Abstract
Vosaroxin is a first-in-class anticancer quinolone derivative that intercalates DNA and inhibits topoisomerase II. This study assessed the safety and tolerability of vosaroxin plus cytarabine in patients with relapsed/refractory acute myeloid leukemia. Escalating vosaroxin doses (10-minute infusion; 10–90 mg/m2; days 1, 4) were given in combination with cytarabine on one of two schedules: schedule A (24-hour continuous intravenous infusion, 400 mg/m2/day, days 1–5) or schedule B (2-hour intravenous infusion, 1 g/m2/day, days 1–5). Following dose escalation, enrollment was expanded at the maximum tolerated dose. Of 110 patients enrolled, 108 received treatment. The maximum tolerated dose of vosaroxin was 80 mg/m2 for schedule A (dose-limiting toxicities: grade 3 bowel obstruction and stomatitis) and was not reached for schedule B (recommended phase 2 dose: 90 mg/m2). In the efficacy population (all patients in first relapse or with primary refractory disease treated with vosaroxin 80–90 mg/m2; n=69), the complete remission rate was 25% and the complete remission/complete remission with incomplete blood count recovery rate was 28%. The 30-day all-cause mortality rate was 2.5% among all patients treated at a dose of 80–90 mg/m2. Based upon these results, a phase 3 trial of vosaroxin plus cytarabine was initiated in patients with relapsed/refractory acute myeloid leukemia.
Introduction
Outcomes for patients with relapsed or refractory acute myeloid leukemia (AML) are generally poor, with such patients having a median survival of less than 1 year.1–4 Multiple single-agent and combination induction therapies show clinical activity in this setting; however, there is no consensus on a standard-of-care regimen. Although therapies targeting specific acquired genetic mutations and defects are in development, broadly cytotoxic chemotherapeutic approaches continue to be a primary treatment modality for patients with relapsed/refractory AML. Several cytarabine-based combination induction regimens have been investigated in the relapsed/refractory setting, often incorporating topoisomerase II inhibitors, such as anthracyclines (daunorubicin, idarubicin), anthracenediones (mitoxantrone), or epipodophyllotoxins (etoposide). These regimens have demonstrated limited efficacy, due to intrinsic or acquired resistance, and substantial toxicity, particularly in older adults.5–9
Vosaroxin is a first-in-class anticancer quinolone derivative that induces replication-dependent DNA damage by intercalating DNA and inhibiting topoisomerase II, leading to apoptosis.10 In contrast to classic topoisomerase II agents, the anticancer activity of vosaroxin results exclusively from intercalation of DNA and inhibition of topoisomerase II.10 Vosaroxin activity in mammalian cells parallels the activity of quinolone antibiotics in prokaryotes, producing site-selective, double-stranded breaks in G/C-rich sequences that are characteristic of quinolone-induced DNA cleavage.10 Importantly, due to the stability of the quinolone backbone, vosaroxin does not produce significant free radicals,11 or the reactive oxygen species implicated in the cumulative cardiotoxicity seen with anthracyclines.10 Additionally, vosaroxin is not a substrate for the P glycoprotein efflux pump, and its activity is independent of p53 family members;11–13 it may, therefore, bypass some mechanisms of chemotherapy resistance. Consistent with this, single-agent activity has been noted in anthracycline-resistant populations,13 including women whose ovarian cancer progressed on liposomal doxorubicin14 and AML patients with refractory/relapsed disease.15 Overall, the characteristics of vosaroxin suggest that it might have a more favorable risk-benefit profile than that of other commonly used agents, such as anthracyclines.
Results from a phase 1b study of single-agent vosaroxin demonstrated an acceptable safety profile and clinical activity in patients with advanced hematologic malignancies, the majority of whom had relapsed/refractory AML.15 Vosaroxin was tolerable on both a weekly schedule [days 1, 8, and 15; maximum tolerated dose (MTD) 72 mg/m2] and a twice-weekly schedule (days 1, 4, 8, and 11; MTD 40 mg/m2). The dose-limiting toxicity (DLT) was stomatitis, and primary non-hematologic toxicities were reversible stomatitis and febrile neutropenia. A complete remission (CR) or CR with incomplete platelet recovery was achieved in five patients (7%), with a median response duration of 3.1 months; an additional 11 patients (15%) achieved a morphologically leukemia-free state (bone marrow blasts < 5%).
Preclinical data suggest that vosaroxin may be combined with other DNA-damaging agents, such as nucleoside analogs, that have different primary mechanisms of action. The combination of vosaroxin with cytarabine produced synergistic cytotoxic effects in human leukemia cell lines and primary AML blasts.12,16 Similarly, in vivo studies demonstrated that vosaroxin and cytarabine were superadditive in ablating bone marrow in normal mice.16 Although preclinical results suggest that this combination may have synergistic antiproliferative effects, an appropriate dosing regimen in humans has not been investigated.
