SUMMARY
Parkinson's disease (PD) is a neurodegenerative disease caused by the loss of dopaminergic neurons in the substantia nigra. PARK2 mutations cause early-onset forms of PD. PARK2 encodes an E3 ubiquitin ligase, Parkin, that can selectively translocate to dysfunctional mitochondria to promote their removal by autophagy. However, Parkin knockout (KO) mice do not display signs of neurodegeneration. To assess Parkin function in vivo, we utilized a mouse model that accumulates dysfunctional mitochondria caused by an accelerated generation of mtDNA mutations (Mutator mice). In the absence of Parkin, dopaminergic neurons in Mutator mice degenerated causing an L-DOPA reversible motor deficit. Other neuronal populations were unaffected. Phosphorylated ubiquitin was increased in the brains of Mutator mice, indicating PINK1-Parkin activation. Parkin loss caused mitochondrial dysfunction and affected the pathogenicity but not the levels of mtDNA somatic mutations. A systemic loss of Parkin synergizes with mitochondrial dysfunction causing dopaminergic neuron death modeling PD pathogenic processes.
INTRODUCTION
Parkinson's disease (PD) is one of the most common progressive, age-related neurodegenerative diseases. Three neuropathological signs characterize PD: the presence of α-synuclein-positive neuronal inclusions known as Lewy bodies, the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SN) that project to the striatum, and the consequent striatal dopamine (DA) depletion causing the disease's classical motor phenotypes (Dauer and Przedborski, 2003). The motor symptoms (rigidity, bradykinesia, postural instability, and tremor) appear when approximately 50%–60% of SN dopaminergic neurons degenerate (Fearnley and Lees, 1991). The majority of PD cases are sporadic, and, to date, therapies remain limited.
There is a strong connection between the role of mitochondrial defects and the pathogenesis of PD. Disruption of oxidative phosphorylation (OXPHOS), particularly complex I, is believed to be an important contributor to neuronal loss in PD (Schapira et al., 1990a, 1990b). Dopaminergic neurons in both PD and aged individuals display dysfunctional mitochondria accumulating high levels of mitochondrial DNA (mtDNA) deletions (Bender et al., 2006; Kraytsberg et al., 2006). Mitochondrial disease patients harboring POLG mutations (the polymerase responsible for mtDNA replication) also accumulate mtDNA mutations in dopaminergic neurons leading to nigrostriatal degeneration (Reeve et al., 2013).
PARK2 is the second most common gene mutated in cases of early-onset familial PD (Kitada et al., 1998), and its mutations have been associated with mitochondrial dysfunction. Recessive mutations are responsible for loss of function of Parkin, disrupting its ubiquitin-ligase activity necessary to target dysfunctional mitochondria for selective, autophagic destruction in a process known as mitophagy (Pickrell and Youle, 2015). Although groups have shown strong evidence that Parkin-mediated mitophagy occurs in neurons (Ashrafi et al., 2014; Bingol et al., 2014), the extensive studies of this pathway in cultured cells have failed to reveal how Parkin contributes to PD pathogenesis and affects dopaminergic neurons in vivo. Parkin-knockout (KO) mice do not show significant motor phenotypes, DA metabolism abnormalities, or signs of nigrostriatal degeneration (Goldberg et al., 2003; Kitada et al., 2009; Perez and Palmiter, 2005). There are also no reported PD mouse models where dopaminergic neurons degenerate without experimentally targeting these neurons specifically (Pickrell et al., 2013). Our data provide insight as to why Parkin-KO mice have no neurodegeneration and show the importance of endogenous Parkin in enforcing mitochondrial quality control to protect dopaminergic neurons.
RESULTS
We hypothesized that the loss of endogenous Parkin may be detrimental to neuronal health in a mouse model with mitochondrial dysfunction. To test this hypothesis, we used mice homozygous for a proofreading deficiency in DNA polymerase γ (Mutator mice) as a model for mitochondrial dysfunction. These mice exhibit a ubiquitous and progressive accumulation of mtDNA mutations (Kujoth et al., 2005; Trifunovic et al., 2004), which leads to a decline in mitochondrial function and an associated premature aging phenotype with a significantly decreased lifespan. Mutator mice lose OXPHOS function in the central nervous system but do not display overt signs of neurodegeneration at 12 months of age, especially dopaminergic neuron loss (Dai et al., 2013; Kujoth et al., 2005; Ross et al., 2010). This model is highly relevant to the changes that occur during aging. Aging is the biggest risk factor for PD, and during aging, mitochondrial function declines and mtDNA mutations accumulate (Cottrell et al., 2000).
Mutator mice were crossed to Parkin-KO animals to obtain Parkin-KO, Mutator, Mutator Parkin-KO, and wild-type mice, respectively (Figures S1A and S1C). Mice were born at the expected Mendelian ratios and appeared normal at birth, except for a small but significant decrease in body weight of these mice lacking Parkin, as previously reported (Kim et al., 2011). Mutator and Mutator Parkin-KO mice had reduced body weight at 48- to 52-weeks-of-age (Figures S1B, S1D, and S1E) and a premature aged appearance (not shown). The loss of Parkin did have a surprising effect of partially rescuing the Mutator mouse's splenomegaly phenotype (Trifunovic et al., 2004) (Figure S1F).
Dopaminergic Neurons Degenerate after the Loss of Endogenous Parkin in a Mouse Model of Mitochondrial Dysfunction
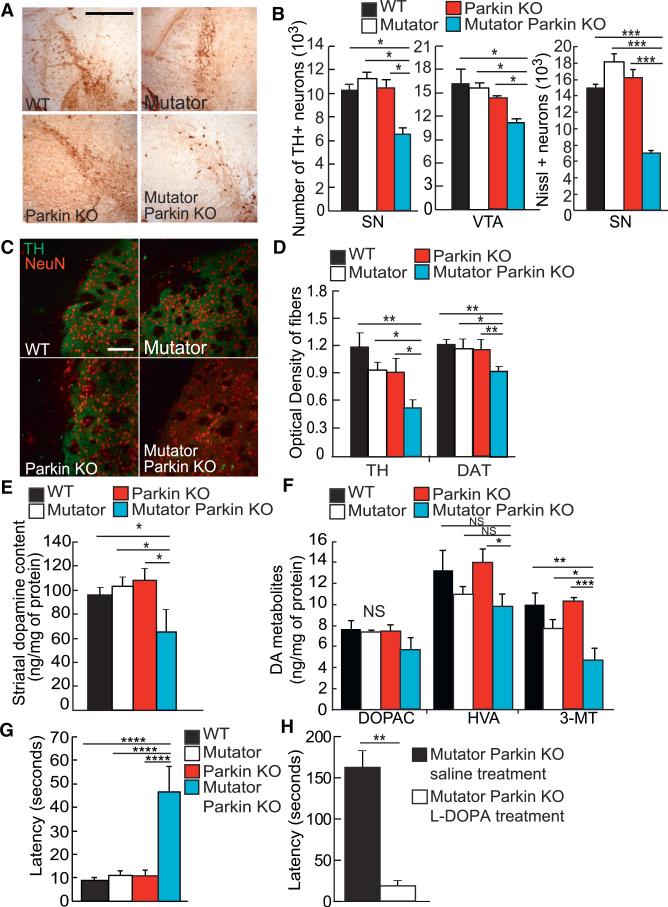
Dopaminergic neurons were quantified from mice sacrificed at 48–52 weeks of age when mitochondria become dysfunctional in the central nervous system of Mutator mice (Ross et al., 2010). Wild-type, Mutator, and Parkin-KO mice displayed no reduction in the amount of TH+ neurons relative to wild-type, as previously reported (Dai et al., 2013; Goldberg et al., 2003). However, Mutator Parkin-KO mice displayed a 40% reduction in TH+ neurons in both the SN and ventral tegmental area (VTA) regions (Figures 1A and 1B). Nissl counts ensured that the loss of TH staining detected in Mutator Parkin-KO mice indicated neuronal death and not a decrease or lack of TH expression (Figure 1B, right). We also analyzed the striatum in order to detect if the axons of these dopaminergic neurons were also affected. We found that Mutator Parkin-KO mice at 12 months of age showed a significant decrease in the optical density of TH+ and DAT+ fibers when compared to wild-type, Parkin-KO, and Mutator mice (Figures 1C and 1D). We checked younger mice before mitochondria had accumulated enough mtDNA mutations to become dysfunctional (Ross et al., 2010). Neither TH+ neurons nor DA striatal fibers were affected in 3-month-old Mutator Parkin-KO mice (Figures S2A–S2D). Thus, endogenous Parkin prevents DA neuron loss in the midbrain from the stress of mitochondrial damage accumulation in Mutator mice.
