shorter cue durations.
Mice were trained on the 3-choice serial reaction-time task with a 1-s cue duration. At this duration, there were no persistent differences between the groups on measures of sustained attention or impulsive behavior. When challenged with shorter cue durations presented randomly, accuracy diminished similarly for both groups (a) but reaction times were significantly slower in MPTP-lesioned mice (b). This slowing was specific to hit reaction times, as time to make a commission error (c) and latency to run to the food well (hopper) following a reinforcer (d) did not differ between groups. No other measures of attention or impulsive behavior were altered significantly by MPTP. Acronyms: RT, reaction time; ITI, intertrial interval. *p < .05.
Fig. 2.
MPTP-lesioned mice lose impulse control when pre-cue duration is doubled.
Mice were trained on the 3-choice serial reaction-time task with randomly-presented pre-cue durations of 3, 4, 5, 6, 7, or 8 s during the “ready” phase. At these values, there were no persistent differences between groups on measures of sustained attention or impulsive behavior. When the pre-cue durations were doubled to 6–16 s, performance accuracy was unaffected or similarly-affected for both groups, as reflected by accuracy and hopper errors (a,c). MPTP also did not slow performance when pre-cue durations were longer, as indicated by normal reaction times and hopper latencies (b,d). Responding during the inter-trial interval, or prematurely during the pre-cue period, did not differ between groups overall (e,f). However, when broken down by pre-cue duration significant differences in premature responding in the food well (hopper) were evident at the two longest values (h). This relationship did not hold for premature responses in the cue holes (g). **p < .01.
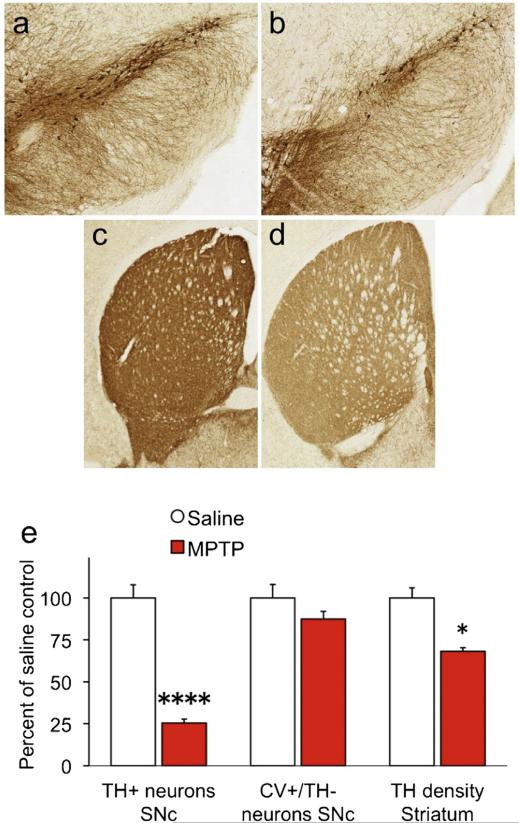
At the completion of the SRT task mice were sacrificed and brains processed for immunohistochemistry or neurochemistry. MPTP induced a significant loss of TH+ neurons in the SNc (Fig. 3a–b) and reduced TH staining in the striatum (Fig. 3c–d). Stereological assessment of SNc neurons showed a 74.5% reduction of TH+ neurons in the SNc [Fig. 3e; F(1,7) = 104.8, p < .0001]. There was no significant difference in CV+ neurons that were negative for TH in the same region [F(6) = 1.9, p = .218]. Densitometric analysis of TH staining in the dorsolateral striatum showed significant reduction in optical density in MPTP-lesioned mice [F(1,11) = 9.6, p = .0103].
Fig. 3.
MPTP selectively destroys TH-positive SNc neurons.
TH-positive neurons were counted in the SNc using unbiased stereology (a,b), and reduction in TH+ striatal terminals was estimated using densitometry (c,d). MPTP reduced TH+ neuronal number 74.5% and reduced TH optical density in the striatum by 31.8% (e). *p < .05; ****p < .0001.
MPTP depleted striatal dopamine by 54.5% [Table 2; F(1,30) = 56.6, p < .0001]. Striatal DOPAC and HVA were also reduced by MPTP [F's > 4.7, p's < .038], and all three metabolite/dopamine ratios in the striatum were increased significantly [λ = 0.125, F(1,30) = 65.2, p < .0001]. Striatal norepinephrine was increased by MPTP [F(1,30) = 7.2, p = .0115] and the DA/NE ratio was significantly reduced [F(1,30) = 26.7, p < .0001], suggesting greater availability of norepinephrine in lesioned mice. None of the catecholamine-related measures in the PFC was significantly affected by MPTP [F's < 3.5, p's > .072]. Serotonin and 5-HIAA were both significantly increased by MPTP in the striatum [F's > 4.3, p's < .046], but 5-HIAA/5-HT was unchanged [F(1,30) = 0.03, p = .855]. In the PFC, 5-HT was unaffected by MPTP [F(1,30) = 0.5, p = .465] but 5-HIAA and 5-HIAA/5-HT were both significantly reduced in lesioned mice [F's > 4.9, p's < .035].
Table 2.