To this end, we conducted an open-label, phase 1b/2 study (NCT00541866) to assess the safety and tolerability of vosaroxin in combination with cytarabine given either as a continuous intravenous infusion or as a short daily infusion in patients with relapsed or refractory AML. This study included a dose-escalation and an expansion phase. In addition, antileukemic activity, pharmacokinetic profile, and potential biomarkers of vosaroxin activity were assessed.
Methods
Further details on the Methods are provided in the Online Supplementary Appendix.
Patients
Patients ≥18 years with advanced relapsed or refractory, de novo or secondary AML were eligible. For the dose-escalation phase, one to three prior induction regimens for AML (including cytarabine) were allowed. Consolidation cycles were not limited. For the expansion phase, eligible patients were in first relapse (had received ≤2 prior induction cycles and achieved first CR lasting ≥3 months) or had primary refractory disease (no CR or CR lasting ≤3 months following ≤2 induction cycles). Study site institutional review boards approved the study protocol (University of South Florida IRB protocol # 106124c). Patients provided informed consent in accordance with the principles of the Declaration of Helsinki.
Study design
Vosaroxin was administered on a day 1, 4 dosing schedule, based on the safety and efficacy profile demonstrated with the twice-weekly schedule in the single-agent phase 1b trial.15 The day 1, 4 regimen was chosen to maximize vosaroxin exposure while accelerating completion of treatment. Patients were enrolled sequentially in successive cohorts to either schedule A (vosaroxin 10–90 mg/m2, 10-min infusion on days 1 and 4 plus cytarabine 400 mg/m2/day, 24-h continuous intravenous infusion on days 1–5) or schedule B (vosaroxin 70–90 mg/m2 10-min infusion on days 1 and 4 plus cytarabine 1 g/m2/day, 2-h intravenous infusion on days 1–5). Patients were enrolled in the dose-escalation phase using a standard 3 + 3 design. Patients with stable disease or reduction in bone marrow blasts without persistent clinically significant toxicity were eligible for one reinduction cycle. Patients with CR or CR with incomplete blood count recovery (CRi) and absolute neutrophil count ≥500 cells/mL were eligible for up to two consolidation cycles.
DLT was evaluated through induction to day 29 or initiation of a second induction cycle, whichever occurred first. The MTD was defined as the highest dose at which none or one of six patients experienced a DLT.
Safety and efficacy assessments
The safety population comprised all patients who received the study drug. The efficacy population included patients with first relapsed or primary refractory AML treated in either schedule at the MTD or recommended phase 2 dose in either study phase. Adverse events were graded by National Cancer Institute Common Terminology Criteria for Adverse Events (v 3.0). Grade 4 neutropenia or thrombocytopenia lasting >8 weeks without residual leukemia was considered a DLT. Responses were assessed using International Working Group criteria.17 Primary endpoints were DLT and determination of MTD in the dose-escalation phase, and combined CR rate (CR + CRi) in the escalation phase.
Pharmacokinetic and pharmacodynamic studies
Plasma vosaroxin concentration–time data were used to determine standard pharmacokinetic parameters and to assess dose proportionality and vosaroxin accumulation. Peripheral blood mononuclear cells were isolated from patients’ blood samples, and levels of pDNA-PKcs and pCHK2 were investigated by western blot analysis.
Statistics
The study was powered to test the null hypothesis that the probability of remission (CR) is ≤0.20. In order to reject the null hypothesis, at least six remissions (CR) had to be observed among 15 patients, or nine remissions among 25 patients, with a one-sided significance level of approximately 0.06 or 0.05, respectively. If the true remission probability was 0.40, the power to reject the null hypothesis was approximately 60% in 15 patients and 73% in 25 patients. The probability of observing at least one adverse event occurring with an underlying frequency of 10% was approximately 78% in 15 patients and 93% in 25 patients.