Figure 1. Dopaminergic Neurons Degenerate after the Loss of Endogenous Parkin in a Mouse Model of Mitochondrial Dysfunction.
(A) Representative images of the substantia nigra detected in midbrain sections after staining with anti-TH antibodies. Scale bar, 500 μm.
(B) The estimated number of TH+ neurons in the substantia nigra and VTA and Nissl+ neurons in the substantia nigra in 48- to 52-week-old mice. n = 4/group.
(C) Representative images of striatum sections stained with anti-TH (green) and anti-NeuN (red) antibodies. Scale bar, 500 μm.
(D) The optical density of TH+ and DAT+ fibers (representative images not shown) when stained with either antibody in striatal sections of 48- to 52-week-old mice. n = 3 to 4 per group.
(E) Basal levels of DA in the striatum of 48- to 52-week-old mice normalized to the amount of protein. n = 5/group.
(F) Basal level of DA degradation metabolites, DOPAC, HVA, and 3-MT, found in the striatum of 48- to 52-week-old mice normalized to the amount of protein. n = 5/group.
(G) Latency time in seconds recorded for 48- to 52-week-old mice performing the pole test. n = 6–8/group.
(H) Latency time in seconds recorded for 56-week-old Mutator Parkin-KO mice performing the pole test. Each individual mouse was scored for a baseline performance for comparison after L-DOPA treatment. n = 4/group.
(A–H) *p < 0.05; **p < 0.01; ***p < 0.001; NS, not significant. Mean ± SEM. ANOVA Tukey's post hoc test.
Another pathological sign of PD is DA depletion in the striatum, which results from the loss of dopaminergic neurons and axonal projections. In agreement with our other results showing a loss of both DA neurons in the SN and striatal dopaminergic fibers (Figures 1A–1D), DA levels were significantly reduced in Mutator Parkin-KO mice (Figure 1E). Furthermore, 3-methoxytyramine (3-MT) but not homovanillic acid (HVA) or 3,4-dihydroxyphenylacetic acid (DOPAC), which are degradation of products of DA metabolism, was decreased in the striatal tissues of Mutator Parkin-KO mice confirming the reduction of DA (Figure 1F). This depletion in DA levels or metabolites was absent in 3-month-old Mutator Parkin-KO mice (Figures S2E and S2F).
The major pathological sign of PD is the presence of Lewy bodies composed of aggregates of several proteins including α-synuclein. We did not detecta-synuclein aggregations in our cohorts. We used mice that overexpress mutated A53T α-synuclein targeted specifically to dopaminergic neurons as a positive control (Figure S2I). There was no increase or presence of neuroinflammatory markers for reactive astrocytes or activated microglia, which are additional pathological indicators of PD, in Mutator Parkin-KO mice or the other groups analyzed (Figures S2G and S2H).
We tested whether or not this degree of DA neuron loss would cause behavioral changes. Because the loss of dopaminergic neurons in man causes motor symptoms, we tested if nigrostriatal-dependent behaviors were affected in Mutator Parkin-KO mice using the pole test. This test, which detects defects in motor coordination, has been used for other PD mouse models, and the behavior is dependent on nigrostriatal function (Matsuura et al., 1997; Pickrell et al., 2011b). Mutator and Parkin-KO mice displayed latency times descending the pole that were comparable to those of wild-type mice (Figure 1G). However, Mutator Parkin-KO mice at 48–52 weeks had significantly higher latency times (Figure 1G). In order to test if the motor coordination defects were specifically due to impairments in the nigrostriatal pathway, we assessed Mutator Parkin-KO mice 4 weeks later before and after L-DOPA treatment. L-DOPA is a precursor in the DA synthesis pathway and is used to ameliorate motor symptoms in PD patients. L-DOPA treatment completely reverted the motor phenotype of the Mutator Parkin-KO mice (Figure 1H; Movie S1), indicating that the climbing impairment was specifically due to the loss of dopaminergic neurons.
Neurons from Different Neuroanatomical Regions Appear Unaffected in Mutator Parkin-KO Mice
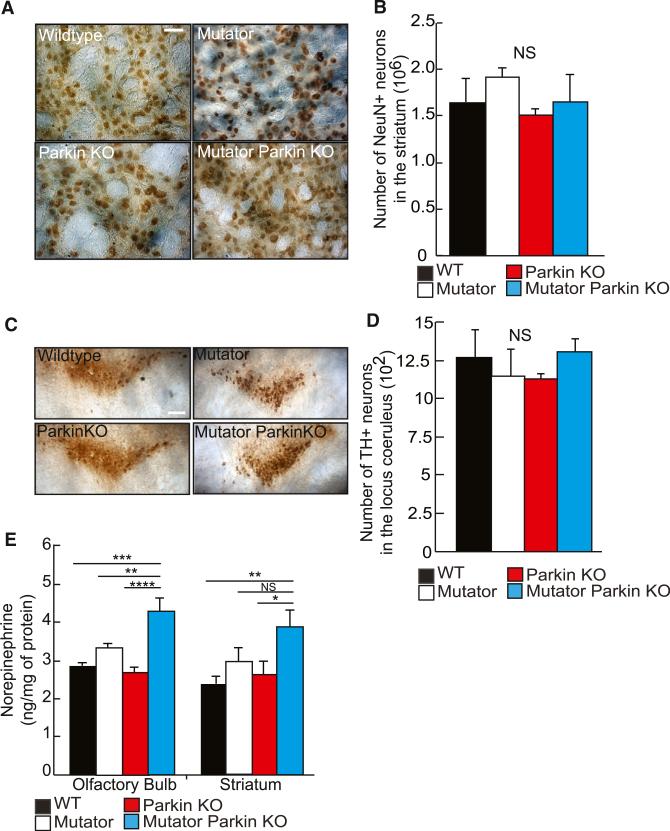
We assessed if other neuronal populations were affected in Mutator Parkin-KO mice. The gross brain weight and appearance of Mutator Parkin-KO mice was identical to that of wild-type mice (Figure S3C). The levels of NeuN protein, a pan neuronal nuclei marker, in the hippocampus and cerebellum of Mutator Parkin-KO mice appeared no different than the other cohorts of mice, indicating that mass neurodegeneration was absent (Figures S3A and S3B).
We also examined specific neuronal populations for evidence of neurodegeneration. GABAergic neurons predominate among neurons in the striatum. Although Mutator Parkin-KO mice displayed a loss of dopaminergic axonal innervation (Figures 1C and 1D) in the striatum, there was no significant difference in the number of NeuN+ (most likely composed of GABAergic neurons) cells in Parkin-KO, Mutator, or Mutator Parkin-KO mice as compared to wild-type controls (Figures 2A and 2B). The locus coeruleus, which contains norepinephrine neurons, is another brain region affected in PD patients (Chan-Palay and Asan, 1989). However, norepinephrine neurons of the locus coeruleus were unaffected in Mutator Parkin-KO mice relative to control mice (Figures 2C and 2D). Surprisingly, norepinephrine levels were significantly elevated in Mutator Parkin-KO mice in the olfactory bulb and striatum, two regions innervated by the locus coeruleus (Figures 2E and S3D). Furthermore, mice exhibited a significant increase in serotonin levels, coincident with DA neurodegeneration, in these same tissues (Figures S3E–S3H). We speculate that this could be a compensatory response to the loss of dopaminergic neurons similar to the increases in serotonin in the striatum previously reported in pharmacological PD models in nonhuman primates and rodents (Boulet et al., 2008; Zhou et al., 1991).
Figure 2. Neurons from Different Neuroanatomical Regions Appear Unaffected in Mutator Parkin-KO Mice.
(A) Representative images of the striatum stained with anti-NeuN antibodies. Scale bar, 20 μM.
(B) The number of NeuN-positive neurons detected in the striatum from mice 48- to 52-week-old. n = 3 to 4/group. NS, not significant. Mean ± SEM. ANOVA Tukey's post hoc test.
(C) Representative images of the locus coeruleus detected in hindbrain sections using anti-TH antibodies. Scale bars, 50 μM.
(D) The number of TH+ neurons counted in the locus coeruleus of 48- to 52-week-old mice. n = 4 to 5/group. NS, not significant. Mean ± SEM.
