In the haploidentical (haplo-) hematopoietic stem cell transplantation (HSCT)1 setting, natural killer (NK) cells play a central role in the successful therapy of high-risk leukemia, including adult acute myeloid leukemia (AML) and pediatric acute lymphoblastic leukemia. Thus, donor-derived allo-reactive NK cells can mediate efficient graft-versus-leukemia (GvL) activity without causing graft-versus-host disease (GvHD).1,2 In this context, it is important to investigate which type of graft manipulation or conditioning regimen may best support a rapid NK-cell recovery and an efficient GvL response following HSCT. In addition, it is necessary to define whether leukemia cells may impair the process of NK-cell generation from HSC as well as the functional capability of mature NK cells. Experimental models of NK-cell differentiation from CD34+ precursors provide a useful tool to analyze the molecular mechanisms involved in this process, under both physiological and pathological conditions. Notably, in vitro models of NK-cell differentiation revealed that CD34+ cells may give rise also to group 3 innate lymphoid cells (ILC3), i.e. cells developmentally related to NK cells involved in defensive function and in lymphoid organ-inducing activity.3–5 In patients receiving HSCT, residual leukemic cells may interfere with the homeostasis of the bone marrow (BM) microenvironment. This effect could be exerted either through cell-to-cell contact or by the release of soluble factors.6,7 Moreover, the conditioning regimen and/or the occurrence of GvHD may sustain an inflammatory response in the BM which may further interfere with the normal hematopoiesis.8 Notably, inflammation has been associated with promotion and progression of solid tumors, and may correlate with a poor prognosis.9 Previous reports showed that serum levels of inflammatory cytokines or chemokines in AML patients may represent a prognostic factor and correlate with the clinical outcome.10,11 In this context, we have previously shown that IL-8 may accelerate in vitro CD34+ cell differentiation towards CD161+ CD56+ (NK) cells, thus suggesting that cytokines or chemokines, released during an inflammatory process, may influence NK-cell differentiation.4
In the present study, in order to address the question as to whether AML blasts may affect NK-cell development, we performed co-cultures of umbilical cord blood (UCB)-CD34+ cells in the absence [control (CTR)] or in the presence of fresh leukemia blasts belonging to different FAB categories. Leukemia blasts were isolated from peripheral blood (PB) or BM of AML patients at diagnosis (Table 1). Cells were plated at graded AML/CD34 cell ratios (1/10, 1/5, and 1/2). UCB samples were obtained from normal full-term neonates after written informed consent from their mothers; AML samples were collected after patient written informed consent. The local Ethical Committee had approved all procedures. Preliminary experiments suggested that, indeed, leukemia cells could interfere with NK-cell differentiation. However, it was difficult to unambiguously distinguish normal precursors undergoing differentiation from fresh AML blasts. Since it was conceivable that the inhibitory effect was, at least in part, mediated by AML-derived soluble factors, trans-well culture experiments were performed in which UCB-CD34+ cells were plated in the lower chambers and AML blasts in the upper chambers.