Results
Disposition, demographics, and baseline characteristics
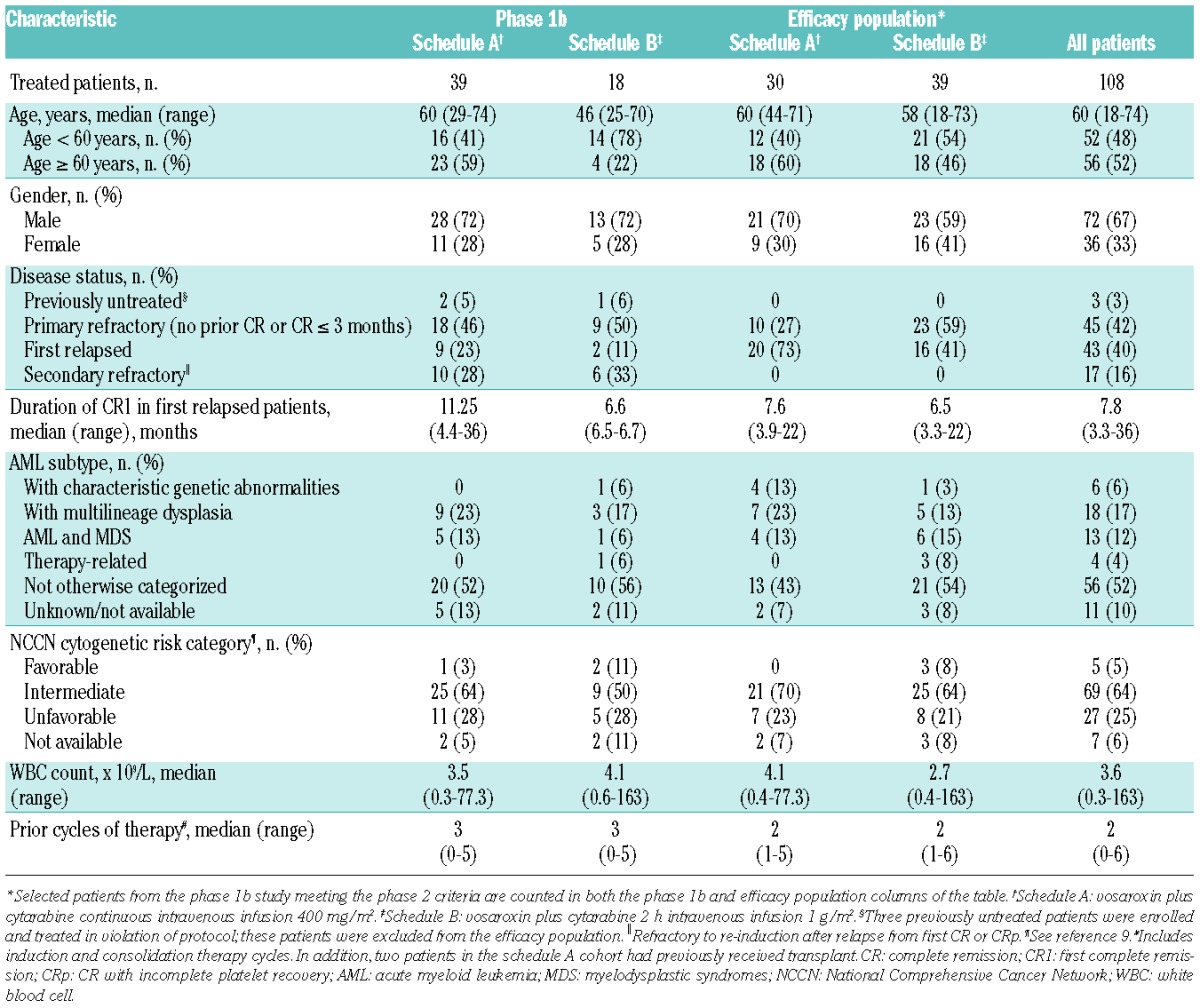
In total, 110 patients were enrolled in the study and 108 received at least one dose of vosaroxin and/or cytarabine, 56 on schedule A and 52 on schedule B (Figure 1). Three treated patients who were enrolled in the phase 1 part of the study did not meet the pre-specified eligibility criteria (they had had no prior treatment for AML); these patients were excluded from the efficacy population, but are included in the safety analyses. Eighty-six patients (78%) received one cycle of study treatment, 17 patients (16%) received two cycles, and five patients (5%) received three cycles. The most common reasons for discontinuation of study treatment were treatment failure (including persistent or recurrent leukemia; 61%) and death (13%). The demographics and baseline characteristics of treated patients are shown in Table 1.
Figure 1.
Patients’ disposition.
Table 1.
Baseline patient and disease characteristics.
Dose-limiting toxicities and maximum tolerated dose
In schedule A, no DLT were observed at doses from 10 to 50 mg/m2; one of seven patients experienced a DLT at 70 mg/m2 (fatal sepsis), one of eight patients experienced a DLT at 80 mg/m2 (grade 3 stomatitis), and two of seven patients experienced a DLT at 90 mg/m2 (grade 3 bowel obstruction and grade 3 stomatitis lasting >7 days). The DLT are summarized in Online Supplementary Table S1. The MTD for schedule A was determined to be vosaroxin 80 mg/m2, days 1 and 4, with cytarabine 400 mg/m2/day by continuous intravenous infusion; the MTD was the recommended phase 2 dose.
In schedule B, no DLT were observed at 70 mg/m2; one of six patients experienced a DLT at 80 mg/m2 (grade 3 odynophagia) and one of six patients experienced a DLT at 90 mg/m2 (grade 3 stomatitis/esophagitis lasting >7 days). The MTD was not reached for schedule B; the highest tested dose, vosaroxin 90 mg/m2, was selected as the recommended phase 2 dose based on rates of remission and vosaroxin plasma concentration-time data.