(E) Basal levels of norepinephrine in the olfactory bulb and striatum of 48- to 52-week-old mice. n = 5 to 6/group.
(A–E) *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; NS, not significant. Mean ± SEM. ANOVA Tukey's post hoc test.
Phosphorylated Ubiquitin at S65 Is Increased during Mitochondrial Dysfunction in the CNS
To corroborate the in vivo function of Parkin in Mutator mice, we assessed PINK1 activity in the brains of Mutator and Mutator Parkin-KO mice. During mitochondrial depolarization, PINK1, a serine/threo-nine-protein kinase, is stabilized on mitochondria (Narendra et al., 2010; Pickrell and Youle, 2015). PINK1 phosphorylates both ubiquitin (Ub) and Parkin at serine 65 leading to Parkin's activation and retention on damaged mitochondria (Kane et al., 2014; Kazlauskaite et al., 2014; Koyano et al., 2014; Ordureau et al., 2014; Wauer et al., 2015). This cascade of events triggers translocation of Parkin specifically to damaged mitochondria causing ubiquitylation of protein targets on the outer mitochondrial membrane, ultimately inducing mitophagy (Pickrell and Youle, 2015).
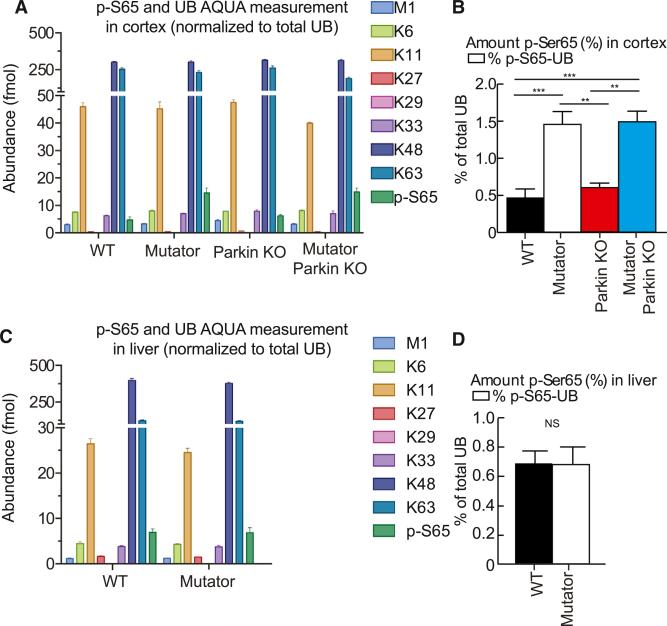
We used a sensitive quantitative mass spectrometry approach to detect phosphorylated ubiquitin at S65 in vivo (Ordureau et al., 2015a). Cortical brain tissue of Mutator mice contains 3.1 times greater levels of phospho-S65-Ub than wild-type cortical tissue. (Figures 3A and 3B). Interestingly, the levels of phospho-S65-Ub in liver tissue did not differ between wild-type or Mutator mice, possibly reflecting differences in the level of expression of PINK1 and/or Parkin (Figures 3C and 3D). Both the liver and brain of Mutator mice accumulate dysfunctional mitochondria (Edgar et al., 2009), whereas the phosphorylation of Ub in response to mitochondrial dysfunction appears to be tissue specific. Additionally, we did not observe a difference between mice with and without Parkin (Figures 3A and 3B), consistent with Parkin functioning downstream of PINK1. Taken together, these data suggest that the PINK1-Parkin pathway is more active in the brains of mice that accumulate dysfunctional mitochondria.
Figure 3. Phosphorylated Ubiquitin at S65 Is Increased during Mitochondrial Dysfunction in the CNS.
(A) UB-AQUA proteomics of total UB, individual UB chain linkages, and phospho-S65 UB in whole-cell cortical lysates. Ubiquitin chain linkage key: blue, M1 linear chains; lime, K6 linkages; gold, K11 chain linkages; red, K27 chain linkages; pink, K29 chain linkages (not quantifiable); violet, K33 chain linkages; navy, K48 chain linkages; aqua, K63 chain linkages; green, p-S65 UB. n = 5/group.
(B) Percentage of phospho-S65 UB normalized to the total percentage of UB in cortical samples. n = 5/group.
(C) UB-AQUA proteomics of total UB, individual UB chain linkages, and phospho-S65 UB in whole-cell liver lysates. Ubiquitin chain linkage key: blue, M1 linear chains; lime, K6 linkages; gold, K11 chain linkages; red, K27 chain linkages; pink, K29 chain linkages (not quantifiable); violet, K33 chain linkages; navy, K48 chain linkages; aqua, K63 chain linkages; green, p-S65 UB. n = 5/group.
(D) Percentage of phospho-S65 UB normalized to the total percentage of UB in liver samples. n = 5/group. (A–D) **p < 0.01; ***p < 0.001; NS, not significant. Mean ± SEM. ANOVA Tukey's post hoc test.
Parkin Does Not Purify the Number of Somatic mtDNA Point Mutations but Affects Their Pathogenicity
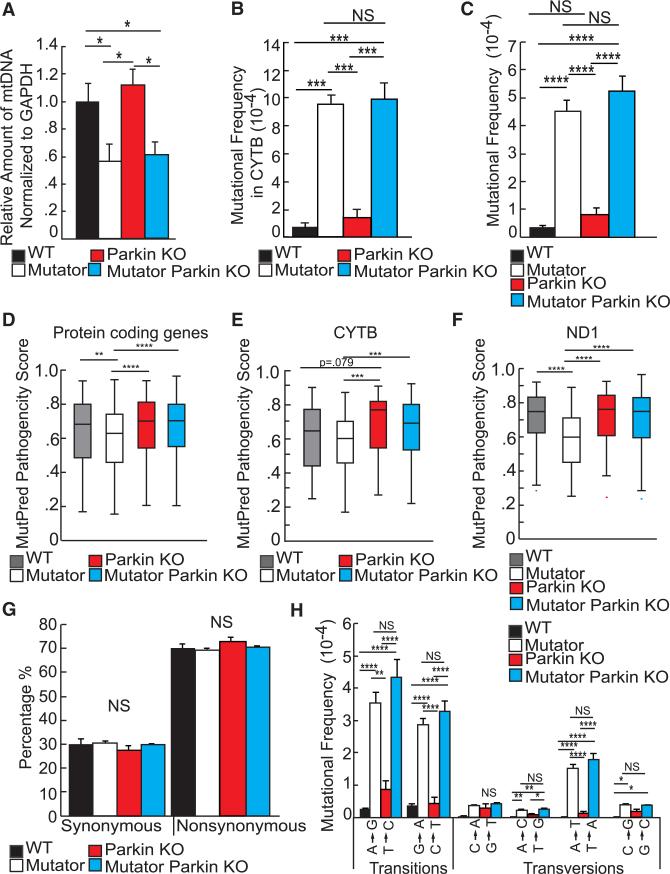
As mtDNA depletion occurs in the brain of Mutator mice (Williams et al., 2010), we quantified mtDNA copy number and found a significant depletion in both Mutator and Mutator Parkin-KO mice as compared to controls, but no significant difference between the two groups (Figure 4A). The excessive cell loss in the Mutator Parkin-KO mice may stem from insufficient autophagy of mitochondria that accumulate deleterious mtDNA mutations. To assess if Mutator Parkin-KO mice accumulate more mtDNA mutations than Mutator mice, we measured the mtDNA mutation rate in the striatum by Sanger sequencing a portion of the mtCYTB gene. We found no difference in the number of mutations generated between Mutator and Mutator Parkin-KO mice (Figure 4B). However, PCR error may mask a difference between the mutation rates using traditional sequencing methods.
Figure 4. Parkin Does Not Purify the Number of Somatic mtDNA Point Mutations but Affects Their Pathogenicity.
(A) Real-time PCR detecting mtDNA copy number using COX1 probes in the striatum of 48- to 52-week-old mice. The nuclear gene GAPDH was probed to normalize variance between samples. n = 5/group.
(B) Sanger sequencing estimating the point mutation rate inferred by analyzing the CYTB gene from 48- to 52-week-old striatal samples. n = 4/group.
(C) Duplex sequencing detecting the point mutation rate of somatic mutations accumulating in 48-to 52-week-old striatal samples. n = 4/group.