Monoamine and metabolite levels in striatum and prefrontal cortex in saline- and MPTP-treated mice.
| Dorsolateral striatum |
Prefrontal cortex |
|||
|---|---|---|---|---|
| Measure | Saline | MPTP | Saline | MPTP |
| Dopamine | 282.11 ± 17.67 | 128.47 ± 10.24**** | 4.12 ± 0.30 | 4.43 ± 0.61 |
| DOPAC | 16.83 ± 0.89 | 9.58 ± 0.70**** | 3.68 ± 0.99 | 2.36 ± 0.89 |
| HVA | 34.15 ± 2.23 | 28.23 ± 1.56* | 3.96 ± 0.39 | 3.59 ± 0.37 |
| 3-MT | 16.74 ± 1.18 | 16.01 ± 1.10 | 3.32 ± 1.08 | 2.09 ± 0.78 |
| DOPAC/DA | 0.06 ± 0.00 | 0.08 ± 0.00*** | 1.19 ± 0.56 | 0.20 ± 0.04 |
| HVA/DA | 0.12 ± 0.01 | 0.23 ± 0.01**** | 7.41 ± 2.66 | 13.95 ± 8.12 |
| 3-MT/DA | 0.06 ± 0.00 | 0.12 ± 0.00**** | 0.71 ± 0.35 | 0.34 ± 0.14 |
| Norepinephrine | 0.14 ± 0.01 | 0.10 ± 0.01* | 0.07 ± 0.01 | 0.08 ± 0.01 |
| DA/NE | 42.07 ± 5.29 | 13.52 ± 1.59**** | 1.60 ± 0.63 | 1.81 ± 0.84 |
| 5-HT | 16.16 ± 0.68 | 18.15 ± 0.61* | 16.00 ± 0.59 | 15.28 ± 0.76 |
| 5-HIAA | 10.17 ± 0.39 | 11.46 ± 0.48* | 5.37 ± 0.34 | 4.24 ± 0.32* |
| 5-HIAA/5-HT | 0.64 ± 0.03 | 0.63 ± 0.02 | 0.34 ± 0.02 | 0.28 ± 0.02* |
All values are expressed in ng/mg tissue except those transformed to correct non-normal distributions as described in theMethods section. Because NE values were reciprocal-transformed, higher values indicate lower levels of NE. Abbreviations: DA, dopamine; NE, norepinephrine; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; 3-MT, 3-methoxytyramine; 5-HT, serotonin; 5-HIAA, 5-hydroxyindoleacetic acid.
p < .05.
p < .001.
p < .0001.
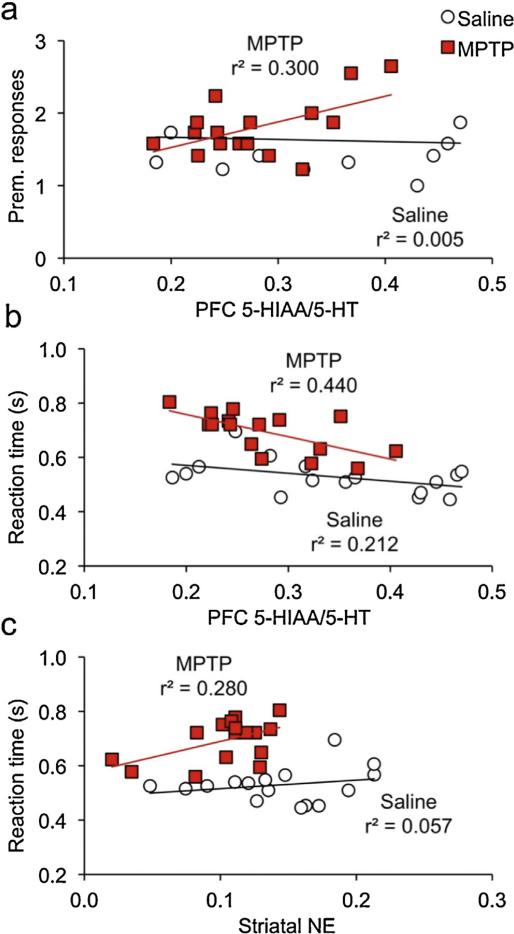
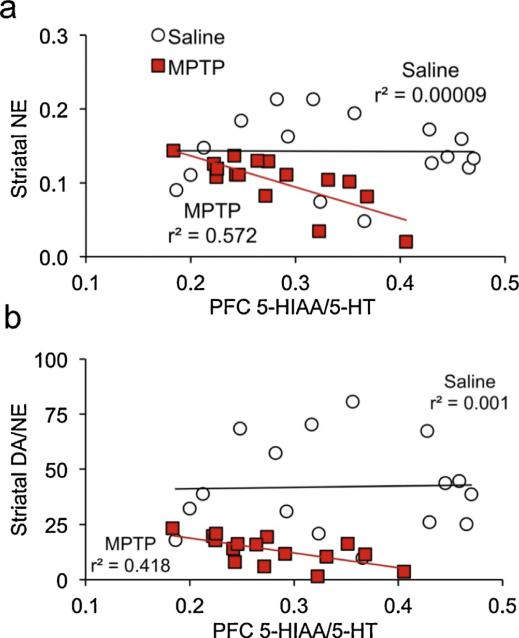
To determine whether monoamine levels were associated with the executive dysfunction exhibited by MPTP-lesioned mice, we conducted correlations between monoamine markers and performance during the two challenge SRT sessions. Specifically, the mean overall reaction time during the short-cue-duration session was used as a measure of sustained attention, and premature responses on trials with pre-cue durations ranging from 14 to 16 s during the long-pre-cue-duration session was used as a measure of impulse control. In the PFC, there were no significant associations between either behavioral measure and any neurochemical measure in the saline control group [|r|'s < .232, p's > .389]. Among MPTP-lesioned mice, PFC 5-HT turnover was significantly correlated with both executive functions (Fig. 4a,b), negatively with sustained attention [Fig. r(15) = −.650, p = .0064] and positively with impulsivity [r(15) = .550, p = .0272]. No other PFC neurochemical measure was associated with either cognitive measure in the MPTP mice [|r|'s < .480, p's > .061]. None of the neurochemical measures in the striatum was significantly correlated with impulse control, in either lesion group [|r|'s < .45, p's > .083]. Striatal norepinephrine was correlated with sustained attention in MPTP-lesioned mice [r(15) = .523, p = .0374; Fig 4c] but not saline controls [r(15) = .208, p = .437]. Striatal DA/NE also had a modest correlation with reaction time in lesioned mice, but it was not statistically significant [r(15) = .487, p = .0556]. No other measure was associated with sustained attention in either group [|r|'s < .411, p's > .113]. Interestingly, of the neurochemical measures in the striatum, only NE and DA/NE were significantly associated with PFC 5-HT/5-HIAA, and in MPTP-lesioned mice [Fig. 5; |r's| > .641; p's < .0074] but not in saline controls [|r's| < .033, p > .904].