Safety
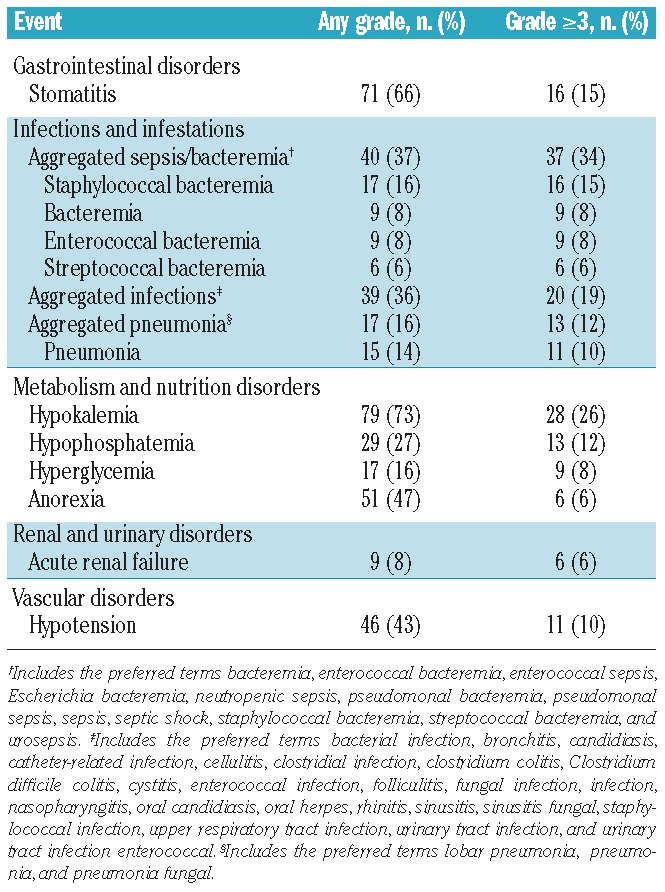
The most common treatment-emergent non-hematologic adverse events of any grade were diarrhea (76%), hypokalemia (73%), nausea (67%), and stomatitis (66%). Grade 3 or 4 non-hematologic adverse events are listed in Table 2. Three patients (3%; all in schedule A) discontinued study treatment due to adverse events, consisting of sepsis in one patient; supraventricular tachycardia, diffuse erythematous rash, and acute renal failure in one patient; and febrile neutropenia and hypotension in one patient. Serious adverse events occurred in 50 patients (46%); the most common serious adverse events included infections such as bacteremia, pneumonia, and sepsis (30%); febrile neutropenia (8%); and stomatitis (4%).
Table 2.
Treatment-emergent non-hematologic adverse events occurring as grade 3 or higher in ≥5% of patients.
At the time of last follow-up, 94 of 108 treated patients had died. Most deaths (81%; n = 76) were due to progressive disease. Among patients who died of causes other than progressive disease, seven died as a result of adverse events that occurred on study, including sepsis (n = 3), and sepsis/multiorgan failure, pneumonia, cardiac arrest, and acute respiratory distress syndrome (n = 1 each). The 30-day all-cause mortality rate was 9.3% (10/108) overall and 2.5% (2/78) for patients treated at the MTD or recommended phase 2 dose; the 60-day all-cause mortality rate was 14.8% (16/108) and 9.0% (7/78), respectively. Comparing schedule A versus schedule B, 30-day mortality was 16% versus 2%, respectively (P=0.0168), and 60-day mortality was 21% versus 8% (P=0.0585).
In the overall population, the median time to recovery of absolute neutrophil count (to >1.0×109/L) in patients who responded to treatment (CR or CRi) was 36 days (range, 20–83 days) and the median time to recovery of platelet count (to >100×109/L) was 34 days (range, 21–49 days). Median recovery times were similar in the efficacy population.
Efficacy
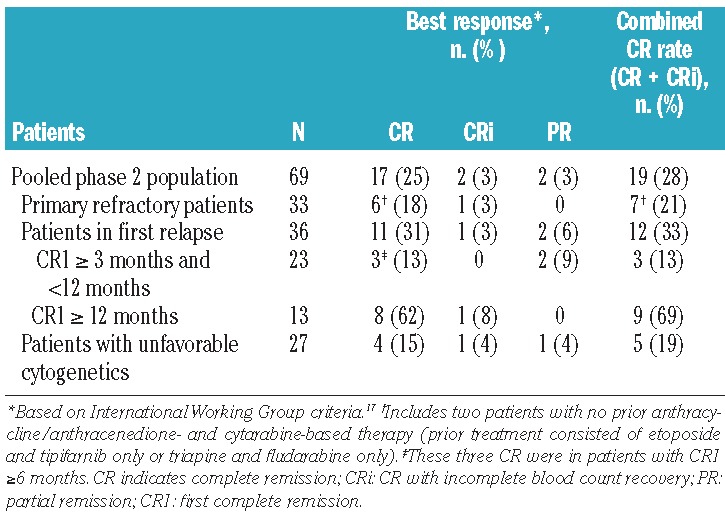
The efficacy population consisted of 69 patients; 30 patients (majority in first relapse) were treated on schedule A and 39 patients (majority with primary refractory disease) were treated on schedule B. In this pooled set of patients, the CR rate was 25% [schedule A: 30% (n = 9); schedule B: 21% (n = 8)], and the combined CR rate (CR + CRi) was 28% [schedule A: 33% (n = 10); schedule B: 23% (n = 9)] (Table 3). Overall, responses were observed with both cytarabine schedules and in both primary refractory patients and patients in first relapse (Table 3). The combined response rate was higher in patients whose first CR lasted ≥12 months (69%) than in patients who experienced early relapse (first CR >3 months and <12 months) (13%) or who had primary refractory AML (21%). All three patients with early relapse who achieved CR had a first CR ≥6 months.