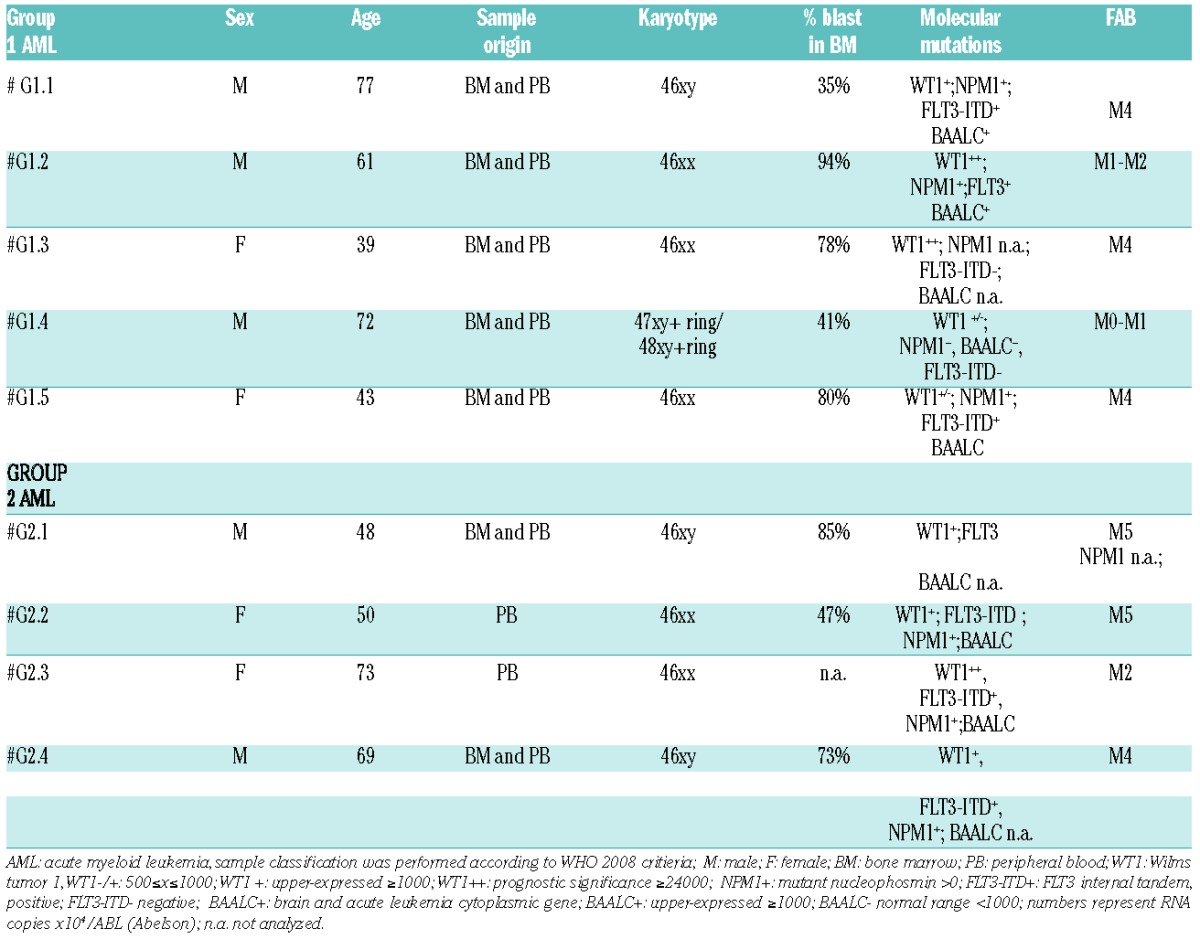
Table 1.
Characteristics of AML samples.
Two groups of AML were identified on the basis of their effect on NK-cell differentiation. Group 1 (G1 AML) did not modify the CD161+ CD56+ (NK) cell recovery. In contrast, Group 2 (G2 AML) displayed a marked inhibitory effect. Inhibition was detectable during the first 20 days of culture in a dose-dependent manner (Figure 1A). Statistical analysis confirmed that Group 2 AML significantly reduced the numbers of CD161+ CD56+ cells recovered from cultures performed at 1/5 AML/CD34 cell ratio after 25 days of culture (Figure 1B). It is worthy of note that we did not observe any increase of apoptotic cells in precursors undergoing differentiation in the presence of G2 AML (data not shown). On the other hand, there was no substantial difference in the recovery of CD161+ CD56+ cells from co-cultures with G1 AML blasts or with normal CD33+ myeloid cells isolated from healthy donors (HD CD33+) to that of controls. Analysis of CD161+ CD56+ cells recovered after 25 days of culture revealed that co-cultures with Group 1 AML contained CD161+ CD56+ cells characterized by a surface phenotype similar to that of controls. In contrast, CD161+ CD56+ cells cultured in the presence of Group 2 AML expressed significantly higher percentages of LFA-1+ and CD94+/NKG2A+ cells than controls (Figure 1C). A modest increase in NKp30, NKp46 and DNAM-1 receptors could be observed only in some experiments (Figure 1C and D). Importantly, the surface expression of NK receptors varied at different AML/CD34 cell ratios (see a representative experiment in Figure 1D). These results indicate that the ratio between leukemic cells and precursors undergoing NK-cell differentiation plays a relevant role in the phenomenon observed (Figure 1D). In G2 AML co-cultures, the ratio between CD56+ LFAℒ1-CD94ℒ/NKG2Aℒcells (that may include both NK-cell precursors and ILC3)5 and CD56+ LFA-1+ CD94+/NKG2A+ cells was modified in favor of the latter (Figures 1D and 2A). Thus, G2 AML may interfere with CD161+ CD56+ cell proliferation mainly by acting on CD56+ LFA−1-CD94−/NKG2A− cells. The increased expression of NK-specific receptors, paralleled by increases of Eomes TF and downregulation of RORγt, supported this possibility (Figure 2A and B). In addition, CD161+CD56+ cells, developed in trans-well co-cultures with G2 AML, expressed higher levels of Granzyme A, B, Perforin and CD107a. CD107a expression was detected upon direct cell interaction with G2 AML blasts. Taken together, these data suggest that CD161+CD56+ NK cells have acquired a more efficient anti-leukemic activity (Figure 2B and C).