Fig. 4.
Inattention and impulsivity are significantly correlated with prefrontal 5-HIAA/5-HT in lesioned mice.
(a,b) Prefrontal 5-HIAA/5-HT was significantly associated with both (a) premature responses emitted on the long-precue challenge session when precue durations exceeded 12 s, and (b) reaction times on the short cue duration challenge session. (c) Striatal NE was also significantly correlated with reaction times on the short cue duration challenge session. All three correlations were statically significant only in MPTP-lesioned mice.
Fig. 5.
Greater striatal NE and DA/NE are associated with higher levels of prefrontal 5-HIAA/5-HT.
(a,b) Striatal NE and DA/NE (but not DA) are significantly and negatively correlated with PFC 5-HIAA/5-HT, in MPTP-lesioned mice only. Note that striatal NE was reciprocal transformed, so that higher values represent lower levels of NE.
4. Discussion
We show here for the first time that MPTP impairs sustained attention and impulse control in mice. MPTP increased impulsive behavior, but performance improved with extended training. Additional impairments in sustained attention and impulse control were evident under more challenging conditions. Neurochemical analyses showed that MPTP significantly reduced striatal dopamine and increased estimates of dopamine turnover, consistent with previous reports of MPTP's effect in mice. Serotonin levels were unchanged by MPTP in the PFC, but 5-HIAA/5-HT was reduced and both measures of executive function were significantly correlated with this ratio in lesioned mice only. Striatal NE and striatal DA/NE were both significantly associated with PFC 5-HIAA/5-HT in lesioned mice only. These results suggest that the executive dysfunction exhibited by MPTP-lesioned mice may be mediated by the availability of striatal norepinephrine and prefrontal serotonin.
With the 1-s cue duration used during training, all mice performed well on baseline. Immediately following injections, MPTP-lesioned mice performed worse than saline controls on every measure of impulse control. By the fifth day of re-training the impairments had abated and lesioned mice were performing as well as before the injections and as well as wild-type mice. This may reflect the recovery of striatal dopamine content that begins within days after lesioning [4,35–37]. Alternately, the return to baseline-level responding may reflect the nature of the task, specifically that reinforced behaviors take on the characteristics of habits with over-training [38–40]. This transition to habit can be disrupted by lesions to the dorsolateral striatum [41,42].
Although baseline performance normalized in MPTP-treated mice with extended training, additional deficits were evident when cognitive demands were increased. On a challenge session with shorter, randomized cue durations, MPTP mice were slower to respond in the correct nose-poke hole, indicative of impaired attention. Two previous studies showed similar results, specifically that 6-OHDA lesions of the dorsal striatum slowed reaction times on a task but did not affect choice accuracy [43,44]. A third study found mixed results when dissociating medial and lateral striatal regions. They showed that showed that quisqualic acid lesions of the medial striatum slowed reaction times and reduced hit rate, but equivalent lesions of the lateral striatum only affected non-cognitive motor components of the task [45]. Our results are not consistent with an interpretation of impaired motor performance. On baseline sessions preceding the challenge session, MPTP-lesioned mice performed the task as well as wild-type mice in all respects. All motor components of the task were the same in the baseline and challenge versions of the tasks; the only difference was the cognitive demand placed on the animal by the shorter cue duration. In addition, other timed components of the task, such as reaction time to commission errors and hopper latency following a hit, were unaffected by the introduction of more challenging task demands. Thus we can be confident that the slower reaction times are not attributable to non-cognitive performance variables.