Table 3.
Response to treatment with vosaroxin plus cytarabine in patients with AML (n = 69).
Among the 108 patients treated with vosaroxin in either schedule across all doses, the CR rate was 22% (n = 24) and the combined remission rate (CR + CRi) was 26% (n = 28). Responses were observed at 20 to 90 mg/m2 in schedule A and at 80 to 90 mg/m2 in schedule B (doses below 70 mg/m2 were not studied in schedule B). Responses were observed across all subtypes of AML (response by AML subtype is presented in Online Supplementary Table S2).
In patients from the efficacy population (n = 69) who achieved CR or CRi (n = 19), the median leukemia-free survival was 25.2 months [95% confidence interval (CI), 5.3 months – not reached] (Figure 2A). The median overall survival was 6.9 months (95% CI, 4.3–10.1 months), with similar survival in patients in first relapse and in those with primary refractory disease (Figure 2B). Eighteen patients (26%) underwent hematopoietic stem cell transplantation; 15 of these patients had achieved remission with the study treatment (12 CR, one CRi, two partial remissions).
Figure 2.
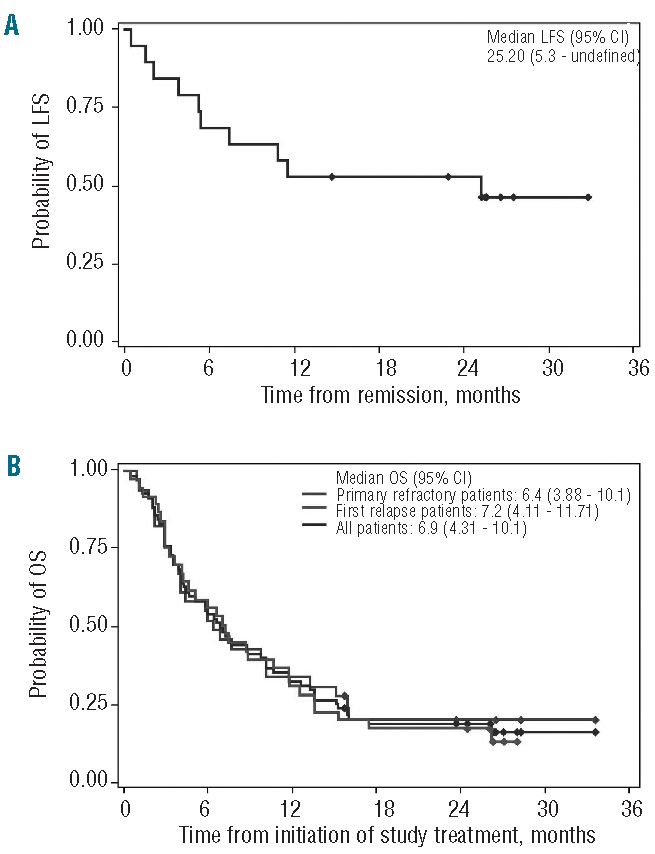
Leukemia-free survival (LFS) and overall survival (OS) in patients with primary refractory and first relapsed AML (efficacy population). (A) LFS in patients with complete response (CR) or CR with incomplete blood count recovery (n = 19); patients were not censored for subsequent therapies such as maintenance therapy or transplantation. (B) OS in the efficacy population (n = 69).
Pharmacokinetic results
Pharmacokinetic profiles for vosaroxin were evaluated in 98 patients in cycle 1. Pharmacokinetic parameters by schedule, dosing day, and dose cohort are presented in Online Supplementary Table S3. After a single, short intravenous infusion (schedule A and schedule B, cycle 1 day 1), plasma vosaroxin concentrations declined in a biphasic manner with a short initial distribution phase followed by a prolonged elimination phase. The average terminal half-life in plasma was approximately 24 h, total body clearance was 4 L/h, and volume of distribution at steady state was 123 L. The drug accumulation ratio on day 4 was approximately 1.2. Vosaroxin exposure (AUC) increased proportionally over doses from 10 to 90 mg/m2, suggesting linear kinetics. At the MTD/recommended phase 2 dose, high vosaroxin plasma concentrations were sustained for prolonged periods (Online Supplementary Figure S1). Concentrations equal to the in vitro EC50 and EC90 concentrations (as assessed in the MV4-11 Flt3ITD AML cell line) were maintained in plasma for approximately 7 days and 3 days, respectively. No statistically significant difference in clearance was noted between males and females (P=0.44). Clearance was lower in patients aged ≥65 years than in those <65 years, with the difference approaching statistical significance (median 3.52 L/h versus 4.18 L/h, P=0.054). The average 24 h renal excretion of vosaroxin and its metabolites N desmethylvosaroxin and O desmethylvosaroxin was 4.46% (3.39%, 1.08%, and 0.127%, respectively) of the total dose infused, suggesting that the primary excretion pathway of vosaroxin is non-renal.