Figure 1.
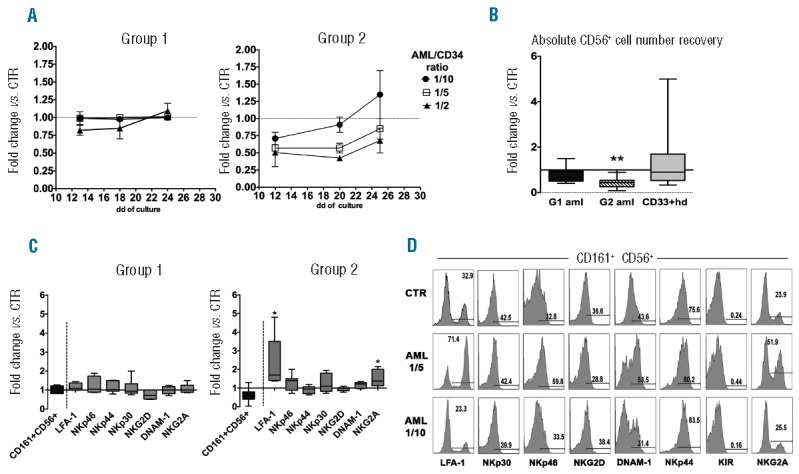
Modulation of in vitro NK-cell differentiation from CD34+ precursors by AML blasts. UCB-derived CD34+ cells were cultured in trans-well plates with medium containing Stem Cell Factor, FMS-like tyrosine kinase 3 ligand, IL-7, IL-15 and IL-21 (cytokine mix medium), in the absence (CTR) or in the presence of human AML blasts. (A) At different time intervals, cells were analyzed for CD161 and CD56 surface expression. Graphics represent the fold change (mean±SEM) of percentages of CD161+CD56+ cells recovered in the presence of AML blasts in comparison with controls, arbitrarily normalized to one. Group 1: 4 independent experiments performed with three different AML. Group 2: 3 independent experiments performed with two different AML. (B) Box and Whisker plot represents the CD161+CD56+ absolute cell number fold changes in cultures performed in the presence of Group 1 AML (8 independent experiments performed with five different G1 AML), Group 2 AML (8 independent experiments performed with four different G2 AML), or in the presence of HD-CD33+ myeloid cells (15 independent experiments performed with 15 different donors) compared to CTR, arbitrarily normalized to one. All cells were plated at 1/5 AML/CD34+ cell ratio. Wilcoxon signed rank test (**P≤0.005). (C) Box and Whisker plot represents the percentages fold change of CD161+CD56+ cells (black box) or CD161+CD56+ NK receptor-positive cells (gray box) after 25 days of co-culture performed in the presence of Group 1 or Group 2 AML (1/5 ratio) in comparison with CTR (normalized to one). Wilcoxon signed rank test (*P≤0.05). (D) Flow-cytometry assays were performed after 25 days of culture to analyze the expression of NK-specific receptors on CD161+CD56+ cells derived from CTR cultures, or from co-cultures with G2 AML at 1/5 and 1/10 AML/CD34+ cell ratio.