(D) Box plot of MutPred prediction scores from mutations detected by Duplex sequencing in striatal samples from mice 48–52 weeks of age. n = 4/group. Number of variants analyzed per genotype: wild-type = 335, Parkin-KO = 448, Mutator = 918, and Mutator Parkin KO = 925.
(E) Box plot of MutPred prediction scores from mutations detected by Duplex sequencing found in CYTB in striatal samples from mice 48–52 weeks of age. n = 4/group. Number of variants analyzed per genotype: wild-type = 43, Parkin-KO = 69, Mutator = 145, and Mutator Parkin-KO = 163.
(F) Box plot of MutPred prediction scores from mutations detected by Duplex sequencing found in ND1 in striatal samples from mice 48–52 weeks of age. n = 4/group. Number of variants analyzed per genotype: wild-type = 51, Parkin-KO = 40, Mutator = 89, and Mutator Parkin-KO = 90.
(G) Percentage of synonymous and non-synonymous mutations detected by Duplex sequencing in 48- to 52-week-old striatal samples. n = 4/group.
(H) Mutational frequency of the types of transitions and transversions in the striatum of 48- to 52-week-old mice. n = 4–6/group.
(A–H) *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; NS, not significant. Mean ± SEM. ANOVA Tukey's post hoc test.
To further assess the ability of Parkin to selectively remove mitochondria harboring deleterious mutated genomes, we performed Duplex Sequencing to increase the sensitivity and coverage and decrease PCR error in sequencing for somatic mtDNA mutations (Kennedy et al., 2013; Schmitt et al., 2012). We found a significant increase in the mutational frequency in Mutator and Mutator Parkin-KO mice relative to wild-type and Parkin-KO mice; however, again there was no difference resulting from the absence of Parkin (Figure 4C). As expected, most of these point mutations generated in Mutator and Mutator Parkin-KO mice were transitions due to the misincorporation of nucleotides during replication due to the defective polymerase γ (Figure 4H).
Although somatic nucleotide base substitutions were not increased in Mutator Parkin-KO mice, it was possible that the types of mutations that accumulate in mice that lacked Parkin were more pathogenic. MutPred software was used to analyze and score the pathogenicity of mutations detected (see Supplemental Experimental Procedures). We observed a slight but significant difference in the median MutPred score between the Mutator and Mutator Parkin-KO group and for two additional genes, ND1 and CYTB, encoding subunits for complexes I and III, respectively (Figures 4D–4F), but not detectably different in the other six individual mtDNA-encoded complex I proteins (Figure S4). Interestingly, endogenous Parkin appears to decrease the MutPred scores in Mutator mice more than in wild-type mice, perhaps because the PINK1-Parkin pathway is activated by mitochondrial stress. The number of point mutations, especially those in protein-coding genes, dictates the phenotype in the Mutator mouse (Edgar et al., 2009) and the loss of Parkin expression increased the predicted pathogenicity of the mutations present. There is no significant difference in the percentage of synonymous and non-synonymous point mutations between groups (Figure 4G). Collectively, these data indicate that Parkin does not select against the quantity of mtDNA mutations but does affect the types of mutations present.
Mitochondrial Dysfunction Is Exacerbated after the Loss of Parkin
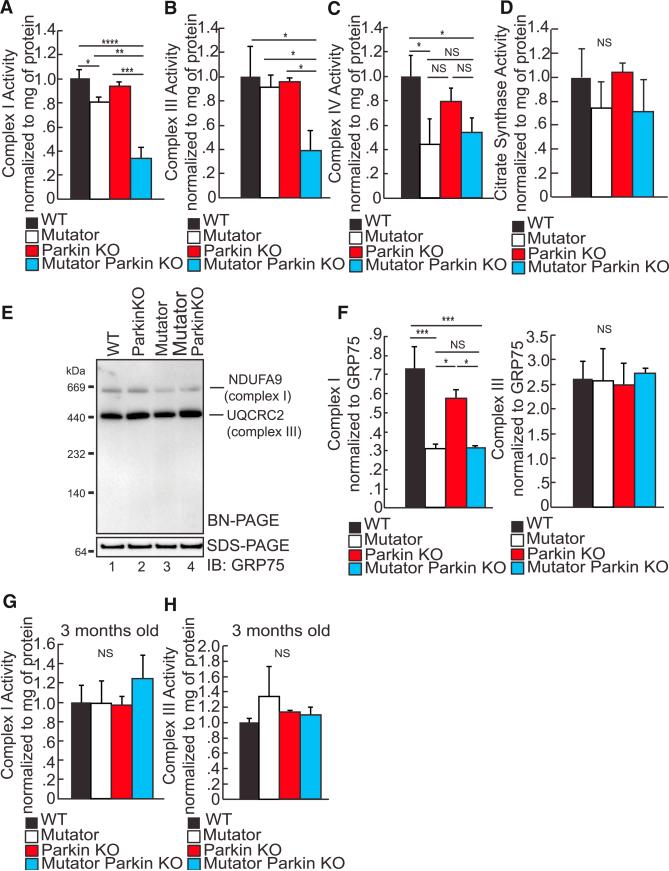
We measured the enzymatic activity of the complexes encoded by mtDNA. A significant defect in the enzymatic activity of complexes I and III was detected in Mutator Parkin-KO mice (Figures 5A and 5B). Complex I was slightly perturbed in Mutator mice, and complex IV activity was decreased in the striatum of both Mutator and Mutator Parkin-KO mice (Figures 5A and 5C). To ensure that this observation was not due to reduced mitochondrial mass, we measured the activity of citrate synthase. Citrate synthase is often used as a surrogate for mitochondrial mass because it is the rate-limiting step in the tricarboxylic acid cycle (TCA) cycle, which means its activity is not dependent on mitochondrial-encoded proteins. We observed no differences in citrate synthase activity between groups, indicating that alterations in mitochondrial mass do not explain the reduced complexes I and III activities (Figure 5D). Importantly, these changes in complexes I and III were not detected in young Mutator Parkin-KO mice (Figures 5G and 5H). In the Mutator Parkin-KO mice, the enzyme defects in complexes I and III correlate with the two mitochondrial-encoded genes with the worst MutPred scores relative to wild-type mice (Figures 4E and 4F). Thus, Mutator Parkin-KO mice not only display specific degeneration of the DA neurons but also biochemically mimic PD in humans (Bender et al., 2006; Schapira et al., 1989, 1990a).
Figure 5. Mitochondrial Dysfunction Is Exacerbated after the Loss of Parkin.
(A) Complex I enzymatic activity assays measuring striatal homogenates of 48- to 52-week-old mice. n = 4 to 5/group.
(B) Complex III enzymatic activity assays measuring striatal homogenates of 48- to 52-week-old mice. n = 4 to 5/group.
(C) Complex IV enzymatic activity assays measuring striatal homogenates of 48- to 52-week-old mice. n = 4 to 5/group.
(D) Citrate synthase activity assays measuring striatal homogenates of 48- to 52-week-old mice. n = 4 to 5/group.
(E) Representative BN-PAGE gel probing for the assembly and stabilization of complex I and III from mitochondria isolated from the striata of 48- to 52-week-old mice.
(F) Quantification of the optical density of BN-PAGE detected complexes I and III detected by western blotting and normalized to GRP75 on a SDS-PAGE gel. n = 5/group.
(G) Complex I enzymatic activity assays measuring striatal homogenates of 12-week-old mice. n = 4 to 5/group.
(H) Complex III enzymatic activity assays measuring striatal homogenates of 12-week-old mice. n = 4 to 5/group.
(A–D and F–H) *p < 0.05; **p < 0.01; ***p < 0.001; NS, not significant. Mean ± SEM. ANOVA Tukey's post hoc test.
To further understand how mitochondrial OXPHOS activity is affected by the loss of Parkin, we performed analysis on the steady-state levels of proteins encoding OXPHOS complexes and their assembly. Performing blue native-PAGE gel analysis, we found that the complex I assembly is perturbed in Mutator and Mutator Parkin-KO striatal mitochondria (Figures 5E and 5F); however, complexes II–V were unaffected (Figures 5E–5F, Figure S5A). We ran western blots of SDS gels to assess the steady-state levels of proteins that encode different complex subunits. COXI, a mtDNA-encoded protein, was slightly decreased in mice harboring the Mutator background (Figure S5B), but we saw no substantial difference in the four mitochondrial subunits tested between Mutator and Mutator Parkin-KO mice (Figure S5B). Taken together, our data are consistent with the idea that the loss of Parkin in the Mutator background results in the persistence of some pathogenic mutations that would otherwise be removed, consistent with a more severe defect in the activity of complexes I and III.