Consistent with previous reports using subchronic MPTP, we observed substantial depletion of striatal dopamine and destruction of TH+ neurons in the SNc of lesioned mice. However, we also observed changes that have not been reported in the literature. Striatal serotonin was significantly increased by MPTP, in contrast to numerous reports showing no change in C57BL/6 strains following subchronic MPTP regimens with cumulative doses ranging from 100 to 240 mg/kg administered over 4–5 days [46–50]. A possible reason for this discrepancy may be the time between lesion and sacrifice in the present study. Subjects are typically sacrificed within a week or two of MPTP injections, but Ansah et al. [48] injected mice with an acute MPTP regimen and examined monoamines 3 weeks or 18 months following the last injection. Striatal 5-HT was unchanged 3 weeks after MPTP lesioning but significantly elevated after 18 months. A similar report by Schneider [51] showed increased striatal 5-HT using a chronic low-dose of MPTP over 38–175 days in monkeys sacrificed 8–60 days following the last injection. Two studies showed decreased 5-HT 1 week following MPTP but normal levels 4 weeks later [52,53]. Similarly, Luthman et al. [54] found increased sprouting of serotonergic terminals in the striatum of adult rats that correlated with dopamine loss, after receiving striatal injections of 6-OHDA as neonates. In the present study 5 weeks elapsed between the last MPTP injection and sacrificing the mice because of the extended SRT training. Thus the change in 5-HT may represent a long-term compensatory response to nigro-striatal lesioning.
In contrast to striatum, 5-HT in the PFC was unchanged by MPTP. However, 5-HIAA and 5-HIAA/5-HT were lower in the PFC of MPTP-lesioned mice. To date there is no published report of similar changes in 5-HIAA/5-HT after any MPTP regimen or intrastriatal 6-OHDA injections in rodents, although Ansah et al. [48] observed reduced PFC 5-HT up to 18 weeks following an acute MPTP regimen in mice. In macaques, Sánchez et al. [55] showed that unilateral striatal lesions significantly reduced serotonin transporter (SERT)-specific [3H]citalopram binding in the ipsilateral frontal cortex and anterior cingulate, compared to equivalent regions on the contralateral (unlesioned) side. Although all the monkeys in that study had undergone ovariectomies 4 years earlier, it demonstrates that that MPTP-induced nigrostriatal damage can interfere with serotonergic signaling in the anterior cortex. 5-HIAA/5-HT is often considered an estimate of serotonin turnover; however, other explanations for changes in the ratio are possible. Nayyar et al. [56] reported a significant and long-lasting reduction in the number of PFC axons labeled for SERT, in mice treated acutely with MPTP. The reduced density was more prominent in thick-beaded SERT-immunoreactive (ir) neurons originating in the median raphe nucleus, as opposed to the fine axons of the dorsal raphe neurons. A number of studies, including Nayyar et al., have shown reduced PFC SERT-ir axons, reduced 5-HT-ir median raphe neurons, or reduced PFC SERT binding capacity in brains from Parkinson's patients [57–60]. Thus the reduced 5-HIAA/5-HT ratio observed in the PFC of MPTP-lesioned mice in the present study may reflect damaged serotonergic axons instead of, or in addition to, altered 5-HT metabolism or uptake. Further investigation is needed to better understand changes to striatal serotonergic function following subchronic MPTP.
It is well-established that executive function is associated with prefrontal cortical activity (e.g., [61,62]), and here we show that prefrontal 5-HIAA/5-HT was the best predictor of executive impairments in MPTP-lesioned mice. Given that 5-HIAA/5-HT may be indicative of the rate of 5-HT turnover and/or damaged serotonergic fibers as noted above, these results suggest that executive function is sensitive to levels of prefrontal synaptic 5-HT in mice with MPTP-induced nigrostriatal lesions. The fact that the relationship was observed only in lesioned mice suggests that under normal conditions there is sufficient 5-HT to mediate good cognitive function in saline control mice. However, the umbrella term “executive function” comprises multiple faculties subserved by different pathways and neurochemical systems. Indeed, a comprehensive review by Bari and Robbins [63] illustrates that even “impulsivity” is too broad a concept to pin on a single neurobiological substrate, and that what we conceive of as impulse control can be mediated by different neurochemical systems depending on the behavioral requirements of the tasks used. Miyazaki et al. [64,65] showed that 5-HT is involved in the response inhibition components of impulse-control tasks, as opposed to the motivational or motor aspects. They showed that dorsal raphe neurons increased firing rate as the delay to reinforcement elapsed, and that impulse control was impaired—at long but not short delays—with intra-raphe infusion of a 5-HT1a receptor antagonist. Simultaneous activation of the dorsal raphe and dorsolateral PFC during delayed reinforcement has also been demonstrated in human subjects [66]. Our data are consistent with these findings. Both groups of mice were able to wait out the delays of 12 s or less, and premature responses did not differ. However, when pre-cue delays were 14 s or greater, MPTP-lesioned mice were unable to refrain from responding. The greater the 5-HIAA/5-HT, suggestive of lower extracellular 5-HT, the greater the number of premature responses. In contrast to impulse control, the noradrenergic system is more closely associated with speed of responding during attentional tasks [63]. The α1 adrenergic receptor antagonist prazosin produces a dose-dependent increase in reaction time on a stop-signal task, and the NE reuptake inhibitor atomoxetine improves reaction times in children with ADHD [67,68]. In the present study both striatal NE and prefrontal 5-HT were significantly correlated with reaction times in MPTP mice, despite slower response times overall in the lesioned group. Sustained attention is typically thought to be mediated by the cholinergic and noradrenergic systems. Specifically, acetylcholine is important for the maintenance of attention in a well-trained task, and NE comes into play when novel stimuli or situations are encountered [69]. Our data are consistent with this. The MPTP-lesioned mice performed well on baseline, but impairments were evident when challenged with novel contingencies. The fact that both striatal NE and striatal DA/NE were significantly correlated with prefrontal 5-HT suggests that these changes may involve prefrontal serotonergic signaling as well. Indeed, contingency changes disrupted impulse control as well as reaction time. Further research is needed to determine whether the relationship between NE and 5-HT underlies the loss of impulse control.