Pharmacodynamic results
A DNA damage response consistent with DNA double-stranded breaks was observed in K562 cells (Online Supplementary Figure S2) and primary AML peripheral blood samples (Figure 3A,B) treated with vosaroxin. Increases in pDNA-PKcs or pCHK2 were evident after 2 h of treatment. Increased levels of pDNA-PKcs and pCHK2 were also observed with cytarabine treatment; however, the pharmacodynamic response to cytarabine treatment occurred more slowly, with increases first becoming evident after 24 h of treatment. Clinical evidence of a mechanism-based pharmacodynamic response to vosaroxin was observed in samples from patients treated with vosaroxin plus cytarabine. Increased levels of pDNA-PKcs and/or pCHK2 were detected within 2 h in 15 of 23 (65%) patients evaluated who received vosaroxin doses ≥ 34 mg/m2 (Figure 3C); CR/CRi was achieved in 60% (9/15) of patients with a pharmacodynamic response, compared with 25% (2/8) of patients without a detectable pharmacodynamic response.
Figure 3.
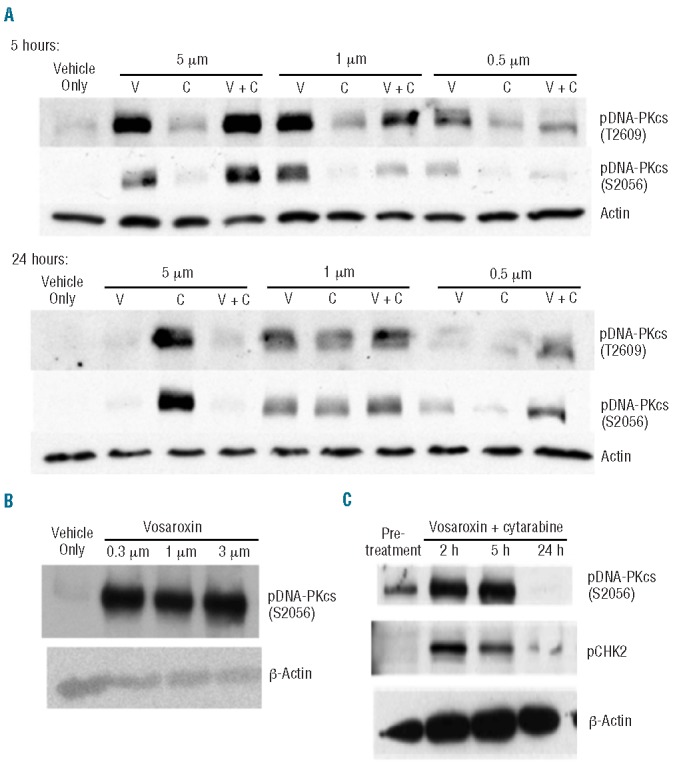
Pharmacodynamic analyses. (A) Peripheral blood mononuclear cells (PBMC) from a patient with acute myeloid leukemia (AML) (80% AML blasts; obtained from AllCells LLC; Emeryville, CA, USA) were treated ex vivo with vosaroxin, cytarabine, both agents combined, or vehicle for 5 or 24 h. Samples were analyzed for the induction of pDNA-PKcs with actin as a loading control. pDNA-PKcs is detectable 5 h after exposure to 0.5 μM vosaroxin, whereas a 24 h exposure is required for a comparable pharmacodynamic response to 1 μM cytarabine. Induction of both pS2056 and pT2609 was observed and pDNA-PKcs S2056 was selected as the pharmacodynamic marker for subsequent analyses. (B) To identify optimal time points for collection of clinical samples, induction of pDNA-PKcs following a 2 h exposure to vosaroxin was examined. PBMC from an AML patient (80% blasts; obtained from AllCells LLC) were treated ex vivo with a dose titration of vosaroxin or vehicle for 2 h. Samples were analyzed for the induction of pDNA-PKcs with actin as a normalizing control. Vosaroxin induced a strong pharmacodynamic response at this time point. (C) Example of changes in pDNA-PKcs and pCHK2 Ievels over time as detected via western blot analysis in PBMC collected from a patient treated with 34 mg/m2 vosaroxin in combination with 400 mg/m2 continuous intravenous cytarabine. Increased levels were observed by 2 h after treatment; this patient achieved a complete remission.
Discussion
Vosaroxin is an anti-cancer quinolone derivative, with a structure and mechanism of action that are well-differentiated from those of the classic topoisomerase II inhibitors (e.g. daunorubicin, idarubicin, mitoxantrone, and etoposide) commonly used in AML treatment in combination with cytarabine. Given the potential advantages of vosaroxin over these agents (lack of formation of free radical and reactive oxygen species, p53-independent activity, and resistance to P-glycoprotein-mediated cellular efflux), it was considered important to test vosaroxin in combination with cytarabine in patients with AML.
