Recurrent somatic mutations in the JAK2, MPL, and CALR genes have been described in patients diagnosed with Philadelphia-negative myeloproliferative neoplasms (MPN), including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). These mutations are generally mutually exclusive, and their profiles in different disease entities are diverse. In PV, JAK2 mutations exist in approximately 95% of patients. However, in ET and PMF patients, JAK2, CALR, and MPL mutations are present at frequencies of approximately 60%, 20%, and 5%, respectively.1
To further understand MPN pathogenesis associated with the ET and PMF induced by different gene alterations, classification and epidemiological examination of patients according to gene alterations have been performed. In ET, the CALR mutation is associated with a lower hemoglobin level, higher platelet count, lower leukocyte count, and younger age compared with the JAK2V617F mutation;2–5 similar characteristics have been observed in PMF patients.6,7 The CALR mutation is also associated with male predominance,2,5 lower thrombosis risk,4,6 and better overall survival;6 however, these characteristics are not always evident and are diverse in some cases. The same gender ratio has been reported in a Chinese cohort of ET patients with JAK2 and CALR mutations.4 The variation between cohorts in the published data most likely reflects different genetic backgrounds in the different ethnic groups that were studied. In addition, because all analyses have been performed in Caucasian populations, with the exception of one study from China,4 the epidemiological evidence regarding the Asian population is limited.
Here, we studied a Japanese MPN cohort that was previously characterized with respect to the JAK2V617F mutation. The cohort consisted of 66 PV, 112 ET, and 23 PMF patients, as defined by the 2008 World Health Organization (WHO) criteria.8 Clinical and laboratory parameters were obtained at the time of first diagnosis or when genomic DNA samples were collected. This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Juntendo University School of Medicine (IRB#2012208 and #2013020).
All patient specimens that had previously been analyzed for the JAK2 mutation8 were assessed for CALR and MPL mutations using polymerase chain reaction (PCR)-based assays and subsequent deep-sequencing. In addition, the specimens that exhibited a JAK2V617F mutant allele frequency below 10% in the previous study were re-evaluated via deep sequencing (Online Supplementary Appendix and Online Supplementary Table S1). Re-evaluation identified one PV and 2 ET patients as negative for the JAK2 mutation who a PCR-based assay had previously identified as JAK2V617F-positive with a low allele frequency. Conversely, JAK2 mutations in MPN patients who were negative for JAK2, MPL, and CALR mutations by PCR-based assays were not identified by deep sequencing (see below for MPL and CALR mutation detection). Thus, JAK2 mutations were found in 64 (97%) PV (including 3 exon 12 mutations), 61 (54.5%) ET, and 11 (47.8%) PMF patients in this cohort (Figure 1A).
Figure 1.
The JAK2, MPL, and CALR mutation frequencies in ET and PMF patients. (A) The JAK2, MPL, and CALR mutation frequencies in ET (n=112) and PMF (n=23) patients are shown. (B) The mutation statuses of different age groups are shown.
The MPLW515K/L mutation was assessed using a newly developed allele-specific PCR technique called dual amplification refractory mutation system PCR (DARMS-PCR) and subsequent capillary electrophoresis.9 All identified MPL mutations were further verified using deep sequencing. In addition, by screening MPN patients who were previously negative for JAK2, MPL, and CALR mutations we identified MPLW515K/L mutations below the detection limit of DARMS-PCR as well as other MPL mutations (MPLW515R).
Collectively, the MPLW515K/L/R mutation was identified in 9 (8.0%) ET and one (4.4%) PMF patients (Table 1 and Figure 1A), which was similar to the frequencies of 3%–8.3% that were found in Caucasian cohorts2,3,5–7,10–13 but different from those in a Chinese cohort with a substantially lower frequency (1.2%).4 We noted that one ET patient exhibited both the MPLW515K (allele frequency 50.7%) and W515L (allele frequency 2.5%) mutations.