Prefrontal serotonergic mediation of impulse control has been demonstrated in Parkinson's disease as well. Parkinson's patients without a diagnosis of impulse control disorder had slower stop-signal reaction times (SSRTs) and more no-go errors than age-matched controls on a choice reaction-time task [70]. When challenged with a dose of the serotonin re-uptake blocker citalopram, SSRTs slowed slightly. However, both measures of impulsivity were significantly correlated with the motor subscore of the Unified Parkinson's Disease Rating Scale (UPDRS). Specifically, patients with more advanced disease had better impulse control under citalopram while those with lower scores tended to worsen. Functional MRI during the reaction time task showed that the activation deficit in patients under placebo compared to controls was limited to the right inferior prefrontal gyrus, when executing a stop-signal or no-go response. The ability of citalopram to enhance this activation was significantly and positively correlated with the UPDRS-motor subscore. A genetic association study showed that an allelic variant of the serotonin transporter gene promoter region was not differentially associated with impulsivity in 404 Parkinson's patients, 58 of whom had been diagnosed with comorbid impulse control disorder [71]. In contrast, variants of the dopamine 3 receptor (DRD3) and NMDA 2B receptor (GRIN2B) genes were both associated with a doubling of the risk of impulsivity. In a separate study using the same 404 patients, Lee et al. [72] examined the 102 T → C variants of the 5-HT receptor subtype 2A gene (HTR2A). They found that among patients on higher medication doses (levodopa equivalents), impulsivity was equally prevalent regardless of genotype. However, in the lower levodopa-dose-equivalent group, patients with the TT variant were 6.9 and 2.8 times more likely than those with CC or CT variants, respectively, to have been diagnosed with impulse control disorder. Together, these studies suggest that impulse control in Parkinson's disease involves multiple neural systems, including prefrontal serotonin, and that the expression of symptoms is affected by drug status and severity of disease.
We have demonstrated that subchronic MPTP induces executive dysfunction. MPTP also induced serotonergic changes in the striatum and PFC, the latter being the best predictor of deficits in attention and impulse control. The fact that the executive deficits were only evident on challenge sessions, along with significant associations between striatal NE markers and prefrontal 5-HIAA/5-HT, suggests that noradrenergic signaling may interact with prefrontal 5-HT to mediate executive function in lesioned mice. Indeed, we observed serotonergic and noradrenergic abnormalities that have not been reported in the literature before following MPTP administration. It is not known whether they may reflect in part changes associated with long-term training on the SRT task, the food restriction regimen, time between lesion and sacrifice, or interactions among them and other factors. Given the importance of maintaining good cognitive function as Parkinson's disease progresses, these relationships warrant further attention.
HIGHLIGHTS.
Mice were trained on a task that measures sustained attention & impulse control.
MPTP impaired executive function when task parameters were made more difficult.
MPTP induced changes in serotonergic and noradrenergic measures.
Executive function was correlated with prefrontal 5-HIAA/5-HT in lesioned mice.
Acknowledgements
Microscopic image production and stereological analyses were conducted at the Neuroscience Institute's Imaging Center at UTHSC (cns.uthsc.edu/imaging-center). HPLC assays were conducted by the Neurochemistry Core at the Vanderbilt Brain Institute. Funding was provided by the National Institutes of Health (NS065063). None of the authors has any conflict of interest or relationship, financial or otherwise, that might be perceived as influencing objectivity.
References
- 1.Ebmeier KP, Calder SA, Crawford JR, Stewart L, Besson JA, Mutch WJ. Clinical features predicting dementia in idiopathic Parkinson's disease: a follow-up study. Neurology. 1990;40:1222–1224. doi: 10.1212/wnl.40.8.1222. [DOI] [PubMed] [Google Scholar]
- 2.McDonald C, Brown GG, Gorell JM. Impaired set-shifting in Parkinson's disease: new evidence from a lexical decision task. J. Clin. Exp. Neuropsychol. 1996;18:793–809. doi: 10.1080/01688639608408303. [DOI] [PubMed] [Google Scholar]
- 3.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130:1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 4.Schneider JS, Yuwiler A. GM1 ganglioside treatment promotes recovery of striatal dopamine concentrations in the mouse model of MPTP-induced parkinsonism. Exp. Neurol. 1989;105:177–183. doi: 10.1016/0014-4886(89)90117-9. [DOI] [PubMed] [Google Scholar]
- 5.Schneider JS, Schroeder JA, Rothblat DS. Differential recovery of sensorimotor function in GM1 ganglioside-treated vs. spontaneously recovered MPTP-treated cats: partial striatal dopaminergic reinnervation vs. neurochemical compensation. Brain Res. 1998;813:82–87. doi: 10.1016/s0006-8993(98)01007-5. [DOI] [PubMed] [Google Scholar]