DISCUSSION
Using the mouse as a model system, our work demonstrates that endogenous Parkin expression protects dopaminergic neurons from mitochondrial dysfunction. Although Parkin expression does not significantly shift heteroplasmic point mutation levels in the brain, it does affect the pathogenicity of these mutations.
Sterky et al. (2011) crossed a different mouse model of mitochondrial dysfunction with the Parkin-KO mouse and reported no exacerbation of the phenotypes. In these mitochondrial transcription factor A (TFAM)-KO/Parkin-KO mice, where TFAM is necessary for mtDNA replication and transcription, there was no worsening of dopaminergic neurodegeneration in the absence of Parkin (Sterky et al., 2011). Parkin mediation of quality control at either the mtDNA level or downstream at the protein level would not be anticipated to rescue mitochondria that cannot replicate their DNA.
The Mutator Parkin-KO displays many phenotypes and biochemical signs reminiscent of human PD. Specifically, we found DA neuron loss, depletion of DA in the striatum, and L-DOPA reversible motor disturbances in aged Mutator Parkin-KO mice. However, we did not detect α-synuclein aggregation or the presence of neuroinflammatory markers in the surviving DA neurons, which are classical pathological indicators of PD. Although neuroinflammatory processes are observed in several neurodegenerative disorders such as Alzheimer's disease (AD) and Huntington's disease (HD), the pathologic role of inflammatory processes contributing to dopaminergic neuron loss in PD is not clear (Hirsch and Hunot, 2009). It is important to note that it is still unclear if PARK2 PD patients have Lewy body pathology (Houlden and Singleton, 2012). Interestingly, a recent paper reports that mutant α-synuclein overexpression induces mitophagy and that loss of Parkin exacerbates this phenotype (Chen et al., 2015), suggesting that synuclein pathology may intersect the PINK1/Parkin pathway related to mitophagy.
We also provide in vivo biochemical evidence that the PINK1-Parkin pathway is active in the brain. It has been difficult to assess this pathway previously due to inadequate antibodies and the unlikelihood that neurons would physiologically undergo the synchronized type of mitophagy induction that can be experimentally induced in cultured cell lines that facilitates the detection of Parkin and LC3 accumulation on mitochondria. Mass spectrometry yields the sensitivity to detect ubiquitin phosphor-ylated specifically at serine 65, the product of the PINK1 kinase, offering a biomarker of PINK1 activity. In brains of Mutator mice with mitochondrial dysfunction, the level of phospho-S65 Ub rises substantially. We did not find an increase in phospho-S65 Ub in Mutator mouse liver above levels detected in wild-type mice. This tissue specificity may shed light on why PINK1 and Parkin monogenic patients develop Parkinsonism. These data also support the notion that Parkin is protecting the Mutator mouse through mitophagy, where PINK1 is well established to activate Parkin activity, than through neuroprotective (Müller-Rischart et al., 2013) or transcriptional activation (Shin et al., 2011), where PINK1 has not been shown to be involved.
Why are dopaminergic neurons specifically affected? Multiple lines of evidence support that DA neurons are under more mitochondrial stress than other neuron types. SN DA neurons, due to their pacemaking activity, undergo mild mitochondrial un-coupling, generating excessive reactive oxygen species, and DA metabolism drives the generation of mtDNA mutations (Guzman et al., 2010; Neuhaus et al., 2014). Loss-of-function mutations in Parkin and PINK1 in Drosophila lead to the accumulation of dysfunctional mitochondria specifically in DA neurons (Burman et al., 2012). Mitochondrial protein turnover is reduced in Parkin and PINK1 mutant Drosophila due to decreased mitophagy (Vincow et al., 2013). This causes the specific degeneration of DA neurons with the sparing of other neuron types (serotonergic) in Parkin mutant Drosophila (Whitworth et al., 2005). Our data are in agreement with this model. However, because the Parkin-KO mice have some degree of mitochondrial dysfunction (Palacino et al., 2004), we cannot completely rule out the possibility that the synthetic phenotype occurs because we have heightened the degree of mitochondrial dysfunction in the Mutator Parkin-KO mouse. Without mitochondrial dysfunction synergizing with the loss of Parkin, dopaminergic neuron health is not at risk in the mouse, which is in contrast to man and Drosophila (Palacino et al., 2004; Perez and Palmiter, 2005; Von Coelln et al., 2004). Our results finally provide an explanation for these puzzling findings and strongly suggest that mitochondrial dysfunction in aged wild-type mice does not reach high enough levels for the loss of Parkin to have a detrimental effect.
MtDNA in the striatum shown here and in liver (Trifunovic et al., 2004) showed no shift toward synonymous relative to non-synonymous point mutations. Furthermore, inherited mtDNA mutations are also unaffected by Parkin (Ma et al., 2014). How can Parkin select against the types of mutations present without also affecting the mutation frequency? We may not be able to detect the subtle differences in mutational frequencies between the Parkin-KO and Parkin+/+ backgrounds, if only a minority of sites result in the type of dysfunction that is recognized by PINK1/Parkin. Overexpression of Parkin reverted heteroplasmy in COXI cybrid cells harboring high levels of a deleterious mutant mtDNA, but was not effective in CYTB, mttRNALeu, and mtATP6 cybrid lines (Gilkerson et al., 2012; Suen et al., 2010). These data may reveal that Parkin affects certain mutations, as we observed that Parkin slightly decreased the pathogenicity of mtDNA protein-encoding genes overall and specifically for some complex I and III genes (Figures 4D–4F).
Potentially counteracting Parkin purification of mitochondria at the DNA level is a recent finding that autophagy and mitochondrial clearance are inhibited in reticulocytes in Mutator mice, and decreased autophagy in Mutator mouse embryonic fibro-blasts is associated with increased mTOR activity (Li-Harms et al., 2015). Wholesale mitophagy may be inhibited in Mutator mice whereas Parkin-mediated piecemeal or selective auto-phagy of mitochondrial debris continues to compensate for mtDNA mutations and rescue mitochondrial function and dopaminergic neuron survival. Evidence by several groups suggests that Parkin removes damaged subcomponents of mitochondria or defective respiratory chain components from mitochondria in human cancer cell lines, neuronal generated iPSCs, and Drosophila (Hämäläinen et al., 2013; McLelland et al., 2014; Vincow et al., 2013; Yang and Yang, 2013).
Our findings suggest how Parkin-mediated quality control and mitochondrial dysfunction contribute to pathogenesis of PD. We find that Parkin protects dopaminergic neurons with high levels of mtDNA mutations. We speculate that the accumulation of mtDNA mutations in DA neurons during aging, a decline in mitochondrial function, and/or the loss of Parkin expression could also be contributing factors for sporadic PD patients.
EXPERIMENTAL PROCEDURES
Animals
The generation of Mutator mice was previously described (obtained from Jackson Laboratories: B6.129S7 (Cg)-Polgtm1Tprol/J) (Kujoth et al., 2005). Mutator mice were crossed with Parkin-KO mice (obtained from Jackson Laboratories: B6.129S4-Park2tm1Shn/J) in which most of exon 3 was replaced in-frame by the coding sequence of EGFP. Exon 3 skipping causes a missense mutation and premature termination at a stop codon in exon 5 following 49 additional out-of-frame amino acid residues (Goldberg et al., 2003). Other mouse strain descriptions are included in the Supplemental Experimental Pro cedures. The nuclear background of all the mouse models described here were C57BL/6J (backcrossed at least ten generations).
Immunohistochemistry
Anesthetized mice were perfused with ice-cold 1× PBS and subsequently sacrificed by cervical dislocation. Brains were isolated, and the regions of interest were dissected using a Mouse Brain Slicer Matrix (for midbrain, the region between −1 mm and −4 mm from Bregma; for striatum, the region between −1 mm and +3 mm from Bregma; for hindbrain, the region between −5 mm and −6 mm from Bregma). Brain segments were submerged in 4% paraformaldehyde at 4°C overnight and cryoprotected in a 30% sucrose solution. Brains were fixed in OCT (TissueTek) and frozen by submersion into 2-methylbutane cooled in liquid nitrogen. For fluorescent immunohistochemistry images, 20 mm frozen sections were used. Slides were permeabilized with 0.4% Triton x-100, blocked with 5% BSA for 1 hr at room temperature (RT) and incubated with the following primary antibodies: anti-tyrosine hydroxylase (Sigma) at 1:500, anti-DA transporter (Sigma) at 1:500, anti-NeuN (Cell Signaling), α-synuclein at 1:250 (BioLegend), and anti-Iba-1 1:1,000 (Wako Chemicals) ON at 4°C. Alexa 488- or 594-conjugated secondary antibodies (Invitrogen) were used for 1 hr at RT in the dark. Slides were then mounted with fluorescent mounting media with DAPI (Vector Laboratories). Images were captured with a LSM510 confocal microscope (Zeiss).