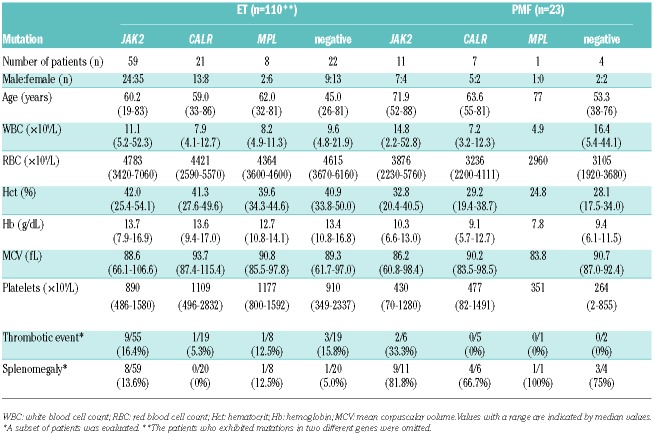
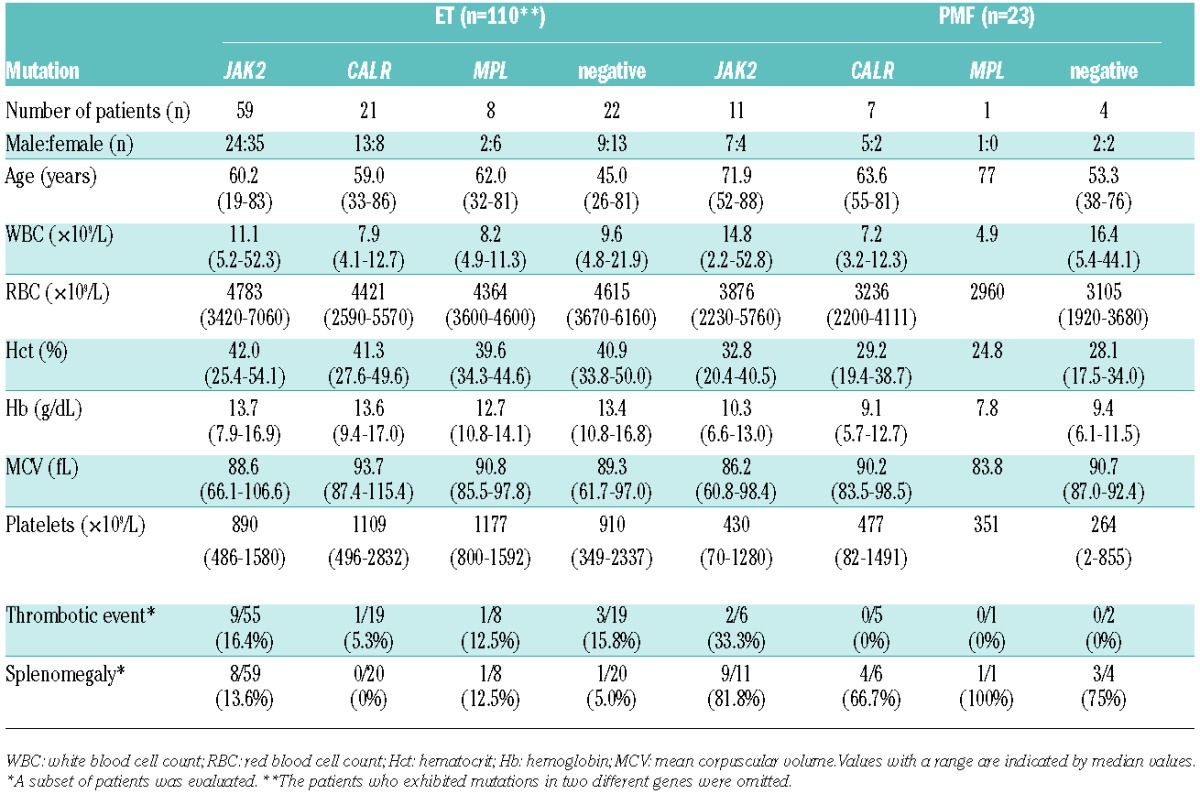
Table 1.
Clinical and hematologic characteristics according to gene mutation status.
The CALR mutation on exon 9 was examined using our in-house fragment analysis method (Online Supplementary Appendix). All identified CALR mutations were confirmed by deep sequencing. CALR mutations were identified in 22 (19.6%) ET and 7 (30.4%) PMF patients (Figure 1A), which was similar to the reported frequency in ET (15.5–28%)2–6 and PMF (25%) patients.7 In contrast, one study examined a Cypriot cohort and identified a frequency of 8.7%,10 but patients with thrombocytosis were not classified according to the WHO 2008 criteria. Unlike the MPL mutation, the CALR mutation was present at similar frequencies in ET patients in both Japanese (19.6%) and Chinese (22.7%)4 cohorts.