Figure 2.
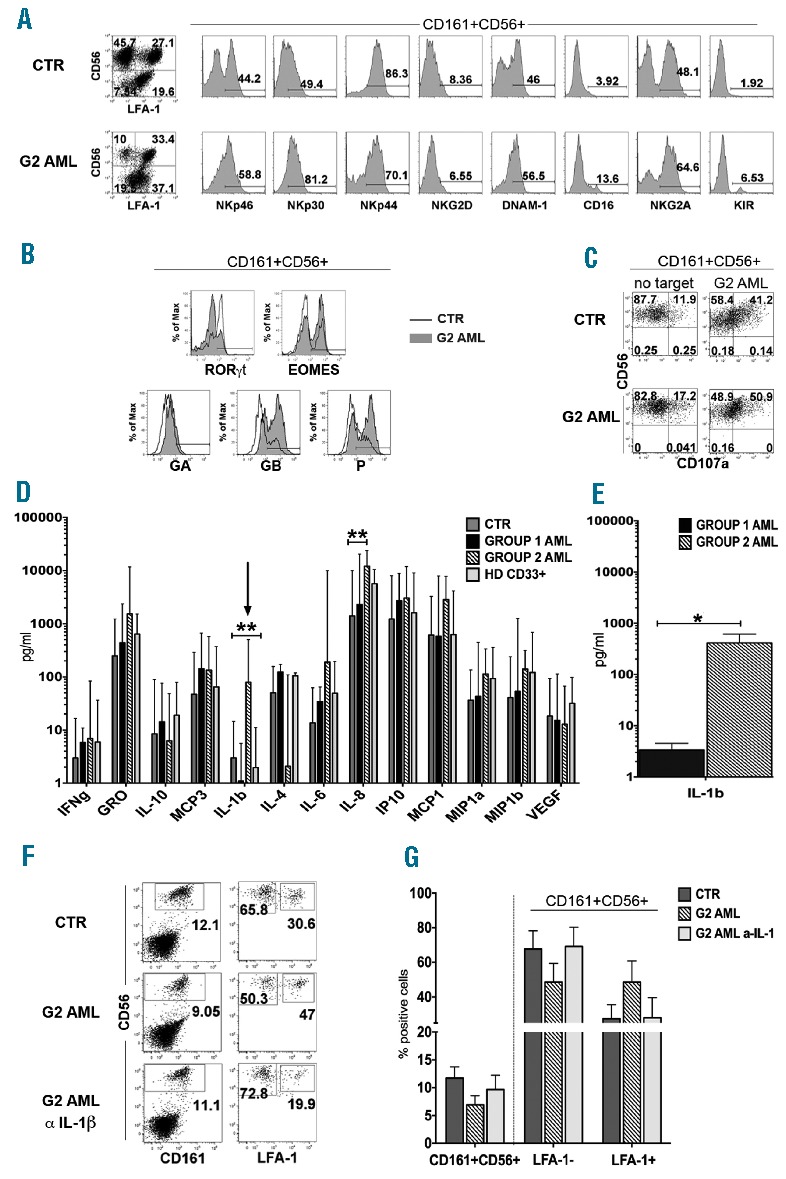
IL-1β-releasing AMLs favor in vitro NK-cell phenotypic and functional maturation. (A) The expression of NK-specific receptors in CD161+CD56+ cells was analyzed after 25 days of culture in the presence of cytokine mix medium alone (CTR) or co-cultured with G2 AML. One representative experiment out of 7 is shown. (B) Flow-cytometry analysis of the indicated TF and of intra-cytoplasmic expression of Granzyme A (GA), Granzyme B (GB) and Perforin (P). Analysis was performed after gating CD161+CD56+ cells. CTR: empty profile; G2 AML: gray profile (#G2.2). One representative experiment out of 4. (C) CD107a analysis was performed after gating on CD161+CD56+ cells, after 3 h incubation with fresh G2 AML (#G2.2). One representative experiment out of 3 performed. (D) After 25 days of culture, SN from CTR cultures, G1 AML, G2 AML and HD-CD33+ cells co-cultures (1/5 cell ratio), were collected and analyzed by ELISA multiplex assay for the indicated cytokine and chemokine. Data are expressed as mean±SEM of pg/mL. IL-1β mean±SEM pg/mL release in the different culture conditions: CTR: 3.83±1.33; G1 AML: 1.94±0.75; G2 AML: 182±68,01; HD CD33+: 4.38±1.84. Multiple column t-test (**P≤0.005). (E) Analysis of IL-1β release by G1 or G2 AML cultured alone for 48 h in the presence of cytokine mix medium. Data are expressed as mean±SEM of pg/mL, obtained by analysis of supernatant (SN) derived from 5 G1 AML and 4 G2 AML. IL-1β mean±SEM pg/mL release: G1 AML: 3.37±1.37; G2 AML: 413,37± 201. (*P<0.05, Mann-Whitney test). (F) Cells were cultured in cytokine mix medium (CTR) with G2 AML in the absence or in the presence of neutralizing anti-IL-1β mAb plus G2 AML. The surface expression of CD161, CD56 and of LFA-1 was evaluated after 15 days of culture. One representative experiment out of 3. (G) Histogram represents CD161+CD56+ cell percentages (left) and percentages of CD161+CD56+LFA-1− and CD161+CD56+ LFA-1+ cells (right) detected in control cultures (CTR) or in G2 AML co-cultures in the absence or in the presence of neutralizing anti-IL-1β mAb. Data are expressed as mean±SEM obtained after 3 independent experiments performed with three different G2 AML.
