In patients with multiple myeloma (MM) not eligible for high-dose therapy and autologous stem cell transplantation (ASCT), the 2 following options are recommended as part of front-line treatment and approved based on data from randomized phase III trials: melphalan/prednisone/thalidomide (MPT), or bortezomib/melphalan/prednisone (VMP).1–2 Lenalidomide combined with low-dose dexamethasone (Len-dex) was recently compared to MPT in a large randomized phase III trial, and showed superiority in terms of progression-free survival (PFS) and overall survival (OS).3 Nevertheless, most patients ultimately relapse and new combinations are needed to improve survival from the time of progression. At the time of first relapse, lenalidomide plus dexamethasone4 or bortezomib plus dexamethasone5 are the most commonly used regimens. Bendamustine is another option and has shown promising results in patients with advanced disease.6 In the present phase II study, we investigated a triplet combination consisting of bortezomib, dexamethasone plus bendamustine (BVD) in elderly patients at the time of first relapse.
Eligible patients were aged 65 years or over and relapse/refractory MM following only one line of prior therapy. The main exclusion criteria were: more than one prior anti-myeloma treatment; front-line ASCT; prior use of bortezomib or bendamustine. All patients provided written informed consent. The study was registered with as NCT01045681 and EudraCT as 2009-012359-91 (www.clinicaltrials.gov).
The BVD regimen consisted of intravenous (IV) bendamustine 70 mg/m2 on days 1 and 8, IV bortezomib 1.3 mg/m2 on days 1, 8, 15 and 22, and oral dexamethasone 20 mg on days 1, 8, 15 and 22. Cycles were repeated every 28 days. Response was evaluated after 4 cycles of BVD. Per protocol, patients achieving partial response (PR) or better received 2 additional cycles followed by a maintenance phase that consisted of 6 BVD cycles given every two months. Overall, the total number of cycles was 12 (6 cycles administered monthly, plus 6 administered every 2 months), and duration of therapy was 72 weeks. Patients who did not reach PR following the first 4 cycles of BVD were excluded from the study.
The primary end point was the overall response rate (ORR) after 4 cycles of BVD. Secondary end points included best response on therapy, PFS, OS and toxicity. Response (centralized assessment) was evaluated using the International Uniform Response Criteria for MM,7 after each treatment cycle, and every two months during the follow-up phase. Centralized fluorescence in situ hybridization (FISH) study at baseline [t(4;14) translocation and del(17p)] was performed. PFS and OS curves were estimated using the Kaplan-Meier method. A multivariate logistic regression analysis according to Cox model was performed to identify predictive factors for response.
From March 2010 to July 2011, a total of 73 patients were enrolled. All patients had received only one line of therapy. This had been MPT in 42 patients (57.5%), Lendex according to the FIRST study3 in 14 (19.2%), melphalan/prednisone in 12 (16.5%), and other regimens in 5 cases (6.8%). The median time from start of first-line therapy to the initial dose of chemotherapy of the BVD regimen was 29 months (range 5–88 months). The median age at inclusion was 76 years (range 66–86 years) (Table 1).
Table 1.
Patients’ base-line characteristics and response to therapy.