Stereological Neuron Counting
Forebrain, midbrain, and hindbrain were isolated as described above. To count dopaminergic neurons, sections were cut starting from Bregma −1 mm; the first 30 slides were discarded (35 mm sections were used). Dopaminergic neurons were counted in every 5th section, with 10–12 total sections counted per individual animal. Norepinephrine neurons were counted in every 4th section, with three sections counted per individual animal starting from Bregma −5.25 mm. NeuN-positive neurons were counted in every 200 μM section striatal sections starting at +1.45 mm (Slow et al., 2003). Stereological counting details are in Supplemental Experimental Procedures. Slides were permeabilized with 0.4% Triton X-100, blocked for 1 hr at RT with normal goat serum (KPL), and incubated with anti-tyrosine hydroxylase- (Immunostar) or anti-NeuN-positive antibodies (Cell Signaling) at 1:500. Secondary conjugated HRP antibodies (KPL) were used for one hour at RT. Experimenter was blinded to genotype of each sample identified by ear tag number. Slides were subsequently incubated with streptavidin-peroxidase (KPL) for 30 min, visualized with 0.05% 3,3′-diaminobenzidine (DAB) for 7 min, and/or costained with Cresyl Violet solution (IHC World) and mounted with glycerol. Representative images were captured with an Axiovert 200 (Zeiss).
OXPHOS Complex Enzyme Activities
Complex I, IV, and citrate synthase activity were measured as previously described (Barrientos et al., 2009). Complex III was measured according to the manufacturer's instructions (Cayman Chemicals). Absorbance was measured with a SpectraMax M2 plate reader (Molecular Devices). Homogenates were prepared from isolated striatum homogenized with a hand-held rotor (VWR) in PBS-containing protease inhibitor cocktail (Roche). Activities were normalized by the amount of protein measured by the Bradford protein assay (BioRad).
Ubiquitin Capture and Proteomics
Ubiquitin and ubiquitylated proteins from cortex extracts (400 μg) or liver extracts (400 μg) were purified using Halo-4xUBAUBQLN1 as described (Ordureau et al., 2014). Briefly, whole-cell extracts were incubated at 4°C for 6 hr with 25 μl of Halo-4xUBAUBQLN1 beads (pack volume) in a final volume adjusted to of 1 ml with lysis buffer (50 mM Tris/HCl [pH 7.5], 1 mM EDTA, 1 mM EGTA, 50 mM NaF, 5 mM sodium pyrophosphate, 10 mM sodium 2-glycerol 1-phosphate, 1 mM sodium orthovanadate, 0.27M sucrose, 1% (v/v) NP-40, 1 μg/ml leupeptin/aprotinin, 0.5 mM 4[2-aminoethyl] benzenesulfonyl fluoride [AEBSF], 50 mM choloracetamide, and phosphatase inhibitor cocktail [Roche]). Following four washes with lysis buffer containing 0.5 M NaCl and five wash in 10 mM Tris (pH 8.0), proteins were released from Halo-4xUBAUBQLN1 beads using 6 M guanidine HCL. Samples were subjected to TCA precipitation and digested overnight at 37°C with Lys-C and trypsin (in 100 mM TEAB, 0.1% Rapigest, 10% ACN). Digests were acidified with equal volume of 5% formic acid (FA) to a pH ~2 for 30 min, dry down, resuspended in 5% FA, and subjected the UB-AQUA proteomics workflow as described below.
UB-AQUA/PRM Proteomics
UB-AQUA/PRM was performed largely as described previously but with several modifications (Ordureau et al., 2015b; Phu et al., 2011). A collection of heavy-labeled reference peptides each containing a single 13C/15N-labeled amino acid was produced at Cell Signaling Technologies and quantified by amino acid analysis. The 19 UB-AQUA reference peptides used for quantitation were previously listed in Ordureau et al. (2014). UB-AQUA peptides from working stocks (in 5% FA) were diluted into the digested sample (in 5% FA) to be analyzed to an optimal final concentration predetermined for individual peptide such that each peptide's intensity would be ranging between 106 and 108. Samples mixed to AQUA peptides were oxidized with 0.1% hydrogen peroxide for 30 min, subjected to C18 StageTip desalting, and re-suspended in 5% FA. Experiments were performed with five independent biological samples and analyzed sequentially by mass spectrometry. Our MS data were collected using a Q Exactive mass spectrometer (Thermo Fisher Scientific) coupled with a Famos Autosampler (LC Packings) and an Accela600 LC pump (Thermo Fisher Scientific). Peptides were separated on a 100-mm i.d. microcapillary column packed with ~0.5 cm of Magic C4 resin (5 μm, 100Å; Michrom Bioresources) followed by ~20 cm of Accucore C18 resin (2.6 μm, 150Å; Thermo Fisher Scientific). Peptides were separated using a 60 min gradient of 3%–25% acetonitrile in 0.125% FA with a flow rate of 300 nl·min−1. The scan sequence began with an Orbitrap full MS1 spectrum with the following parameters: resolution of 70,000, scan range of 200–1,000 Thomson (Th), AGC target of 1 × 106, maximum injection time of 250 ms, and profile spectrum data type. This scan was followed by 12 targeted MS2 scans selected from a scheduled inclusion list with a 5 min retention time window. Each targeted MS2 scan consisted of high-energy collision dissociation (HCD) with the following parameters: resolution of 35,000, AGC of 1 × 106, maximum injection time of 200 ms, isolation window of 1 Th, normalized collision energy (NCE) of 27, and profile spectrum data type. Raw files were searched and precursor and fragment ions quantified using Skyline version 3.1 (MacLean et al., 2010). Data generated from Skyline was exported into a Microsoft Excel spread sheet and GraphPad Prism for further analysis as previously described (Ordureau et al., 2014). Total UB amount was determined as the average of the total UB calculated for each individual locus (Phu et al., 2011). Samples were normalized to total amount of UB (1,000 fmol).
Duplex Sequencing
Duplex Sequencing was performed as previously described (Kennedy et al., 2014; Schmitt et al., 2012) with several modifications. Briefly, 500 ng of total DNA was sonicated in 60 μl of nuclease-free ddH2O and subjected to end-repair and 3′-dA-tailing using the NEBNext Ultra End-Repair/dA-Tailing Module (New England Biolabs) according to the vendor's instructions. A 20:1 molar excess of Duplex Sequencing adapters was ligated to the sample DNA using the NEBNext Ultra Ligation Module (New England Biolabs). After ligation, bead cleaning and PCR amplification were performed as previously described (Kennedy et al., 2014), and the mtDNA was isolated using the Agilent SureSelectXT Target Enrichment System (Agilent) with probes specific for the mouse mitochondrial genome following the manufacturer's instructions. The captured DNA samples were then sequenced on an Illumina HiSeq2500 using 101 bp paired-end sequencing. After sequencing, the reads were aligned against the mouse genome (GRCm38) and processed using a custom software workflow described previously (Kennedy et al., 2014). Reads not uniquely mapping to the mitochondrial genome were excluded from further analysis. After processing, we called de novo somatic mutations by using a clonality cutoff that excluded variants occurring at a frequency of >1% and positions with <100× depth. Mutations were scored only once at each position of the genome.
BN PAGE Gels
Mitochondrial isolation from striatal tissue has been previously described (Pickrell et al., 2011a). In brief, anesthetized animals were killed immediately and regionally dissected brain regions were homogenized in a Dounce glass homogenizer (eight to ten strokes) in SEE buffer (250 mM sucrose, 10 mM HEPES [pH 7.4], 0.5 mM EDTA [pH 7.4], and 0.5 mM EGTA) with the addition of 50 mg/ml BSA and protease inhibitor mixture (Roche) before use. The homogenate was spun on a Sorvall Superspeed RC2-B centrifuge at 4° C at 2,000 × g for 5 min. The supernatant was removed and spun at 12,000 × g for 8 min. The pellet was resuspended in SEE buffer and respun. A final spin of the resuspended pellet was performed in 250 mM sucrose. The final pellet was resuspended in mitochondria incubation buffer (pH 7.2) (130 mM KCl, 2 mM KH2PO4, 2 mM MgCl2, 10 mM HEPES, and 1 mM EDTA). Proteins in the final suspension were measured using the Bradford methodology with a BSA standard curve using Bio-Rad Protein Assay dye (Bio-Rad).