- 6.Schneider JS, DiStefano L. Oral administration of semisynthetic sphingolipids promotes recovery of striatal dopamine concentrations in a murine model of parkinsonism. Neurology. 1994;44:748–750. doi: 10.1212/wnl.44.4.748. [DOI] [PubMed] [Google Scholar]
- 7.Pope-Coleman A, Tinker JP, Schneider JS. Effects of GM1 ganglioside treatment on pre- and postsynaptic dopaminergic markers in the striatum of parkinsonian monkeys. Synapse. 2000;36:120–128. doi: 10.1002/(SICI)1098-2396(200005)36:2<120::AID-SYN5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 8.Pope-Coleman A, Schneider JS. Effects of chronic GM1 ganglioside treatment on cognitieve and motor deficits in a slowly progressing model of parkinsonism in non-human primates. Restor. Neurol. Neurosci. 1998;12:255–266. [PubMed] [Google Scholar]
- 9.Braungart E, Gerlach M, Riederer P, Baumeister R, Hoener MC. Caenorhabditis elegans MPP+ model of Parkinson's disease for high-throughput drug screenings. Neurodegener. Dis. 2004;1:175–183. doi: 10.1159/000080983. [DOI] [PubMed] [Google Scholar]
- 10.Sedelis M, Schwarting RK, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson's disease. Behav. Brain Res. 2001;125:109–125. doi: 10.1016/s0166-4328(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 11.Jakowec MW, Petzinger GM. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned model of parkinson's disease, with emphasis on mice and nonhuman primates. Comp. Med. 2004;54:497–513. [PubMed] [Google Scholar]
- 12.Dhanushkodi A, Akano EO, Roguski EE, Xue Y, Rao SK, Matta SG, et al. A single intramuscular injection of rAAV-mediated mutant erythropoietin protects against MPTP-induced parkinsonism. Genes Brain Behav. 2013;12:224–233. doi: 10.1111/gbb.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald MP. Methods and models of the non-motor symptoms of Parkinson disease. In: LeDoux MS, editor. Movement Disorders: Genetics and Models. Elsevier Academic Press; Amsterdam: 2015. [Google Scholar]
- 14.Schintu N, Frau L, Ibba M, Garau A, Carboni E, Carta AR. Progressive dopaminergic degeneration in the chronic MPTPp mouse model of Parkinson's disease. Neurotox. Res. 2009;16:127–139. doi: 10.1007/s12640-009-9061-x. [DOI] [PubMed] [Google Scholar]
- 15.Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, et al. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. Eur. J. Neurosci. 2009;29:954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- 16.Carta AR, Carboni E, Spiga S. The MPTP/probenecid model of progressive Parkinson's disease. Methods Mol. Biol. 2013;964:295–308. doi: 10.1007/978-1-62703-251-3_17. [DOI] [PubMed] [Google Scholar]
- 17.Szabo S, Brown A, Pihan G, Dali H, Neumeyer JL. Duodenal ulcer induced by MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) Proc. Soc. Exp. Biol. Med. 1985;180:567–571. doi: 10.3181/00379727-180-rc3. [DOI] [PubMed] [Google Scholar]
- 18.Natale G, Kastsiushenka O, Fulceri F, Ruggieri S, Paparelli A, Fornai F. MPTP-induced parkinsonism extends to a subclass of TH-positive neurons in the gut. Brain Res. 2010;1355:195–206. doi: 10.1016/j.brainres.2010.07.076. [DOI] [PubMed] [Google Scholar]
- 19.Barraud Q, Lambrecq V, Forni C, McGuire S, Hill M, Bioulac B, et al. Sleep disorders in Parkinson's disease: the contribution of the MPTP non-human primate model. Exp. Neurol. 2009;219:574–582. doi: 10.1016/j.expneurol.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Schneider JS, Roeltgen DP. Delayed matching-to-sample, object retrieval, and discrimination reversal deficits in chronic low dose MPTP-treated monkeys. Brain Res. 1993;615:351–354. doi: 10.1016/0006-8993(93)90049-s. [DOI] [PubMed] [Google Scholar]
- 21.Schneider JS, Smith MG, DiStefano L, Berrian J. GM1 ganglioside treatment partially reverses the nigrostriatal dopamine defect in the weaver mutant mouse. Brain Res. 1994;636:353–356. doi: 10.1016/0006-8993(94)91037-5. [DOI] [PubMed] [Google Scholar]
- 22.Stern Y, Tetrud JW, Martin WR, Kutner SJ, Langston JW. Cognitive change following MPTP exposure. Neurology. 1990;40:261–264. doi: 10.1212/wnl.40.2.261. [DOI] [PubMed] [Google Scholar]
- 23.Decamp E, Schneider JS. Attention and executive function deficits in chronic low-dose MPTP-treated non-human primates. Eur. J. Neurosci. 2004;20:1371–1378. doi: 10.1111/j.1460-9568.2004.03586.x. [DOI] [PubMed] [Google Scholar]
- 24.Decamp E, Schneider JS. Effects of nicotinic therapies on attention and executive functions in chronic low-dose MPTP-treated monkeys. Eur. J. Neurosci. 2006;24:2098–2104. doi: 10.1111/j.1460-9568.2006.05077.x. [DOI] [PubMed] [Google Scholar]
- 25.Rane P, Shields J, Heffernan M, Guo Y, Akbarian S, King JA. The histone deacetylase inhibitor, sodium butyrate, alleviates cognitive deficits in pre-motor stage PD. Neuropharmacology. 2012;62:2409–2412. doi: 10.1016/j.neuropharm.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J. Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald MP, Wong R, Goldstein G, Weintraub B, Cheng SY, Crawley JN. Hyperactivity and learning deficits in transgenic mice bearing a human mutant thyroid hormone β1 receptor gene. Learn. Mem. 1998;5:289–301. [PMC free article] [PubMed] [Google Scholar]