In order to identify factors possibly responsible for the modulatory effect on NK-cell differentiation, we performed a multiplex assay allowing detection of a large panel of cytokines and chemokines in culture supernatant (SN). Figure 2D shows that SN derived from co-cultures with G2 AML contained significantly higher concentrations of IL-1 β as compared either with CTR or with co-cultures containing G1 AML or HD CD33+ cells. On the other hand, IL-8 concentrations were significantly higher only when compared with control cultures.
Analysis of SN derived from AML cells, cultured alone, revealed that G2 AML blasts produced significantly higher amounts of IL-1 β compared to G1 AML (Figure 2E). In order to verify whether IL-1 β was involved in inhibition of NK-cell differentiation, experiments were performed in which neutralizing anti-IL-1 β mAb was added to G2 AML co-cultures. In these experiments, both the percentages and the phenotypic properties of CD161+CD56+ cells recovered in the presence of neutralizing mAb were similar to those of control cultures in the absence of G2 AML (Figure 2F and G). Addition of neutralizing anti-IL-1β mAb to control cultures did not induce relevant changes in CD161+CD56+ cell recovery (mean±SEM from 4 independent experiments: CTR=15.8±3.1%; CTR+anti-IL-1β mAb=14.7±2.26%).
Although we cannot exclude the possibility that other inhibitory factors may contribute to the modulation of NK-cell differentiation, the finding that the numbers of CD161+CD56+ cells recovered after treatment with neutralizing anti-IL-1β mAb were comparable to those of control cultures, suggested that IL-1β secreted by G2 AML plays a predominant role in the inhibitory effect and is responsible for the different effect in comparison with G1 AML.