To examine the integrity of the individual mitochondrial respiratory complexes, blue native electrophoresis was performed with 60 μg of isolated mitochondria from striatum. Mitochondria were treated with 1% lauryl maltoside (Sigma) for 20 min on ice. The solubilized material was cleared by centrifugation for 20 min at 14,000 × g. One third of the cleared material was loaded on SDS-PAGE to assess the total protein levels, while the rest was loaded on a 4%–16% acrylamide gradient BN-PAGE gel (Invitrogen). Both gels were transferred to PVDF membranes and incubated with antibodies against the individual respiratory complexes.
Membranes were blocked in 5% milk for 1 hr at RT. Primary antibodies used were anti-NDUFA9 1:500, anti-SDHB 1:1,000, anti-UQCRC2 1:4,000, anti-MTCOXI 1:1,000, and anti-ATP5A 1:5,000 (all from Abcam). For total protein analysis by SDS-PAGE, the antibodies against Grp75 (1:1,000) were used. Primary antibodies were incubated overnight at 4° C. Secondary antibodies were used at 1:5,000 concentrations 1 hr RT. Blots were exposed with ECL Prime (GE Healthcare) detected with the ChemiDoc system (BioRad). Semiquantitative optical density measurements of band intensity were taken with Image Lab software (BioRad).
Approvals
All mice procedures were performed according to a protocol approved by the National Institutes of Health NINDS Institutional Animal Care and Use Committee. Mice were housed in a virus-antigen-free facility at the NIH Division of Veterinary Resources in a 12 hr light/dark cycle at RT and fed ad libitum with a standard rodent diet.
Statistics
A one-way ANOVA was performed for multiple comparisons with Tukey post hoc analysis; p ≤ 0.05 determined significance. The number of observations/animals used in each experimental series was included in the figure legends.
Supplementary Material
Highlights.
Parkin preferentially protects dopaminergic neurons from mitochondrial stress
Phosphorylated-S65 ubiquitin is increased in the brain after mitochondrial stress
Parkin affects the pathogenicity not quantity of somatic mtDNA point mutations
Loss of Parkin exacerbates mitochondrial dysfunction in neurons
ACKNOWLEDGMENTS
This work was supported in part by the National Institutes of Health Grants NINDS intramural program (R.J.Y.), Parkinson's Disease Foundation Fellowship PDF-FBS-1216 and intramural NIGMS Postdoctoral Research Associate Fellowship (PRAT) (A.M.P.), Edward R. and Anne G. Lefler Center Postdoctoral Fellowship (A.O.), NINDS Grant R37NS083524 (J.W.H.), and the Genetic Approaches to Aging Training Grant NIA T32-AG000057 (S.R.K.).
We would like to thank the NIH DNA Sequencing and Computational Biology Core and NINDS/NIH Light Microscopy Core. We also would like to thank Drs. Yoshiyuki Wakabayashi and Shireen Sarraf for technical advice and Dr. Huaibin Cai for access to his StereoInvestigator workstation. The authors declare no competing financial interests.
Footnotes
AUTHOR CONTRIBUTIONS
A.M.P., C.-H.H., and R.J.Y. designed research. A.M.P. and C.-H.H. performed all research unless specifically noted. D.P.S. performed and analyzed BN-PAGE experiments. S.R.K. and J.G.H. performed and analyzed Duplex Sequencing experiments. A.O. performed and analyzed proteomic experiments under the direction of J.W.H. A.M.P., C.-H.H., and R.J.Y. analyzed all data including those specifically noted. A.M.P. and R.J.Y. wrote the paper.
SUPPLEMENTAL INFORMATION
Supplemental Information includes five figures, one movie, and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2015.06.034.
REFERENCES
- Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J. Cell Biol. 2014;206:655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos A, Fontanesi F, Diaz F. Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using polarography and spectrophotometric enzyme assays. Curr. Protoc. Hum. Genet. 2009 doi: 10.1002/0471142905.hg1903s63. http://dx.doi.org/10.1002/0471142905.hg1903s63. [DOI] [PMC free article] [PubMed]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- Bingol B, Tea JS, Phu L, Reichelt M, Bakalarski CE, Song Q, Foreman O, Kirkpatrick DS, Sheng M. The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature. 2014;510:370–375. doi: 10.1038/nature13418. [DOI] [PubMed] [Google Scholar]
- Boulet S, Mounayar S, Poupard A, Bertrand A, Jan C, Pessiglione M, Hirsch EC, Feuerstein C, François C, Féger J, et al. Behavioral recovery in MPTP-treated monkeys: neurochemical mechanisms studied by intrastriatal microdialysis. J. Neurosci. 2008;28:9575–9584. doi: 10.1523/JNEUROSCI.3465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman JL, Yu S, Poole AC, Decal RB, Pallanck L. Analysis of neural subtypes reveals selective mitochondrial dysfunction in dopaminergic neurons from parkin mutants. Proc. Natl. Acad. Sci. USA. 2012;109:10438–10443. doi: 10.1073/pnas.1120688109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V, Asan E. Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer type and in Parkinson's disease with and without dementia and depression. J. Comp. Neurol. 1989;287:373–392. doi: 10.1002/cne.902870308. [DOI] [PubMed] [Google Scholar]
- Chen L, Xie Z, Turkson S, Zhuang X. A53T human α-synuclein overexpression in transgenic mice induces pervasive mitochondria macroautophagy defects preceding dopamine neuron degeneration. J. Neurosci. 2015;35:890–905. doi: 10.1523/JNEUROSCI.0089-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell DA, Blakely EL, Borthwick GM, Johnson MA, Taylor GA, Brierley EJ, Ince PG, Turnbull DM. Role of mitochondrial DNA mutations in disease and aging. Ann. N Y Acad. Sci. 2000;908:199–207. doi: 10.1111/j.1749-6632.2000.tb06647.x. [DOI] [PubMed] [Google Scholar]
- Dai Y, Kiselak T, Clark J, Clore E, Zheng K, Cheng A, Kujoth GC, Prolla TA, Maratos-Flier E, Simon DK. Behavioral and metabolic characterization of heterozygous and homozygous POLG mutator mice. Mitochondrion. 2013;13:282–291. doi: 10.1016/j.mito.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Edgar D, Shabalina I, Camara Y, Wredenberg A, Calvaruso MA, Nijtmans L, Nedergaard J, Cannon B, Larsson NG, Trifunovic A. Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell Metab. 2009;10:131–138. doi: 10.1016/j.cmet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Gilkerson RW, De Vries RL, Lebot P, Wikstrom JD, Torgyekes E, Shirihai OS, Przedborski S, Schon EA. Mitochondrial auto-phagy in cells with mtDNA mutations results from synergistic loss of trans-membrane potential and mTORC1 inhibition. Hum. Mol. Genet. 2012;21:978–990. doi: 10.1093/hmg/ddr529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J. Biol. Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468:696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen RH, Manninen T, Koivumäki H, Kislin M, Otonkoski T, Suomalainen A. Tissue- and cell-type-specific manifestations of heteroplasmic mtDNA 3243A>G mutation in human induced pluripotent stem cell-derived disease model. Proc. Natl. Acad. Sci. USA. 2013;110:E3622–E3630. doi: 10.1073/pnas.1311660110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Houlden H, Singleton AB. The genetics and neuropathology of Parkinson's disease. Acta Neuropathol. 2012;124:325–338. doi: 10.1007/s00401-012-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, Banerjee S, Youle RJ. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J. Cell Biol. 2014;205:143–153. doi: 10.1083/jcb.201402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskaite A, Kondapalli C, Gourlay R, Campbell DG, Ritorto MS, Hofmann K, Alessi DR, Knebel A, Trost M, Muqit MM. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem. J. 2014;460:127–139. doi: 10.1042/BJ20140334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SR, Salk JJ, Schmitt MW, Loeb LA. Ultra-sensitive sequencing reveals an age-related increase in somatic mitochondrial mutations that are inconsistent with oxidative damage. PLoS Genet. 2013;9:e1003794. doi: 10.1371/journal.pgen.1003794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SR, Schmitt MW, Fox EJ, Kohrn BF, Salk JJ, Ahn EH, Prindle MJ, Kuong KJ, Shen JC, Risques RA, Loeb LA. Detecting ultralow-frequency mutations by Duplex Sequencing. Nat. Protoc. 