- 28.Siesser WB, Cheng SY, McDonald MP. Hyperactivity, impaired learning on a vigilance task, and a differential response to methylphenidate in the TRβPV knock-in mouse. Psychopharmacology (Berl) 2005;181:653–663. doi: 10.1007/s00213-005-0024-5. [DOI] [PubMed] [Google Scholar]
- 29.Siesser WB, Zhao J, Miller LR, Cheng SY, McDonald MP. Transgenic mice expressing a human mutant β1 thyroid receptor are hyperactive, impulsive, and inattentive. Genes Brain Behav. 2006;5:282–297. doi: 10.1111/j.1601-183X.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- 30.Bernardo A, McCord M, Troen AM, Allison JD, McDonald MP. Impaired spatial memory in APP-overexpressing mice on a homocysteinemia-inducing diet, Neurobiol. Aging. 2007;28:1195–1205. doi: 10.1016/j.neurobiolaging.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 31.Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe + PSEN1 ΔE9 bigenic mouse model of Alzheimer's disease. Genes Brain Behav. 2007;6:54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- 32.Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, et al. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J. Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- 33.Flanigan TJ, Xue Y, Kishan Rao S, Dhanushkodi A, McDonald MP. Abnormal vibrissa-related behavior and loss of barrel field inhibitory neurons in 5×FAD transgenics. Genes Brain Behav. 2014;13:488–500. doi: 10.1111/gbb.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cramer D. Basic Statistics for Social Research. Routledge; New York: 1997. [Google Scholar]
- 35.Bohn MC, Marciano F, Cupit L, Gash DM. Recovery of dopaminergic fibers in striatum of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mouse is enhanced by grafts of adrenal medulla. Prog. Brain Res. 1988;78:535–542. doi: 10.1016/s0079-6123(08)60328-3. [DOI] [PubMed] [Google Scholar]
- 36.Bezard E, Gross CE. Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog. Neurobiol. 1998;55:93–116. doi: 10.1016/s0301-0082(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 37.Mitsumoto Y, Watanabe A, Mori A, Koga N. Spontaneous regeneration of nigrostriatal dopaminergic neurons in MPTP-treated C57BL/6 mice. Biochem. Biophys. Res. Commun. 1998;248:660–663. doi: 10.1006/bbrc.1998.8986. [DOI] [PubMed] [Google Scholar]
- 38.Dickinson A. Actions and habits: the development of behavioral autonomy. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 1985;308:78. [Google Scholar]
- 39.Boakes RA. The role of repetition in transforming actions into habits: the contribution of John Watson and contemporary research into a persistent theme. Mex. J. Behav. Anal. 1993;19:67–90. [Google Scholar]
- 40.Coutureau E, Killcross S. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav. Brain Res. 2003;146:167–174. doi: 10.1016/j.bbr.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 41.Lingawi NW, Balleine BW. Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits. J. Neurosci. 2012;32:1073–1081. doi: 10.1523/JNEUROSCI.4806-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinn JJ, Pittenger C, Lee AS, Pierson JL, Taylor JR. Striatum-dependent habits are insensitive to both increases and decreases in reinforcer value in mice. Eur. J. Neurosci. 2013;37:1012–1021. doi: 10.1111/ejn.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turle-Lorenzo N, Maurin B, Puma C, Chezaubernard C, Morain P, Baunez C, et al. The dopamine agonist piribedil with l-DOPA improves attentional dysfunction: relevance for Parkinson's disease. J. Pharmacol. Exp. Ther. 2006;319:914–923. doi: 10.1124/jpet.106.109207. [DOI] [PubMed] [Google Scholar]
- 44.Domenger D, Schwarting RK. Effects of neostriatal 6-OHDA lesion on performance in a rat sequential reaction time task. Neurosci. Lett. 2008;444:212–216. doi: 10.1016/j.neulet.2008.08.048. [DOI] [PubMed] [Google Scholar]
- 45.Rogers RD, Baunez C, Everitt BJ, Robbins TW. Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behav. Neurosci. 2001;115:799–811. doi: 10.1037//0735-7044.115.4.799. [DOI] [PubMed] [Google Scholar]
- 46.Rousselet E, Joubert C, Callebert J, Parain K, Tremblay L, Orieux G, et al. Behavioral changes are not directly related to striatal monoamine levels, number of nigral neurons, or dose of parkinsonian toxin MPTP in mice. Neurobiol. Dis. 2003;14:218–228. doi: 10.1016/s0969-9961(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 47.Luchtman DW, Shao D, Song C. Behavior, neurotransmitters and inflammation in three regimens of the MPTP mouse model of Parkinson's disease. Physiol. Behav. 2009;98:130–138. doi: 10.1016/j.physbeh.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 48.Ansah TA, Ferguson MC, Nayyar T, Deutch AY. Age- and duration-dependent effects of MPTP on cortical serotonin systems. Neurosci. Lett. 2011;504:160–164. doi: 10.1016/j.neulet.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pain S, Gochard A, Bodard S, Gulhan Z, Prunier-Aesch C, Chalon S. Toxicity of MPTP on neurotransmission in three mouse models of Parkinson's disease. Exp. Toxicol. Pathol. 2013;65:689–694. doi: 10.1016/j.etp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Saitoh K, Abe K, Chiba T, Katagiri N, Saitoh T, Horiguchi Y, et al. Properties of 3-methyl-TIQ and 3-methyl-N-propargyl-TIQ for preventing MPTP-induced parkinsonism-like symptoms in mice. Pharmacol. Rep. PR. 2013;65:1204–1212. doi: 10.1016/s1734-1140(13)71478-6. [DOI] [PubMed] [Google Scholar]