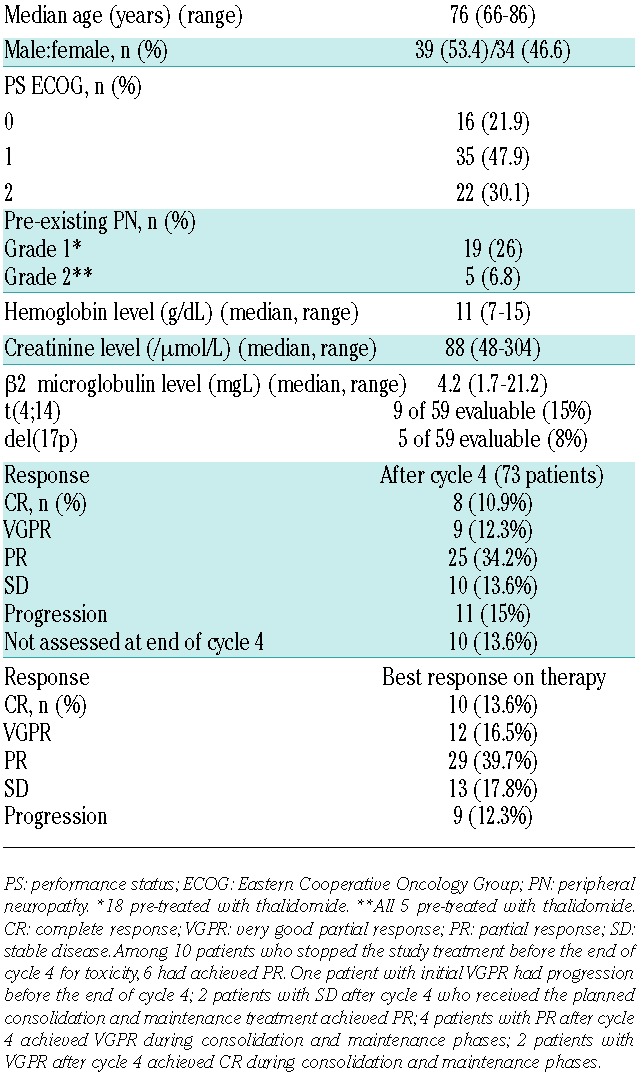
Forty-two patients of 73 (57.6%) achieved at least PR after 4 cycles of BVD: a complete response (CR) was seen in 8 (10.9%), a very good partial response (VGPR) in 12 (16.5%), and a PR in 29 patients (39.7%). The remaining 31 patients had either stable disease (n=10, 13.6%), progressive disease (n=11, 15%), or premature study treatment interruption for toxicity (n=10, 13.6%) respectively. These 31 patients were excluded from the trial, except 3 patients in stable disease with clinical improvement who received the planned additional cycles according to study design. Best ORR assessed during treatment was 69.8% (Table 1). Time to best response was 1.9 months (range 0.9–19.3 months). The median number of cycles administered was 7 (range 1–12). Twenty-six patients of 40 (65%) who started the maintenance phase completed the planned 12 cycles of therapy.
At the cut-off date of July 2013, with a median follow up of 15.7 months, the median PFS (Figure 1A) was 10.8 months (95%CI: 7.0–18.2 months). Thirty-seven patients (50.6%) had died: 6 from treatment-related toxicity (5 infections, one renal failure), and 31 from disease progression. Early death, within the first three months of therapy, occurred in 7 patients (9.5%), which was due to progressive disease in 3, infection in 3, and renal failure in one patient. The median OS (Figure 1B) was 23 months (95%CI: 15.4–27.5 months).
Figure 1.
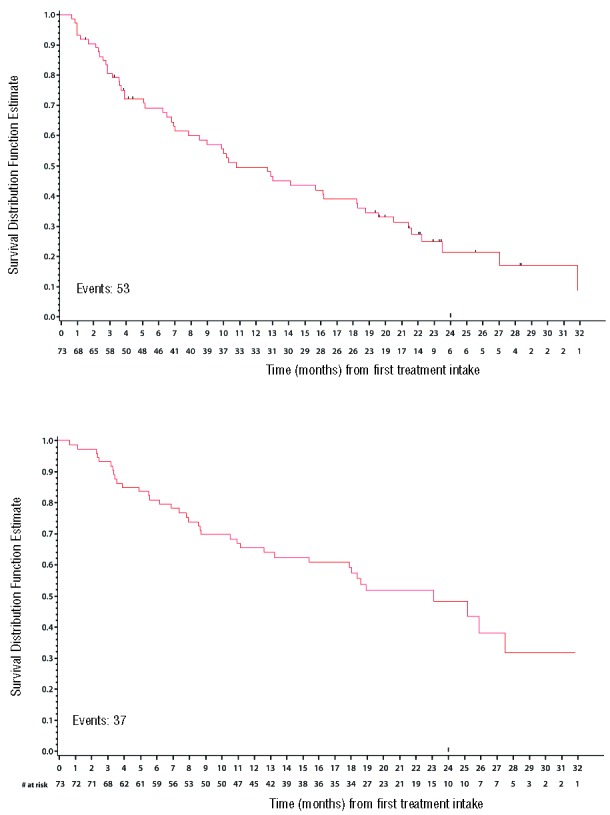
(A). Progression-free survival. (B). Overall survival.