Previous reports showed that mature NK cells isolated from peripheral blood of AML patients displayed a low expression of major activating NK receptors resulting in an impaired cytolytic activity.12–15 In this context, it has been shown that sera derived from human AML patients may contain microvesicles bearing TGF-β on their surface TGF-β, and can induce modulation of the surface expression of activating NK receptors resulting in impairment of NK-cell function.14 This effect was not detectable under our experimental condition, most likely due to the use of a trans-well system in which microvescicles cannot diffuse from upper to lower chambers containing cell precursors. Notably, previous reports regarding inhibitory AML-mediated effect focused on PB mature NK cells, and not on differentiating NK-cell precursors, which may display a different susceptibility to AML-derived inhibitory factors. In this context, further experiments are in progress to better characterize the effects of AML and IL-1β on NK-cell development. On the basis of our present data, it is conceivable that IL-1β produced by AML blasts may affect proliferation of NK-cell precursors at early stages of differentiation in the BM. Our present data are relevant in the context of HSCT in which IL-1β released by AML blasts, residual after the conditioning regimen, may alter the BM microenvironment displaying a bidirectional effect, on the one hand, by limiting proliferation of NK-cell precursors, it would impair NK-cell recovery. On the other hand, IL-1β would promote differentiation of CD34+ precursors towards mature, competent NK cells. However, due to the low numbers of NK cells recovered, the overall effect is likely to be detrimental, primarily in patients with high residual leukemic burden or in rapidly relapsing AML.
Acknowledgments
Authors would like to thank Fabio Guolo, IRCCS AOU San Martino-IST, Genova, for providing detailed information on AML samples.
Footnotes
Funding: the following Institutions for their financial support: AIRC (Associazione Italiana per la Ricerca sul Cancro), IG project n. 10225 (L.M.), and Special Project 5x1000 n. 9962 (L.M.); Ricerca Finalizzata RF-IG-2008–CUP G21J11000040001 (M.C.M.); 5x1000 Min. Sal. 2011(M.C.M.). Elisa Montaldo is a recipient of a fellowship awarded by FIRC (Fondazione Italiana per la Ricerca sul Cancro).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Moretta L, Locatelli F, Pende D, Marcenaro E, Mingari MC, Moretta A. Killer Ig-like receptor-mediated control of natural killer cell allore-activity in haploidentical hematopoietic stem cell transplantation. Blood. 2011;117(3):764–771. [DOI] [PubMed] [Google Scholar]
- 2.Locatelli F, Pende D, Mingari MC, et al. Cellular and molecular basis of haploidentical hematopoietic stem cell transplantation in the successful treatment of high-risk leukemias: role of alloreactive NK cells. Front Immunol. 2013;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J, Freud AG, Caligiuri MA. Location and cellular stages of natural killer cell development. Trends Immunol. 2013;34(12):573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montaldo E, Vitale C, Cottalasso F, et al. Human NK cells at early stages of differentiation produce CXCL8 and express CD161 molecule that functions as an activating receptor. Blood. 2012; 119(17):3987–3996. [DOI] [PubMed] [Google Scholar]
- 5.Montaldo E, Vacca P, Moretta L, Mingari MC. Development of human natural killer cells and other innate lymphoid cells. Semin Immunol. 2014;26(2):107–113. [DOI] [PubMed] [Google Scholar]
- 6.Mussai F, De Santo C, Abu-Dayyeh I, et al. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood. 2013;122(5):749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curti A, Trabanelli S, Salvestrini V, Baccarani M, Lemoli RM. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: focus on hematology. Blood. 2009;113(11):2394–2401. [DOI] [PubMed] [Google Scholar]
- 8.Ayala F, Dewar R, Kieran M, Kalluri R. Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia. 2009;23(12):2233–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsimberidou AM, Estey E, Wen S, et al. The prognostic significance of cytokine levels in newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndromes. Cancer. 2008;113(7):1605–1613. [DOI] [PubMed] [Google Scholar]
- 11.Kornblau SM, McCue D, Singh N, Chen W, Estrov Z, Coombes KR. Recurrent expression signatures of cytokines and chemokines are present and are independently prognostic in acute myelogenous leukemia and myelodysplasia. Blood. 2010;116(20):4251–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costello RT, Sivori S, Marcenaro E, et al. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99(10):3661–3667. [DOI] [PubMed] [Google Scholar]
- 13.Carlsten M, Baumann BC, Simonsson M, et al. Reduced DNAM-1 expression on bone marrow NK cells associated with impaired killing of CD34+ blasts in myelodysplastic syndrome. Leukemia. 2010;24(9):1607–1616. [DOI] [PubMed] [Google Scholar]
- 14.Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 2011; 96(9):1302–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stringaris K, Sekine T, Khoder A, et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica. 2014; 99(5):836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]