In this study, vosaroxin in combination with cytarabine demonstrated antileukemic activity and an acceptable risk-benefit profile in patients with relapsed or refractory AML. Patients were selected for clinical resistance to the combination of cytarabine and anthracyclines or anthracenediones; in addition, they were often older (median age 60 years) and had received as many as six prior cycles of therapy. Furthermore, most patients (89%) had intermediate or unfavorable cytogenetic risk status. In the efficacy population, vosaroxin plus cytarabine produced a combined CR rate of 28% with activity in both the primary refractory and first-relapse populations (combined CR rates of 21% and 33%, respectively). Responses were observed across all AML subtypes; however, meaningful comparisons of response rate between subtypes cannot be made due to the small number of patients with certain subtypes.
Stem cell transplantation is recommended when possible following induction therapy in relapsed AML patients; in this study, 18 patients (26%) subsequently proceeded to allogeneic stem cell transplantation, many of whom achieved CR or CRi. Early mortality was low (2.5% all-cause 30-day mortality in patients treated at the MTD or recommended phase 2 dose) in this heavily treated population. Although this study was not designed to compare mortality rates between schedules, the difference in mortality between schedules A and B supported in part the decision to select schedule B for further evaluation.
Responses were observed over a broad range of vosaroxin doses in both tested schedules. The observed CR rate was lower with schedule B; however, a higher proportion of patients had primary refractory disease, and the median duration of first CR was shorter among patients treated with schedule B, so the ability to compare the cohorts is limited. Vosaroxin doses higher than 90 mg/m2 were not explored, as the rates of remission were encouraging at this dose, and plasma concentrations remained above the in vitro 50% inhibitory concentration16 for over a week.
Other investigational drugs in the refractory/relapsed setting have produced higher response rates in combination with cytarabine than those observed in the present study but were associated with increased short-term mortality. For example, in a phase 3 placebo-controlled trial investigating laromustine plus high-dose cytarabine (HDAC) compared with HDAC alone,18 HDAC and laromustine induced more CR than did HDAC and placebo (35% versus 19%; P=0.005). However, the study was halted due to higher mortality in the laromustine group (30-day mortality rate of 11% versus 2% in the HDAC/placebo group; P=0.016), which compromised any potential survival advantage from achievement of CR. Similarly, in a phase 3, placebo-controlled trial comparing clofarabine plus cytarabine versus cytarabine alone, the CR rate was improved with clofarabine (35% versus 18%; P<0.01) but the 30 day mortality was higher in the clofarabine arm, and overall survival was not improved.19 These studies demonstrate that safety and tolerability are as important as antileukemic activity for successful treatment of relapsed/refractory AML.
The toxicity observed with vosaroxin plus cytarabine in this study was acceptable and generally manageable, with few clinically significant cardiac, respiratory, renal, and hepatic adverse effects. An increased risk of infection due to myelosuppression is expected in this population, and treating clinicians were accustomed to monitoring patients and providing supportive care, as appropriate. Grade 3 or 4 stomatitis was a relatively common adverse event, but did not lead to treatment discontinuation in any patient. Both dosing schedules were found to be acceptable from a tolerability/feasibility standpoint; however, stomatitis was noted to be less frequent with short infusions than with continuous intravenous administration of cytarabine.
With both dosing schedules, the pharmacokinetic profile described for vosaroxin was comparable to that observed with single-agent vosaroxin in advanced hematologic malignancies,15 suggesting that the pharmacokinetics of vosaroxin is unaffected by co-administration of cytarabine. In pharmacodynamic analyses, mechanism-based activation of a DNA damage response consistent with double-stranded DNA breaks was observed in peripheral blood mononuclear cells from patients treated with vosaroxin doses of 34 mg/m2 or higher; the CR rate was higher among pharmacodynamic responders, but establishment of a relationship between DNA damage response and clinical activity requires study in a larger number of patients. While not performed in this study, it may be of interest in future studies to stratify patients by extent and/or intensity of prior cytarabine exposure to assess the impact, if any, on response to vosaroxin.
The results of the present study, together with considerations of clinical practicality, provided the rationale for selecting the dose regimen of vosaroxin 90 mg/m2 as a 10-min infusion on days 1 and 4, plus cytarabine 1 g/m2 as a 2-h intravenous infusion on days 1–5 as the recommended dose for further study in a phase 3 setting. Based on the clinical activity of the combination regimen in this study, as well as previous studies of vosaroxin in patients with relapsed/refractory AML, a multinational, randomized, double-blind, placebo-controlled, phase 3 study in first-relapsed or refractory AML (the VALOR trial) was initiated20 (NCT01191801).
In summary, these results support further investigation of vosaroxin combined with cytarabine for the treatment of patients with relapsed or refractory AML. The combination has clinically significant activity in patients previously treated with other topoisomerase II inhibitors, and is well tolerated with low rates of early mortality and organ toxicity.