Regarding high-risk cytogenetics, 7 of 9 patients with t(4;14) responded (2 CR, 4 VGPR and one PR); one progressed on therapy, and the last patient died during cycle 2 from infection. The median OS was 23 months (2–36). Only 1 of 5 patients harboring del(17p) responded to the BVD regimen; the remaining 4 patients progressed on treatment.
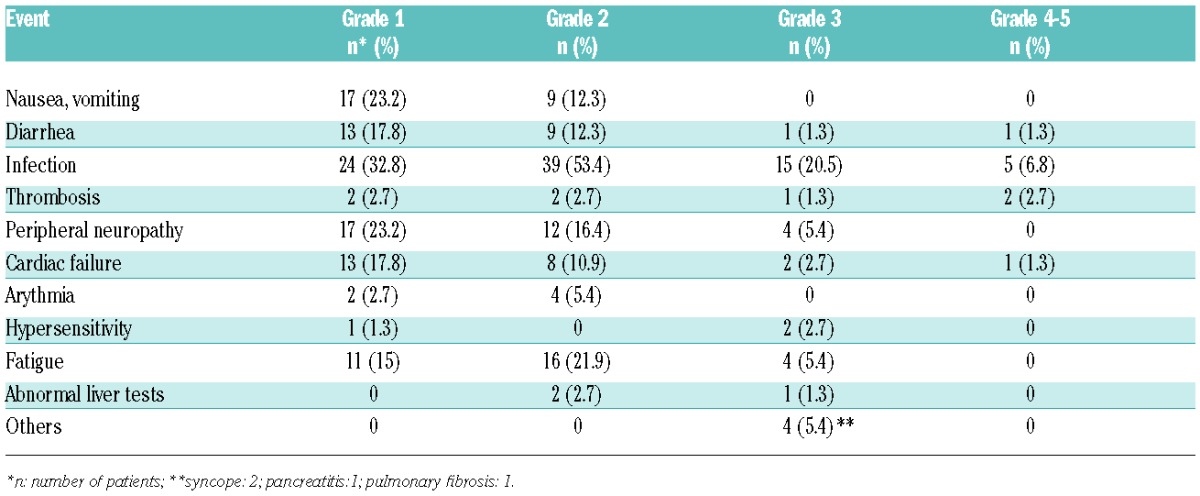
The most common adverse events (AEs) are summarized in Table 2. Grade 3 and 4 AEs occurred in 48 patients (65.7%). Few patients experienced grade 3 and 4 hematologic toxicity: neutropenia was seen in 15 (20.5%), thrombocytopenia in 7 (9.5%), and anemia in 4 patients (5.5%), respectively. The most common grade 3–4 AE was infection: 20 episodes in 17 patients (23.2%); infection was responsible for death in 5 cases. All patients who died from infection were 70 years or older (range 70–81 years). Significant peripheral neuropathy (PN) was reported in 16 patients (21.9%): grade 2 in 12, and grade 3 in 4 patients, respectively. Overall, these AEs led to dose modification in 33 patients (45.2%). The dose of bortezomib was decreased in 19 patients (26%), mainly due to PN (12 patients) and thrombocytopenia (2 patients). Bendamustine dose reduction occurred in 19 patients (26%), mostly because of hematologic toxicity: neutropenia in 11, and thrombocytopenia in 6 cases.
Table 2.
Non-hematologic adverse events.
In univariate analysis, adverse predictive factors for response were PS = 2 (P=0.004), and del(17p) (P=0.03). The multivariate regression model identified PS = 2 as the single factor predicting for a lower response rate (HR 0.306; 95%CI: 0.16–0.59; P=0.0004).