CALR mutations (n=29) were present in the following distribution: 11 type 1 (c.1092_1143del), 8 type 2 (c.1154_1155insTTGTC), one type 4 (c.1102_1135del), one type 22 (c.1120_1123del), one type 28 (c.1131_1152del), 2 type 33 (c.1154_1155insATGTC), and one type 34 (c.1154delinsCTTGTC) mutation, as well as four novel mutations (type 42–45; c.1100_1133del, c.1126_1144del, c.1153_1154insTCTGT, and c.1148_1154>GAC) (Online Supplementary Table S2). The CALR mutation in PMF patients is limited to types 1 and 2, and the type 1 mutation (n=6) is more frequent than the type 2 mutation (n=1), as observed in a Caucasian PMF cohort.3 All novel CALR mutations generate a frame shift that converts the C-terminal amino acids from negatively to positively charged, as is the case for other mutations (Online Supplementary Table S2).
Although JAK2, MPL, and CALR mutations have been proposed to be mutually exclusive, we identified one ET patient with JAK2V617F and MPLW515L mutations and one ET patient with JAK2V617F and CALR mutations, which was consistent with recent reports that described a rare concomitant mutation of these gene mutations in Caucasian7,14 and Chinese4 cohorts. Finally, patient specimens that were negative for JAK2V617F, MPLW515K/L, and CALR exon 9 mutations by PCR-based assays were analyzed by deep sequencing of all the JAK2, MPL, and CALR exons (Online Supplementary Appendix). This analysis identified 22 (19.6%) ET and 4 (17.4%) PMF “triple-negative” patients (Figure 1A).
We compared the hematologic and clinical features of patients who were classified according to mutation status (Table 1), with the exception of 2 ET patients who harbored concurrent JAK2 and MPL or CALR mutations (see above). In the ET patients, compared with the JAK2V617F mutation, the presence of the CALR or MPL mutation was associated with lower leukocyte and higher platelet counts (Table 1). The CALR mutation was also associated with a lower red blood cell count. These hematologic features are consistent with the features reported for different ethnic groups.2–5 We observed a trend of male dominance among the ET patients with CALR mutations (male to female ratio 13:8) compared with the patients with JAK2 mutations (male to female ratio 24:35), which is consistent with findings in Caucasian cohorts but inconsistent with findings in a Chinese cohort.4
We determined that the triple-negative ET patients (mean age 45.0 years old) were strikingly younger than the patients with other genotypes (Table 1 and Figure 1B). Adjusted P values for multiple comparisons of ages between triple-negative and other genotypes such as mutated JAK2, CALR, or MPL by Tukey-Kramer test were <0.001, 0.015, and 0.037, respectively. The mean age of the triple-negative ET patients exhibited a wide variation between the cohorts, ranging from 42 to 53 years.3–5 The difference in age between the triple-negative patients and patients with other genotypes was more than 14 years, which was only observed in our cohort. In other cohorts, the difference in the ages of the triple-negative patients with the youngest age and patients with other genotypes with the second youngest age was, at most, five years.15 Despite a lack of known clonal gene mutations, the triple-negative patients’ bone marrow biopsies indicated apparent proliferation of megakaryocytes with a large and mature morphology, and their clinical characteristics corresponded to the WHO 2008 criteria for ET. Although it was a small cohort, we observed a very similar phenomenon in PMF patients; the difference in the ages of the triple-negative patients and patients with other genotypes was more than ten years (Table 1). Patients exhibiting myelofibrosis with no clonal mutations (“triple-negative”) were diagnosed as PMF by excluding other diseases such as myelodysplasia through confirming no dysplasia in erythroid and/or myeloid lineages on bone marrow biopsy, chronic myeloid leukemia through defining Bcr-Abl negativity by FISH or PCR, and other diseases including autoimmune disorders through examining clinical records. This finding suggests that triple-negative ET and PMF patients in Japan have a distinct genetic background that facilitates the acceleration of disease onset compared with the Caucasian population.