Acknowledgments
The authors would like to thank Jennifer Smith for statistical input, Dan Combs for performing pharmacokinetic analyses, Andy Conroy and Nguyen Tan for conducting pharmacodynamic analyses, and Craig Berman, MD, for his role as Medical Monitor. This study was sponsored by Sunesis Pharmaceuticals, Inc.
The authors would like to thank Janis Leonoudakis, PhD, of Powered 4 Significance LLC for medical writing assistance. Funding for manuscript development was provided by Sunesis Pharmaceuticals, Inc.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23(9):1969–1978. [DOI] [PubMed] [Google Scholar]
- 2.Keating MJ, Kantarjian H, Smith TL, et al. Response to salvage therapy and survival after relapse in acute myelogenous leukemia. J Clin Oncol. 1989;7(8):1071–1080. [DOI] [PubMed] [Google Scholar]
- 3.Leopold LH, Willemze R. The treatment of acute myeloid leukemia in first relapse: a comprehensive review of the literature. Leuk Lymphoma. 2002;43(9):1715–1727. [DOI] [PubMed] [Google Scholar]
- 4.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–1062. [DOI] [PubMed] [Google Scholar]
- 5.Estey EH, Kantarjian HM. Therapy for acute myeloid leukemia. In: Hoffman R, Benz EJ, Jr, Shattil S, et al., eds. Hematology: Basic Principles and Practice. Philadelphia: Churchill Livingstone; 2004:1099–1120. [Google Scholar]
- 6.Eisenbeis CF, Larson RA. Chemotherapy of acute leukemias in adults. In: Perry MC, ed. The Chemotherapy Source Book. Philadelphia: Lippincott Williams and Wilkins; 2001:824–838. [Google Scholar]
- 7.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106(4):1154–1163. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: acute myeloid leukemia. v2.2012. Available at: http://www.nccn.org/professionals/physician_gls/pdf/aml.pdf Accessed August 16, 2012.
- 9.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331(14):896–903. [DOI] [PubMed] [Google Scholar]
- 10.Hawtin RE, Stockett DE, Byl JA, et al. Voreloxin is an anticancer quinolone derivative that intercalates DNA and poisons topoisomerase II. PLoS One. 2010;5(4): e10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evanchik MJ, Allen D, Yoburn JC, Silverman JA, Hoch U. Metabolism of (+)-1,4-dihydro-7-(trans-3-methoxy-4-methylamino-1-pyrrolidinyl)-4-oxo-1-(2-thiaz olyl)-1,8-naphthyridine-3-carboxylic acid (voreloxin; formerly SNS-595), a novel replication-dependent DNA-damaging agent. Drug Metab Dispos. 2009;37(3):594–601. [DOI] [PubMed] [Google Scholar]
- 12.Walsby EJ, Coles SJ, Knapper S, Burnett AK. The topoisomerase II inhibitor voreloxin causes cell cycle arrest and apoptosis in myeloid leukemia cells and acts in synergy with cytarabine. Haematologica. 2011;96(3): 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoch U, Lynch J, Sato Y, et al. Voreloxin, formerly SNS-595, has potent activity against a broad panel of cancer cell lines and in vivo tumor models. Cancer Chemother Pharmacol. 2009;64(1):53–65. [DOI] [PubMed] [Google Scholar]
- 14.Hirte HW, McGuire WP, III, Edwards RP, et al. Final results of a phase II study of voreloxin in women with platinum-resistant ovarian cancer. J Clin Oncol. 2010;28(15s): (suppl; abstr 5002). [Google Scholar]
- 15.Lancet JE, Ravandi F, Ricklis RM, et al. A phase Ib study of vosaroxin, an anticancer quinolone derivative, in patients with relapsed or refractory acute leukemia. Leukemia. 2011;25(12):1808–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scatena CD, Kumer JL, Arbitrario JP, et al. Voreloxin, a first-in-class anticancer quinolone derivative, acts synergistically with cytarabine in vitro and induces bone marrow aplasia in vivo. Cancer Chemother Pharmacol. 2010;66(5):881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003; 21(24):4642–4649. [DOI] [PubMed] [Google Scholar]
- 18.Giles F, Vey N, DeAngelo D, et al. Phase 3 randomized, placebo-controlled, double-blind study of high-dose continuous infusion cytarabine alone or with laromustine (VNP40101M) in patients with acute myeloid leukemia in first relapse. Blood. 2009;114(19):4027–4033. [DOI] [PubMed] [Google Scholar]
- 19.Faderl S, Wetzler M, Rizzieri D, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol. 2012;30(20):2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta CR, Michelson G, Salganik M, et al. Adaptive design of VALOR, a phase III trial of vosaroxin or placebo in combination with cytarabine for patients with first relapsed or refractory acute myeloid leukemia [abstract]. J Clin Oncol. 2011;29(suppl): Abstract TPS201. [Google Scholar]