The aim of our study was to evaluate both the safety and efficacy of a triplet combination consisting of bendamustine / bortezomib /dexamethasone in a very homogeneous cohort of patients older than 65 years of age, with disease relapsing or progressing after only one prior line of therapy. Since MPT is one of the combinations considered a standard of care for the front-line treatment of elderly patients (not eligible for ASCT),1 and lenalidomide / low-dose dexamethasone, although not yet approved, also presents a very promising combination as part of first-line therapy in the same group of patients,3 it is, therefore, logical to evaluate IMiD-free combination strategies that include bortezomib at the time of first relapse. The combination of bendamustine, which has previously demonstrated efficacy in the relapse setting,6 with bortezomib and dexamethasone was, therefore, considered to be a good candidate to be tested in elderly patients at the time of first relapse.
Our study shows that the BVD combination was able to induce a rapid and high response rate (almost 70%). Although we must exercise caution when comparing data on response rates of phase II and III trials in the relapse setting, our results compare favorably with those achieved with bortezomib-dexamethasone, showing an ORR between 50%5 and 54.6%,8 or with lenalidomide-dexamethasone.4 The best options for future treatment of relapsed MM are likely to consist of triplet combinations, which have, up to now, been reported in only a few phase III trials.9 Nevertheless, results from a number of phase II trials of triplet combinations are available. Recently, Ludwig reported the results of the BVD combination in 79 patients with relapsed MM.10 Interestingly, overall their findings are similar to ours: the ORR was 61%, with a PFS of ten months, with the notable exception that Ludwig et al. allowed the inclusion of patients previously exposed to bortezomib, previously treated with ASCT, and having received more than one prior line of therapy. The duration of therapy was also different, with the use of 8 consecutive monthly cycles of BVD. Offidani and the Italian group also evaluated the BVD regimen with exactly the same schedule of administration as used in our study.11 They also enrolled patients treated with ASCT as part of front-line therapy, patients previously exposed to bortezomib, and patients who had experienced more than one relapse. The ORR reported by Offidani11 is identical to that in our study (71.5%), while the median PFS was longer (15.5 months). Another potential advantage of the BVD combination is that it can be safely administered in patients with renal failure since neither bortezomib nor bendamustine require dose adaptations in this setting.12
In our population, the toxicity of the BVD combination was manageable. A minority of patients interrupted treatment because of intolerance. The incidence of severe hematologic toxicity was moderate. Serious infection was the major side effect.
With the widespread use of front-line MPT in elderly patients, and in the near future of Len-dex, our results, obtained in a homogeneous population of patients treated at the time of first relapse, suggest that the BVD combination could form the basis of future trials.
Footnotes
Trial registrations: Clinicaltrials.gov identifier NCT01045681; EudraCT identifier 2009-012359-91.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Fayers PM, Palumbo A, Hulin C, et al. Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood. 2011;118(5):1239–1247. [DOI] [PubMed] [Google Scholar]
- 2.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. [DOI] [PubMed] [Google Scholar]
- 3.Benboubker L, Dimopoulos M, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906–917. [DOI] [PubMed] [Google Scholar]
- 4.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. [DOI] [PubMed] [Google Scholar]
- 5.Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed and refractory multiple myeloma. Br J Haematol. 2004;127(2):165–172. [DOI] [PubMed] [Google Scholar]
- 6.Damaj G, Malard F, Hulin C, et al. Efficacy of bendamustine in relapsed/refractory myeloma patients: Results from the French compassionate use program. Leuk Lymphoma. 2012;53(4):632–634. [DOI] [PubMed] [Google Scholar]
- 7.Durie BGM, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(12):1467–1473. [DOI] [PubMed] [Google Scholar]
- 8.San Miguel J, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014:15(11):1195–1206. [DOI] [PubMed] [Google Scholar]
- 9.Lonial S, Kaufman JL. The era of combination therapy in myeloma. J Clin Oncol. 2012:30(20):2434–2436. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig H, Kasparu H, Leitgeb C, et al. Bendamustine-bortezomib-dexamethasone is an active and well tolerated regimen in patients with relapsed or refractory multiple myeloma. Blood. 2014; 123(7):985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Offidani M, Corvatta L, Maracci L, et al. Efficacy and tolerability of bendamustine, bortezomib and dexamethasone in patients with relapsed-refractory multiple myeloma: a phase II study. Blood Cancer J. 2013;3.e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lentzch S. Bendamustine: the remedy that came in from the cold. Blood. 2014;123(7):948–950. [DOI] [PubMed] [Google Scholar]