- 51.Schneider JS. Chronic exposure to low doses of MPTP. II. Neurochemical and pathological consequences in cognitively-impaired, motor asymptomatic monkeys. Brain Res. 1990;534:25–36. doi: 10.1016/0006-8993(90)90108-n. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura S, Fukuda H, Hara K, Fukuyama H, Kameyama M. Biochemical aspects of Parkinson-dementia complex. Eur. Neurol. 1988;28(Suppl. 1):24–28. doi: 10.1159/000209731. [DOI] [PubMed] [Google Scholar]
- 53.Rozas G, Liste I, Guerra MJ, Labandeira-Garcia JL. Sprouting of the serotonergic afferents into striatum after selective lesion of the dopaminergic system by MPTP in adult mice. Neurosci. Lett. 1998;245:151–154. doi: 10.1016/s0304-3940(98)00198-0. [DOI] [PubMed] [Google Scholar]
- 54.Luthman J, Bolioli B, Tsutsumi T, Verhofstad A, Jonsson G. Sprouting of striatal serotonin nerve terminals following selective lesions of nigro-striatal dopamine neurons in neonatal rat. Brain Res. Bull. 1987;19:269–274. doi: 10.1016/0361-9230(87)90092-x. [DOI] [PubMed] [Google Scholar]
- 55.Sánchez MG, Morissette M, Di Paolo T. Estradiol and brain serotonin reuptake transporter in long-term ovariectomized parkinsonian monkeys. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;45:170–177. doi: 10.1016/j.pnpbp.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Nayyar T, Bubser M, Ferguson MC, Neely MD, Shawn Goodwin J, Montine TJ, et al. Cortical serotonin and norepinephrine denervation in parkinsonism: preferential loss of the beaded serotonin innervation. Eur. J. Neurosci. 2009;30:207–216. doi: 10.1111/j.1460-9568.2009.06806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halliday GM, Li YW, Blumbergs PC, Joh TH, Cotton RG, Howe PR, et al. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson's disease. Ann. Neurol. 1990;27:373–385. doi: 10.1002/ana.410270405. [DOI] [PubMed] [Google Scholar]
- 58.Haapaniemi TH, Ahonen A, Torniainen P, Sotaniemi KA, Myllyla VV. [123I]beta-CIT SPECT demonstrates decreased brain dopamine and serotonin transporter levels in untreated parkinsonian patients. Mov. Disord. 2001;16:124–130. doi: 10.1002/1531-8257(200101)16:1<124::aid-mds1007>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 59.Chinaglia G, Landwehrmeyer B, Probst A, Palacios JM. Serotoninergic terminal transporters are differentially affected in Parkinson's disease and progressive supranuclear palsy: an autoradiographic study with [3H]citalopram. Neuroscience. 1993;54:691–699. doi: 10.1016/0306-4522(93)90240-g. [DOI] [PubMed] [Google Scholar]
- 60.Guttman M, Boileau I, Warsh J, Saint-Cyr JA, Ginovart N, McCluskey T, et al. Brain serotonin transporter binding in non-depressed patients with Parkinson's disease. Eur. J. Neurol. 2007;14:523–528. doi: 10.1111/j.1468-1331.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- 61.Bari A, Mar AC, Theobald DE, Elands SA, Oganya KC, Eagle DM, et al. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J. Neurosci. 2011;31:9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006;139:865–876. doi: 10.1016/j.neuroscience.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 63.Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 64.Miyazaki KW, Miyazaki K, Doya K. Activation of dorsal raphe serotonin neurons is necessary for waiting for delayed rewards. J. Neurosci. 2012;32:10451–10457. doi: 10.1523/JNEUROSCI.0915-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miyazaki K, Miyazaki KW, Doya K. The role of serotonin in the regulation of patience and impulsivity. Mol. Neurobiol. 2012;45:213–224. doi: 10.1007/s12035-012-8232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat. Neurosci. 2004;7:887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 67.Bari A, Robbins TW. Noradrenergic versus dopaminergic modulation of impulsivity, attention and monitoring behaviour in rats performing the stop-signal task: possible relevance to ADHD. Psychopharmacology (Berl) 2013;230:89–111. doi: 10.1007/s00213-013-3141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kratz O, Studer P, Baack J, Malcherek S, Erbe K, Moll GH, Heinrich H. Differential effects of methylphenidate and atomoxetine on attentional processes in children with ADHD: an event-related potential study using the Attention Network Test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;37:81–89. doi: 10.1016/j.pnpbp.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Dalley JW, McGaughy J, O'Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J. Neurosci. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T, et al. Selective serotonin reuptake inhibition modulates response inhibition in Parkinson's disease. Brain. 2014;137:1145–1155. doi: 10.1093/brain/awu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee JY, Lee EK, Park SS, Lim JY, Kim HJ, Kim JS, et al. Association of DRD3 and GRIN2B with impulse control and related behaviors in Parkinson's disease. Mov. Disord. 2009;24:1803–1810. doi: 10.1002/mds.22678. [DOI] [PubMed] [Google Scholar]
- 72.Lee JY, Jeon BS, Kim HJ, Park SS. Genetic variant of HTR2A associates with risk of impulse control and repetitive behaviors in Parkinson's disease. Parkinsonism Relat. Disord. 2012;18:76–78. doi: 10.1016/j.parkreldis.2011.08.009. [DOI] [PubMed] [Google Scholar]