In summary, we have shown that the mutation profile of our Japanese cohort of ET and PMF patients is comparable to that of Caucasian cohorts. However, the frequency of the MPL mutation differs between Asian populations, as demonstrated by the differences found between our cohort and a Chinese cohort. We identified four novel CALR mutations, all of which generate an altered C-terminus sequence that is commonly observed in patients with other CALR mutations, which implies that they are genuine mutations. The triple-negative ET and PMF patients were significantly younger than the patients with other genotypes in our cohort. The magnitude of this difference in Japan is much larger than that in other cohorts, which implies the presence of ethnic differences in ET and PMF development in triple-negative cases. Further genetic analysis would help clarify the genetic factors associated with ET development other than JAK2, MPL, and CALR mutations.
Acknowledgments
We thank Kazuhiko Ikeda (Fukushima Medical University), Nobuyoshi Hanaoka (Wakayama Medical University), Toshiro Kurokawa (Toyama Red Cross Hospital), Hideo Harigae (Tohoku University), Takayuki Ikezoe (Kochi University), Jun Murakami (University of Toyama), Kensuke Usuki (NTT Kanto Medical Center), Keita Kirito (University of Yamanashi), and Takao Hirano (Juntendo Nerima Hospital) for providing patient specimens and clinical information. We also thank Joe Matsuoka (Clinical Research Support Center, Juntendo University Graduate School of Medicine); Satoshi Tsuneda and Yuji Sekiguchi for their generous support and encouragement; Kyoko Kubo, Kazuko Kawamura, Junko Enomoto, and Megumi Hasegawa for providing secretarial assistance; and other members of the Department of Hematology for providing support in this study. We also acknowledge the Laboratory of Molecular and Biochemical Research, Research Support Center, Juntendo University Graduate School of Medicine.
Footnotes
Funding: This work was funded in part by JSPS (http://www.jsps.go.jp/english/e-grants/) KAKENHI grant #25860416 (SM). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Cazzola M, Kralovics R. From Janus kinase 2 to calreticulin: the clinically relevant genomic landscape of myeloproliferative neoplasms. Blood. 2014;123(24):3714–3719. [DOI] [PubMed] [Google Scholar]
- 2.Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014;123(10):1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tefferi A, Lasho TL, Finke C, et al. Type 1 vs. type 2 calreticulin mutations in primary myelofibrosis: differences in phenotype and prognostic impact. Leukemia. 2014;28(7):1568–1570. [DOI] [PubMed] [Google Scholar]
- 4.Fu R, Xuan M, Zhou Y, et al. Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia. 2014;28(9):1912–1914. [DOI] [PubMed] [Google Scholar]
- 5.Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014;123(10):1552–1555. [DOI] [PubMed] [Google Scholar]
- 6.Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013; 369(25):2379–2390. [DOI] [PubMed] [Google Scholar]
- 7.Tefferi A, Lasho TL, Finke CM, et al. CALR vs. JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014;28(7):1472–1477. [DOI] [PubMed] [Google Scholar]
- 8.Edahiro Y, Morishita S, Takahashi K, et al. JAK2V617F mutation status and allele burden in classical Ph-negative myeloproliferative neoplasms in Japan. Int J Hematol. 2014;99(5):625–634. [DOI] [PubMed] [Google Scholar]
- 9.Takei H, Morishita S, Araki M, et al. Detection of MPLW515L/K mutations and determination of allele frequencies with a single-tube PCR assay. PloS one. 2014; 9(8):e104958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi J, Nicolaou KA, Nicolaidou V, et al. Calreticulin gene exon 9 frameshift mutations in patients with thrombocytosis. Leukemia. 2014;28(5):1152–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108(10):3472–3476. [DOI] [PubMed] [Google Scholar]
- 12.Rumi E, Pietra D, Guglielmelli P, et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood. 2013;121(21):4388–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guglielmelli P, Pancrazzi A, Bergamaschi G, et al. Anaemia characterises patients with myelofibrosis harbouring Mpl mutation. Br J Haematol. 2007;137(3):244–247. [DOI] [PubMed] [Google Scholar]
- 14.Lundberg P, Karow A, Nienhold R, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123(14):2220–2228. [DOI] [PubMed] [Google Scholar]
- 15.Tefferi A, Wassie EA, Guglielmelli P, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: A collaborative study of 1027 patients. AM J Hematol. 2014;89(8):E121–124. [DOI] [PubMed] [Google Scholar]