2014;9:2586–2606. doi: 10.1038/nprot.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Stevens MV, Akter MH, Rusk SE, Huang RJ, Cohen A, Noguchi A, Springer D, Bocharov AV, Eggerman TL, et al. Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J. Clin. Invest. 2011;121:3701–3712. doi: 10.1172/JCI44736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kitada T, Tong Y, Gautier CA, Shen J. Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. J. Neurochem. 2009;111:696–702. doi: 10.1111/j.1471-4159.2009.06350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, Kimura Y, Tsuchiya H, Yoshihara H, Hirokawa T, et al. Ubiquitin is phosphor-ylated by PINK1 to activate parkin. Nature. 2014;510:162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat. Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Li-Harms X, Milasta S, Lynch J, Wright C, Joshi A, Iyengar R, Neale G, Wang X, Wang YD, Prolla TA, et al. Mito-protective autophagy is impaired in erythroid cells of aged mtDNA-mutator mice. Blood. 2015;125:162–174. doi: 10.1182/blood-2014-07-586396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Xu H, O'Farrell PH. Transmission of mitochondrial mutations and action of purifying selection in Drosophila melanogaster. Nat. Genet. 2014;46:393–397. doi: 10.1038/ng.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Kabuto H, Makino H, Ogawa N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J. Neurosci. Methods. 1997;73:45–48. doi: 10.1016/s0165-0270(96)02211-x. [DOI] [PubMed] [Google Scholar]
- McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014;33:282–295. doi: 10.1002/embj.201385902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Rischart AK, Pilsl A, Beaudette P, Patra M, Hadian K, Funke M, Peis R, Deinlein A, Schweimer C, Kuhn PH, et al. The E3 ligase parkin maintains mitochondrial integrity by increasing linear ubiquitination of NEMO. Mol. Cell. 2013;49:908–921. doi: 10.1016/j.molcel.2013.01.036. [DOI] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus JF, Baris OR, Hess S, Moser N, Schröder H, Chinta SJ, Andersen JK, Kloppenburg P, Wiesner RJ. Catecholamine metabolism drives generation of mitochondrial DNA deletions in dopaminergic neurons. Brain. 2014;137:354–365. doi: 10.1093/brain/awt291. [DOI] [PubMed] [Google Scholar]
- Ordureau A, Sarraf SA, Duda DM, Heo JM, Jedrychowski MP, Sviderskiy VO, Olszewski JL, Koerber JT, Xie T, Beausoleil SA, et al. Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol. Cell. 2014;56:360–375. doi: 10.1016/j.molcel.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordureau A, Heo JM, Duda DM, Paulo JA, Olszewski JL, Yanishevski D, Rinehart J, Schulman BA, Harper JW. Defining roles of PARKIN and ubiquitin phosphorylation by PINK1 in mitochondrial quality control using a ubiquitin replacement strategy. Proc. Natl. Acad. Sci. USA. 2015a;112:6637–6642. doi: 10.1073/pnas.1506593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordureau A, Münch C, Harper JW. Quantifying Ubiquitin Signaling. Mol. Cell. 2015b;58:660–676. doi: 10.1016/j.molcel.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J. Biol. Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc. Natl. Acad. Sci. USA. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phu L, Izrael-Tomasevic A, Matsumoto ML, Bustos D, Dynek JN, Fedorova AV, Bakalarski CE, Arnott D, Deshayes K, Dixit VM, et al. Improved quantitative mass spectrometry methods for characterizing complex ubiquitin signals. Mol. Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.003756. http://dx.doi.org/10.1074/mcp.M110.003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Fukui H, Wang X, Pinto M, Moraes CT. The striatum is highly susceptible to mitochondrial oxidative phosphorylation dys-functions. J. Neurosci. 2011a;31:9895–9904. doi: 10.1523/JNEUROSCI.6223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Pinto M, Hida A, Moraes CT. Striatal dysfunctions associated with mitochondrial DNA damage in dopaminergic neurons in a mouse model of Parkinson's disease. J. Neurosci. 2011b;31:17649–17658. doi: 10.1523/JNEUROSCI.4871-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Pinto M, Moraes CT. Mouse models of Parkinson's disease associated with mitochondrial dysfunction. Mol. Cell. Neurosci. 2013;55:87–94. doi: 10.1016/j.mcn.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve A, Meagher M, Lax N, Simcox E, Hepplewhite P, Jaros E, Turnbull D. The impact of pathogenic mitochondrial DNA mutations on substantia nigra neurons. J. Neurosci. 2013;33:10790–10801. doi: 10.1523/JNEUROSCI.3525-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JM, Öberg J, Brené S, Coppotelli G, Terzioglu M, Pernold K, Goiny M, Sitnikov R, Kehr J, Trifunovic A, et al. High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc. Natl. Acad. Sci. USA. 2010;107:20087–20092. doi: 10.1073/pnas.1008189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. J. Neurochem. 1990a;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD. Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson's disease. J. Neurochem. 1990b;55:2142–2145. doi: 10.1111/j.1471-4159.1990.tb05809.x. [DOI] [PubMed] [Google Scholar]
- Schmitt MW, Kennedy SR, Salk JJ, Fox EJ, Hiatt JB, Loeb LA. Detection of ultra-rare mutations by next-generation sequencing. Proc. Natl. Acad. Sci. USA. 2012;109:14508–14513. doi: 10.1073/pnas.1208715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1a contributes to neurodegeneration in Parkinson's disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slow EJ, van Raamsdonk J, Rogers D, Coleman SH, Graham RK, Deng Y, Oh R, Bissada N, Hossain SM, Yang YZ, et al. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum. Mol. Genet. 2003;12:1555–1567. doi: 10.1093/hmg/ddg169. [DOI] [PubMed] [Google Scholar]
- Sterky FH, Lee S, Wibom R, Olson L, Larsson NG. Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc. Natl. Acad. Sci. USA. 2011;108:12937–12942. doi: 10.1073/pnas.1103295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen DF, Narendra DP, Tanaka A, Manfredi G, Youle RJ. Parkin overexpression selects against a deleterious mtDNA mutation in heteroplasmic cybrid cells. Proc. Natl. Acad. Sci. USA. 2010;107:11835–11840. doi: 10.1073/pnas.0914569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, Wibom R, et al. Premature ageing in mice expressing defective mitochondrial DNA polymer-ase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- Vincow ES, Merrihew G, Thomas RE, Shulman NJ, Beyer RP, MacCoss MJ, Pallanck LJ. The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc. Natl. Acad. Sci. USA. 2013;110:6400–6405. doi: 10.1073/pnas.1221132110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Coelln R, Thomas B, Savitt JM, Lim KL, Sasaki M, Hess EJ, Dawson VL, Dawson TM. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc. Natl. Acad. Sci. USA. 2004;101:10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauer T, Swatek KN, Wagstaff JL, Gladkova C, Pruneda JN, Michel MA, Gersch M, Johnson CM, Freund SM, Komander D. Ubiquitin Ser65 phosphorylation affects ubiquitin structure, chain assembly and hydrolysis. EMBO J. 2015;34:307–325. doi: 10.15252/embj.201489847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth AJ, Theodore DA, Greene JC, Benes H, Wes PD, Pallanck LJ. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson's disease. Proc. Natl. Acad. Sci. USA. 2005;102:8024–8029. doi: 10.1073/pnas.0501078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SL, Huang J, Edwards YJ, Ulloa RH, Dillon LM, Prolla TA, Vance JM, Moraes CT, Züchner S. The mtDNA mutation spectrum of the progeroid Polg mutator mouse includes abundant control region multimers. Cell Metab. 2010;12:675–682. doi: 10.1016/j.cmet.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JY, Yang WY. Bit-by-bit autophagic removal of parkin-labelled mitochondria. Nat. Commun. 2013;4:2428. doi: 10.1038/ncomms3428. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Bledsoe S, Murphy J. Serotonergic sprouting is induced by dopamine-lesion in substantia nigra of adult rat brain. Brain Res. 1991;556:108–116. doi: 10.1016/0006-8993(91)90553-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.